Abstract
Sialic acid binding immunoglobulin-like lectin 8 (Siglec-8), which exists in 2 isoforms including one possessing cytoplasmic tyrosine motifs, is expressed only on human eosinophils, basophils, and mast cells. Until now, its function was unknown. Here we define a novel function of Siglec-8 on eosinophils. Siglec-8 cross-linking with antibodies rapidly generated caspase-3–like activity and reduced eosinophil viability through induction of apoptosis. The pancaspase inhibitor benzyloxycarbonyl (Cbz)–Val-Ala-Asp-(Ome)-fluoromethyl ketone (zVAD-FMK) completely blocked this response, implicating caspases in Siglec-8 cross-linking–induced apoptosis. Eosinophil survival-promoting cytokines such as interleukin 5 (IL-5) and granulocyte-macrophage colony-stimulating factor (GM-CSF) failed to block apoptosis and instead enhanced the sensitivity of eosinophils to undergo apoptosis in response to Siglec-8 antibody. Siglec-8 activation may provide a useful therapeutic approach to reduce numbers of eosinophils (and perhaps basophils and mast cells) in disease states where these cells are important.
Introduction
Eosinophils, through release of preformed and newly generated mediators, are considered key effector cells in several diseases. Their recruitment and activation are regarded as central to the pathophysiology of allergic disorders, including asthma.1-3 Besides selective migration, increased cell survival and decreased apoptosis have been proposed as mechanisms contributing to tissue-specific accumulation of these inflammatory cells.4 Hence, therapeutic efforts in the area of allergic inflammation continue to focus on the development of agents to suppress eosinophil recruitment, activation, and survival.
As a result of molecular efforts to identify novel, specific markers expressed on eosinophils, our group and others recently discovered Siglec-8, formerly called SAF-2.5,6 Sialic acid binding immunoglobulin-like lectins (Siglecs) belong to the immunoglobulin (Ig) supergene family and characteristically have an N-terminal V-set domain that binds sialic acid.7 Although originally isolated from a human eosinophil cDNA library by random expressed sequence tag sequencing, Siglec-8 is also expressed on human basophils and mast cells5 and exists in 2 isoforms with identical extracellular and transmembrane sequences. One isoform (Siglec-8) has a short cytoplasmic tail with no known signaling sequences, while the other, Siglec-8 long form (Siglec-8L), has a longer cytoplasmic tail containing 2 tyrosine-based signaling motifs.8,9 Although the function of Siglec-8 and Siglec-8L, and indeed most Siglecs, is unknown, the cytoplasmic region of Siglec-8L contains one consensus immunoreceptor tyrosine-based inhibitory motif (ITIM) and a signaling lymphocyte activation molecule (SLAM)–like motif, suggesting that Siglec-8L may possess signal transduction activity.7-9 Indeed, for some Siglecs, such as Siglec-3 and Siglec-7 (p75/AIRM-1), antibody cross-linking has been shown to inhibit proliferation and survival of myeloid leukemic cells.10-12 Therefore we hypothesized that ligation of Siglec-8 would inhibit eosinophil survival, and we present data that show a proapoptotic effect with antibody-mediated cross-linking.
Materials and methods
Antibodies and recombinant proteins
Murine monoclonal IgG1 isotype antibodies (Abs) recognizing Siglec-8 (2E2, 2C4, and 9G4) were generated using previously described methods5 and were endotoxin-free (ie, below the detection level of < 0.01 EU/mL as determined by amebocyte lysate assay; BioWhittaker, Walkersville, MD). Unless noted otherwise, these monoclonal Abs (mAbs) were used at saturating concentrations (2.5 μg/mL). Normal sheep serum and goat antisheep horseradish peroxidase–linked Abs were from Calbiochem (La Jolla, CA). Goat antirabbit horseradish peroxidase–linked Ab was from Amersham Pharmacia Biotech (Piscataway, NJ). Polyclonal intact and F(ab′)2 goat antimouse IgG (heavy and light chain) were purchased from Caltag Laboratories (Burlingame, CA). Recombinant human interleukin 5 (IL-5) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were from R&D Systems (Minneapolis, MN). Mouse antihuman CD44 mAb (clone J-173, IgG1), anti-Fas/CD95 (clone 7C11, IgM), and anti-CD18 mAb (clone 195N, IgG1) were from Beckman-Coulter (Hialeah, FL). Rabbit polyclonal IgG polyhistidine His-1 Ab (mHis6 Ab) was from Santa Cruz Biotechnology (Santa Cruz, CA). IgG and IgM isotype control Abs were from Sigma-Aldrich (St Louis, MO).
Eosinophil purification
Eosinophils from normal, allergic, and hypereosinophilic donors were purified from peripheral blood after density-gradient centrifugation using Percoll (Pharmacia, Upsala, Sweden) for removal of mononuclear cells, followed by lysis of red blood cells and immunomagnetic removal of neutrophils as described.13 Eosinophil purity was consistently higher than 98%, with neutrophils being the only contaminating cells. The viability of freshly isolated eosinophils was higher than 99%, as determined by erythrosin-B dye exclusion.
Cell culture
Jurkat cells and purified peripheral blood eosinophils were cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, UT), 100 U/mL penicillin G, and 0.1 mg/mL streptomycin sulfate (Invitrogen–Life Technologies, Grand Island, NY). Eosinophils were harvested at different time points over a period of 2 to 72 hours after coculture with murine Siglec-8 mAb in the presence or absence of polyclonal goat antimouse IgG Ab used for secondary cross-linking. In some experiments, intact and F(ab′)2 goat antimouse IgG were compared to elucidate any effect of Fc on eosinophil apoptosis. For controls, cells were incubated with medium alone or CD44 mAb (2.5 μg/mL) in the presence or absence of secondary cross-linking Ab and with or without IL-5 or GM-CSF (1-30 ng/mL). In priming experiments, eosinophils were first preincubated with IL-5 or GM-CSF (30 ng/mL) for 24 hours, then various Abs were added for an additional 24 hours of culture before analysis of apoptosis. In some experiments, cells were preincubated for 30 minutes at 37°C with the general caspase inhibitor benzyloxycarbonyl (Cbz)–Val-Ala-Asp-(Ome)-fluoromethyl ketone (zVAD-FMK)14 (25 μM; R&D Systems) before addition of various Abs to the cultures.
Analysis of viability and apoptosis in cultured cells
In certain experiments, viability of cultured eosinophils was determined by erythrosin dye exclusion as assessed by light microscopy.13,15 For other experiments, morphologic analysis using established light microscopic criteria was performed.15 Briefly, cytocentrifugation preparations were stained with Leukostat (Fisher Diagnostics, Pittsburgh, PA) to reveal nuclear morphology. Apoptotic cells were detected by the condensed and rounded appearance of their nuclei under light microscopy. Cells exhibiting apoptotic nuclei were enumerated in different fields in a blinded manner using a random coded order. At least 500 total cells were counted per slide. Cells were then photographed using a Zeiss Axioscope microscope (Oberkochen, Germany) at × 400 magnification. In addition to light microscopic techniques, cell cycle analysis was performed using propidium iodide (PI) staining (50 μg/mL) of fixed, permeabilized (70% EtOH, 4°C, 30 minutes), and RNase treated (RNase A, 0.05 mg/mL, 37°C, 30 minutes) eosinophils. Stained cells were then analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA) as described previously.16 Finally, annexin-V labeling was used to detect apoptosis in eosinophils.15,17
Western blot analysis
HEK293T cells (5 × 106) were transfected by electroporation with pcDNA6/V5-His vector (Invitrogen–Life Technologies) encoding flag-tagged Siglec-8L (His6-Siglec-8L DNA; kindly provided by Dr Jian Ni at Human Genome Sciences, Gaithersburg, MD). Blasticidin-resistant cells were cloned by limiting dilution and were genotyped by polymerase chain reaction (PCR) using Siglec-8L–specific primers.18 The clones with stable integration of the His6-Siglec-8L transgene were subsequently grown in the continuous presence of 1 μg/mL blasticidin. HEK293T transfectants encoding the short form of Siglec-8 were kindly provided by Drs Kristy Kikly and John White (GlaxoSmithKline, King of Prussia, PA). Equal numbers of eosinophils or Siglec-8L–HEK293T transfectants (106 cells/mL) were resuspended in Tris buffer (10 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4; 150 mM NaCl; 5 mM EDTA [ethylenediaminetetraacetic acid]) containing 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, and protease inhibitor tablets (Roche Diagnostics, Mannheim, Germany). Protein lysates were then fractionated over 8% sodium dodecyl sulfate (SDS)–polyacrylamide, transferred onto polyvinylidene difluoride membranes, and probed for Siglec-8L using anti-His tag polyclonal Ab (1:500 dilution) or both Siglec-8 and Siglec-8L using sheep polyclonal Ab6 (1:300 dilution), kindly provided by Dr Paul Crocker (University of Dundee, Dundee, Scotland, United Kingdom). Bound antibodies were then detected using peroxidase-coupled secondary Abs, followed by analysis using a chemiluminescence system (ECL; Amersham, Arlington Heights, IL).
Evaluation of caspase-3–like activity by spectrofluorimetry
Caspase-3–like proteases (caspase-3, -6, -7) have distinct roles in the execution phase of apoptosis.19 Activation of caspase-3–like proteases that have a substrate specificity for the amino acid sequence Asp-Glu-Val-Asp (DEVD) was evaluated by the ability of eosinophil protein extracts to cleave the specific 7-amino-4-methylcoumarin–derived substrate, Z-DEVD-AMC (Z representing a benzyloxycarbonyl group), using the fluorimetric EnzChek Assay System (Molecular Probes, Eugene, OR). Eosinophils from 3 separate donors were cultured for 24 hours in medium alone and Siglec-8 cross-linking conditions (106 cells/mL). Eosinophils were harvested and lysates were prepared according to the manufacturer's instructions. Total protein extracts from lysates of 106 cells were assayed in triplicate by incubating at room temperature for 30 minutes with 10 μM substrate Z-DEVD-AMC, in the presence or absence of the caspase-3–like protease inhibitor, Ac-DEVD-CHO (20 μM). AMC release was then monitored on a CytoFlor 4000 spectrofluorimeter with filter settings at 350 nm for excitation and 460 nm for emission using Cytoflor V.4.2.1 software (both from Applied Biosystems, Stafford, TX). Results were calculated by comparison with a standard curve of AMC and were expressed in pM/106 cells.
Statistical analysis
Data are presented as mean ± SEM unless otherwise indicated. Statistical significance was determined by 1-way or 2-way analysis of variance (ANOVA), as appropriate, and values were considered significant at P values less than .05.
Results
Eosinophils express Siglec-8 and Siglec-8L
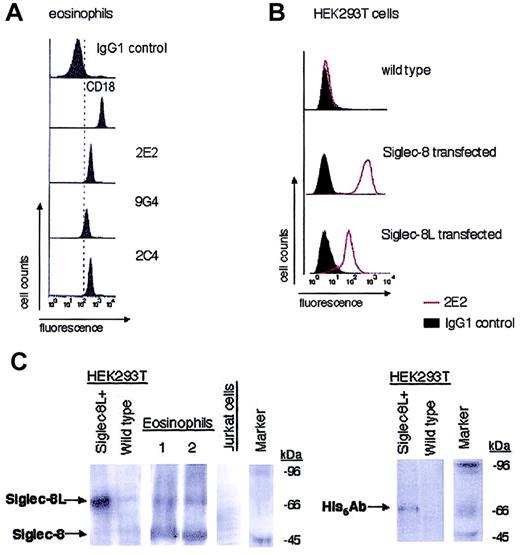
Figure 1A demonstrates the expression of Siglec-8 on eosinophils using flow cytometry with 3 different murine monoclonal antibodies, 2E2, 2C4, and 9G4. These antibodies recognize the extracellular portion of the Siglec-8 molecule,5 so they do not distinguish between Siglec-8 and Siglec-8L. The staining intensity of eosinophils with 2C4 and 2E2 was identical, while 9G4 stained less intensely. These results were consistent among multiple different eosinophil donors studied (data not shown). Furthermore, the specificity of 2E2 mAb is shown in Figure 1B, using untransfected wild-type versus Siglec-8– and Siglec-8L–transfected HEK293T cells. Similar results were obtained using 2C4 and 9G4 mAbs (data not shown). In separate experiments, we determined that the saturating concentration for all 3 mAbs was 2.5 μg/mL (data not shown).
Expression of Siglec-8 and Siglec-8L by eosinophils and transfected cell lines. Purified peripheral blood eosinophils (A) and HEK293T cells stably transfected with Siglec-8 or Siglec-8L (B) were analyzed by flow cytometry for Siglec-8 surface expression using murine monoclonal antibodies (2E2, 2C4, and/or 9G4). Histograms shown are representative of 3 to 4 experiments with identical results. Monoclonal reagents used as positive (CD18) and negative controls are also shown. (C) Western blot analysis of human peripheral blood eosinophils that were obtained from 2 different atopic donors. Lysates from these cells, as well as from Siglec-8L–transfected HEK293T cells, were generated. Equal amounts of protein from each sample were analyzed by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with sheep antihuman polyclonal Siglec-8 Ab or His6 Ab. Wild-type HEK293T cells and Jurkat T cells were used as negative controls. Data are representative of 3 different experiments with similar results. Marker indicates molecular weight standard markers.
Expression of Siglec-8 and Siglec-8L by eosinophils and transfected cell lines. Purified peripheral blood eosinophils (A) and HEK293T cells stably transfected with Siglec-8 or Siglec-8L (B) were analyzed by flow cytometry for Siglec-8 surface expression using murine monoclonal antibodies (2E2, 2C4, and/or 9G4). Histograms shown are representative of 3 to 4 experiments with identical results. Monoclonal reagents used as positive (CD18) and negative controls are also shown. (C) Western blot analysis of human peripheral blood eosinophils that were obtained from 2 different atopic donors. Lysates from these cells, as well as from Siglec-8L–transfected HEK293T cells, were generated. Equal amounts of protein from each sample were analyzed by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with sheep antihuman polyclonal Siglec-8 Ab or His6 Ab. Wild-type HEK293T cells and Jurkat T cells were used as negative controls. Data are representative of 3 different experiments with similar results. Marker indicates molecular weight standard markers.
We next performed Western blotting experiments to determine the expression of Siglec-8 and Siglec-8L isoforms in eosinophils. In initial experiments, the Siglec-8 mAbs were used, but they failed to work in Western blots performed under reducing, denaturating conditions (data not shown). Therefore, specific Siglec-8 polyclonal antisera was obtained and lysates from highly purified eosinophils, His6-Siglec-8L–transfected HEK293T cells, and wild-type HEK293T cells were prepared and probed using Siglec-8 polyclonal Ab or His6 Ab. Use of His6 Ab with Siglec-8L–transfected HEK293T cell lysates enabled us to determine the molecular weight of Siglec-8L. Figure 1C (left panel) shows that transfected cells express Siglec-8L protein at 75 kDa. Slightly fainter but similar bands of 75 kDa were detected in eosinophil lysates obtained from different individuals, including hypereosinophilic, atopic asthmatic, and nonasthmatic donors (Figure 1C, right panels, and data not shown). Additionally, a broad prominent band was detected at 48 kDa, the expected molecular weight of the short form of Siglec-8. As negative controls, human peripheral blood neutrophils (not shown), Jurkat cells, and wild-type HEK293T cells did not express either Siglec-8 or Siglec-8L. In addition, membranes were stripped and reprobed with normal sheep serum or IgG1 isotype control Ab and no positive bands were detected (data not shown). Thus, human eosinophils express both Siglec-8 isoforms.
Siglec-8 cross-linking reduces eosinophil viability in a time- and concentration-dependent manner
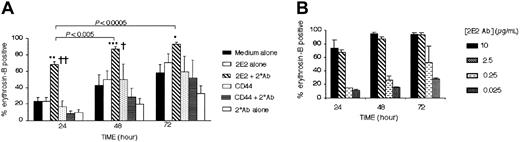
Based on the knowledge that Siglec-8L has tyrosine-based inhibitory consensus sequences, we hypothesized that incubation of eosinophils with Siglec-8 antibody might have inhibitory effects on eosinophil survival. Using specific murine monoclonal antibodies against Siglec-8 (all subsequent uses of the term “Siglec-8” will refer to both isoforms unless specified otherwise) and a secondary polyclonal antimouse antibody to enhance cross-linking, we determined whether Siglec-8 ligation inhibited eosinophil survival in vitro. As shown in Figure 2, Siglec-8 cross-linking with 2E2 mAb plus a secondary polyclonal Ab induced a significant increase in eosinophil death. Determined at 24 hours of culture, for example, the percentage of eosinophil death induced by Siglec-8 cross-linking (68% ± 4%) was significantly higher than that induced by medium alone (23% ± 4%, P < .0005), CD44 control cross-linking conditions (9% ± 2%, P < .0005), or Siglec-8 Ab alone (23% ± 5%, P < .0005). The effect of Siglec-8 cross-linking was time-dependent, with the levels of eosinophil death increasing to more than 90% by 48 to 72 hours of culture.
Siglec-8 cross-linking induces eosinophil death. Purified peripheral blood eosinophils were cultured under the indicated conditions. Viability was assessed using erythrosin-B dye exclusion. (A) Note that induction of cell death requires the presence of both 2E2 and secondary (2°) Abs. Data are mean ± SEM from 6 experiments. *P ≤ .05; **P ≤ .0005; ***P ≤ .005 for comparison of medium control versus 2E2 + 2° Ab. †P ≤ .05; ††P < .0005 for comparison of 2E2 Ab alone versus 2E2 + 2° Ab. (B) Siglec-8–mediated eosinophil apoptosis was concentration-dependent. Maximal apoptosis was observed at concentrations equal to or greater than 2.5 μg/mL 2E2 mAb (mean ± SEM; n = 4).
Siglec-8 cross-linking induces eosinophil death. Purified peripheral blood eosinophils were cultured under the indicated conditions. Viability was assessed using erythrosin-B dye exclusion. (A) Note that induction of cell death requires the presence of both 2E2 and secondary (2°) Abs. Data are mean ± SEM from 6 experiments. *P ≤ .05; **P ≤ .0005; ***P ≤ .005 for comparison of medium control versus 2E2 + 2° Ab. †P ≤ .05; ††P < .0005 for comparison of 2E2 Ab alone versus 2E2 + 2° Ab. (B) Siglec-8–mediated eosinophil apoptosis was concentration-dependent. Maximal apoptosis was observed at concentrations equal to or greater than 2.5 μg/mL 2E2 mAb (mean ± SEM; n = 4).
Also shown is the fact that secondary antibody cross-linking was absolutely necessary, because culture with 2E2 alone, in the absence of secondary cross-linking polyclonal Ab, did not induce eosinophil death (Figure 2A). Moreover, all Siglec-8 antibodies (2E2, 9G4, and 2C4, each at 2.5 μg/mL) were equally effective in the absence or presence of a secondary cross-linking Ab (data not shown). As shown in Figure 2B, the percentage of cell death was optimal using saturating concentrations of 2E2 Ab (≥ 2.5 μg/mL), while concentrations equal to or lower than 0.25 μg/mL had no functional effect. Thus, the concentrations giving optimal labeling for flow cytometry were also optimal for induction of apoptosis.
Siglec-8 cross-linking reduces eosinophil viability through induction of apoptosis
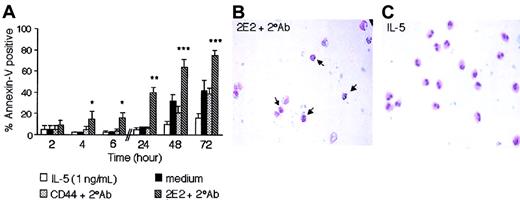
Eosinophil death induced by Siglec-8 cross-linking, as assessed by dye exclusion, was already approximately 70% after only 24 hours of culture. To further explore the effects of Siglec-8 cross-linking on eosinophil death and to more carefully examine the kinetics, annexin-V staining was used to distinguish apoptosis from necrosis at various time points. Figure 3A demonstrates a significant increase (P ≤ .005) in annexin-V+ eosinophils as early as 4 hours of culture with Siglec-8 cross-linking (15% ± 7%), compared with CD44 control cross-linking (5% ± 3%) or medium control (3% ± 1%), indicating a rapid apoptotic effect (n = 6). Figure 3A also demonstrates that the Siglec-8 effect became even more pronounced by 24 to 72 hours of culture, especially when compared with effects of the survival-promoting cytokine IL-5.
Siglec-8 ligation induces eosinophil apoptosis. (A) Eosinophils were cultured as indicated, harvested, and analyzed by flow cytometry for annexin-V labeling. Data are mean ± SEM from 6 experiments. (*P ≤ .005; **P ≤ .0005; ***P ≤ .05 for comparison of medium control versus 2E2 + 2° Ab). (B-C) Morphologic changes in eosinophils consistent with apoptosis were observed after 24 hours of 2E2 cross-linking (B) but not after 24 hours of culture in IL-5 (1 ng/mL; panel C). Note in panel B the decreased cytoplasmic volume and condensed, rounded nuclei (arrows) compared with eosinophils in panel C. Data in B-C are representative of 6 experiments (original magnification, × 400).
Siglec-8 ligation induces eosinophil apoptosis. (A) Eosinophils were cultured as indicated, harvested, and analyzed by flow cytometry for annexin-V labeling. Data are mean ± SEM from 6 experiments. (*P ≤ .005; **P ≤ .0005; ***P ≤ .05 for comparison of medium control versus 2E2 + 2° Ab). (B-C) Morphologic changes in eosinophils consistent with apoptosis were observed after 24 hours of 2E2 cross-linking (B) but not after 24 hours of culture in IL-5 (1 ng/mL; panel C). Note in panel B the decreased cytoplasmic volume and condensed, rounded nuclei (arrows) compared with eosinophils in panel C. Data in B-C are representative of 6 experiments (original magnification, × 400).
For an additional assessment of apoptosis induced by Siglec-8 cross-linking, light microscopic examination of eosinophils was performed. After 24 hours of culture, Siglec-8 cross-linking on eosinophils resulted in changes in morphology characterized by reduced cell volume, loss of cytoplasmic content, and condensation of nuclei typical of apoptosis (Figure 3B). This was rarely seen in cells cultured with IL-5 (Figure 3C) or control CD44 cross-linking (data not shown). As an average from 4 experiments, the percentage of eosinophils displaying morphologic characteristics of apoptosis with Siglec-8 cross-linking was 43% ± 15%, compared with 10% ± 2% and 10% ± 1% with medium or CD44 Ab cross-linking, respectively (P ≤ .05). In parallel experiments, we also studied DNA fragmentation in permeabilized eosinophils, using PI staining in fixed cells. Siglec-8 cross-linking for 24 hours increased DNA fragmentation (48% hypodiploid DNA staining, compared with 18% and 21% with medium alone or CD44 cross-linking, respectively [n = 1]). These data provide multiple lines of evidence demonstrating apoptosis induced by Siglec-8 cross-linking.
Cross-linking with intact or F(ab′)2 polyclonal secondary antibody induces eosinophil apoptosis
FcγRII (CD32) is an ITIM-bearing receptor and is expressed by eosinophils, where it has been shown to regulate various functions, including survival.20 Although cross-linking of an isotype-matched CD44 mAb provided an appropriate control for Siglec-8 cross-linking studies and argues against Fc effects, experiments were performed to determine whether the Fc portion of the cross-linking polyclonal antibody was involved in Siglec-8 cross-linking–induced eosinophil apoptosis. Both F(ab′)2 and intact polyclonal secondary antibodies had similar effects on Siglec-8–mediated eosinophil apoptosis. Used with Siglec-8 Ab for 24 hours of culture, eosinophil apoptosis with F(ab′)2 or intact polyclonal secondary antibody was 39% ± 9% and 43% ± 8%, respectively, while apoptosis was 7% ± 2% with F(ab′)2 used alone (n = 4). Thus, the Fc portion of the cross-linking antibody is not involved in Siglec-8–induced eosinophil apoptosis.
The role of caspases in Siglec-8–induced eosinophil apoptosis
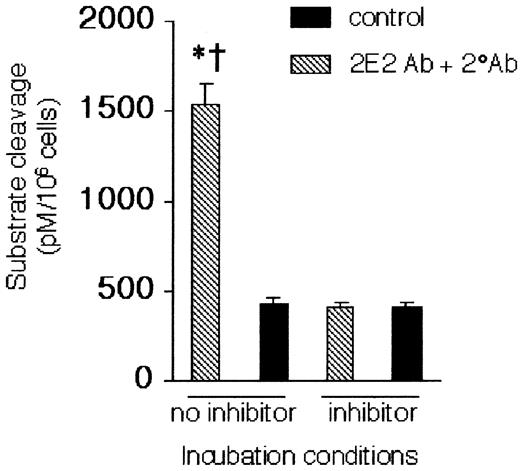
As determined by cleavage of the Z-DEVD-AMC substrate, caspase-3–like activity in eosinophil lysates was significantly augmented by Siglec-8 cross-linking. Preincubation of these protein extracts with the caspase-3–like protease inhibitor Ac-DEVD-CHO completely blocked Z-DEVD-AMC cleavage (Figure 4). To determine whether Siglec-8–mediated apoptosis was caspase-dependent, Siglec-8 cross-linking–induced eosinophil apoptosis was examined in the presence or absence of the general caspase inhibitor zVAD-FMK.14 As measured by annexin-V staining (Figure 5), zVAD-FMK completely inhibited Siglec-8 cross-linking–induced apoptosis but had no effect on spontaneous eosinophil apoptosis.
Caspase-3–like activity is activated by Siglec-8 cross-linking. Cells were cultured for 24 hours under control or cross-linking conditions, as indicated, and analyzed for caspase-3–like activity in the presence and absence of the caspase-3 inhibitor Ac-DEVD-CHO (20 μM). Data are mean ± SEM from 3 experiments. *P ≤ .05 for comparison of 2E2 + 2° Ab versus medium control; †P ≤ .05 for comparison of 2E2 + 2° Ab versus 2E2 + 2° Ab + inhibitor.
Caspase-3–like activity is activated by Siglec-8 cross-linking. Cells were cultured for 24 hours under control or cross-linking conditions, as indicated, and analyzed for caspase-3–like activity in the presence and absence of the caspase-3 inhibitor Ac-DEVD-CHO (20 μM). Data are mean ± SEM from 3 experiments. *P ≤ .05 for comparison of 2E2 + 2° Ab versus medium control; †P ≤ .05 for comparison of 2E2 + 2° Ab versus 2E2 + 2° Ab + inhibitor.
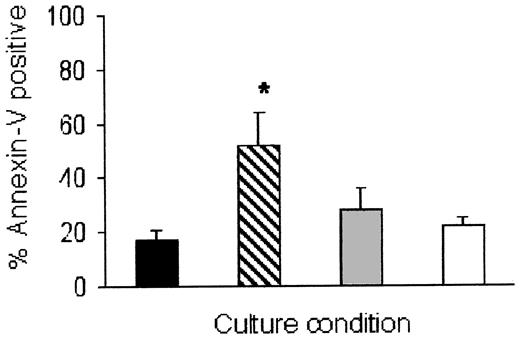
Eosinophil apoptosis induced by Siglec-8 cross-linking is inhibited by the pancaspase antagonist zVAD-FMK. Cells were cultured for 24 hours, then analyzed for annexin-V staining as in Figure 3. Data are mean ± SEM from 4 experiments. *P ≤ .05 for comparison of 2E2 + 2° Ab (▨) versus 2E2 + 2° Ab + zVAD-FMK (▦). ▪ indicates medium alone; □, medium + zVAD-FMK.
Eosinophil apoptosis induced by Siglec-8 cross-linking is inhibited by the pancaspase antagonist zVAD-FMK. Cells were cultured for 24 hours, then analyzed for annexin-V staining as in Figure 3. Data are mean ± SEM from 4 experiments. *P ≤ .05 for comparison of 2E2 + 2° Ab (▨) versus 2E2 + 2° Ab + zVAD-FMK (▦). ▪ indicates medium alone; □, medium + zVAD-FMK.
Effect of IL-5 and GM-CSF on Siglec-8 antibody–induced eosinophil apoptosis
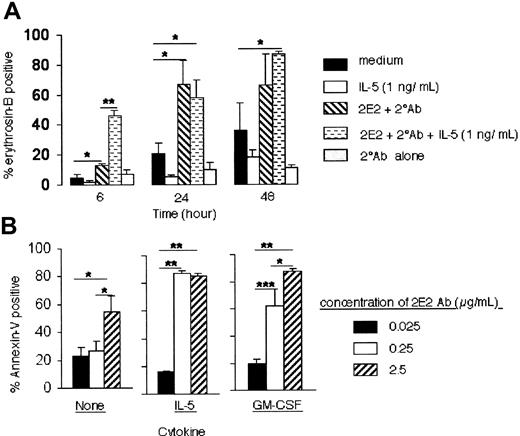
IL-5 and GM-CSF are potent and specific antiapoptotic cytokines for eosinophils,4 and their expression is increased at sites of allergic inflammation in the airways.2,21,22 When eosinophils are cultured in the absence of survival-promoting cytokines, they rapidly undergo apoptosis; in the presence of these cytokines, their survival can be maintained for weeks.4 Therefore, we examined the effect of IL-5 and GM-CSF on Siglec-8 cross-linking–induced eosinophil apoptosis. When IL-5 (1 ng/mL) was added simultaneously with Siglec-8 cross-linking antibodies at the beginning of the culture, the cytokine could not override the Siglec-8 cross-linking–induced cell death. In fact, at 6 hours (Figure 6A), the level of eosinophil apoptosis induced by Siglec-8 cross-linking in the presence of 1 ng/mL of IL-5 (46% ± 4%) was significantly enhanced compared with Siglec-8 cross-linking alone (12% ± 1%, P < .0001, n = 4). Similar results were obtained using higher concentrations of IL-5 or GM-CSF (10-30 ng/mL; data not shown).
Effect of IL-5 and GM-CSF on Siglec-8 cross-linking–induced eosinophil death. (A) IL-5 (1 ng/mL) was added at the beginning of the cell culture and viability was determined at various time points as indicated. Data are from 4 to 6 experiments. *P < .05; **P < .0005. (B) IL-5 or GM-CSF (each at 30 ng/mL) reduces the concentration of Siglec-8 mAb needed to induce maximal eosinophil apoptosis. Eosinophils were initially cultured with IL-5 or GM-CSF in the presence of secondary Ab and the indicated concentrations of 2E2. After 24 hours, apoptosis was analyzed using annexin-V staining. Data are presented as mean ± SEM from 4 experiments. *P < .05; **P < .0005; ***P < .005.
Effect of IL-5 and GM-CSF on Siglec-8 cross-linking–induced eosinophil death. (A) IL-5 (1 ng/mL) was added at the beginning of the cell culture and viability was determined at various time points as indicated. Data are from 4 to 6 experiments. *P < .05; **P < .0005. (B) IL-5 or GM-CSF (each at 30 ng/mL) reduces the concentration of Siglec-8 mAb needed to induce maximal eosinophil apoptosis. Eosinophils were initially cultured with IL-5 or GM-CSF in the presence of secondary Ab and the indicated concentrations of 2E2. After 24 hours, apoptosis was analyzed using annexin-V staining. Data are presented as mean ± SEM from 4 experiments. *P < .05; **P < .0005; ***P < .005.
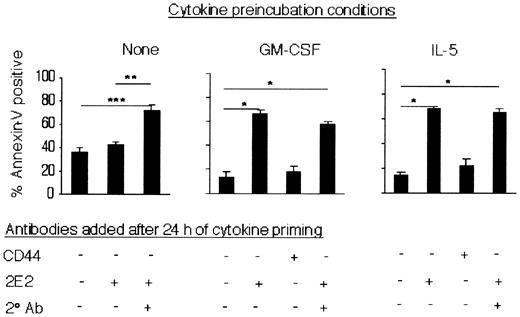
To determine whether these cytokines were enhancing eosinophil sensitivity to undergo apoptosis induced by Siglec-8 ligation, experiments were performed in which cells were cultured with saturating to subsaturating concentrations of Siglec-8 mAb in the presence or absence of IL-5, GM-CSF (30 ng/mL), or secondary Ab. Addition of IL-5 or GM-CSF markedly enhanced eosinophil apoptosis even when subsaturating concentrations of Siglec-8 mAb (0.25 μg/mL) were used (Figure 6B). These data suggest that the presence of IL-5 or GM-CSF rendered eosinophils more sensitive to the Siglec-8 cross-linking-induced apoptotic effect. Therefore, one additional set of experiments was performed to determine whether eosinophil priming with these cytokines, prior to addition of Siglec-8 cross-linking antibodies, enhanced the proapoptotic effect. Eosinophils were preincubated with IL-5 or GM-CSF for 24 hours, then mAbs were added and cells were cultured for an additional 24 hours, after which apoptosis was analyzed. Remarkably, cytokine priming (4-30 ng/mL) led to a profound proapoptotic response in the presence of Siglec-8 mAb alone (Figure 7 and data not shown), a response not seen in the absence of cytokines (Figures 2 and 7). Note that the percentage of apoptosis in the presence of IL-5 alone or CD44 control mAb alone was 14% ± 3% or 22% ± 6%, respectively (Figure 7; n = 6). Levels of apoptosis with 2E2 mAb alone (68% ± 2%) in IL-5–primed eosinophils were similar to those seen in unprimed eosinophils exposed to both 2E2 and secondary Ab (72% ± 5%). Upon priming, however, apoptosis was maximally induced by mAb alone and further secondary antibody cross-linking did not lead to further induction of apoptosis (65% ± 3%). Similar results were obtained using GM-CSF (Figure 7).
IL-5 or GM-CSF priming enhances eosinophil apoptosis in response to Siglec-8 mAb. Eosinophils were preincubated with or without IL-5 or GM-CSF (each at 30 ng/mL) for 24 hours. Antibodies (2.5 μg/mL) were then added to the cultures, as indicated, and apoptosis was analyzed using annexin-V staining 24 hours later. Data are presented as mean ± SEM from 4 experiments. *P < .0005; **P ≤ .005; ***P < .05.
IL-5 or GM-CSF priming enhances eosinophil apoptosis in response to Siglec-8 mAb. Eosinophils were preincubated with or without IL-5 or GM-CSF (each at 30 ng/mL) for 24 hours. Antibodies (2.5 μg/mL) were then added to the cultures, as indicated, and apoptosis was analyzed using annexin-V staining 24 hours later. Data are presented as mean ± SEM from 4 experiments. *P < .0005; **P ≤ .005; ***P < .05.
To explore how long it takes to induce cytokine priming of this response, shorter durations of cytokine preincubation were tested. After 3 hours of IL-5 priming, levels of apoptosis with CD44 mAb alone, 2E2 mAb alone, or 2E2 mAb plus secondary Ab were 29% ± 5%, 58% ± 8%, or 63% ± 5%, respectively (mean ± range; n = 2). Thus, priming of eosinophils for as little as 3 hours was sufficient to induce a maximal priming effect, namely that the Siglec-8 mAb alone induced maximal apoptosis and the secondary cross-linking antibody was no longer needed. These findings suggest that cytokines are enhancing not the rate of apoptosis but the ability of the cross-linking to induce apoptosis. In additional experiments we determined whether IL-5 or GM-CSF (30 ng/mL for 24 hours) altered the surface expression or cellular content of Siglec-8 or Siglec-8L. By flow cytometry, there was no change in total surface Siglec-8 and Siglec-8L, and by Western blotting, no differences were observed in the relative amounts of the isoforms (data not shown). Thus, cytokine priming does not increase relative amounts of Siglec-8L, the isoform likely to possess signal transduction activity.
Discussion
Data from the present studies have, for the first time, defined the structure and the function of Siglec-8 on human eosinophils. Western blotting demonstrated expression of both Siglec-8 short form and Siglec-8L, the latter isoform containing 2 tyrosine-based motifs associated with signaling properties in other cells. Three different Siglec-8 antibodies (2E2, 2C4, and 9G4), each raised against the extracellular portion of Siglec-8, were equally capable of inducing rapid and profound eosinophil apoptosis. Data shown in Figure 1A suggest that at least 2 different epitopes are recognized, because the staining intensity with 2C4 and 2E2 was identical, while the 9G4 mAb consistently stained less intensely. The apoptotic effect required the presence of a secondary antimouse cross-linking antibody in freshly isolated peripheral blood eosinophils, while the presence of IL-5 or GM-CSF, usually considered eosinophil survival-promoting cytokines, actually enhanced the ability of Siglec-8 cross-linking to induce apoptosis. In fact, when eosinophils were primed with cytokines, the secondary cross-linking antibody was no longer required for full induction of apoptosis. Caspase-3–like activity is induced by Siglec-8 cross-linking, and based on pharmacologic manipulations with the pancaspase inhibitor zVAD-FMK, caspases were shown to be involved in the apoptosis mechanism.
Previous studies have shown that Siglec-8 is expressed exclusively on eosinophils, mast cells, and basophils.5,6 Siglec-10 is the only other member of the Siglec family expressed on eosinophils, albeit at much lower levels than on monocytes and natural killer (NK) cells.9,23 Thus, it appears that Siglec-8 is most specific for eosinophils. Initial studies of Siglec-8 suggested that the molecule had an uncharacteristically short cytoplasmic tail with no known signaling motifs.5,6 However, a subsequent report by Foussias et al demonstrated that Siglec-8 exists in another isoform, designated Siglec-8L.8 Siglec-8L was identical to Siglec-8 in its extracellular domain but possessed a longer cytoplasmic domain with 2 tyrosine-based signaling motifs, one resembling a classical ITIM (ILV)xYxx(LV) found in many Siglecs, and another with similarity to a motif found in CD154 (SLAM) TxYxx(IV).8,24-26 Recent studies have confirmed the existence of Siglec-8L, and a re-evaluation of eosinophils, basophils, and mast cells by reverse transcriptase polymerase chain reaction (RT-PCR), including the same donor from which the eosinophil mRNAwas isolated for the original report, verified that these cells express both Siglec-8 and Siglec-8L.18,27 From the results of immunoblotting studies, we were able for the first time to identify both Siglec-8 and Siglec-8L protein in eosinophil lysates, with relative molecular masses of 45 kDa and 75 kDa, respectively (Figure 1C). Since the observed molecular mass of Siglec-8L was slightly greater than that predicted from the amino acid sequence of the mature protein (approximately 55 kDa), it is likely that at least one of the 4 potential N-linked glycosylation sites5,6 is occupied by carbohydrate. The protein band designated as Siglec-8 was always more prominent than that of Siglec-8L, which may suggest relatively higher expression of Siglec-8 than of Siglec-8L. Furthermore, in eosinophil lysates that were prepared from different donors with different hypereosinophilic disorders, similar intensities of Siglec-8 and Siglec-8L bands were detected, suggesting that Siglec-8 expression was not specific to the disease state studied. At this point, however, the functional relevance of the ratio of expression of short versus long forms of Siglec-8 is unknown.
Siglec cytoplasmic domains typically contain multiple tyrosine residues, including consensus ITIMs.8 The presence of ITIMs is a common feature of many other receptors, such as CD32 (in particular, FcγRIIb), killer cell Ig-like receptors (KIRs), and gp49B1.28-30 Among Siglecs, signaling involving downstream phosphatases such as SHP-1, SHP-2, and SLAM-associated proteins has been shown in association with ITIMs and SLAM, respectively.7,24-26 Examples of functional consequences mediated via Siglecs include studies in which antibody cross-linking of CD33 induced apoptosis of leukemic cells.11,12
Our finding that Siglec-8 cross-linking induces eosinophil apoptosis has several implications. First, our data suggest that extensive cross-linking with secondary Ab is necessary in fresh cells to induce eosinophil apoptosis, indicating that dimerization or formation of small aggregates of surface Siglec-8 is insufficient to trigger an apoptotic signal. More extensive aggregation, in the presence of a cross-linking secondary Ab, may occur by forming bigger Siglec-8 clusters. Aggregation has been reported to trigger a variety of inhibitory receptors, including CD32 and the cell-death receptor Fas/CD95.28,31 Because neither Siglec-8 isoform has a recognizable death domain, another possibility is that antibody-mediated cross-linking induces coaggregation with another cell surface receptor, such as CD32 or Fas.13,20 A role for CD32 seems unlikely, because both intact and F(ab′)2 secondary antibodies were equally effective and anti-CD44, used as a binding control mAb, did not induce apoptosis. While Fas engagement has been shown to induce eosinophil apoptosis, the effect is much slower and is partially reversed by IL-5 and GM-CSF,13 which is clearly not the case with Siglec-8 ligation. Together, these observations argue strongly against the possibility of CD32 or Fas involvement in the Siglec-8 response.
A second implication of this work stems from the observations that caspase-3–like proteases were activated by Siglec-8 cross-linking (Figure 4) and the pancaspase inhibitor zVAD-FMK rescued these cells from apoptosis, indicating the involvement of caspases in the Siglec-8 signaling pathway (Figure 5). Caspases are proteases that have been implicated as universal apoptosis effectors associated with downstream events of cell death in many cell types, including eosinophils.32 While our findings suggest the divergence of the proximal mechanisms involved in Siglec-8 and Fas signaling, it is clear that distally they use caspases and common death pathways. Besides activation through death receptors, caspases can also be activated through mechanisms that are independent of death receptors. In this case, different exogenous factors (eg, oxidative stress, drugs, viruses) may change mitochondrial permeability, leading to “intrinsic” activation of caspases.33,34 Efforts are currently under way to delineate the natural sequence of these events in Siglec-8–mediated apoptosis.
A final implication of this work was the intriguing finding that in the presence of survival-promoting cytokines, lower levels of cross-linking became effective in inducing apoptosis. Indeed, after cytokine priming, Siglec-8 mAb alone was sufficient to induce eosinophil apoptosis (Figure 7). In most situations, IL-5 and GM-CSF inhibit eosinophil apoptosis by altering expression of Bcl-xL and caspases.32,35 This is true for spontaneous apoptosis, a caspase-dependent event, as well as apoptosis induced by Fas and glucocorticoids.13,36 To date, the only eosinophil apoptosis pathway in which cytokines enhance the response involves CD69.37
One potential limitation of our study, and indeed of functional studies of virtually all Siglecs, is the need for antibodies to engage the receptor. Siglecs are known to recognize sialylated structures as counterreceptors.7,38 Like other Siglecs, Siglec-8 mediates sialic acid–dependent binding of human red blood cells (RBCs),5,6 possibly via α2,6-linked sialic acid, but its exact physiologic ligands remain to be defined. Given the proapoptotic effect of Siglec-8 ligation, it is tempting to speculate that eosinophils may encounter ligands in tissues where eosinophils are unwanted or their elimination is required, such as in noninflamed tissues or in spleen. An alternative hypothesis is that the ligand is found on certain pathogens, such as helminthic parasites, and engagement induces a “suicidal” yet beneficial eosinophil host response leading to local release of toxic eosinophil granules, akin to the “kamikaze” hypothesis put forth more than 2 decades ago.39 Further investigation of the functional consequences of Siglec-8 engagement will be needed to define its exact role in eosinophil host defense mechanisms. Regardless, Siglec-8 remains of particular interest in the context of allergic diseases because of its restricted expression on eosinophils, basophils, and mast cells and the fact that the chromosomal location of most Siglecs, on 19q13.3-13.4, has shown linkage with asthma.40-42 The proapoptotic function defined in this study may offer a new therapeutic strategy for allergic and other diseases, in which stimulation of Siglec-8 or its relevant signaling pathways may effectively reduce or eliminate eosinophils (and perhaps mast cells and basophils as well).
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-10-3058.
Supported by grant AI41472 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Robert Schleimer for helpful discussions; Carol Bickel and Mary Brummet for technical assistance; Drs John White, Kristy Kikly, Jian Ni, and Paul Crocker for Siglec-8 reagents and cell lines; and Bonnie Hebden for assistance in the preparation of the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal