Cytokine gene-modified tumor cells have increased immunogenicity and retain the antigenic repertoire of a particular neoplasia. However, practical concerns have led to an increased interest in allogeneic gene-transduced bystander cells as a broader source of cytokines for autologous tumor cell–based vaccines. Here, we show that allogeneic B78H1 major histocompatibility complex (MHC) class I–negative and –positive (H-2Kb– and Db-transfected) cells induced cytotoxic T lymphocytes (CTLs) and protection in BALB/c mice at comparable levels in response to a challenge with C26 (H-2d) colon carcinoma cells sharing the tumor-associated antigen envelope glycoprotein 70 (env-gp70) with both cell lines. Class I–negative B78H1 cells transduced to express interleukin-12 (IL-12) and mixed with autologous A20 tumor cells led to eradication of preestablished A20 lymphoma in 50% or 100% of treated mice after 3 or 4 vaccinations, respectively, whereas A20 cells alone or mixed with nontransduced B78H1 cured none or 50% of mice after 3 or 4 vaccinations, respectively. Immunization with the IL-12–producing bystander cell line increased tumor-specific proliferation and type 1 cytokine production by CD4+ T cells. By contrast, CD4 T-cell function appeared impaired after immunization with A20 cells alone or mixed with B78H1 cells. Indeed, only CD4+ T cells from IL-12–treated mice could be restimulated with anti-OX40 monoclonal antibody (mAb) in place of a fourth cellular boost. Moreover, the IL-12–based tumor vaccine induced expansion of tumor-specific interferon-γ (IFN-γ)–producing CD8+ T cells. These results are clinically relevant for the development of feasible IL-12 cancer vaccines based on engineered class I–negative bystander cells.
Introduction
Therapeutic interventions based on conventional or, more frequently, high-dose chemotherapy have significantly improved partial and complete remission rates, especially for hematologic malignancies such as chronic and acute leukemia. However, many patients have minimal residual disease, which is often resistant to further pharmacologic treatments and ultimately leads to disease relapse and progression. Novel approaches are being investigated to improve the clinical outcome of these patients, and treatments with high compliance such as immunotherapy are desirable. Attempts to effectively prime and sustain antitumor immunity have recently provided promising preclinical and clinical results.1
The phenomenon of graft versus leukemia that follows allogeneic bone marrow transplantation provides the best evidence that the immune system has the capacity to eradicate leukemia.2Alloreactive T cells appear to be the most important mediators of the graft versus leukemia in humans,3 but a more restricted antileukemia immune response is well documented in murine leukemia models.4 Specific leukemia-associated antigens including PRAME, Wilms tumor gene, and proteinase 3, have been identified in humans,5-7 but their relevance as leukemia rejection antigens is not clear. In that context, tumor vaccination approaches based on whole tumor cells are attractive, because they provide the entire antigenic repertoire of that neoplasm.
Transduction of genes encoding cytokines or costimulatory molecules can greatly improve the immunogenicity of tumor cells intended for vaccine use. Gene-modified tumor vaccines can alter the microenvironment of the vaccination site such that tumor cell–mediated immunosuppression is countered and critical stimuli are provided for activation of an effective antitumor immune response. However, translation of this simple approach to the clinical setting is hampered by complex technical and practical obstacles concerning the choice of transducing vector or the use of autologous versus allogeneic tumor cells. A fully autologous approach may be limited by the availability of tumor cell lines and by the need to genetically modify tumor cells from every patient, a costly and labor-intensive task. A possible alternative is the use of allogeneic cell lines, which were initially used as a general stimulator of the immune response and then as source of shared antigens recognized by T or B lymphocytes.8,9 Recently, allogeneic granulocyte-macrophage colony-stimulating factor (GM-CSF) tumor cell vaccine has been studied in a phase 1 trial against pancreatic cancer. The vaccine effect was evaluated for delayed-type hypersensitivity response against autologous tumors.10Moreover, melanoma-specific CD8+ T cells can be activated by dendritic cells (DCs) loaded with killed allogeneic cell lines sharing melanoma-associated tumor antigens.11 A third formulation of a tumor cell–based vaccine utilizes autologous tumor cells mixed with genetically modified bystander cells, which can be fibroblast- or tumor-derived. A bystander cell–based approach is readily applicable to leukemia, because the target cells are abundant and easily accessible in the bloodstream or bone marrow. Although human fibroblasts obtained from cancer patients have been used in clinical trials,12,13 a universal gene-modified bystander tumor cell line admixed with leukemia cells is likely the easiest approach in patients.14
Gene transfer of DNA encoding an allogeneic major histocompatibility complex (MHC) class I protein has been reported to stimulate an effective antitumor immune response in melanoma patients15; however, the precise role of alloreactivity in the induction of antitumor immunity has not been extensively investigated. In particular, little is known about the effect of allogeneic MHC class I molecules expressed by immunizing tumor cells on the activation of tumor-specific cytotoxic T lymphocytes (CTLs) and on the induction of a protective antitumor immunity.
Among the cytokine genes known to enhance tumor-cell vaccine immunogenicity, interleukin-12 (IL-12) is likely the most potent.16,17 IL-12 is a heterodimeric cytokine produced by antigen-presenting cells (APCs) that bridges innate and adaptive immunity. IL-12 exerts its biologic effects on T cells by mediating the differentiation of CD4+ T lymphocytes into interferon-γ (IFN-γ)–secreting cells and of CD8+ cells into CTLs. Moreover, IL-12 enhances the cytolytic activity of natural killer (NK) cells and induces IFN-γ production by macrophages. IL-12 has been used successfully in the treatment of murine tumors,17,18and its combination with allogeneic tumor cell vaccination has been reported to completely prevent tumor development in a murine model of spontaneous mammary carcinoma.19 However, because the systemic administration of recombinant IL-12 in cancer patients has been associated with a significant toxicity,20 therapeutic strategies based on local delivery of IL-12 are still considered.
In the present study, we tested (1) the effect of MHC class I alloreactivity on recognition and rejection of bystander tumor cells and (2) the therapeutic value of bystander cells transduced to express IL-12 or not transduced and admixed with irradiated A20 lymphoma cells in treating disseminated autologous tumors.
Materials and methods
Mice
Eight- to 10-week-old BALB/c mice were obtained from Charles River (Calco, Italy) and maintained at the Istituto Nazionale Tumori under standard conditions according to institutional guidelines.
Tumor cells
B78H1 is a MHC class I–negative, C57BL/6-derived amelanotic clone of B16 melanoma.21 C26 is a murine colon adenocarcinoma cell line derived from BALB/c mice treated withN-nitroso-N-methylurethane.22 Murine B78H1 and C26 tumor cells were cultured in Dulbecco modified Eagle medium (DMEM) (GIBCO, Paisley, Great Britain) supplemented with 10% fetal calf serum (FCS) (GIBCO), penicillin/streptomycin (50 U/mL),l-glutamine (2 mM), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (Whittaker Bioproducts, Walkersville, MD), and nonessential amino acids (Whittaker Bioproducts) and grown at 37°C in a 5% CO2 atmosphere. BALB/c-derived A20 B-cell lymphoma was obtained from the American Type Culture Collection (Rockville, MD) and maintained in RPMI 1640 (Whittaker Bioproducts) supplemented with 10% FCS (GIBCO), penicillin/streptomycin (50 U/mL),l-glutamine (2 mM), HEPES buffer (Whittaker Bioproducts), nonessential amino acids (Whittaker Bioproducts), and 2-mercaptoethanol (50 mM).
Gene transduction
B78H1 cells were transduced with the polycistronic Lp40Ip35 SN retroviral vector encoding both p35 and p40 murine IL-12 subunits as described.23 Single G418-resistant colonies were isolated, expanded into cell lines, and injected into mice or subjected to further analysis. Murine IL-12 concentration was determined by a 2-site sandwich enzyme-linked immunosorbent assay (ELISA) using monoclonal antibody (mAb) 9A5 to detect p70 and peroxidase-conjugated mAb 5C3-POD to detect p40 (kindly provided by Dr Luciano Adorini, Roche Milano Ricerche, Milan, Italy). G418-resistant B78H1 cells transfected with LXSN vector alone were used as controls, designated hereafter as B78H1 cells.
An H-2b–negative B78H1 cell line transfected with H-2Kb and H-2Db genes24 was kindly provided by Dr Pierluigi Lollini, Bologna, Italy.
Vaccinations
On day 0, A20 cells were harvested from in vitro culture and washed twice in saline solution and injected intravenously (105 cells in a total volume of 0.4 mL) into the tail vein of the mice. On days 1, 5, 15, and 30 after tumor challenge, mice were vaccinated subcutaneously in the left flank with either 106A20 cells alone or mixed with 106 B78H1 bystander cells genetically modified to produce different amounts of IL-12. In some experiments, the fourth immunization with cellular vaccine was replaced by intraperitoneal administration of 200 μg anti-OX40 mAb, produced by the hybridoma OX86 (European Collection of Cell Cultures, Wiltshire, United Kingdom). In selected experiments, bystander MHC class I–negative B78H1 cells were compared with a MHC class I–positive B78H1 variant obtained by transduction with H-2b molecules. Before injection, A20 and bystander cells used for vaccination were irradiated with 15 000 and 5000 rad (150 to 50 Gy), respectively. Mice were monitored twice weekly and killed after the development of tumor.
Mixed lymphocyte tumor culture (MLTC) and cell-mediated lympholysis (CML) assays
MLTC was performed in RPMI 1640 supplemented with 10% FCS (Hyclone, Logan, UT). To test CTL induction against tumor-associated antigen (TAA) gp70, mice were injected once into the right footpad with 5 × 106 irradiated class I–negative or –positive B78H1 cells. After 5 days, lymphocytes from popliteal lymph nodes were collected and used as responder cells. Because C26 and B78H1 cells share the TAA murine leukemia virus envelope glycoprotein 70 (env-gp70), previously irradiated C26 cells were used as stimulators. In some experiments, responder cells were stimulated with AH1, the synthetic gp70-derived peptide 423-431 (SPSYVYHQF).25 In assays of tumor-specific CTL generation after bystander cell–based vaccination, splenocytes obtained from immunized or nonimmunized mice were used as responders cells and irradiated A20 cells as stimulators.
Responders and stimulators were suspended to 2.5 × 105and 2.5 × 104 cells per milliliter, respectively, in culture medium supplemented with IL-2 (20 U/mL) and mixed in a total volume of 2 mL in 24-well plates (Costar, Cambridge, MA). Cultures were incubated for 5 days in a humidified atmosphere of 5% CO2in air. At the end of the culture period, viable and activated lymphoid cells were counted using the trypan blue exclusion method and used as effectors in a standard 4-hour 51chromium (51Cr) release assay against target cells.
Cytokine release
CD4+ T cells were purified from splenocytes on a MiniMacs high-gradient magnetic separation column (Myltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer's instructions. Purity of the cell fractions was further analyzed by flow cytometry of a gated population set on scatter properties using FACScan equipment (Becton Dickinson, San José, CA). A minimum of 10 000 events was collected in list mode on FACScan software. T cells were mixed in triplicate with irradiated A20 tumor cells to 5 × 104 and 12.5 × 103 cells per well, respectively, in flat-bottom 96-well plates (Costar). As a negative control, CD4+ T cells were cultured in medium alone. Supernatants were collected 5 days later, and IL-4, IL-5, IL-10, GM-CSF, IL-2, and IFN-γ production was measured by ELISA using matched pairs of mAbs (Pharmingen, San Diego, CA).
Proliferation assay
Highly purified CD4+ T cells (5 × 104per well) from the different experimental groups were cocultured with irradiated A20 cells (12.5 × 103 per well) in triplicate U-bottom 96-well plates. Expression of I-Ad by A20 cells was confirmed by fluorescence-activated cell sorter (FACS) analysis. Cells were pulsed with [3H]thymidine (1 μCi [0.037 MBq] per well) (Amersham Pharmacia Biotech, Piscataway, NJ) after 4 days and harvested 18 hours later with a Tomtec cell harvester. [3H]thymidine incorporation was measured as counts per minute (cpm) on a Wallac 1205 β-counter.
ELISPOT assay for IFN-γ release
To detect tumor-specific IFN-γ–releasing effector cells in the different experimental groups, an enzyme-linked immunospot (ELISPOT) was performed. CD4− spleen cells obtained from CD4+ T-cell purification (see “Cytokine release”) were stimulated with anti-CD3 mAb (10 μg/mL) coated on the plates. After overnight incubation, collected cells were added (105 cells per well) to plates precoated with 10 μg/mL of a primary anti–IFN-γ mAb (Endogen, Woburn, MA) in the presence or absence of irradiated A20 cells (104cells per well). After 18 hours, wells were extensively washed and incubated with biotinylated second mAb to IFN-γ (Endogen) (1μg/mL) for 2 hours, washed again, and stained with AEC staining kit (Sigma, St Louis, MO). A more than 2-fold increase over the number of spots induced by medium alone was considered an enhanced response.
Statistical analysis
Results are expressed as mean ± SD. Where indicated, differences were compared using the Student t test.
Results
MHC class I molecules on allogeneic tumor cells do not change induction of CTLs against TAA or protection from tumor challenge
To test whether allogeneic class I expression by immunizing tumor cells affects CTL generation against TAA, we immunized BALB/c mice (H-2d) with C57BL/6-derived MHC class I–negative B78H1 and with its class I–positive variant, obtained by H-2b gene transduction. Because B78H1 and the BALB/c colon carcinoma C26 share the env-encoded TAA gp70 (data not shown), lymphocytes from immunized mice were restimulated in vitro with irradiated C26 cells and tested for cytotoxicity against C26 (Figure 1A) or autologous blasts pulsed with the gp-70–derived immunogenic peptide AH1 (Figure 1B). CTLs obtained from mice immunized with class I–positive and –negative tumors lysed specific target cells with equal efficiency. As expected, only class I–positive tumors induced CTL against C57BL/6 (H-2b)–derived blasts (Figure 1C), whereas no lysis against syngeneic BALB/c-derived blasts was observed (Figure 1D). These data demonstrate that in our model, expression of MHC class I molecules by allogeneic tumor cells does not influence induction of CTLs against a TAA.
MHC class I–negative allogeneic cell line induces tumor-specific CTLs as efficiently as its class I–positive counterpart.
Effector cells were obtained from popliteal lymph nodes after footpad immunization of BALB/c mice with allogeneic class I–negative (♦) or class I–positive B78H1 (■) cells, both of which share the TAA env-gp70 with C26 cells. After a 5-day restimulation in vitro with C26 cells (A-B,D) or with class I–positive B78H1 cells (C), lymphocytes were tested in a standard 4-hour 51Cr release assay. Target cells are C26 tumor cells (A), BALB/c-derived blasts previously pulsed (B) or not (D) with AH1 peptide, and C57BL/6-derived blasts (C). Data shown are representative of 3 independent experiments with similar results.
MHC class I–negative allogeneic cell line induces tumor-specific CTLs as efficiently as its class I–positive counterpart.
Effector cells were obtained from popliteal lymph nodes after footpad immunization of BALB/c mice with allogeneic class I–negative (♦) or class I–positive B78H1 (■) cells, both of which share the TAA env-gp70 with C26 cells. After a 5-day restimulation in vitro with C26 cells (A-B,D) or with class I–positive B78H1 cells (C), lymphocytes were tested in a standard 4-hour 51Cr release assay. Target cells are C26 tumor cells (A), BALB/c-derived blasts previously pulsed (B) or not (D) with AH1 peptide, and C57BL/6-derived blasts (C). Data shown are representative of 3 independent experiments with similar results.
To examine the contribution of MHC class I molecules expressed by allogeneic immunizing tumor cells to protective antitumor immunity, BALB/c mice were inoculated subcutaneously 1, 2, or 5 times with irradiated B78H1 or with the class I–positive variant and challenged with live C26 tumor cells. While repeated alloimmunization accelerated rejection of an allogeneic skin graft (data not shown), protection against a challenge with syngeneic C26 tumor cells was progressively reduced (Figure 2). Indeed, the initial difference in protection (Figure 2A) between immunization with MHC class I–positive and –negative cells was lost after the second immunization (Figure 2B), and any residual protection disappeared after 5 rounds of vaccination (Figure 2C). Thus, MHC class I–negative allogeneic cells do not show any apparent disadvantage over their MHC class I–positive counterparts in terms of CTL induction and tumor protection.
Induction of protective antitumor immunity is not affected by MHC class I expression in allogeneic tumor vaccine after repeated immunizations.
BALB/c mice were inoculated subcutaneously with 5 × 106irradiated wild-type (■) or class I–positive B78H1(○) cells and challenged 14 days after the last immunization with 5 × 104 live C26 tumor cells. Number of vaccination rounds is indicated. Control mice were challenged with C26 cells without previous immunization (▪). Ten mice were included in each group.
Induction of protective antitumor immunity is not affected by MHC class I expression in allogeneic tumor vaccine after repeated immunizations.
BALB/c mice were inoculated subcutaneously with 5 × 106irradiated wild-type (■) or class I–positive B78H1(○) cells and challenged 14 days after the last immunization with 5 × 104 live C26 tumor cells. Number of vaccination rounds is indicated. Control mice were challenged with C26 cells without previous immunization (▪). Ten mice were included in each group.
MHC class I molecules expressed by allogeneic bystander cells do not enhance eradication of a preestablished autologous lymphoma
BALB/c mice intravenously injected with live A20 lymphoma cells and subsequently vaccinated 4 times with irradiated A20 cells alone and together with wild-type class I–negative or variant class I–positive B78H1 cells showed no significant differences in tumor-free survival (Figure 3). Thus, MHC class I–negative allogeneic tumors appear to be suitable bystander cells to express cytokine or costimulatory molecules that enhance immunogenicity to autologous tumor cells in a vaccine combination.
Allogeneic MHC class I expression on bystander tumor cells does not improve long-term survival of lymphoma-bearing mice.
On day 0, BALB/c mice were injected intravenously with 105live A20 tumor cells. On days 1, 5, 15, and 30, tumor-bearing mice were subcutaneously vaccinated with irradiated 106 A20 cells alone or together with 106 allogeneic class I–positive or –negative B78H1 cells. A group of tumor-bearing mice was left unvaccinated. Mice were monitored weekly for the development of tumor. Fourteen mice were included in each group.
Allogeneic MHC class I expression on bystander tumor cells does not improve long-term survival of lymphoma-bearing mice.
On day 0, BALB/c mice were injected intravenously with 105live A20 tumor cells. On days 1, 5, 15, and 30, tumor-bearing mice were subcutaneously vaccinated with irradiated 106 A20 cells alone or together with 106 allogeneic class I–positive or –negative B78H1 cells. A group of tumor-bearing mice was left unvaccinated. Mice were monitored weekly for the development of tumor. Fourteen mice were included in each group.
IL-12 gene transduction of bystander cells provides therapeutic efficacy to autologous tumor vaccine
Retroviral vector-mediated transduction of B78H1 cells with IL-12 genes gave rise to clones producing different amounts of IL-12. Three clones were selected and designated IL-12 400, IL-12 16 000, and IL-12 25 000 depending on the amount of IL-12 produced (picograms per milliliter). IL-12 clones show the same morphology as parental cells and do not express MHC class I molecules, as confirmed by FACS analysis (data not shown).
BALB/c mice were injected intravenously with 105 live A20 cells on day 0 and subcutaneously vaccinated with 106irradiated A20 cells combined with 106 B78H1 or B78H1–IL-12 25 000 cells on days 1, 5, and 15. Mice immunized with A20 cells alone or together with B78H1 cells died within 50 days after tumor challenge, as did control nonvaccinated mice. By contrast, vaccination with A20 cells plus B78H1–IL-12 25 000 cells cured 50% of treated mice (Figure 4A). An additional boost at day 30 enhanced therapeutic efficacy by 50% in every vaccinated group (Figure 4B). All mice treated with the combination of A20 and B78H1–IL-12 cells became tumor-free, whereas immunization with A20 cells alone or combined with B78H1 cells resulted in 50% tumor-free survival. To test whether the amount of IL-12 produced by the allogeneic bystander cell line critically influences the induction of protective antitumor immunity, A20 tumor-bearing BALB/c mice were vaccinated on days 1, 5, 15, and 30 with irradiated A20 cells plus IL-12 400, IL-12 16 000, and IL-12 25 000 B78H1 cells. Bystander cells producing IL-12 25 000 and IL-12 16 000 showed a similar therapeutic effect, whereas those producing IL-12 400 did not differ from vector-transduced bystander cells in curing A20-bearing mice (Figure 4C).
Immunization with autologous tumor cells mixed with allogeneic IL-12–producing bystander cells eradicates preestablished syngeneic lymphoma.
A20 tumor-bearing BALB/c mice were vaccinated subcutaneously on days 1, 5, 15 (A-C), and 30 (B-C) after tumor challenge. Irradiated tumor cells used as vaccine are indicated. A group of tumor-bearing mice was left unvaccinated. Tumor-free survival was evaluated over an observation period of 4 months. Fourteen mice were included in each group.
Immunization with autologous tumor cells mixed with allogeneic IL-12–producing bystander cells eradicates preestablished syngeneic lymphoma.
A20 tumor-bearing BALB/c mice were vaccinated subcutaneously on days 1, 5, 15 (A-C), and 30 (B-C) after tumor challenge. Irradiated tumor cells used as vaccine are indicated. A group of tumor-bearing mice was left unvaccinated. Tumor-free survival was evaluated over an observation period of 4 months. Fourteen mice were included in each group.
These data demonstrate that syngeneic wild-type A20 cells combined with IL-12–producing allogeneic cells can cure mice with preestablished A20 lymphoma and that the level of IL-12 available at the vaccination site is critical.
CD4+ T cells are induced to proliferate and to produce a spectrum of cytokines in mice immunized with A20 cells plus allogeneic IL-12–secreting bystander cells
The role of IL-12 produced by the allogeneic class I–negative bystander cells in generating antitumor immunity was evaluated in vitro using lymphocytes collected from hemisplenectomized mice 5 days after the last immunization, and in vivo the same mice were maintained to follow their survival. As described in “Materials and methods,” splenocytes were used to assess T-cell proliferation, CTL induction, and cytokine production. Freshly isolated spleen cells were enriched in CD4+ T cells, and the positive and negative fractions were used to evaluate CD4 and CD8 functions, respectively. FACS analysis indicated purity of CD4+ T-cell fraction at more than 90%, and the negative fraction contained up to 15% CD8+ T cells, with B lymphocytes and monocytes comprising the remaining cells. Purified CD4+ T lymphocytes from mice immunized with A20 cells plus B78H1–IL-12 bystander cells proliferated more efficiently than CD4+ T cells from mice immunized with A20 cells alone or together with B78H1 cells (Figure5) (P = .035). Interestingly, freshly isolated CD4+ T cells from nonimmunized tumor-bearing mice displayed low-level spontaneous proliferation but increased their proliferation rate upon restimulation with irradiated A20 cells in vitro. No response to A20 cell restimulation was observed in mice immunized with A20 cells alone or combined with B78H1 cells, whereas a strong proliferative response was detected in cells of mice immunized with A20 cells plus B78H1–IL-12 cells. These results suggest that systemic A20 lymphoma per se is not capable of inducing CD4+ T-cell anergy. However, anergy is an active process that requires restimulation; repeated immunizations with irradiated A20 cells alone or combined with allogeneic B78H1 cells resulted in a diminished capacity of CD4+ T cells to respond to tumor antigens, a capacity that was partially restored by IL-12 in the tumor vaccine formulation. Moreover, the pattern of cytokine release in response to irradiated A20 cells (Figure6), measured by ELISA, showed that CD4+ T lymphocytes produced IL-4, IL-5, and IL-10 from every vaccinated group independently from vaccine composition, whereas only CD4+ T cells from mice immunized with A20 plus B78H1-IL12 cells produced considerable amounts of IFN-γ, GM-CSF, and IL-2. Thus, immunization with A20 plus B78H1–IL-12 cells induced CD4+ T cells producing type 1 cytokines without down-modulating those secreting type 2 cytokines in response to the autologous tumor.
Autologous tumor cells plus IL-12–secreting bystander cells induced CD4+ T cells to proliferate upon in vitro stimulation with syngeneic tumor.
After 4 immunizations splenocytes from the indicated experimental groups were collected and purified CD4+ T cells were incubated for 5 days in the absence (■) or presence (▪) of irradiated A20 cells. [3H] incorporation is given as counts per minute (cpm) minus values for medium alone. Incorporation of irradiated A20 cells alone was comparable to that in medium. Data are represented as means (+ SDs) of the T-cell cpm per well from 3 mice in each group, analyzed individually.
Autologous tumor cells plus IL-12–secreting bystander cells induced CD4+ T cells to proliferate upon in vitro stimulation with syngeneic tumor.
After 4 immunizations splenocytes from the indicated experimental groups were collected and purified CD4+ T cells were incubated for 5 days in the absence (■) or presence (▪) of irradiated A20 cells. [3H] incorporation is given as counts per minute (cpm) minus values for medium alone. Incorporation of irradiated A20 cells alone was comparable to that in medium. Data are represented as means (+ SDs) of the T-cell cpm per well from 3 mice in each group, analyzed individually.
Immunization with autologous plus IL-12–secreting allogeneic tumor cells induces Th1 and Th2 immune responses.
Highly purified CD4+ T cells from the different experimental groups were evaluated for cytokine release after 5-day in vitro restimulation with irradiated A20 cells. T cells were collected after 4 immunizations. Negligible cytokine production was observed in the presence of irradiated A20 cells alone. Data are given as means (+ SDs) of groups of 3 mice, analyzed individually.
Immunization with autologous plus IL-12–secreting allogeneic tumor cells induces Th1 and Th2 immune responses.
Highly purified CD4+ T cells from the different experimental groups were evaluated for cytokine release after 5-day in vitro restimulation with irradiated A20 cells. T cells were collected after 4 immunizations. Negligible cytokine production was observed in the presence of irradiated A20 cells alone. Data are given as means (+ SDs) of groups of 3 mice, analyzed individually.
IL-12–secreting tumor vaccine in vivo expands tumor-specific CD4− T cells
CD8+ T-cell function was investigated in either freshly isolated or in vitro–stimulated T cells. Freshly isolated CD4− spleen cells were tested by ELISPOT assay for the presence of IFN-γ–producing cells. This technique has the advantage of avoiding potential biases caused by ex vivo expansion, thus more accurately reflecting the in vivo immune response. Cells purified from vaccinated and nonvaccinated mice were incubated overnight in the presence of coated anti-CD3 mAb. In this way, although IFN-γ production by CD4− spleen cells other than CD8+ T cells cannot be completely excluded, the triggering of the T-cell receptor by anti-CD3 is likely to preferentially induce IFN-γ secretion by CD8+ T cells. As shown in Figure7, T cells from mice immunized with A20 cells plus IL-12–secreting bystander cells showed a significantly enhanced response to A20 tumor cells (4-fold increase in number of spots per 105 CD4− cells as compared with nonstimulated cells) (P = .012). Unlike CD4+ T cells, CD8+ cells from the other experimental groups did not appear to be completely impaired, because a weak response to A20 was detectable (Figure 7). Indeed, analysis of splenocytes from immunized and nonimmunized mice for CTL activity against A20 cells after 5-day in vitro restimulation revealed no differences among the different groups of immunized mice, while stimulation of spleen cells from nonvaccinated mice resulted in a lower but significant lysis of target cells (Figure 8). Given the higher frequency of IFN-γ–producing CD4− T cells in mice vaccinated with A20 plus IL-12–producing bystander cells, IL-12–mediated stimulation in vivo results in an expansion of tumor-specific CD8+ T cells. Such an effect on CD8+ T cells may be direct or mediated by other components of the immune response.
Immunization with autologous tumor plus IL-12–secreting allogeneic bystander cells induces expansion of tumor-specific IFN-γ–secreting CD4− T cells.
Tumor-specific IFN-γ–secreting cells were quantified using an ELISPOT assay and expressed as number of spots per 105CD4− spleen cells obtained from each experimental group. Stimulated cells (▪) were compared with cells resuspended in medium alone (■). Data are represented as means (+ SDs) of groups of 3 mice, analyzed individually.
Immunization with autologous tumor plus IL-12–secreting allogeneic bystander cells induces expansion of tumor-specific IFN-γ–secreting CD4− T cells.
Tumor-specific IFN-γ–secreting cells were quantified using an ELISPOT assay and expressed as number of spots per 105CD4− spleen cells obtained from each experimental group. Stimulated cells (▪) were compared with cells resuspended in medium alone (■). Data are represented as means (+ SDs) of groups of 3 mice, analyzed individually.
CTLs generated from immunized mice are equally efficient in killing A20 tumor cells.
Cytotoxic activity was evaluated after 5 days of in vitro restimulation of total spleen cells with irradiated A20 cells, as described. A20 cells represent target cells. Vaccine formulation is indicated. The percentage of lysis of BALB/c blasts as negative controls was below 10% in each experimental group. Data shown are representative of the results obtained from 3 mice for each group, analyzed individually.
CTLs generated from immunized mice are equally efficient in killing A20 tumor cells.
Cytotoxic activity was evaluated after 5 days of in vitro restimulation of total spleen cells with irradiated A20 cells, as described. A20 cells represent target cells. Vaccine formulation is indicated. The percentage of lysis of BALB/c blasts as negative controls was below 10% in each experimental group. Data shown are representative of the results obtained from 3 mice for each group, analyzed individually.
These data indicate that the extent of IFN-γ production correlates better than CTL with the therapeutic outcome and that CD4 cells participate in maintaining elevated levels of IFN-γ. IL-12 plays an important role in expanding these lymphocyte subpopulations and, in terms of therapeutic activity, accounts for 50% of overall survival (Figure 4).
IL-12 production by tumor vaccine enhances the recruitment of NK cells and DCs at the site of vaccination
To test whether NK cells may participate in the therapeutic effect of IL-12–producing tumor vaccine, splenocytes were collected after 3 rounds of vaccinations and tested against NK-sensitive YAC cells. None of the vaccinated individual mice showed increased NK activity regardless the vaccine formulation (3 mice per group); NK-mediated cytotoxicity was detected only in splenocyts from mice injected with poly(IC) (data not shown). These data might suggest that systemic, late activation of NK cells does not occur. Differently, NK cells may take part in the early phase of immune stimulation. To be capable of collecting and testing NK cells shortly after priming, BALB/c mice were injected intraperitoneally with 1 × 106 live A20 cells and, the day after, were vaccinated with irradiated A20 cells, A20 cells plus B78H1, and A20 cells plus B78H1 producing a high or low amount of IL-12 by the same intraperitoneal route. The number and the activity of NK cells infiltrating the peritoneum were evaluated 3 days after vaccination. FACS analysis revealed that vaccination with A20 plus B78H1 cells recruited more NK cells than A20 cells alone (Figure9), thus confirming that the lack of MHC class I expression on immunizing tumor cells is critical to induce the recruitment of NK cells.26 However, IL-12 production by bystander cells further increased the number of NK cells and conferred the ability to kill YAC cells in a dose-dependent manner (Figure 9, insert).
IL-12 gene transduction of bystander cells increases local infiltration of NK cells and DCs.
BALB/c mice were injected intraperitoneally with 106 live A20 cells. The following day, mice were intraperitoneally vaccinated with the indicated irradiated cell tumor vaccines. On day 3 after vaccination, peritoneal exudate cells (PECs) were collected, counted, and analyzed by FACS for DC (■) and NK (▪) infiltration (main graph). PECs were also evaluated for NK activity in a 4-hour51Cr release assay against YAC target cells. PECs were from mice vaccinated with the following: A20 cells (♦), A20 plus B78H1 cells (▪), A20 plus B78H1–IL-12400 cells (▴), and A20 plus B78H1–IL-1225000 cells (✖). Three individual mice were analyzed in each group.
IL-12 gene transduction of bystander cells increases local infiltration of NK cells and DCs.
BALB/c mice were injected intraperitoneally with 106 live A20 cells. The following day, mice were intraperitoneally vaccinated with the indicated irradiated cell tumor vaccines. On day 3 after vaccination, peritoneal exudate cells (PECs) were collected, counted, and analyzed by FACS for DC (■) and NK (▪) infiltration (main graph). PECs were also evaluated for NK activity in a 4-hour51Cr release assay against YAC target cells. PECs were from mice vaccinated with the following: A20 cells (♦), A20 plus B78H1 cells (▪), A20 plus B78H1–IL-12400 cells (▴), and A20 plus B78H1–IL-1225000 cells (✖). Three individual mice were analyzed in each group.
We also evaluated the number of dendritic cells (DCs) infiltrating the site of vaccination. As shown in Figure 9, the addition of bystander cells to A20 cells increased the absolute number of CD11c+cells. The IL-12 effect was threshold dependent, because only bystander cells producing the higher amount of IL-12 further increased DC infiltration.
These data suggest that the therapeutic effect of vaccination with A20 cells plus B78H1–IL-12 25 000 does not depend on a direct NK-mediated cytotoxicity against disseminated A20 cells. Rather, IL-12 produced by bystander cells may critically enhance the recruitment of both NK cells and DCs at the site of vaccination, thus favoring cross-presentation of TAA, an effect maximized by a treshold amount of IL-12.
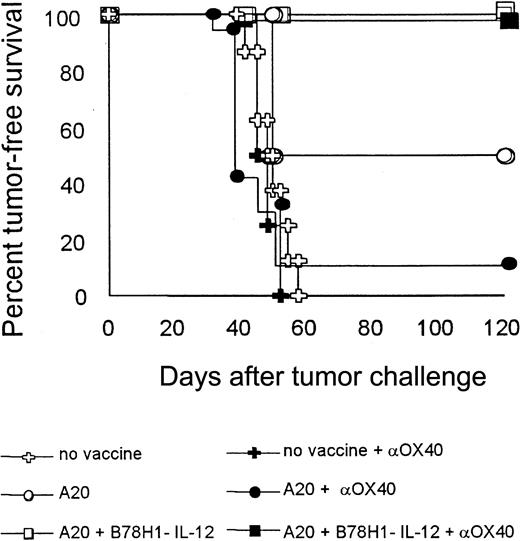
Boosting T cells through the OX40 engagement has the same therapeutic effect as a tumor cell vaccine in mice pretreated with autologous tumor plus IL-12–secreting bystander cells
To better correlate therapeutic outcome with the effect of IL-12 produced by tumor vaccine on CD4+ T cells, the fourth cellular boost was replaced by the systemic administration of an agonistic anti-OX40 mAb. OX40 triggering of CD4+ T cells promotes their activation, clonal expansion, and protection from apoptosis.27 Administration of the anti-OX40 mAb instead of cellular vaccine was expected to have a therapeutic effect only in the presence of a pool of activated CD4+ T cells. As shown in Figure 10, the mAb boosted the host response to the same extent as the cellular vaccine (A20 plus B78H1–IL-12 cells), conferring 100% protection. By contrast, 12.5% and 50% of mice vaccinated with A20 cells alone were tumor free using anti-OX40 mAb or irradiated A20 cells, respectively, as the fourth boost. Isotype control mAb was ineffective (not shown).
Boosting T-cell response through OX40 engagement can substitute cellular vaccine in mice previously treated with autologous tumor plus IL-12–secreting bystander cells.
On days 1, 5, and 15 after tumor challenge, BALB/c mice were immunized with the indicated irradiated tumor vaccines. On day 30, mice received an additional dose of cellular vaccine (open symbols) or were injected intraperitoneally with 200 μg anti-OX40 mAb (closed symbols). As controls, nonvaccinated tumor-bearing mice were left untreated or received mAb against OX40. Mice were evaluated for tumor-free survival over a 4-month observation period. Eight mice were included in each group.
Boosting T-cell response through OX40 engagement can substitute cellular vaccine in mice previously treated with autologous tumor plus IL-12–secreting bystander cells.
On days 1, 5, and 15 after tumor challenge, BALB/c mice were immunized with the indicated irradiated tumor vaccines. On day 30, mice received an additional dose of cellular vaccine (open symbols) or were injected intraperitoneally with 200 μg anti-OX40 mAb (closed symbols). As controls, nonvaccinated tumor-bearing mice were left untreated or received mAb against OX40. Mice were evaluated for tumor-free survival over a 4-month observation period. Eight mice were included in each group.
These data suggest that the contribution of IL-12 to the therapeutic effect of a cellular vaccine rests mainly in the activation of a CD4+ T-cell–mediated immune response and that suboptimal CD4 activation, probably during the initial courses of vaccination, may eventually reduce the therapeutic benefit.
Discussion
Appropriate design of a tumor vaccine based on allogeneic tumor cells requires knowledge of the precise contribution of alloreactivity to the induction of antitumor immunity. Immunization with allogeneic tumors may provide a favorable cytokine milieu by helping antigen presentation, but expansion of alloreactive T cells might overcome the tumor-specific response, especially in the case of repeated immunizations. In particular, the expression of MHC class I molecules by the immunizing tumor cells can critically affect the tumor-specific immune response by reducing the recruitment of NK cells to the vaccine site.26 Indeed, NK cells may favor the induction of a potent antitumor immune response not only through the local production of IFN-γ28 but also by interacting with other fundamental tumor-infiltrating cell components such as DCs28,29 to favor cross-priming of NK-killed tumor cell debris.30 In a syngeneic model, MHC class I expression by immunizing tumor cells had a negative effect on the subsequent response to class I–negative tumor challenge, likely due to reduced NK cell activation.31 On the other hand, MHC class I–negative tumors can efficiently prime CTLs that are useless against MHC class I–negative targets.32 Although cross-priming is a widely accepted mechanism of tumor-associated immune response,33,34 with one notable exception,35several clinical studies based on cytokine-transduced allogeneic tumor cells were careful to match one HLA allele between vaccine and patient.36 Perhaps the most striking approach to testing alloreactivity as a means to inducing tumor rejection is direct tumor injection with allogeneic HLA-B7 cDNA,15 an approach that has apparently exhausted the initial emphasis37 although clinical phase 2 studies are still in progress.
Allogeneic MHC class I–positive tumor cells were a required component of a cellular vaccine that, together with systemic IL-12, prevented mammary carcinogenesis in HER-2/neu transgenic mice.19 Our efforts to identify a genetically modified bystander cell line to be admixed with autologous tumor cells led us to investigate whether MHC class I expression on the bystander is advantageous or detrimental for recognition of TAA expressed by the bystander or the autologous tumor. Our results indicate that despite a different pattern of allorecognition, an allogeneic class I–negative cell line has the same capacity to activate tumor-specific CTLs as its class I–positive counterpart. Moreover, immunization with MHC class I–positive allogeneic tumor cells in combination with the autologous tumor did not enhance antitumor immunity as compared with class I–negative bystander cells. Given its low immunogenicity against alloantigens, an allogeneic MHC class I–negative cell line is likely a more suitable bystander cell for the following reasons: (1) A weak allogeneic response is unlikely to alter the desired microenvironment created by the cytokine gene introduced in the vaccine formulation; (2) repeated immunizations with a strongly alloreactive cytokine-producing bystander cell might decrease the efficacy of a tumor vaccine by accelerating the destruction of immunizing tumor cells, thereby limiting the duration of cytokine secretion; and (3) a cytokine-modified class I–negative tumor vaccine may provide a favorable microenvironment for recruitment and activation of NK cells, which play an important role in controlling tumor cell clones that have lost MHC class I expression and are not sensitive to CD8+T-cell–mediated killing.
Levitsky and collaborators14 reported that the characteristics of leukocytes infiltrating the vaccine site of mice injected with a mixture of irradiated autologous tumor and an allogeneic class I–negative B78H1 GM-CSF–producing bystander cell line were similar to those observed by using autologous GM-CSF–transduced tumor cells. Those data indicate that the overall histology of the vaccine site is determined mainly by the cytokine milieu and not by the presence of weakly alloreactive tumor cells. We introduced IL-12 genes in place of GM-CSF in B78H1 cells. Such B78H1–IL-12 cells have been reported to be heavily infiltrated by NK cells and DCs when injected into syngeneic B6 mice.38Accordingly, by injecting both A20 cells and cell vaccines into the peritoneum of BALB/c mice we were capable of collecting increasing numbers of NK cells and DCs depending on the amount of IL-12. Systemically, B78H1–IL-12 cells in combination with irradiated A20 cells cured more mice bearing disseminated A20 lymphoma than autologous tumor cells alone or together with the empty vector-transduced allogeneic cells. We observed a threshold effect of IL-12, which probably parallels the differential display of leukocyte infiltration and function noted above.
The A20 B-cell lymphoma has been associated with significant impairment of tumor-specific CD4+ T cells during tumor progression.39 In particular, cross-presentation of a surrogate tumor antigen such as the transfected influenza hemagglutinin gene product by APCs rather than direct presentation by live A20 cells has been proposed as the major mechanism responsible for decreased CD4+ T-cell–mediated proliferation and decreased IL-2 production in response to in vitro exposure to the hemagglutinin TAA.40 Accordingly, our results indicate that CD4+ T cells from mice repeatedly injected with irradiated A20 cells and probably undergoing cross-priming have a blunted proliferation and IL-2 release response when restimulated in vitro with A20 tumor cells. By contrast, immunization with A20 cells plus B78H1–IL-12 increased the proliferation rate and IL-2 secretion of CD4+ T cells in response to in vitro restimulation with A20 cells. Note that only CD4+ T cells from mice immunized with the IL-12–secreting bystander cell line produced IFN-γ, GM-CSF, and IL-2 in addition to IL-4, IL-5, and IL-10; only the latter were induced by immunization with A20 cells alone or together with B78H1. Maximal protective immunity depends on the simultaneous induction of Th1 and Th2 immune responses, both of which are necessary for efficient destruction of tumor cells.41 Our results point to the capacity of IL-12 to counteract the tolerogenic effect on CD4+ T cells by APCs loaded with A20-derived tumor antigens. Interestingly, although assessment of freshly isolated CD4− spleen cells revealed a higher frequency of IFN-γ–producing cells in mice immunized with A20 plus B78H1–IL-12 cells, CTL activity against A20 target cells after short-term ex vivo expansion did not differ in the vaccinated groups. These data suggest that CD8+ T cells are functionally competent in all experimental groups, but their in vivo expansion is elicited by repeated immunizations with a tumor vaccine secreting IL-12. Given the critical role of T helper cells in inducing and sustaining tumor-specific CTLs,42 it is likely that IL-12–mediated prevention of CD4+ T-cell impairment significantly favors CD8+ T-cell function in vivo. Consistent with the hypothesis that efficacious CD4 T-cell activation occurs only in the A20 plus B78H1–IL-12 group, substitution of the fourth cellular boost with agonistic mAb against OX40 was possible only in this group. Indeed, OX40 is activated primarily in CD4+ T cells, where its cross-linking by OX40L increases proliferation and cytokine production, including IL-2 and IFN-γ.26 Moreover, OX40 engagement can break existing tolerance,43 protects T cells from apoptosis,44 and favors induction of T-cell memory.45
In conclusion, we have demonstrated the therapeutic efficacy of IL-12 in the formulation of a clinically feasible tumor cell–based vaccine consisting of nonmanipulated autologous tumor and a gene-transduced, MHC class I–negative allogeneic cell line. Such results have implications for the use of IL-12 in the treatment of hematologic malignancies such as leukemia in which considerable numbers of tumor cells are available for mixing with a gene-modified allogeneic cell line. Moreover, in our lymphoma model, IL-12 appears to sustain antitumor immunity by eliciting CD4+ T-cell–mediated immune response.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-03-0991.
Supported by the Italian Association for the Research against Cancer (AIRC) and Consiglio Nazionale delle Ricerche (CNR) Finalized Project on Biothechnology (PF49). A.C. is a recipient of a visiting fellowship from Italian Association Against Leukemia (AIL).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mario P. Colombo, Immunotherapy and Gene Therapy Unit, Istituto Nazionale per lo Studio e la Cura dei Tumori, Via Venezian 1, 20133, Milan, Italy; e-mail:mcolombo@istitutotumori.mi.it.





![Fig. 5. Autologous tumor cells plus IL-12–secreting bystander cells induced CD4+ T cells to proliferate upon in vitro stimulation with syngeneic tumor. / After 4 immunizations splenocytes from the indicated experimental groups were collected and purified CD4+ T cells were incubated for 5 days in the absence (■) or presence (▪) of irradiated A20 cells. [3H] incorporation is given as counts per minute (cpm) minus values for medium alone. Incorporation of irradiated A20 cells alone was comparable to that in medium. Data are represented as means (+ SDs) of the T-cell cpm per well from 3 mice in each group, analyzed individually.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-03-0991/4/m_h80233650005.jpeg?Expires=1767779193&Signature=Zo2qArnp7oevnI9EWgKcycLOs~g3k4dYle8~o2fd4cgSMwwXADUxY5FtqGHNFg2JonTNvDTJk35cOmp6FgfY32WVo5sP-tlHh80YFmaRfHV56fY0LY0uMiu4FAeAQEGuFBb2viBDXwNG9zcxvcPuF3iAwFSMHdM6~CGWckU-HY74-~jHCaJklkBIEYHfiabVgl~Mlgoac9pQ8jY7hz9UU4jw01xWciFQXm1Z2PSRHehNLm1Vw2yFP-FqYCp-O~YtJdsDtsUix5v2MAYIYjGKslpdWkkAetDCEYLsJ16SpG2g0NgukuB2A9wRYdMnY~viUS4GsSWTyPOkeZ~JXiEcew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal