Abstract
Recent findings have challenged the current view of Plasmodium falciparum (P falciparum) blood-stage biology by demonstrating the cytoadhesion of early ring-stage–infected erythrocytes (rIEs) to host endothelial cells and placental syncytiotrophoblasts. The adhesion of rIEs was observed only in parasites that bind to the placenta via chondroitin sulfate A (CSA). In this work, a panel of mouse monoclonal antibodies (mAbs) that specifically inhibit cytoadhesion of rIEs but not of mature IEs was generated The previously described ring surface protein 2 (RSP-2), a 42-kDa protein, was identified as the target of the ring-stage–specific mAbs. Time course surface fluorescence experiments revealed a short overlap (approximately 4 hours) of expression between RSP-2 and P falciparum erythrocyte membrane protein 1 (PfEMP1). Their consecutive expression enables IEs to adhere to endothelial cells during the entire blood-stage cycle. During this study, a new phenotype was detected in parasite cultures, the adhesion of normal erythrocytes (nEs) to endothelial cells. All adherent nEs were coated with RSP-2. Immunolocalization studies show that RSP-2 is a rhoptry-derived protein that is discharged onto the erythrocyte membrane during contact with merozoites. Our results identify RSP-2 as a key molecule in sequestration of young blood-stage forms and nEs to endothelial cells.
Introduction
The unique ability of Plasmodium falciparum (P falciparum)–infected erythrocytes (IEs) to adhere to the endothelial cells of microvessels and placental syncytiotrophoblasts was previously attributed exclusively to mature IE stages (trophozoite and schizonts).1 This dogma has been challenged by the recent discovery that ring-stage IEs (rIEs) can bind to endothelial cells and syncytiotrophoblasts in a PfEMP1-independent manner to an as-yet-unknown receptor.2 Adhesion of rIEs is seen only in parasites that will acquire the chondroitin sulfate A (CSA)–binding phenotype at the trophozoite stage (a particular adhesion phenotype is seen in parasites that bind to placental syncytiotrophoblasts).3 This finding points to a specific subpopulation of IEs that sequentially displays 2 different adhesion phenotypes during the 48-hour blood-stage cycle. This raises the possibility that noncirculating, and therefore cryptic, parasite subpopulations could be present in patients with malaria.Aprevious study on sequestration of P falciparum in the human brain supported the idea that ring-stage IEs may cytoadhere in patients other than pregnant women and play a role in severe malaria.4 In this study, all developmental stages were observed in brain vessels of patients dying from cerebral malaria, and several vessels contained large numbers of rIEs. The underrepresentation of specific virulent adhesive phenotypes in the bloodstream may have a major impact on clinical studies based on peripheral blood–stage parasites.
The search for molecules involved in the binding of ring-stage parasites revealed the presence of 2 novel ring-stage surface proteins named RSP-1 and RSP-2. Their expression during the blood-stage cycle coincides with the observed change in adhesive phenotype, thus suggesting a role for RSP-1 or RSP-2 in rIE adhesion.2 In order to investigate this novel adhesive phenotype, we developed mouse monoclonal antibodies (mAbs) that react specifically with intact rIEs using a new immunization procedure.5 These mAbs allowed us to identify RSP-2 as an important molecule in the adhesion process and revealed the mechanism used to transfer RSP2 to the surface of rIEs. An unexpected finding is that RSP-2 is found on a large number of normal erythrocytes (nEs). In placental isolates, noninfected erythrocytes that carry RSP-2 on their surface (nERSP-2) show a similar adhesion phenotype to rIEs under flow conditions using endothelial cells and are significantly more resistant to shear stress than PfEMP1-mediated IE binding.
Materials and methods
Parasites
We used strains BXII, HB3, FCBR, Suk, H, IBR, FCR3CSA, and 6 Cameroonian clinical isolates (nos. 24, 42, 42DJ, 193, and 939) eluted from human placentas with a soluble CSA.6 CSA or CD36 and intercellular adhesion molecule-1 (ICAM-1) adhesive phenotypes were selected from the FCR3 strain by panning on the Saimiri monkey brain microvascular endothelial cells Sc17, ScC2, and Sc3A4 as described elsewhere.7 Cytoadhesion assays using Saimiri brain endothelial cell (SBEC) Sc1D cell lines were performed as described.8 Parasites were grown under standard culture conditions,8 replacing the 10% human serum with 0.25% Albumax (Life Technology, Paris, France). For some experiments parasites were synchronized (± 2 hours) (if not otherwise mentioned) by repeated 5% sorbitol treatment.
Development of monoclonal antibodies
To develop antibodies against parasite-specific surface proteins of rIEs, we immunized BALB/c mice, which had been rendered B-cell immunotolerant against normal human O– red blood cells, with synchronized rIEs of the CSA phenotype (rIECSA) as described earlier.5 Two days after the third boost the mice were killed, their spleen cells fused with P3U1 cells and distributed in 96-well flat bottom plates.9,10 Screening for antibodies directed against the surface rIECSA was done by liquid immunofluorescence assays (L-IFA). Cloning by limited dilution and expansion of positive clones was performed as described elsewhere.9,10
Purification of mAbs using modified protein A (MAPS, Bio-Rad, Marne la Coquette, France) and isotyping with the ImmunoPure monoclonal antibody isotyping Kit-I (Pierce, Rockford, IL) was done following the manufacturer's recommendations. Three mAbs, B4 (IgG2a), C10 (IgG2a), and D10 (IgG3), were selected for their functional differences and further characterized.
Polyclonal human anti–P falciparum sera pool
Nineteen individual immune sera from multiparous Senegalese women were analyzed individually by L-IFA. Sera positive in surface IFA with rIEs were pooled at equivalent parts. This pool was used to immunoprecipitate RSP-1 and RSP-2 I125-labeled extracts from rIEs. The human sera were obtained from Dr O. Garraud from the Pasteur Institute of Dakar, Senegal, in conformation with the procedure recommended by the ethical committee of the Pasteur Institute.
Immunofluorescence assays
mAbs were assessed by L-IFA at +4°C and on air-dried (AD-IFA) parasites, selected for binding to CSA, CD36, and ICAM-1 as described.5 To assess the surface exposure of the antigen, rIEs were preincubated with 100 μg/mL trypsin or chymotrypsin before adding the mAbs.2 The dynamics of RSP-2 and PfEMP1CSA expression were assessed by L-IFA using synchronized IECSA (± 2 hours) incubated with 40 μg/mL DAPI (4′6-diamidino-2-phenylindole 2HCl). After washing, the IEs were incubated with 10 μg/mL purified mAb B4, washed, and bound antibodies detected with the goat (Fab)′2 Alexa Fluor 488–labeled antimouse IgG. IECSA were incubated a second time for 30 minutes at 4°C with 10 μg/mL purified anti-PfEMP1CSA mAb 1B4/D4,5 which was covalently labeled to Alexa Fluor 594 (Molecular Probes) following the manufacturer's recommendations, washed, and then analyzed. The percentage of RSP-2 surface-stained rIEs and nEs was assessed by L-IFA using synchronized FCR3CSA cultures, 4 hours after reinvasion, at a parasitemia of 5%, 10%, and 20%. Results from 4 individual experiments were expressed as the arithmetic mean ± 2 SD of labeled rIEs and nEs versus total number of erythrocytes and of labeled rIEs versus all rIEs. For the AD-IFA, a monolayer of IEs was washed twice with phosphate-buffered saline (PBS) pH7.2 and coincubated for 30 minutes at +37°C with mAb culture supernatant or 0.1 to 10 μg/mL purified mAbs in the presence of 1 μg/mL DAPI. After washing, bound antibodies were revealed with the Alexa Fluor 488–labeled antimouse IgG. In all experiments, the IF staining was analyzed with a Nikon E800 microscope and images acquired with a DXM 1200 Nikon camera. As negative controls, either P3U1 culture supernatant or unrelated mouse IgG isotypes (Sigma, St Quentin Fallavier, France) were used.
To assess whether physical interaction between nEs and merozoites is prerequisite for RSP-2 transfer to the erythrocyte surface, highly synchronized mature stage IEs and nEs were coincubated, but separated physically by a membrane barrier. Mature-stage IEs of the CSA, CD36, and ICAM-1 phenotypes were concentrated by gelatin flotation, washed twice with culture medium, and deposited in the upper chamber of 6-well plates, separated from the nEs at the bottom of the chamber by a low-protein binding polyester membrane with a pore size of 0.4 μm (Transwell-Clear, Corning Costar, Cambridge). After 10 hours of coincubation under the same conditions as a standard culture, nEs were recovered and assessed by L-IFA for the presence of RSP-2.
Immunoprecipitation of 35S-methionin and 125I surface–labeled IE extracts
Synchronized mIEs, previously selected by panning on Sc17, C2, and 3A4 for the CSA, CD36, and ICAM-1 binding phenotypes, respectively,7 were cultivated in the presence of 2 mCi (74 MBq) 35S-methionin (Amersham, France). The culture was stopped at 0 hour or 8 hours and proteins extracted using Triton X-100. In some cases L-1-Tosylamide-2-phenylethyl chloromethyl ketone (TPCK)–trypsin and Nα-p-Tosyl-L-Lysine chloromethyl ketone hydrochloride (TLCK)–α-chymotrypsin (Sigma) treatments were performed before the protein extractions.
125I surface labeling of rIEs (> 10% parasitemia) was performed as previously described.11 Protein extracts were prepared in PBS with protease inhibitors containing Triton X-100 and 2% sodium dodecyl sulfate (SDS). Extracts were immunoprecipitated with mAbs. The sera pool from multiparous Cameroonian women (as described6 ) was used as a positive control and unrelated mouse IgG of the same isotypes (Sigma) as a negative control for the mAbs. IgG immune complexes were recovered by incubation with protein G sepharose (Amersham-Pharmacia, Orsay, France) and separated by 5%-10% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), dried, and exposed on a Kodak MR film.
Western blot of rIE Triton X-100 extracts
Proteins from Triton X-100 rIE extracts were separated by 12.5% SDS-PAGE, transferred to nitrocellulose membranes, and blocked in 5% skimmed milk in PBS pH7.2 for 2 hours at room temperature. Anti–RSP-2 mAbs at 20 μg/mL were incubated at room temperature for 1 hour. Membranes were washed twice with 5% milk in PBS and incubated for 1 hour with goat anti–mouse IgG (H+L)-AP-Conjugate (Bio-Rad). After 2 additional washes, proteins were revealed with Alkaline Phosphatase Conjugate Substrate Kit (Bio-Rad).
Static cytoadhesion inhibition and disruption assays
Cytoadhesion inhibition and disruption assays were performed on Sc1D cells grown to confluence on 12-dot IFA slides as described elsewhere8 by assessing the laboratory CSA-binding strains FCR3, FCBR, BXII, SUK, HB, and IBR, and the placenta isolates nos. 24, 42,42DJ, 193, and 939. In short, 20 μL mixture of synchronized rIEs with nEs or mature blood–stage infected erythrocytes (mIEs) from corresponding cultures at a parasitemia of 4%-15% for a total 107 IE/mL RPMI 1640, pH6.8 were layered per dot of confluent Sc1D cells. For the inhibition assay, 20 μL each mAb at a concentration of 0.2 to 200 μg/mL was deposited simultaneously with rIEs or IEs and corresponding nEs/dot for 2 hours at +37°C. For the disruption assay the same mAbs were added after a 1-hour preincubation of IEs and nEs with endothelial cells for an additional 1 hour. Unbound erythrocytes were removed by vigorously washing the slides in RPMI 1640, pH6.8. Bound IEs and nEs were fixed with 0.25% glutaraldehyde in PBS pH7.2. The inhibition and disruption of cytoadhesion were expressed as bound IEs and nE/mm2 of confluent endothelial cells in the presence of mAbs, in comparison to unrelated mouse IgG of the same IgG isotype (Sigma).
Inhibition and shear stress resistance of adherent rIEs and nEs under flow conditions
Different concentrations (0.2-200 μg/mL) of anti–RSP-2 mAbs were mixed with 107/mL 8 ± 2–hour-old rIEs and flowed through a microslide (VD/3530-050; Camlab, Cambridge, United Kingdom) over a confluent, 3-day-old monolayer of Sc1D cells at 0.05 Pascal (Pa), which is the shear stress that mimics conditions in a placenta. Results are expressed as the arithmetic mean ± SD of the percentage of inhibition of cytoadhesion in 3 individual experiments. As a control we used unrelated homologous mouse IgG.
To evaluate the resistance of increasing shear stresses, synchronized 4 ± 2–hour-old rIEs and nEs from the same culture were passed through a microslide at a final hematocrit of 25% in RPMI 1640, pH 7 for 10 minutes at 0.05 Pa over a 3-day-old confluent Sc1D cell layer.12 After rinsing with the same medium for 10 minutes at 0.05 Pa to remove residual unbound erythrocytes, the flow rate was increased for 10 minutes each time to gradually obtain wall shear stresses from 0.05 Pa to 2.6 Pa. Normal blood circulation through a normal placenta corresponds to 0.05 Pa, while 0.1 Pa corresponds to a wall shear stress in postvenule capillaries.13 Experiments were repeated 3 times and expressed as the arithmetic mean percentage ± SD of bound rIEs and nEs for each shear stress increment.
Functional and chronological differentiation between RSP-2 and PfEMP1CSA in a parasite cytoadhesion inhibition assay under flow conditions
To compare the resistance of rIEs and mature IEs at various shear stresses, we used a new cytoadhesion assay on SBEC 1D and placental tissues (B.T. et al, manuscript in preparation). In short, 4 serial 7-μm normal human placenta cryosections (each approximately 4 mm × 4 mm) of the maternal compartment were mounted on a microscopic slide 75-25 mm and fixed with 0.5% paraformaldehyde in PBS at pH 7.2 for 2 hours at room temperature. After washing several times with PBS and then with RPMI 1640 pH 6.8, the slide is mounted on a perusing chamber (Immunetics, Cambridge, MA). An equipart mixture of 8 ± 4–hour-old synchronized rIECSA (including nEs from the same culture) and 34 ± 4–hour-old IECSA at a final hematocrit of 25% in RPMI 1640, pH 7.2 were then passed through the chamber for 10 minutes at 0.05 Pa. After rinsing for 10 minutes with medium at the same shear stress, 100 μg/mL of a 50-kDa CSA (Fluka, France) dissolved in RPMI or 200 μg/mL mAbs was flushed through the chamber for 10 minutes at the same shear stress. Slides from 3 experiments were recovered and stained by Giemsa. Mature IEs, rIEs, and nEs were counted and the result expressed as the arithmetic mean percentage of bound erythrocytes ± SD/20 fields of an X-100 oil objective (Nikon Plan Apo, Japan) as described elsewhere.6
Merozoite invasion inhibition assay
Inhibition of merozoite invasion by mAbs was assessed by slightly modifying a technique described elsewhere.14 200 μL/well of a mIE highly synchronized suspension (2.2% final hematocrit in RPMI + 0.5% Albumax) were incubated in the above-mentioned culture conditions in 96-well flat-bottom plates (NUNC, VW International, Strasbourg, France) with 100 μL 9.5 to 300 μg/mL mAbs. Added 4 hours after invasion was 1 μCi (0.037 MBq) 3H-hypoxanthine, with a specific activity of 14.1 Ci/mmol (52.17 × 1010 Bq/mmol) (NEN Products, Dreiech, Germany). Cultures were freeze/thawed 24 hours later to lyse the erythrocytes, and the contents of each well were collected on standard filter microplates (Unifilter GF/B; Packard Instrument, Meriden, CT) and washed using a cell harvester (FilterMat Cell harvester; Packard Instrument). Filters were dried and 25 μL scintillation cocktail (Microscint; Packard Instrument) was added to each well. Incorporation was analyzed using a scintillation counter (Top Count; Packard Instrument). As a positive control we used 1 M chloroquine, and as a negative control unrelated mouse IgG of the same isotype. The percentage of inhibition was calculated using the t test by comparing the count-per-minute values of controls with those obtained in the presence of mAbs.
Results
Generation of specific monoclonal antibodies against the surface of rIEs
Mice were immunized with rIEs from the FCR3 strain (selected for CSA binding) using a novel immunization procedure (see “Materials and methods”). Animals that responded to rIE surface antigens were identified using L-IFA and chosen to produce mAbs. Obtained were 3 mAbs that recognize a parasite surface antigen present on rIEs and inhibit adhesion of rIEs to endothelial cells. mAbs B4 (IgG2a), C10 (IgG2a), and D10 (IgG3) were used to study the molecule(s) involved in rIE adhesion. The antigen was detectable at the surface of ring stages immediately after merozoite release (Figure 1A), whereas no labeling was seen in mature parasite stages (> 20 hours) (Figure 1D). However, mAbs B4, C10, and D10 reacted only with about 30% of rIEs using L-IFA. AD-IFA results indicated that a fraction of the antigen is internalized during merozoite invasion (Figure 1E), and de novo expression occurs during the early trophozoite stage (Figure 1F). Later, in the schizont stage, the antigen accumulates in rhoptries, where it can be identified by its typical double-dot staining (Figure 1G). This was confirmed using a rhoptry-specific marker (RAP1, data not shown). At the very end of the blood-stage cycle before red blood cell disruption and merozoite release, the target of the mAbs seems to be translocated to the merozoite surface (Figure 1H).
A rhoptry-derived P falciparum parasite molecule binds to the surface of normal and infected erythrocytes. Staining of nEs and IEs from FCR3CSA parasites using mAb B4 (green) in IFA. Parasite DNA is stained with DAPI (blue). (A-D) L-IFA analysis. (E-H) AD-IFA analysis. (A) Merozoite binding to the membrane of an nE stained with anti–RSP-2. (B) Surface staining of an nE (left) and rIE (right). (C) Transfer of RSP-2 from merozoite to the entire erythrocyte surface. (D) A mature trophozoite-stage parasite is not stained by mAb B4. AD-IFA using B4 in young rIEs. (E) Endoplasmic reticulum (ER) in 26 ± 2–hour-old parasites. (F) Rhoptries in 34 ± 2–hour-old schizont stage (G) and surface of free merozoites at 44 ± 2 hours (H). Scale bars measure 10 μm.
A rhoptry-derived P falciparum parasite molecule binds to the surface of normal and infected erythrocytes. Staining of nEs and IEs from FCR3CSA parasites using mAb B4 (green) in IFA. Parasite DNA is stained with DAPI (blue). (A-D) L-IFA analysis. (E-H) AD-IFA analysis. (A) Merozoite binding to the membrane of an nE stained with anti–RSP-2. (B) Surface staining of an nE (left) and rIE (right). (C) Transfer of RSP-2 from merozoite to the entire erythrocyte surface. (D) A mature trophozoite-stage parasite is not stained by mAb B4. AD-IFA using B4 in young rIEs. (E) Endoplasmic reticulum (ER) in 26 ± 2–hour-old parasites. (F) Rhoptries in 34 ± 2–hour-old schizont stage (G) and surface of free merozoites at 44 ± 2 hours (H). Scale bars measure 10 μm.
Surprisingly, nEs also were stained by all 3 mAbs (Figure 1B). At elevated parasitemia (5%-10%) approximately 30% of all erythrocytes were positive using L-IFA. Surface labeling of rIEs and nEs was completely abolished by trypsin or chymotrypsin treatment (data not shown). Performing L-IFA with synchronized IEs at a 6-hour interval over the entire blood-stage cycle revealed that the antigen was initially discharged on to the erythrocyte membrane surface during contact with a merozoite (Figure 1C). The antigen is then dispersed, probably by lateral movement, over the entire surface of rIEs and nEs. We conclude that the presence of the same antigen on nEs is a direct consequence of aborted merozoite invasion. We then asked the question whether the rIE surface antigen could be released before the attachment of the merozoite to the erythrocyte membrane. We cocultured synchronized schizont parasite stage (CD36, ICAM-1, and CSA adhesion phenotypes) for 18 hours in a 2-chamber culture system, separating nEs from synchronized late-stage infected erythrocytes using a polyester membrane with a pore size of 0.4 μm that allows diffusion of large proteins. Under such culture conditions we were not able to detect a signal on nEs using mAbs B4, C10, and D10 (data not shown). This experiment and the fact that only a fraction of nEs is RSP-2 tagged strongly suggest that contact is necessary for transfer of the antigen.
mAbs detect a protease-sensitive 42-kDa surface protein that forms a complex with 2 other proteins
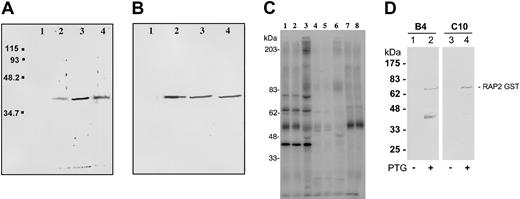
Western blot analysis using Triton X-100 parasite protein extracts (schizonts and rIEs) indicated that each mAb (B4, C10, and D10) recognized a 42-kDa protein (Figure 2A). A molecule of identical size was detected by SDS-PAGE in FCR3 parasite extracts of the adhesion phenotypes CSA, CD36, and ICAM-1 (Figure 2B).
Anti–RSP-2 mAbs recognize a 42-kDa molecule. (A) Western blot analysis using mAbs B4, C10, and D10. Triton X-100 soluble protein extract of rIECSA was analyzed. Lane 1, negative control IgG2a; lane 2, anti–RSP-2 C10; lane 3, anti–RSP-2 B4; and lane 4, anti–RSP-2 D10. (B) Western blot with anti–RSP-2 mAb B4 and Triton X-100 protein extracts. Lane 1, nE; lane 2, IECSA; lane 3, IECD36; and lane 4, IEICAM-1. (C) Immunoprecipitation of Triton X-100 extracts of surface-iodinated FCR3CSA and FCR3CD36 rIEs using mAb B4. Lane 1, surface-iodinated FCR3CSA rIEs immunoprecipitated by B4; lane 2, FCR3CD36 immunoprecipitated by B4; lane 3, FCR3CSA immunoprecipitated by a pool of immune sera from pregnant women (Senegal); lane 4, FCR3CSA trypsin treatment (100 μg/mL) before immunoprecipitation by B4; lane 5, FCR3CD36 trypsin treatment before immunoprecipitation by B4; lane 6, FCR3CSA trypsin treatment before immunoprecipitation by a pool of immune sera from pregnant women. Controls, FCR3CSA immunoprecipitation with no mAbs (lane 7) and by anti-PfEMP1 (lane 8). (D) Western blot analysis of E coli extracts expressing RAP-2 GST fusion proteins. Lanes 1 and 3, noninduced cultures; lanes 2 and 4, IPTG-induced cultures. Lanes 1 and 2, mAb B4; lanes 3 and 4, mAb C10. The RAP-2 GST-predicted molecular weight is 71 kDa. The lower band (lane 2) is a degradation product.
Anti–RSP-2 mAbs recognize a 42-kDa molecule. (A) Western blot analysis using mAbs B4, C10, and D10. Triton X-100 soluble protein extract of rIECSA was analyzed. Lane 1, negative control IgG2a; lane 2, anti–RSP-2 C10; lane 3, anti–RSP-2 B4; and lane 4, anti–RSP-2 D10. (B) Western blot with anti–RSP-2 mAb B4 and Triton X-100 protein extracts. Lane 1, nE; lane 2, IECSA; lane 3, IECD36; and lane 4, IEICAM-1. (C) Immunoprecipitation of Triton X-100 extracts of surface-iodinated FCR3CSA and FCR3CD36 rIEs using mAb B4. Lane 1, surface-iodinated FCR3CSA rIEs immunoprecipitated by B4; lane 2, FCR3CD36 immunoprecipitated by B4; lane 3, FCR3CSA immunoprecipitated by a pool of immune sera from pregnant women (Senegal); lane 4, FCR3CSA trypsin treatment (100 μg/mL) before immunoprecipitation by B4; lane 5, FCR3CD36 trypsin treatment before immunoprecipitation by B4; lane 6, FCR3CSA trypsin treatment before immunoprecipitation by a pool of immune sera from pregnant women. Controls, FCR3CSA immunoprecipitation with no mAbs (lane 7) and by anti-PfEMP1 (lane 8). (D) Western blot analysis of E coli extracts expressing RAP-2 GST fusion proteins. Lanes 1 and 3, noninduced cultures; lanes 2 and 4, IPTG-induced cultures. Lanes 1 and 2, mAb B4; lanes 3 and 4, mAb C10. The RAP-2 GST-predicted molecular weight is 71 kDa. The lower band (lane 2) is a degradation product.
The mAbs immunoprecipitated proteins of molecular weights of approximately 79, 72, and 42 kDa (data not shown) from S35methionin-labeled Triton X-100 rIE extracts. The same set of proteins was recognized by a sera pool from multiparous Senegalese women and also was immunoprecipitated from merozoite extracts of different adhesion phenotypes (CSA, CD36, and ICAM-1) (data not shown).
mAbs B4, C10, and D10 immunoprecipitated 3 proteins from 125I-surface–labeled rIE extracts. A major band was seen at approximately 42 kDa and 2 weaker bands of approximately 79 and 72 kDa. mAb B4 is shown as an example in Figure 2C, lanes 1-2. A pool of immune sera from multiparous Senegalese women immunoprecipitated an additional band (doublet) of approximately 200 kDa (lane 3), which we had described earlier and probably corresponds to RSP-1. These proteins are trypsin sensitive at similar concentrations described for RSP-1 and RSP-22 (lanes 4-6).
Our results show that mAbs B4, C10, and D10 are specifically directed against a protein that has the characteristic features of RSP-2.2 The absence of the 79 and 72 kDa protein in Western blots suggests that these proteins form a complex with the 42-kDa protein in the rhoptries, which is disrupted in the presence of SDS. A similar RSP-2 protein complex is observed at the surface of rIEs. The fact that mAbs B4 and C10 recognize a 42-kDa molecule of a rhoptry protein complex suggested that RSP-2 could correspond to a protein identified earlier as rhoptry associated protein 2′ (RAP2).15,16 To address this question, we expressed RAP2 as glutathione-S–transferase (GST) fusion protein in Escherichia coli. mAbs B4 and C10 recognize the 71-kDa RAP-2 fusion protein by Western blot (Figure 2D). No reaction was seen with GST alone (data not shown). Consequently, we conclude that RSP-2 is encoded by the RAP2 gene.
Inhibition of cytoadhesion in RSP-2–positive rIEs and nEs
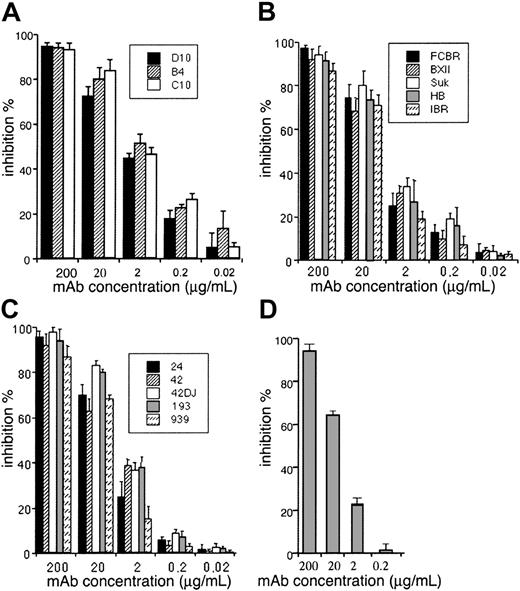
To confirm the involvement of RSP-2 in cytoadhesion of rIEs and nEs to CSA, we used cytoadhesion inhibition and dissociation assays under static and flow conditions using the Saimiri brain endothelial cell line Sc1D and placenta cryosections.6 Only RSP-2–positive rIEs and nEs of parasites selected for the CSA phenotype were able to cytoadhere to endothelial cells or syncytiotrophoblasts under static and flow conditions. The mAbs inhibited cytoadhesion of these synchronized rIEs and nEs by more than 94% at a concentration of 200 μg/mL and was still significant (approximately 50%) at a concentration of 2 μg/mL (Figure 3A). The enzymatic digestion of RSP-2 with trypsin or chymotrypsin abolished this adhesion capacity completely (not shown). This result confirmed the L-IFA and immunoprecipitation results using 125I-labeled rIE and nE membrane extracts and clearly highlights the role of RSP-2 as the cytoadhesion ligand for the rIE CSA phenotype. In addition, the mAbs inhibited the adhesion of rIE CSA phenotypes equally well from 5 genetically distinct laboratory strains (Figure 3B) and 5 distinct CSA binding parasite populations isolated from the placentas of 5 Cameroonian women (Figure 3C). The inhibition of rIE and nE cytoadhesion using the mAbs was confirmed by performing the inhibition assay under flow conditions at 0.05 Pa, which corresponds to shear stress observed in the placenta (Figure 3D).
Inhibition of cytoadhesion of FCR3 rIECSA to endothelial cells using anti–RSP-2 mAbs. 40μL suspension of 107 rIECSA/mL was incubated for 2 hours at 37°C on a confluent monolayer of Sc1D cells grown on 12 dot IFA slides in the presence of different concentrations of mAbs. Slides were washed and adherent rIEs counted. Results are expressed as mean ± SD percent inhibition of control. (A) Inhibition of rIECSA cytoadhesion in the presence of different concentrations of mAbs B4, C10, and D10. (B) Inhibition of rIECSA cytoadhesion of laboratory strains from different geographical areas, FCBR, BXII, SUK, HB, and IBR to Sc1D. (C) Placental isolates from Cameroonian women by anti–RSP-2 mAb B4. (D) Inhibition of rIECSA cytoadhesion to Sc1D by different concentrations of mAb B4 mixed together with 2 × 107 rIECSA under flow conditions at 0.05 Pa.
Inhibition of cytoadhesion of FCR3 rIECSA to endothelial cells using anti–RSP-2 mAbs. 40μL suspension of 107 rIECSA/mL was incubated for 2 hours at 37°C on a confluent monolayer of Sc1D cells grown on 12 dot IFA slides in the presence of different concentrations of mAbs. Slides were washed and adherent rIEs counted. Results are expressed as mean ± SD percent inhibition of control. (A) Inhibition of rIECSA cytoadhesion in the presence of different concentrations of mAbs B4, C10, and D10. (B) Inhibition of rIECSA cytoadhesion of laboratory strains from different geographical areas, FCBR, BXII, SUK, HB, and IBR to Sc1D. (C) Placental isolates from Cameroonian women by anti–RSP-2 mAb B4. (D) Inhibition of rIECSA cytoadhesion to Sc1D by different concentrations of mAb B4 mixed together with 2 × 107 rIECSA under flow conditions at 0.05 Pa.
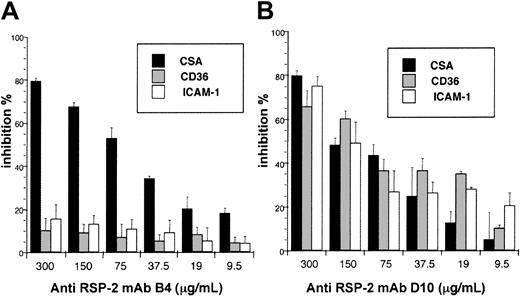
To establish that the interaction of RSP-2 with the host cell was not a low-affinity or nonspecific binding event, we performed a desequestration study by exposing adherent RSP-2–positive erythrocytes to increasing shear stress. Synchronized RSP-2/tagged rIEs and nEs were passed over cryosections of normal human placentas at 0.05 Pa. The subsequent increase of the shear stress from 0.05 Pa to more than 3 Pa revealed an extremely strong interaction with syncytiotrophoblasts. Up to 50% of bound erythrocytes resisted a wall shear stress of around 2.1 Pa, and about 25% of bound erythrocytes resisted to a wall shear stress exceeding 3 Pa at pH 6.8 (Figure 4). This is roughly the same resistance to shear stress as found for mature IEs of the CSA phenotype at pH 6.8. RSP-2 adhesion resists much higher shear stress than the CD36 and ICAM-1 phenotypes.12 Such high levels of shear stress probably are never encountered in the placenta or in microvasculature. We show that RSP-2 is the molecule involved in ring-stage cytoadhesion, and our results also underline the possibility that this phenotype may sequester in target organs other than the placenta.
Desequestration of cytoadherent rIECSA to Sc1D cells under flow conditions. Cytoadhesion was performed by flowing of 2 × 107 rIECSA over a confluent monolayer of Sc1D grown in microslides at 0.05 Pa before rinsing at various shear stresses with culture medium. Results of residual cytoadherent rIECSA are expressed as mean ± SD percent inhibition of control for each shear stress increment starting at 0.05 Pa.
Desequestration of cytoadherent rIECSA to Sc1D cells under flow conditions. Cytoadhesion was performed by flowing of 2 × 107 rIECSA over a confluent monolayer of Sc1D grown in microslides at 0.05 Pa before rinsing at various shear stresses with culture medium. Results of residual cytoadherent rIECSA are expressed as mean ± SD percent inhibition of control for each shear stress increment starting at 0.05 Pa.
Chronology of RSP-2 and PfEMP1CSA surface expression and stage-specific cytoadhesion under flow conditions
To define the difference between RSP-2 and PfEMP1CSA-mediated cytoadhesion more precisely, we performed colocalization L-IFA assays on highly synchronized parasites using anti–RSP-2 and PfEMP1CSA-specific mAbs. The same IE samples were coincubated with fluorescein isothiocyanate (FITC)–labeled anti–RSP-2 and rhodamine-labeled anti-PfEMP1CSA. Figure 5A shows a RSP-2–positive staining (green) of the surface of rIEs (< 16 hours) and young trophozoites (16-20 hours). No staining was detectable in mature trophozoites (> 20 hours).
Expression profile of RSP-2 and PfEMP1 at the erythrocyte surface during the blood-stage cycle. RSP-2CSA was stained with mAb B4 (green) and PfEMP1CSA with mAb 1B4/D4 (red) by L-IFA on IE. Parasite nuclei are stained by DAPI (blue). (A) Positive anti–RSP-2 staining on synchronized rIEs (< 16 hours after invasion) and early trophozoites (16 to 20 hours after invasion). No surface staining was detectable on mature forms (> 20 hours after invasion). (B) Absence of PfEMP1 staining on rings (< 16 hours) but strong IFA signal with early trophozoite and mature stages. (C) Superposition of anti–RSP-2 and anti-PfEMP1 staining shows colocalization in early trophozoite stages. Scale bar measures 10 μm.
Expression profile of RSP-2 and PfEMP1 at the erythrocyte surface during the blood-stage cycle. RSP-2CSA was stained with mAb B4 (green) and PfEMP1CSA with mAb 1B4/D4 (red) by L-IFA on IE. Parasite nuclei are stained by DAPI (blue). (A) Positive anti–RSP-2 staining on synchronized rIEs (< 16 hours after invasion) and early trophozoites (16 to 20 hours after invasion). No surface staining was detectable on mature forms (> 20 hours after invasion). (B) Absence of PfEMP1 staining on rings (< 16 hours) but strong IFA signal with early trophozoite and mature stages. (C) Superposition of anti–RSP-2 and anti-PfEMP1 staining shows colocalization in early trophozoite stages. Scale bar measures 10 μm.
mAb directed against PfEMP1CSA (panel B) shows specific surface staining (red) from young trophozoites on. Merging panels A and B demonstrates a positive surface labeling of anti–RSP-2 and anti-PfEMP1CSA on the same IEs for approximately 4 hours (panel C). These results suggest that both adhesion ligands can be present on the same IE for at least a few hours during the switch from RSP-2 to PfEMP1CSA. The sequential surface expression of RSP-2, then RSP-2 and PfEMP1CSA, followed by only PfEMP1CSA during the parasite cycle, provides the CSA-binding parasites with the capacity to remain sequestered during the whole blood-stage cycle.
Adhesion to the same endothelial cells via 2 distinct parasite adhesion ligands was demonstrated using rIECSA and mature IECSA in adhesion assays using Sc1D cells under flow conditions at 0.05 Pa. Equal parts of rIECSA/nECSA mature IECSA were passed over the endothelial cells. All populations bind to the Sc1D cells in comparable numbers (Figure 6A). Flushing with anti–RSP-2 B4 at 200 μg/mL resulted in the desequestration of rIECSA/nECSA but did not effect mature IECSA (Figure 6B). Flushing with CSA (100 μg/mL final concentration) removed only the mature forms but not the rIECSA/nECSA (Figure 6C). The combination of anti–RSP-2 and CSA eliminated all blood-stage forms as shown in Figure 6D.
Blocking of rIECSA and mature IECSA adhesion to Sc1D cells in the presence of specific inhibitors. A culture of rIECSA and mature IECSA parasites (equal parasitemia) was passed over confluent Sc1D cells in microslides at 0.05 Pa. (A) Sc1D cells with bound rIECSA and mature IECSA. (B) Flushing with 2 mL 100 μg/mL anti–RSP-2 mAb B4 in culture medium. (C) Flushing with 2 mL 100 μg/mL CSA dissolved in culture medium. (D) nE, rIECSA, and mature IECSA were desequestrated using both inhibitors. Scale bar measures 10 μm.
Blocking of rIECSA and mature IECSA adhesion to Sc1D cells in the presence of specific inhibitors. A culture of rIECSA and mature IECSA parasites (equal parasitemia) was passed over confluent Sc1D cells in microslides at 0.05 Pa. (A) Sc1D cells with bound rIECSA and mature IECSA. (B) Flushing with 2 mL 100 μg/mL anti–RSP-2 mAb B4 in culture medium. (C) Flushing with 2 mL 100 μg/mL CSA dissolved in culture medium. (D) nE, rIECSA, and mature IECSA were desequestrated using both inhibitors. Scale bar measures 10 μm.
Inhibition of merozoite invasion by mAbs
Since AD-IFA analysis indicates that RSP-2 seems to be on the merozoite surface at the end of schizogony, we suspected that anti–RSP-2 mAbs may also affect merozoite invasion. To address this question we incubated mAbs B4, C10, and D10 and unrelated IgG of the same isotype with highly synchronized IEs (36 hours after invasion) and measured the incorporation of 3H hypoxanthine (see “Materials and methods”). The results indicated that mAb B4 efficiently inhibited merozoite invasion of the CSA phenotype but not of CD36 or ICAM-1–selected parasites (Figure 7A), whereas mAb D10 inhibited the merozoite invasion of the phenotypes CSA, CD36, and ICAM-1 at similar levels (Figure 7B). mAb C10 had no such inhibitory effect at high protein concentrations (not shown). The results indicated that the mAbs are directed against different epitopes on the RSP-2 molecule.
Inhibition of merozoite invasion using anti–RSP-2 mAbs. A suspension of synchronized mature IEs of the adhesion phenotypes CSA, CD36, and ICAM-1 in 200 μL/well at a final hematocrit of 2% in culture medium were incubated in 96-well flat-bottom plates together with 10 μL mAbs B4 (A) and D10 (B) at different concentrations. Four hours after invasion 100 μCi (3.7 MBq) of 3H-hypoxanthine was added and the incorporated radioactivity counted 24 hours later. The percentage (±SD) of inhibition was calculated using the t test by comparing the count-per-minute values of controls with those obtained in the presence of mAbs.
Inhibition of merozoite invasion using anti–RSP-2 mAbs. A suspension of synchronized mature IEs of the adhesion phenotypes CSA, CD36, and ICAM-1 in 200 μL/well at a final hematocrit of 2% in culture medium were incubated in 96-well flat-bottom plates together with 10 μL mAbs B4 (A) and D10 (B) at different concentrations. Four hours after invasion 100 μCi (3.7 MBq) of 3H-hypoxanthine was added and the incorporated radioactivity counted 24 hours later. The percentage (±SD) of inhibition was calculated using the t test by comparing the count-per-minute values of controls with those obtained in the presence of mAbs.
Discussion
In a previous study, 2 novel ring-surface molecules (RSP-1 and RSP-2) had been identified and linked to rIE adhesion to endothelial cells and placental syncytiotrophoblasts. Here we have developed mAbs that efficiently inhibit the adhesion of ring-stage parasites. The mAbs recognize a trypsin-sensitive and iodinatable parasite protein of approximately 42 kDa, which has similar features to the previously described RSP-2. None of the mAbs were directed against RSP-1, a 200-kDa molecule. Cytoadhesion has been observed in parasites that will express the CSA-binding phenotype later at the trophozoite stage but not in CD36 or ICAM-1–binding parasites.2 However, reactivity with mAbs strongly suggests the surface exposure of RSP-2 on rIEs selected for CSA, CD36, and ICAM-1. Such a functional difference may reflect structural and/or conformational differences between RSP-2 molecules expressed in parasites with distinct PfEMP1 molecules. It remains unclear if ring adhesion is exclusively associated with CSA binding parasites or if it is linked to other variant adhesion phenotypes.
The synthesis of the mature IE surface adhesion molecule PfEMP1 begins very early during the ring stage and gets to the erythrocyte membrane in young trophozoites via a parasite-specific intracellular trafficking pathway. One obvious question is, what mechanism does the RSP-2 use to be transported to the surface of rIEs immediately after the reinvasion process? We show that de novo synthesis of RSP-2 protein begins during the early trophozoite stage in the parasite endoplasmatic reticulum, from where the molecule is transported into the rhoptries. IFA analysis indicates that at least some protein material is translocated to the merozoite surface shortly before the rupture of the schizont. RSP-2 is detectable only on the surface of a subpopulation of erythrocytes after the reinvasion process. Labeling with anti–RSP-2 progressively disappears at 16 to 20 hours in rIEs and nEs, indicating a possible overlap of expression between RSP-2 and PfEMP1 in rIEs.11,17 This is supported by 2 independent experiments. First, anti–RSP-2 and anti-PfEMP1CSA mAbs colocalize in young trophozoites for a short period (16-20 hours). Second, cytoadhesion of young trophozoites on endothelial cells or placenta cryosections cannot be inhibited by more than 25% with anti–RSP-2 or CSA, whereas the same reagents specifically inhibit cytoadhesion by more than 90% in young rings (anti–RSP-2) or mature blood–stages (CSA). We therefore concluded that both parasite-encoded surface ligands interact with different endothelial host receptors at the same time and that both ligands remained fully functional during this overlapping expression period (Figure 6).
These data clearly imply that the sequential appearance of 2 distinct adhesion ligands can lead to parasite sequestration to the same host cell during the entire parasite life cycle. Our work demonstrates that placental syncitiotrophoblasts and some subpopulations of endothelial cells express the as-yet-unidentified RSP-2 receptor in addition to CSA. It can therefore be assumed that the CSA-binding IEs have the capacity to propagate as a cryptic blood-stage in some tissues or organs. Ring-stage–mediated cytoadherence is likely to have important pathological consequences and may participate in the obstruction of blood vessels in the brain of patients with malaria who died.4
An important finding in this work is the discovery of adhesion of nEs to endothelial cells. mAbs B4, C10, and D10 detect RSP-2 on the surface of nEs, suggesting that the RSP-2 protein complex mediates binding to endothelial cells in in vitro binding assays (CSA phenotype). Adhesion of nEs has not been reported yet in target organs of sequestration in humans. It will be interesting to investigate the potential role of RSP-2–tagged nEs in malaria pathology using mAbs B4, C10, and D10.
The percentage of RSP-2–tagged erythrocytes depends on the parasitemia. Up to 30% tagged nEs are observed at elevated parasitemia (5%-20%). L-IFA studies using synchronized IEs during the reinvasion demonstrated that after an initial contact with the erythrocyte surface, a large percentage of merozoites are not able to complete the invasion process (a model is shown in Figure 8).
Schematic model of the different types of RSP-2–tagged nEs and rIEs and their adhesive tropism during the blood-stage cycle. (A) Parasites of the CSA-binding phenotype nE that carry RSP-2 on their surface cytoadhere to endothelial cells and placental syncytiotrophoblasts via an unknown receptor. RSP-2–tagged nEs are potential targets marked for destruction by the host immune response in the presence of anti–RSP-2 antibodies. (B) rIEs of parasites selected for CSA binding will cytoadhere, and during a short period of the blood-stage cycle (early trophozoites), both RSP-2 and PfEMP1 are present on the surface of IEs and can bind to 2 distinct host receptors at the same time. These parasites probably are not present in the peripheral blood circulation.
Schematic model of the different types of RSP-2–tagged nEs and rIEs and their adhesive tropism during the blood-stage cycle. (A) Parasites of the CSA-binding phenotype nE that carry RSP-2 on their surface cytoadhere to endothelial cells and placental syncytiotrophoblasts via an unknown receptor. RSP-2–tagged nEs are potential targets marked for destruction by the host immune response in the presence of anti–RSP-2 antibodies. (B) rIEs of parasites selected for CSA binding will cytoadhere, and during a short period of the blood-stage cycle (early trophozoites), both RSP-2 and PfEMP1 are present on the surface of IEs and can bind to 2 distinct host receptors at the same time. These parasites probably are not present in the peripheral blood circulation.
We assume that the presence of RSP-2 on the surface of nEs and rIEs is the consequence of aborted merozoite invasion. Experimental evidence shows that RSP-2 is not released into the medium before or during the merozoite invasion process. Normal red blood cells that were cocultured with synchronized mIEs in the same compartment separated by a permeable membrane did not acquire any detectable RSP-2 on the surface of nEs, supporting that contact is essential for the acquisition of the molecule (see model presented in Figure 8). It remains unclear whether this is due to defective merozoites or to merozoites expressing alternative invasion pathways that are not compatible with invasion of erythrocytes used for in vitro culture of P falciparum. Phenotypic diversity of merozoites expressing different surface molecules within the same schizont has been illustrated for Plasmodium yoelii.18
Tagging RSP-2–positive red blood cells with antibodies is likely to result in their clearance from the circulation during passage through the spleen by interacting with Fc receptors. Massive removal of RSP-2–positive erythrocytes and particularly of nEs that carry RSP-2 from the blood circulation is likely to cause severe anemia in patients with malaria. The destruction of antibody-tagged nEs is possible during the first 20 hours of each blood-stage cycle. RSP-2–positive nEs can reach elevated levels of approximately 30% during in vitro culture at parasitemias of 5%-10%. Interestingly, anemia is a major factor of pathology observed in young children and primigravid women infected with P falciparum in holoendemic areas.19 The implication of RSP-2 in anemia is effectively supported by the presence of anti–RSP-2 antibodies in sera from pregnant women, adults, and children (data not shown). We speculate that in the presence of anti–RSP-2 antibodies in combination with high parasitemias (frequently observed in children and women during first pregnancy), anemia might develop due to the elimination of RSP-2–tagged erythrocytes.
Immunoprecipitation of extracts of surface-iodinated nEs and rIEs using the anti–RSP-2 mAbs revealed that RSP-2 is complexed with 2 other trypsin-sensitive molecules. Although the molecular weights of the associated proteins on the surface changes slightly to those seen in the rhoptries, it is feasible that modifications occurring during the invasion process are involved. The RSP-2 protein complex recognized by the mAbs B4, C10, and D10 had features similar to the previously described rhoptry complex composed of RAP-1, RAP-2, and RAP-3.15,16 The mAbs recognize a recombinant RAP-2 GST fusion protein, indicating that RSP-2 is encoded by the RAP-2 gene. Analysis of mutant RAP-1 parasites confirmed the identity of RSP-2 and RAP-2 (Sterkers et al, manuscript in preparation).
In conclusion, we have identified and characterized the molecule involved in rIE adhesion to different target organs involved in severe malaria and have detected a novel type of adhesion by noninfected erythrocytes. The newly acquired sticky phenotype of nEs is mediated by a rhoptry-derived protein complex shed by merozoites to the erythrocyte surface during the invasion process. These findings are relevant for vaccine development, since antibodies against RSP-2/RAP-2 target important steps in parasite development such as merozoite invasion and the adhesion of rIEs to host cells. Our data indicate that the same antibodies also could have a role in pathology by inducing anemia. We now have the tools to investigate the role of the RSP-2 complex in the development of anemia.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-12-3710.
Supported by grants from the Ministère de l'Education Nationale de la Recherche et de la Technologie (MENRT) Paludisme (PAL) PAL+ 2000 program, PAL+ 2002 program, Direction Générale pour l'Armement/Projet d'Etude Amont (DGA/PEA) (contract no. 980814), Direction des Systèmes des Forces et de Prospectives/Service de Stratégies Techniques et de Technologies Communes (DSP/STTC) (contract no. 0134020), and the European Union for Research and Technical Development (contract no. QLK2-CT2000-00109), which include postdoctoral fellowships for F.T.M.C. and B.T. and PhD fellowship for Y.S. The authors would like to thank Conselho Nacional de Desenvolvimento Cientifico e Tecnologico Foundation (CNPq)–Brazil for an additional-year fellowship for F.T.M.C. (200076/95-7). J.B.L.D. is a PhD fellow supported by grants from Bourses et Stages Gabon-BGE 1998-753 and FRM-FDT20010920048/1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Bruno Pradines for technical assistance with the merozoite inhibition assay, Dr Olivier Garraud for the human sera, and Dr. Lindsay Pirrit for help with the manuscript.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal