Angiogenesis is a prominent feature of a number of inflammatory human diseases, including rheumatoid arthritis, psoriasis, and cutaneous delayed-type hypersensitivity (DTH) reactions. Up-regulation of placental growth factor (PlGF), a member of the vascular endothelial growth factor (VEGF) family, has been found in several conditions associated with pathologic angiogenesis; however, its distinct role in the control of angiogenesis has remained unclear. To directly investigate the biologic function of PlGF in cutaneous inflammation and angiogenesis, DTH reactions were investigated in the ear skin of wild-type mice, of PlGF-deficient mice, and of transgenic mice with targeted overexpression of human PlGF-2 in epidermal keratinocytes, driven by a keratin 14 promoter expression construct. Chronic transgenic delivery of PlGF-2 to murine epidermis resulted in a significantly increased inflammatory response, associated with more pronounced vascular enlargement, edema, and inflammatory cell infiltration than seen in wild-type mice. Conversely, PlGF deficiency resulted in a diminished and abbreviated inflammatory response, together with a reduction of inflammatory angiogenesis and edema formation. VEGF expression was up-regulated at a comparable level in the inflamed skin of all genotypes. These findings reveal that placental growth factor plays a critical role in the control of cutaneous inflammation, and they suggest inhibition of PlGF bioactivity as a potential new approach for anti-inflammatory therapy.
Introduction
Angiogenesis and inflammation are closely linked,1 and increased vascularity is a prominent feature of a number of inflammatory human diseases, including rheumatoid arthritis2 and psoriasis.3,4 Whereas tissue repair and tumor growth are predominantly associated with sprouting angiogenesis (ie, the outgrowth of new capillaries from preexisting vessels),5-7 the predominant type of angiogenesis observed during inflammation consists of vascular enlargement of preexisting vessels rather than the formation of new blood vessels.3,8However, endothelial cell proliferation and vascular hyperpermeability are shared by both types of angiogenesis, and enlarged and hyperpermeable dermal microvessels are also a consistent feature of the skin inflammation associated with delayed-type hypersensitivity reactions.8,9 The significance of angiogenesis for cutaneous inflammation is supported by the recent finding that deficiency of the endogenous angiogenesis inhibitor thrombospondin-2 was associated with enhanced and prolonged cutaneous delayed-type hypersensitivity reactions.9 Moreover, several proangiogenic factors that are most likely involved in the inflammatory vascular response have been identified, including angiopoietins,10 members of the CXC-chemokine family,11 and vascular endothelial growth factor (VEGF).4,12 13
Placental growth factor (PlGF) is a member of the VEGF family of growth factors, encoding for a protein with an approximately 50% identity to VEGF in the platelet-derived growth factor (PDGF)–like domain.14 PlGF occurs in at least 3 isoforms, PlGF-1 (PlGF149), PlGF-2 (PlGF170), and PlGF-3 (PlGF221),15,16due to alternative mRNA splicing. A highly basic 21–amino acid insertion in the carboxy-terminal region of PlGF-2 results in high heparin-binding affinity, whereas PlGF-1 or PlGF-3 do not bind heparin. PlGF-1 binds to the VEGF receptor-1 (VEGFR-1) that is predominantly expressed by vascular endothelial cells; PlGF-2 additionally binds neuropilin-1, a receptor for the collapsin/semaphorin family of proteins that is expressed by neuronal and nonneuronal cells including endothelial cells.17,18 Whereas PlGF has been originally identified in the placenta, where it has been proposed to control trophoblast growth and differentiation,14,19 PlGF is also expressed during vascular development20 and PlGF expression has been detected in several other organs including the heart and the lung.21 We have previously found that PlGF-1 and PlGF-2, but not PlGF-3, were also expressed in human skin cells and in angiogenic human skin,22 and increased PlGF mRNA expression has been recently detected during murine and human cutaneous wound healing.23,24 Despite this descriptive evidence for a role of PlGF in the control of angiogenesis, and despite the recent findings that the presence of PlGF might be needed for distinct VEGF effects to occur,24 the distinct biologic role of increased PlGF concentrations on angiogenesis and its effects on inflammatory reactions have remained unclear.
We aimed to characterize directly the biologic function for PlGF in cutaneous inflammation under conditions of increased or absent epidermal PlGF expression. Therefore, we established transgenic mice with targeted overexpression of human PlGF-2 in epidermal keratinocytes, using a keratin 14 promoter transgene construct25 that we had previously applied for the successful establishment of transgenic mice overexpressing the angiogenesis factor VEGF.26 We then evaluated cutaneous delayed-type hypersensitivity (DTH) reactions induced by topical application of oxazolone, an established experimental model for skin inflammation,27 in wild-type and in PlGF-2–overexpressing transgenic mice. Moreover, DTH reactions were also studied in previously established mice deficient in PlGF.24 Here, we report that PlGF expression is up-regulated in acute cutaneous inflammation and that chronic transgenic delivery of the PlGF-2 gene to murine epidermis results in a significantly enhanced inflammatory response, associated with enhanced vascular enlargement, edema formation, and inflammatory cell infiltration. The proinflammatory effects of transgenic PlGF-2 were not mediated through modulation of the levels of endogenous PlGF or VEGF protein expression. Conversely, PlGF deficiency resulted in a diminished and abbreviated inflammatory response, together with a reduction of inflammatory angiogenesis and edema formation. These findings identify a previously unknown function of placental growth factor that plays a critical role in the induction of cutaneous inflammation, and they suggest inhibition of PlGF bioactivity as a potential new approach for anti-inflammatory therapy.
Materials and methods
Generation of PlGF-2 transgenic and PlGF-deficient mice
A 533-bp human PlGF-2 cDNA, comprising the full coding sequence, was obtained by polymerase chain reaction (PCR) of Marathon-ready human placenta cDNA (Clontech, Palo Alto, CA) using the following primers: forward primer, 5′-GGATCCTGAGAAGATGCCGGTCATGAG-3′ (nucleotides 5 to 26 of the humanPlGF-2 sequence, GenBank accession no. S72960, and additional BamHI site), and reverse primer, 5′-GAATTCCAAGGGGTGGGTTACCTCCG-3′ (nucleotides 538 to 517 and additionalEcoRI site). The PlGF-2 cDNA was cloned into a pCRII TOPO vector (Invitrogen, San Diego, CA). After restriction digestion with BamHI, the PlGF-2 cDNA fragment was ligated into a BamHI-digested pGEM-3Z transgene vector containing a keratin 14 expression cassette (kindly provided by Dr E. Fuchs, University of Chicago). The correct sequence and orientation of the PlGF-2 insert were verified by restriction mapping and by direct sequencing using the Sanger dideoxy method. A 5.2 kbKpnI-HindIII fragment was used to generate transgenic mice as described.26 Transgenic founders were detected by Southern blot analysis of BamHI-digested genomic tail DNA using a 32P-labeled PlGF-2 cDNA as a probe. For rapid identification, genomic tail DNA was subjected to PCR as described previously.7,26 Transgenic lines were established on the FVB genetic background. PlGF-deficient mice were generated on the 129/Sv genetic background as previously described.24 All animal studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Cell culture
Primary epidermal keratinocytes were isolated from the skin of neonatal PlGF-2 transgenic mice and of wild-type littermates as described previously.28 Cells were resuspended in minimal essential medium (Life Technologies, Grand Island, NY), supplemented with 4% fetal calf serum and 10 ng/mL epidermal growth factor (Becton Dickinson, Bedford, MA) at low calcium concentration (0.05 nM), and were plated onto collagen type 1–coated (50 μg/mL; Collagen, Palo Alto, CA) culture dishes. Human dermal microvascular endothelial cells were isolated from human neonatal foreskins and were maintained in culture as described previously.29
RNA isolation and Northern blot analyses
Total RNA was isolated from cultured mouse keratinocytes or from the skin of 3-week-old mice as previously described,7using the RNeasy kit (Qiagen, Chatsworth, CA). Samples of RNA (10 μg each) were fractionated by electrophoresis on 1% agarose formaldehyde gels and were transferred to Biotrans nylon supported membranes (ICN Pharmaceuticals, Costa Mesa, CA). 32P-radiolabeled cDNA probes were prepared with a random primed synthesis kit (Multiprime; Amersham, Arlington Heights, IL). Messenger RNAs for PlGF-2 were detected with a 533-bp cDNA probe, and a 2.0-kb human β-actin cDNA probe (Clontech) was used as a control for equal RNA loading. Blots were washed at high stringency as described30 and were exposed to X-OMAT film (Kodak, Rochester, NY).
Western blot analysis and ELISA assays
Skin biopsies were obtained from the ventral and dorsal skin of 3-week-old PlGF-2 transgenic mice (n = 3) or wild-type littermates (n = 3) and were homogenized in lysis buffer containing 50 mM Tris (tris(hydroxymethyl)- aminomethane) (pH 7.4), 150 mM NaCl, 0.02M phenylmethylsulfonyl fluoride, 50 μg/mL leupeptin, and 50 μg/mL aprotinin.31 Lysates were also obtained from cultured fifth-passage human dermal microvascular endothelial cells.29 The samples were concentrated 10-fold by Centricon-10 columns (Amicon, Beverly, MA). A total of 50 μg of protein per sample was analyzed by denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with a polyclonal antibody against human PlGF-2 (Zymed Laboratories, San Francisco, CA). Skin lysates were also obtained from unchallenged or challenged ear skin (see next paragraph) at 24 hours after oxazolone challenge (n = 5 for each genotype). Human PlGF and murine VEGF levels were quantified with commercially available enzyme-linked immunosorbent assays (ELISAs; R&D Systems, Minneapolis, MN). For detection of murine PlGF, an ELISA was developed using a rat antimouse PlGF monoclonal antibody for capture and a biotinylated goat antimouse PlGF antibody for detection (R&D Systems).24Experiments were performed at least twice with comparable results. Statistical analyses were performed using the unpaired Studentt test.
Induction of delayed-type hypersensitivity reactions
Delayed-type hypersensitivity (DTH) reactions were induced in the skin of 8-week-old male PlGF-2 transgenic mice (n = 9), PlGF-deficient mice (n = 8), and wild-type FVB (n = 9) or 129Sv mice (n = 8), as previously described.9,27 The mice were sensitized by topical application of 2% oxazolone (4-ethoxymethylene-2-phenyl-2-oxazalone-5-1; Sigma, St Louis, MO) solution in acetone–olive oil (4:1 vol/vol) to the shaved abdomen (50 μL) and to each paw (5 μL). Five days after sensitization, the right ears were challenged by topical application of 20 μL (10 μL to each side) of a 1% oxazolone solution, whereas the left ears were treated with vehicle alone. The ear thickness was measured daily for up to 7 days, as a parameter for the extent of inflammation, using the mouse ear-swelling test.32 Statistical analysis was performed using the unpaired Student t test.
Histology, immunohistochemistry, and in situ hybridization
Mice of each experimental group were killed 24 hours after oxazolone challenge (n = 3 per group). Two hours before humane killing, mice received intraperitoneal injections of 40 mM 5-bromodeoxyuridine (BrdU, Sigma). One half of each ear was fixed for 1 hour in 10% formalin and was processed and embedded in paraffin. The other half was embedded in OCT compound (Sakura Finetek, Torrance, CA) and was snap-frozen in liquid nitrogen. Immunohistochemical stainings were performed on 6 μm cryostat sections as previously described,33 using rat monoclonal antibodies against mouse CD4, CD8, CD31, BrdU (all from Pharmingen, San Diego, CA), or F4/80 antigen (Serotec, Raleigh, NC) as a murine macrophage marker.34 In situ hybridization was performed on 6-μm paraffin sections as described.30 RNA probes to humanPlGF-2 were transcribed from a pCRII TOPO vector containing a 533-bp PCR fragment that included the full coding region of human PlGF-2.
Vascular perfusions and computer-assisted, morphometric analysis of blood vessels
Eight-week-old PlGF-2 transgenic mice (n = 3) and wild-type littermates (n = 3) were anesthetized with a mixture of ketamine (800 μg/10 g body weight ketamine; Fort Dodge Laboratories, Fort Dodge, IA) and avertin (0.5 μg/10 g body weight 2,2,2,-tribromoethanol in 2.5% t-amyl alcohol; Sigma), and perfusions were performed via the left ventricle as described.35 Mice were perfused with fluoresceine-labeled Lycopersicon esculentumlectin (Vector Laboratories, Burlingame, CA) at a constant pressure of 120 mm Hg, leading to lectin binding toN-acetyl-D-glucosamine residues on the luminal surface of vascular endothelial cells.36 The architecture of the vasculature was visualized in whole mounts of mouse ears of 6-week-old mice (n = 3 per group) as described,7 using a Nikon E-600 fluorescence microscope (Nikon, Melville, NY). For computer-assisted, morphometric analysis of blood vessels, representative CD31-stained cryostat sections of ear skin obtained from each experimental group (n = 3 per group) were analyzed using a Nikon E-600 microscope. Images were captured with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI), and morphometric analyses were performed using the IP-LAB software (Scanalytics, Billerica, MA). Three different × 60 fields in each section were examined, and the number of vessels per square millimeter and the average vessel size were determined as described.37 The 2-sided unpaired Student t test was used for statistical analyses.
Miles vascular permeability assay
Miles assays were performed as described previously.7,38 Evans blue dye (100 μL of a 1% solution in 0.9% NaCl) was injected into the tail vein of wild-type FVB mice (n = 6). After 10 minutes, human VEGF165, VEGF/PlGF heterodimer, or PlGF (100 ng in 50 μL phosphate-buffered saline [PBS]; all from R&D Systems) were injected intradermally into the shaved back skin. After 20 minutes, the animals were killed and an area of skin that included the blue spot resulting from leakage of the dye was removed. Evans blue dye was extracted from the skin by incubation with formamide for 4 days at room temperature, and the absorbance of extracted dye was measured at 620 nm using a spectrophotometer.39 The unpaired Student ttest was used for statistical analysis.
Results
Targeted overexpression of PlGF-2 in the skin of transgenic mice
To investigate the biologic role of PlGF in cutaneous inflammation, we first established a new transgenic mouse model for the targeted overexpression of human PlGF-2 in the skin using a keratin 14 (K14) promoter expression vector containing the complete coding sequence of the human PlGF-2 gene (Figure1A). The K14 promoter predominantly targets transgene expression to basal epidermal keratinocytes and to outer root sheath keratinocytes of hair follicles.7,25 26Southern blot and PCR analysis of genomic DNA revealed transgene incorporation in 3 founder mice, and 2 independent transgenic lines were established on the FVB genetic background. PlGF-2 transgenic mice were fertile and did not display any obvious phenotypic abnormalities. Efficient transgene mRNA expression was confirmed by Northern blot analysis of total RNA extracted from skin lysates obtained from wild-type and from PlGF-2 transgenic mice. Transgene PlGF-2mRNA expression was strongly increased over the moderate expression of endogenous (murine) PlGF-2 (Figure 1B). Similarly, cultured epidermal keratinocytes, derived from the skin of neonatal PlGF-2 transgenic mice, showed highly increased levels of PlGF-2 mRNA expression (Figure 1B).
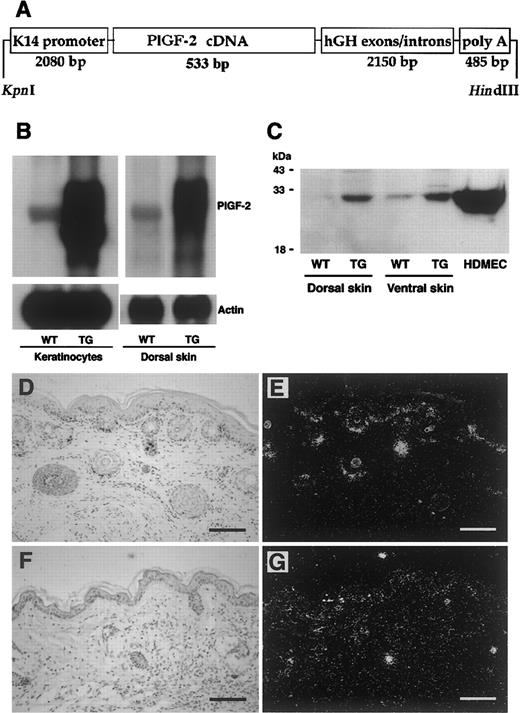
Targeted expression of transgenic PIGF-2 in epidermal keratinocytes.
(A) Schematic representation of the K14–PlGF-2 transgene construct. A 533-bp human PlGF-2 cDNA fragment was ligated to the BamHI restriction site of the keratin 14 promoter cassette. (B) Overexpression of PlGF-2 mRNA in the dorsal skin of 3-week-old K14–PlGF-2 transgenic mice and in cultured epidermal keratinocytes isolated from PIGF-2 transgenic mice was confirmed by Northern blot analysis. Hybridization with a murine β-actin probe served as control for equal loading. (C) Western blot analysis of skin lysates demonstrates the presence of the intact PlGF-2 protein in PlGF-2 transgenic (TG) skin. Low levels of endogenous PlGF-2 were detected in wild-type (WT) skin. Conditioned media obtained from human dermal microvascular endothelial cells (HDMEC) served as positive controls. (D-E) Targeted overexpression of the K14–PlGF-2 transgene in the basal epidermal keratinocyte layer and in outer root sheath keratinocytes of hair follicles was confirmed by in situ hybridization in 2-week-old transgenic mice. (F-G) Low-level epidermal expression of the K14–PlGF-2 transgene was observed by in situ hybridization in adult, 8-week-old transgenic mice. Bright-field (D,F) and dark-field (E,G) micrographs. Scale bars = 50 μm.
Targeted expression of transgenic PIGF-2 in epidermal keratinocytes.
(A) Schematic representation of the K14–PlGF-2 transgene construct. A 533-bp human PlGF-2 cDNA fragment was ligated to the BamHI restriction site of the keratin 14 promoter cassette. (B) Overexpression of PlGF-2 mRNA in the dorsal skin of 3-week-old K14–PlGF-2 transgenic mice and in cultured epidermal keratinocytes isolated from PIGF-2 transgenic mice was confirmed by Northern blot analysis. Hybridization with a murine β-actin probe served as control for equal loading. (C) Western blot analysis of skin lysates demonstrates the presence of the intact PlGF-2 protein in PlGF-2 transgenic (TG) skin. Low levels of endogenous PlGF-2 were detected in wild-type (WT) skin. Conditioned media obtained from human dermal microvascular endothelial cells (HDMEC) served as positive controls. (D-E) Targeted overexpression of the K14–PlGF-2 transgene in the basal epidermal keratinocyte layer and in outer root sheath keratinocytes of hair follicles was confirmed by in situ hybridization in 2-week-old transgenic mice. (F-G) Low-level epidermal expression of the K14–PlGF-2 transgene was observed by in situ hybridization in adult, 8-week-old transgenic mice. Bright-field (D,F) and dark-field (E,G) micrographs. Scale bars = 50 μm.
To study whether skin-specific transgene mRNA expression resulted in enhanced levels of PlGF-2 protein in the skin, lysates were obtained from the dorsal and ventral skin of PlGF-2 transgenic mice and wild-type littermates and were subjected to Western blotting with an anti–PlGF-2 antibody. Highly increased levels of PlGF-2 protein were detected in lysates obtained from the skin of transgenic mice as compared with low-level expression in the skin of wild-type littermates (Figure 1C). To further quantify the levels of PlGF-2 protein expression and to confirm efficient secretion of transgenic PlGF-2, the total amount of PlGF-2 protein in mouse skin and in keratinocyte culture supernatants was assayed by a human PlGF-2 ELISA assay. This ELISA assay is specific for human PlGF-2 and does not detect murine PlGF. PlGF-2 was detected in skin lysates (221.8 ± 28.9 pg/mg) and in keratinocyte-conditioned media (156.6 ± 4.4 pg/mg protein) obtained from transgenic mice but not in wild-type skin or keratinocyte-conditioned media, confirming efficient protein translation and secretion. In situ hybridization confirmed targetedPlGF-2 mRNA expression in basal keratinocytes of the epidermis and in outer root sheath keratinocytes of hair follicles in 2-week-old transgenic mice (Figure 1D-E). In contrast, we found low levels of transgene mRNA expression in adult murine skin (Figure 1F-G).
Normal skin structure, vascularization, and vascular permeability in adult PlGF-2 transgenic mice
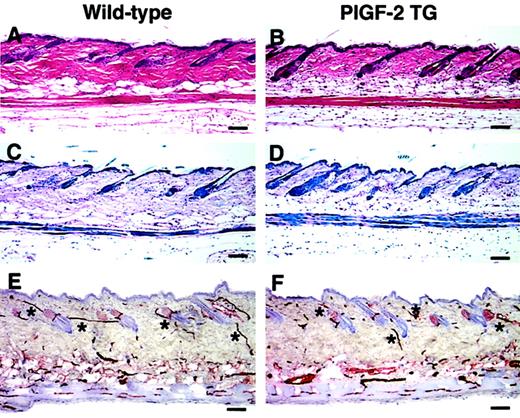
No obvious phenotypic abnormalities of the skin were observed in PlGF-2 transgenic mice as compared with wild-type littermates. Although we detected a slight increase in skin vascularity of transgenic mice up to 7 days of age (data not shown), the histologic analysis of the skin of adult transgenic mice showed no major differences in the thickness and morphology of the epidermis and the dermis (Figure 2A-D). Moreover, the analysis of whole mounts of ear skin after vascular perfusion with the lectinLycopersicon esculentum revealed a comparable branching pattern and architecture of blood vessels in the skin of PlGF-2 transgenic mice (data not shown). Accordingly, immunostains for the endothelial junction molecule CD31 (platelet endothelial cell adhesion molecule 1 [PECAM-1]) did not show any major difference in the amount of vascularization of the skin of PlGF-2 transgenic mice as compared with wild-type littermates (Figure 2E,F). Using computer-assisted morphometric analysis of CD31-stained skin sections, no significant differences in the vascular density or the average vessel size were observed in the normal skin of adult transgenic mice (55 ± 2 vessels per square millimeter; 438 ± 27 μm2) as compared with wild-type skin (56 ± 3 vessels per square millimeter; 525 ± 54 μm2).
Normal skin structure and vascularization in adult PlGF-2 transgenic and wild-type mice.
Histologic analysis of the back skin of 8-week-old PlGF-2 transgenic mice (B,D) showed no major differences in the thickness and morphology of the epidermis and the dermis as compared with wild-type littermates (A,C). (A-B) Hematoxylin and eosin stains; (C-D) trichrome stains. CD31 stains demonstrated comparable vascularization of the skin of PlGF-2 transgenic mice (F) and of wild-type littermates (E). *Cutaneous vessels. Scale bars = 50 μm.
Normal skin structure and vascularization in adult PlGF-2 transgenic and wild-type mice.
Histologic analysis of the back skin of 8-week-old PlGF-2 transgenic mice (B,D) showed no major differences in the thickness and morphology of the epidermis and the dermis as compared with wild-type littermates (A,C). (A-B) Hematoxylin and eosin stains; (C-D) trichrome stains. CD31 stains demonstrated comparable vascularization of the skin of PlGF-2 transgenic mice (F) and of wild-type littermates (E). *Cutaneous vessels. Scale bars = 50 μm.
Because it has been previously suggested that PlGF and VEGF might play synergistic roles in the induction of vascular permeability,40 we next investigated whether VEGF might more potently induce vascular leakage in PlGF-2 transgenic mice. We performed a modified Miles vascular permeability assay using intravenous injection of the blue dye Evans blue followed by intradermal injection of recombinant human VEGF. Spectrophotometric measurements of the amount of extravasated Evans blue revealed a more than 2-fold, significant increase of vascular permeability after injection of VEGF into wild-type mice (optical density [OD]620: 1.799 ± 0.238; mean ± SEM;P < .05) as compared with baseline levels observed after injection of phosphate-buffered saline (0.77 ± 0.038). However, a comparable induction of vascular leakage was found in PlGF-2 transgenic skin (VEGF: 1.919 ± 0.41; PBS: 0.809 ± 0.023;P < .05).
Increased delayed-type hypersensitivity reactions in PlGF-2 transgenic mice
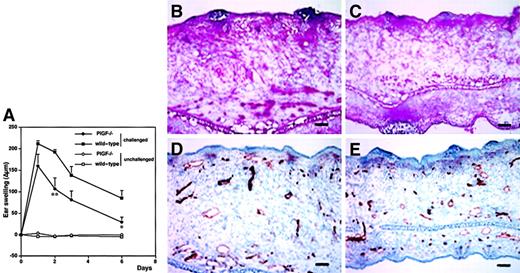
To investigate the biologic role of PlGF in cutaneous inflammation, delayed-type hypersensitivity (DTH) reactions were elicited in PlGF-2 transgenic and wild-type mice by using oxazolone as a sensitizing agent. Twenty-four hours after antigen challenge, PlGF-2 transgenic mice showed significantly increased ear swelling as compared with wild-type mice (P < .01; Figure3A). Macroscopically, ear swelling and redness were clearly enhanced in the challenged ear of transgenic mice as compared with wild-type littermates (Figure 3B-C). These differences were even more pronounced after 48 hours (P < .01; Figure3A). At 7 days after antigen challenge, the extent of ear swelling was less pronounced in both genotypes; however, PlGF-2 transgenic mice still showed increased ear thickness (Figure 3A). No differences in the thickness of ears that were only sensitized, but not challenged, were found in PlGF-2 transgenic mice (Figure 3A).
Increased and prolonged ear swelling in DTH reactions elicited in PlGF-2 transgenic mice.
DTH reactions were induced in the ear skin of PlGF-2-transgenic and wild-type mice using oxazolone. (A) Ear swelling is expressed as the increase (Δ) over the original ear thickness in micrometers. PlGF-2 transgenic mice (●) showed a significantly increased ear swelling (P < .01) 24 hours after challenge as compared with wild-type mice (▪). Moreover, the ear swelling in PlGF-2 transgenic mice persisted longer than in wild-type mice. Unchallenged mice: ○, PlGF-2 transgenic mice; ■, wild-type mice. Data are expressed as means ± SEMs. **P < .01. Macroscopically visible increase of ear swelling and erythema in PlGF-2 transgenic mice (C) as compared with wild-type mice (B) at 24 hours after oxazolone challenge.
Increased and prolonged ear swelling in DTH reactions elicited in PlGF-2 transgenic mice.
DTH reactions were induced in the ear skin of PlGF-2-transgenic and wild-type mice using oxazolone. (A) Ear swelling is expressed as the increase (Δ) over the original ear thickness in micrometers. PlGF-2 transgenic mice (●) showed a significantly increased ear swelling (P < .01) 24 hours after challenge as compared with wild-type mice (▪). Moreover, the ear swelling in PlGF-2 transgenic mice persisted longer than in wild-type mice. Unchallenged mice: ○, PlGF-2 transgenic mice; ■, wild-type mice. Data are expressed as means ± SEMs. **P < .01. Macroscopically visible increase of ear swelling and erythema in PlGF-2 transgenic mice (C) as compared with wild-type mice (B) at 24 hours after oxazolone challenge.
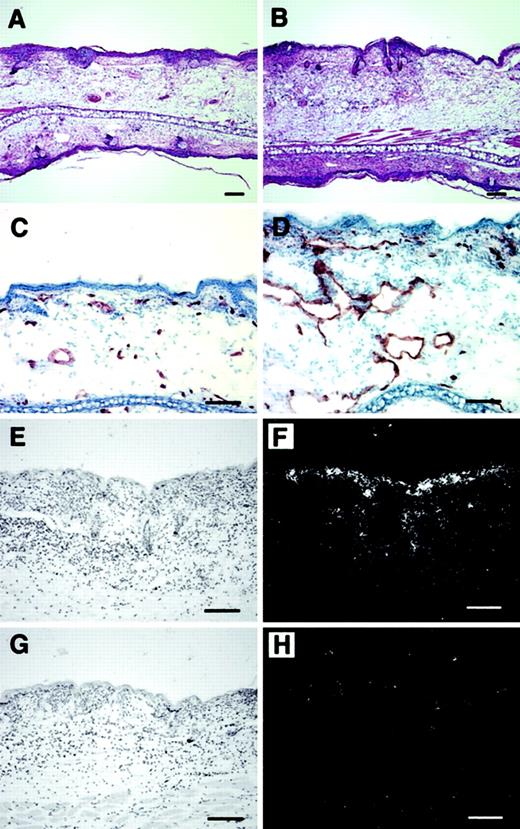
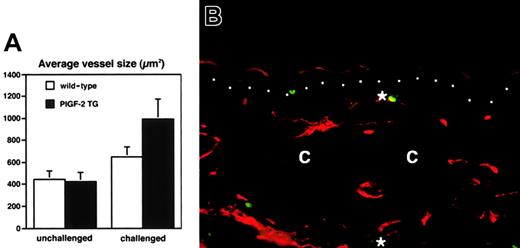
Histologic analysis revealed increased edema and inflammatory cell infiltration in the inflamed skin of PlGF-2 transgenic mice at 24 hours after antigen challenge (Figure 4A-B). Accumulation of neutrophils in the epidermis and upper dermis was found in both transgenic and wild-type mice; however, these changes were more pronounced in PlGF-2 transgenic mice (Figure 4A-B). The inflammatory cell infiltrate consisted mainly of neutrophils and macrophages, as visualized by immunostaining for F4/80, in both genotypes (data not shown). No major differences were found in the numbers of CD4 or CD8 immunopositive cells between wild-type and PlGF-2 transgenic mice (data not shown). The blood vessels in the inflamed ear skin of wild-type mice were found to be enlarged when compared with untreated skin, as evaluated by staining of ear skin with an antibody to the endothelial junction molecule CD31 (Figure 4C). However, the extent of inflammatory vessel enlargement was more pronounced in PlGF-2 transgenic mice (Figure 4D). Computer-assisted morphometric analysis of CD31-stained sections confirmed these findings (Figure5). Whereas no significant differences were found in the average vessel size in unchallenged skin of PlGF-2 transgenic (425.8 ± 83.6 μm2) versus wild-type mice (446.9 ± 74.1 μm2), the average vessel size in the inflamed skin of transgenic mice at 24 hours after challenge was markedly increased (991.0 ± 184.8 μm2) over wild-type mice (646.6 ± 89 μm2;P < .05). The observed vascular enlargement was not simply due to vessel dilation, because double immunofluorescence stains for the vascular marker CD31 and the proliferation marker BrdU revealed proliferation of several vascular endothelial cells in the inflamed skin of PlGF-2 transgenic mice at 24 hours after challenge (Figure 5B).
Increased angiogenesis and inflammation in cutaneous DTH reactions elicited in PlGF-2 transgenic mice.
Increased edema formation and inflammatory infiltration in the skin of PlGF-2 transgenic mice (B) at 24 hours after antigen challenge as compared with wild-type mice (A). Hematoxylin and eosin stains. CD31 stains demonstrated enhanced angiogenesis at 24 hours after challenge in PlGF-2 transgenic mice (D) as compared with wild-type mice (C). (E-H) In situ hybridization confirmed high levels of human PlGF-2 mRNA expression in transgenic epidermal keratinocytes at 24 hours after challenge (E-F), whereas no hybridization signal for human PlGF-2 was detected in wild-type mice (G-H). Bright-field (E,G) and dark-field (F,H) micrographs. Scale bars = 50 μm.
Increased angiogenesis and inflammation in cutaneous DTH reactions elicited in PlGF-2 transgenic mice.
Increased edema formation and inflammatory infiltration in the skin of PlGF-2 transgenic mice (B) at 24 hours after antigen challenge as compared with wild-type mice (A). Hematoxylin and eosin stains. CD31 stains demonstrated enhanced angiogenesis at 24 hours after challenge in PlGF-2 transgenic mice (D) as compared with wild-type mice (C). (E-H) In situ hybridization confirmed high levels of human PlGF-2 mRNA expression in transgenic epidermal keratinocytes at 24 hours after challenge (E-F), whereas no hybridization signal for human PlGF-2 was detected in wild-type mice (G-H). Bright-field (E,G) and dark-field (F,H) micrographs. Scale bars = 50 μm.
Increased vascular remodeling and vascular proliferation in the inflamed skin of PlGF-2 transgenic mice.
(A) Computer-assisted morphometric analysis of CD31-stained tissue sections revealed a moderately increased average vessel size at 24 hours after oxazolone challenge in the ear skin of wild-type mice (■). In contrast, cutaneous vessels in the inflamed skin of PlGF-2 transgenic mice (▪) were significantly larger than in wild-type mice after 24 hours. (B) Double immunofluorescence staining of inflamed skin of PlGF-2 transgenic mice at 24 hours after challenge for the vascular marker CD31 (red) and the proliferation marker BrdU (green) reveals proliferation of vascular endothelial cells (*). Dotted line indicates border between epidermis and dermis; c, cartilage. Original magnification × 30.
Increased vascular remodeling and vascular proliferation in the inflamed skin of PlGF-2 transgenic mice.
(A) Computer-assisted morphometric analysis of CD31-stained tissue sections revealed a moderately increased average vessel size at 24 hours after oxazolone challenge in the ear skin of wild-type mice (■). In contrast, cutaneous vessels in the inflamed skin of PlGF-2 transgenic mice (▪) were significantly larger than in wild-type mice after 24 hours. (B) Double immunofluorescence staining of inflamed skin of PlGF-2 transgenic mice at 24 hours after challenge for the vascular marker CD31 (red) and the proliferation marker BrdU (green) reveals proliferation of vascular endothelial cells (*). Dotted line indicates border between epidermis and dermis; c, cartilage. Original magnification × 30.
In situ hybridization studies revealed that the PlGF-2 mRNA expression by epidermal keratinocytes was greatly enhanced in the inflamed skin of transgenic mice at 24 hours after challenge (Figure 5E-F) as compared with wild-type mice (Figure 5G,H). To investigate whether enhanced PlGF-2 mRNA expression also resulted in increased PlGF-2 protein expression in inflamed transgenic skin, we next quantified the levels of PlGF-2 protein in lysates obtained from the unchallenged and the inflamed ear skin of transgenic mice. We found that the amount of PlGF-2 protein was 5.4-fold increased in inflamed transgenic skin (3.2 ± 0.12 ng/mg) as compared with the unchallenged skin (0.58 ± 0.03 ng/mg; P < .001).
Diminished and abbreviated DTH reactions in PlGF-deficient mice
Our results obtained in PlGF-2 transgenic mice strongly indicated that PlGF acts as a proinflammatory mediator in the skin and that the levels of epidermal PlGF directly affect cutaneous vessel size and permeability. To investigate whether PlGF is in fact necessary for the initiation and maintenance of the cutaneous inflammatory response, we next induced oxazolone-mediated DTH reactions in the skin of PlGF-deficient mice. PlGF deficiency resulted in markedly reduced inflammation after oxazolone challenge and in significantly reduced ear swelling (P < .01 at 48 hours after challenge; Figure6A). Moreover, the course of the cutaneous inflammatory reaction was significantly shortened as compared with age-matched wild-type mice (P < .05; day 6). Histologic analyses revealed a greatly reduced edema in the inflamed ear skin of PlGF-deficient mice (Figure 6C) as compared with wild-type controls (Figure 6B). Moreover, the infiltration of inflammatory cells into the inflamed skin of PlGF-deficient mice was markedly diminished (Figure 6B-C). Vascular stains with an anti-CD31 antibody revealed that the characteristically enlarged inflammatory blood vessels regularly observed in wild-type mice (Figure 6D) were absent in PlGF-deficient mice (Figure 6E).
Decreased and abbreviated ear swelling and diminished angiogenesis in DTH reactions elicited in PlGF-2–deficient mice.
DTH reactions were induced in the ear skin of PlGF-2–deficient and wild-type mice using oxazolone. (A). Ear swelling is expressed as the increase (Δ) over the original ear thickness in micrometers. PlGF-2–deficient mice (●) showed a significantly decreased ear swelling (P < .01 at 48 hours; P < .05 at 24 hours, 3 and 6 days after challenge) as compared with wild-type mice (▪). Moreover, the duration of ear swelling in PlGF-2–deficient mice was abbreviated. Unchallenged mice: ○, PlGF-2–deficient mice; ■, wild-type mice. Data are expressed as means ± SEMs. **P < .01. Diminished edema formation and inflammatory infiltration in the skin of PlGF-2–deficient mice (C) at 24 hours after antigen challenge as compared with wild-type mice (B). CD31 stains demonstrated absence of large angiogenic vessels 24 hours after challenge in PlGF-2–deficient mice (E) as compared with wild-type mice (D). (B-C) Hematoxylin and eosin stains. (D-E) CD31 stains. Scale bars = 50 μm.
Decreased and abbreviated ear swelling and diminished angiogenesis in DTH reactions elicited in PlGF-2–deficient mice.
DTH reactions were induced in the ear skin of PlGF-2–deficient and wild-type mice using oxazolone. (A). Ear swelling is expressed as the increase (Δ) over the original ear thickness in micrometers. PlGF-2–deficient mice (●) showed a significantly decreased ear swelling (P < .01 at 48 hours; P < .05 at 24 hours, 3 and 6 days after challenge) as compared with wild-type mice (▪). Moreover, the duration of ear swelling in PlGF-2–deficient mice was abbreviated. Unchallenged mice: ○, PlGF-2–deficient mice; ■, wild-type mice. Data are expressed as means ± SEMs. **P < .01. Diminished edema formation and inflammatory infiltration in the skin of PlGF-2–deficient mice (C) at 24 hours after antigen challenge as compared with wild-type mice (B). CD31 stains demonstrated absence of large angiogenic vessels 24 hours after challenge in PlGF-2–deficient mice (E) as compared with wild-type mice (D). (B-C) Hematoxylin and eosin stains. (D-E) CD31 stains. Scale bars = 50 μm.
Comparable up-regulation of endogenous VEGF expression in the inflamed skin of PlGF-2 transgenic, PlGF-deficient, and wild-type mice
To evaluate whether the enhanced inflammatory and vascular response observed in transgenic mice with targeted epidermal overexpression of PlGF-2 might have been mediated by enhanced levels of endogenous VEGF expression, we next obtained skin lysates from both unchallenged and challenged ear skin of PlGF-2 transgenic and wild-type mice. Using specific ELISA assays for murine VEGF, we found a strong up-regulation of VEGF protein levels in the inflamed skin of wild-type mice (11.6 ± 1.7 pg/mg) as compared with low levels in unchallenged skin that were below the detection limit of the assay. No further up-regulation of endogenous VEGF levels was detected in the unchallenged (below detection limit) or in the inflamed ear skin (7.4 ± 0.7 pg/mg) of PlGF-2 transgenic mice as compared with wild-type mice. Similarly, endogenous murine PlGF-2 levels were below detection limit in lysates obtained from the unchallenged ear skin of both wild-type and PlGF-2 transgenic mice, and a comparable up-regulation of endogenous murine PlGF protein levels was found in the inflamed ear skins (24 hours after oxazolone challenge) of wild-type (24.7 ± 4.3 pg/mg) and transgenic mice (24.0 ± 1.9 pg/mg). Importantly, VEGF protein levels in the inflamed ear skin of PlGF-deficient mice (7.7 ± 1.0 pg/mg) were comparable to those detected in wild-type and PlGF-2 transgenic mice. No endogenous PlGF was detected in lysates obtained from unchallenged or inflamed ear skin of PlGF-deficient mice, confirming the efficient abrogation of PlGF expression. These results indicate that the specific effects of targeted PlGF-2 overexpression or of PlGF deficiency on cutaneous inflammation were not mediated by modulation of VEGF expression.
Induction of vascular hyperpermeability by PlGF
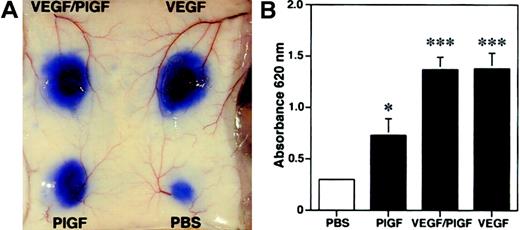
Because we found increased edema formation in PlGF transgenic mice and reduced inflammatory ear swelling in PlGF-deficient mice, we next investigated whether PlGF might directly induce vascular hyperpermeability. We performed a modified Miles vascular permeability assay using intravenous injection of the blue dye Evans blue followed by intradermal injection of recombinant human PlGF, VEGF, or PlGF/VEGF heterodimers. PlGF potently induced vascular hyperpermeability in mouse skin, as evidenced by the increased leakage of Evans blue (Figure7A). However, PlGF was a less potent inducer of vascular leakage than either VEGF or VEGF/PlGF heterodimers (Figure 7A). Spectrophotometric measurements of the amount of extravasated Evans blue revealed a more than 2-fold, significant increase of vascular permeability after injection of PlGF (OD620: 0.73 ± 0.16; mean ± SEM;P < .05) as compared with baseline levels observed after injection of phosphate-buffered saline (0.29 ± 0.02; Figure 7B). In comparison, injection of VEGF165 or VEGF/PlGF heterodimer resulted in a more than 4-fold induction of vascular leakage (VEGF165, 1.38 ± 0.15, P < .001; VEGF/PlGF, 1.37 ± 0.12, P < .001). These findings indicate that PlGF has the capacity to directly enhance cutaneous vascular permeability in vivo.
Enhanced vascular leakage after intradermal injection of PlGF.
PlGF, VEGF/PlGF heterodimer, VEGF (100 ng), or phosphate-buffered saline (PBS) were injected intradermally into the skin of wild-type mice after intravenous injection of Evans blue. (A) PlGF induced vascular hyperpermeability, as demonstrated by extravasation of Evans blue. The effect of VEGF and VEGF/PlGF on vascular leakage was even more pronounced. (B) To quantify the plasma extravasation, Evans blue was extracted from the skin. PlGF injection increased the plasma extravasation by more than 2-fold, whereas a more than 4-fold increase was observed with VEGF and VEGF/PlGF heterodimer when compared with PBS controls. Data are expressed as means ± SDs (n = 5). *P < .05; ***P < .001.
Enhanced vascular leakage after intradermal injection of PlGF.
PlGF, VEGF/PlGF heterodimer, VEGF (100 ng), or phosphate-buffered saline (PBS) were injected intradermally into the skin of wild-type mice after intravenous injection of Evans blue. (A) PlGF induced vascular hyperpermeability, as demonstrated by extravasation of Evans blue. The effect of VEGF and VEGF/PlGF on vascular leakage was even more pronounced. (B) To quantify the plasma extravasation, Evans blue was extracted from the skin. PlGF injection increased the plasma extravasation by more than 2-fold, whereas a more than 4-fold increase was observed with VEGF and VEGF/PlGF heterodimer when compared with PBS controls. Data are expressed as means ± SDs (n = 5). *P < .05; ***P < .001.
Discussion
Whereas it is well established that angiogenesis is of critical importance for tumor progression and tissue repair,6recent evidence indicates that angiogenesis also plays a major role in the pathogenesis of inflammatory diseases1 and that several proangiogenic mediators are up-regulated in inflamed tissue.11,13 In particular, vascular endothelial growth factor (VEGF) and its receptors are overexpressed in several inflammatory diseases.13 However, the expression of other members of the VEGF family of angiogenesis factors and their distinct biologic roles have not been directly tested in experimental inflammatory models. We used an established experimental model for skin inflammation27 to induce delayed-type hypersensitivity (DTH) reactions in mouse ear skin by sensitization and challenge with the contact sensitizer oxazolone. Our results reveal that, similar to the previously reported up-regulation of VEGF,12 the expression of placental growth factor (PlGF), an additional member of the VEGF family, was up-regulated during inflammation. The observed up-regulation of PlGF is in agreement with our recent findings of increased PlGF expression in several inflammatory human skin diseases (M.D. et al, unpublished results).
To test directly the role of PlGF during inflammation, we established transgenic mice with targeted overexpression of human PlGF-2 in the skin. Efficient transgene mRNA and protein expression were confirmed by Northern and Western blot analyses of lysates obtained from the skin of PlGF-2 transgenic mice. As previously shown for other transgenic models that used the K14 promoter for transgene expression,7,26expression of the human PlGF-2 transgene was specifically targeted to basal epidermal keratinocytes and to outer root sheath follicular keratinocytes, in accordance with the previously reported expression pattern of keratin 14.25 Although we found a slightly increased skin vascularization during the first postnatal days (data not shown), no significant increase of skin vascular density or of vascular size was detected in adult mice, and the overall histoarchitecture of the skin of wild-type and PlGF-2 transgenic mice was indistinguishable. These results are most likely related to the strong expression of the K14 promoter during the postnatal period25 with resulting strong expression ofPlGF-2 mRNA, whereas we found only low-levelPlGF-2 mRNA expression in the normal skin of adult transgenic mice. Additional support for a dose dependency of the effects of PlGF on cutaneous angiogenesis stems from results that adenoviral gene transfer into mouse skin resulted in increased vessel formation.41 However, these findings indicated that PlGF-2 is a less potent angiogenesis factor in vivo than VEGF, because we and others have previously shown that transgenic overexpression of VEGF in mouse skin, using the same keratin 14 promoter construct, resulted in persistent, potently increased cutaneous vascularity and in visible redness of the skin of adult transgenic mice.26,42Accordingly, we found that the architecture and the branching pattern of cutaneous blood vessels, as evaluated after systemic perfusion with the lectin Lycopersicon esculentum, were comparable in adult wild-type and in PlGF-2 transgenic mice, indicating that PlGF-2 overexpression, in contrast to VEGF overexpression,42 did not affect normal vascular development. The lack of a direct effect of PlGF overexpression on developmental vasculogenesis and angiogenesis is in agreement with a recent study that demonstrated that vascular development was unaffected in PlGF-deficient mice.24
To test our hypothesis that PlGF might predominantly act as a proinflammatory mediator, we next induced cutaneous DTH reactions in mice with targeted PlGF-2 overexpression in the skin. PlGF-2 transgenic mice showed significantly increased ear swelling and cutaneous edema after challenge with oxazolone, associated with pronounced enlargement of blood vessels and enhanced inflammatory infiltration. Moreover, the duration of the inflammatory response was prolonged in PlGF-2 transgenic mice as compared with wild-type mice. Although the potential development of antibodies against human PlGF-2 was not investigated in this model, the pronounced proinflammatory effects observed in adult K14/PlGF-2 transgenic mice suggest that transgenic mice did not produce substantial amounts of blocking antihuman PlGF-2 antibodies. Together with the observed increase of enlarged angiogenic vessels in the inflamed skin of PlGF-2 transgenic mice, our findings indicated that PlGF plays an important role in the induction of cutaneous inflammation and edema formation through stimulation of vascular remodeling.
To further quantify the effects of PlGF-2 on inflammatory angiogenesis, we performed computer-assisted morphometric analyses of tissue sections stained for the endothelial junction molecule CD31.43These analyses revealed a significant increase of the average vessel size in the inflamed skin of PlGF-2 transgenic mice as compared with wild-type mice. In contrast, no major differences in the vascular density (number of vessels per area unit) were detected in the inflamed skin of both genotypes. These findings are in agreement with the previously reported vascular abnormalities in inflammatory skin diseases. In particular, psoriatic skin lesions are characterized by elongation and enlargement of cutaneous microvessels without the formation of new vessel sprouts.3 Whereas angiogenesis has traditionally been defined as the sprouting of new capillaries from preexisting vessels,5 it has been recently proposed that a second type of angiogenesis (“remodeling/vascular enlargement type”) predominantly involves enlargement and elongation of preexisting vessels.44 Our results confirm that inflammatory cutaneous angiogenesis predominantly involves vascular enlargement, similar to the angiogenesis that occurs physiologically during the growth phase of the hair cycle,45 but not vascular sprouting, which occurs during embryogenesis, carcinogenesis, and tissue repair. The increase in the size of inflamed vessels observed in PlGF-2 transgenic mice indicates an important role of PlGF in the regulation of inflammatory vascular remodeling that involved endothelial cell proliferation.
The critical role of PlGF in the mediation of cutaneous inflammation and vascular enlargement was confirmed by studies of DTH reactions in the skin of PlGF-deficient mice. We found that the extent of edema and inflammatory cell infiltration was greatly diminished in the skin of PlGF-deficient mice as compared with wild-type mice. These results support the previously suggested concept that VEGF-mediated angiogenesis is dependent on the presence of PlGF in the tissue,24 because high VEGF levels are detected in cutaneous DTH reactions.12 However, PlGF deficiency only partially inhibited the cutaneous inflammatory reaction, indicating that either VEGF accounts for only a part of the proinflammatory and hyperpermeability-inducing activity in DTH reactions or that only a part of VEGF's activities are dependent on the presence of PlGF. Future studies in PlGF-deficient/VEGF transgenic mice might provide further insight into the functional in vivo interdependence of VEGF and PlGF.
To investigate whether the observed differences of cutaneous inflammation and edema formation in PlGF transgenic and PlGF-deficient mice might have been caused by direct or indirect effects on endogenous VEGF expression, we compared the VEGF protein levels in skin lysates obtained from the inflamed skin of the different genotypes. Quantitative ELISA analysis of endogenous murine VEGF tissue levels did not detect any significant differences between wild-type, PlGF transgenic, and PlGF-deficient mice. Together with the finding that the endogenous PlGF expression levels were also comparable in the inflamed skin of wild-type and PlGF-2 transgenic mice, these results indicate that PlGF plays a direct role in the control of the cutaneous inflammatory response. Further evidence for a direct role of PlGF in promoting inflammation and edema formation stems from the results of Miles vascular permeability assays performed in mouse skin demonstrating that intradermal injection of recombinant PlGF potently induced vascular leakage of cutaneous blood vessels although with a lower potency than VEGF. The exact mechanisms of action of PlGF on vascular endothelial cells remain at present unclear. However, the following nonexclusive mechanisms might contribute to the angiogenic and proinflammatory effects of PlGF: (1) PlGF could act directly through the Flt-1 receptor and induce unique intracellular signals that are distinct from those mediated through the Flk1 receptor by VEGF; (2) PlGF may displace VEGF from the Flt-1 receptor and thereby make more VEGF available for activation of the Flk1 receptor40; (3) PlGF activation of Flt1 may induce a cross-talk with Flk1 on endothelial cells (P.C. et al, unpublished results); (4) PlGF may recruit Flt1-positive bone marrow–derived progenitor cells that may be involved in vessel growth and inflammation.41,46 47
Taken together, our findings reveal a novel biologic function of placental growth factor in the control of cutaneous inflammation, vascular leakage, and edema formation, and they suggest that inhibition of PlGF bioactivity might represent a novel approach for the treatment of inflammatory diseases. Because underexpression or overexpression of PlGF did not affect normal vascular development or function, PlGF can be considered as a prototype “stress” angiogenic factor, suggesting that therapeutic blockade of such a molecule might be safer than blockade of molecules that are essential for vascular maintenance.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-05-1516.
Supported by National Institutes of Health/National Cancer Institute grants CA91861, CA69184, and CA86410 (M.D.); American Cancer Society Research Project grant 99-23901 (M.D.); and the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co Ltd Agreement (M.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Detmar, Cutaneous Biology Research Center, Department of Dermatology, Massachusetts General Hospital, Building 149, 13th St, Charlestown, MA 02129; e-mail:michael.detmar@cbrc2.mgh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal