Abstract
Initially considered to be of natural killer (NK)–cell origin, CD4+ CD56+ blastic tumors (BTs) of skin have recently been proposed to be of dendritic cell lineage. We have previously described BTs with transformation to myelomonocytic leukemia. Here we report expression of the lymphoid proto-oncogene TCL1 in 10 (83%) of 12 BTs and in lymph node plasmacytoid dendritic cells (DC2s). TCL1 was also expressed in myelomonocytic blasts of 3 transformed BT cases but not in true NK-cell tumors (n = 18), de novo acute myelomonocytic leukemias (1 of 14, 7%), or mature T-cell malignancies (1 of 112, < 1%), with the exception of T-prolymphocytic leukemia (T-PLL). All BT cases were also positive for the DC2-associated marker CD123. These results further support derivation of BTs from DC2s, and demonstrate that TCL1 expression in this tumor is common to the immature blastoid, lymphoid-appearing, and subsequent myelomonocytic phases of this disease.
Introduction
CD4+ CD56+ blastic tumor (BT) is a recently recognized highly aggressive tumor presenting in skin, usually with lymph node and bone marrow involvement, often terminating in a leukemic phase.1 Based on lymphoid morphology, frequent expression of natural killer (NK) cell–associated antigens (eg, CD2, CD7), and the absence of T-cell receptor or surface CD3, these tumors had been considered to be of NK-cell origin2-4 and were designated as blastic NK-cell tumors in the World Health Organization (WHO) classification.5
However, the CD4+ CD56+ BTs express markers typical of plasmacytoid dendritic cells (also known as DC2s), particularly CD123/interleukin-3 (IL-3) receptor δ-chain.6,7 Based on the emergence of typical myelomonocytic leukemias in a subset of BT cases, we have suggested that these tumors may also have monocytic or multilineage potential.1 This is consistent with the finding that DC2s are sometimes increased in patients with myelomonocytic leukemias and can be derived from monocytes in vitro under certain conditions and may undergo conversion in vivo to myeloid-type DCs.8-11
To investigate the histogenesis of CD4+ CD56+ BTs, we tested markers of various hematopoietic lineages. We report here that BTs and DC2s share the expression of CD123 and the lymphoid proto-oncogene product TCL1. TCL1 expression was previously thought to be restricted to B cells, some immature T cells, and most cases of T-cell prolymphocytic leukemia (T-PLL), in which it is activated as a consequence of chromosomal rearrangements involving 14q32.1.12-16 TCL1 is a protein of unknown function that has recently been shown to bind in vitro to the AKT family of serine/threonine kinases and to promote AKT kinase activation through oligomerization and increased AKT transphosphorylation.17-20
Study design
The study was conducted according to the MD Anderson Cancer Center institutional review board–approved protocol, and cases were selected using WHO criteria for blastic NK-cell lymphoma, which required initial cutaneous presentation as well as CD4 and CD56 positivity and absence of surface CD3.5 TCL1 expression was analyzed by Western blot and/or immunohistochemistry using a TCL1 rabbit antiserum.13 TCL1 specificity was confirmed by Western blot using extracts of T-PLL cells with cytogenetically documented rearrangements of the TCL1 locus. Phosphorylated AKT (pAKT) was detected using 2 phospho-specific antisera directed against the Ser473 and Thr308 epitopes of AKT-1, -2, and -3 (pAKT; Cell Signaling Technology, Beverly, MA). Monoclonal antibodies were used to detect terminal deoxynucleotidyl transferase (TdT-NCL; Novocastra, Newcastle-upon-Tyne, England), cutaneous lymphocyte antigen (CLA, HECA-452; Pharmingen, San Diego, CA), CD3 (Dako, Carpinteria, CA), CD4 (1F6; Novocastra), CD20 (L26; Dako), CD45RA (4KB5; Dako), CD56 (1B6; Novocastra), CD68 (KP1; Dako), CD117/c-kit (104D2; Dako), and CD123 (6H6; eBiosciences, San Diego, CA).
Tumor cells were obtained by Ficoll-Hypaque density centrifugation of peripheral blood (PB). Cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane and detected using the enhanced chemiluminescence system (Amersham, Piscataway, NJ). BC-1 (B-cell large cell lymphoma line) and NALM-6 (B-cell lymphoblastic line) were used as negative and positive controls for TCL1, respectively, and Jurkat (T-lymphoblastic cell line) was used as the positive control for pAKT. Immunohisto-chemical staining was performed on formalin-fixed tissue sections using avidin-biotin-peroxidase reagents (LSAB+ kit; Dako) using a previously validated T-cell and NK cell lymphoma tissue array,21 with positive immunostaining results confirmed on whole-tissue sections.
Results and discussion
CD123 and TCL1 expression is characteristic of CD4+ CD56+ BTs
Described previously were 6 BT cases;1 here we added 6 additional cases having similar features, including initial cutaneous presentation with lymphoid morphology (Figure 1A, inset), expression of CD4 and CD56 (Figure 1A), and absence of CD3, myeloperoxidase, and CD117. CD68 was positive in 6 of 8 cases tested. All but one case lacked monoclonal T-cell receptor gamma gene rearrangements by polymerase chain reaction analysis. As previously described, we noted in 10 (83%) of 12 cases variable immunohisto-chemical positivity for TdT, which was strongest in tumor cells in 4 involved lymph node biopsy specimens (Figure 1B). Subsequently, 3 BT patients developed increasing blood myelomonocytic blasts terminating in acute myeloid leukemia (AML) 13, 15, and 22 months after initial cutaneous presentation. These phenotypically distinct blasts in each case were cytochemically positive for butyrate esterase and myeloperoxidase and were TdT-negative. Cytogenetic findings in 6 cases are listed in Table 1.
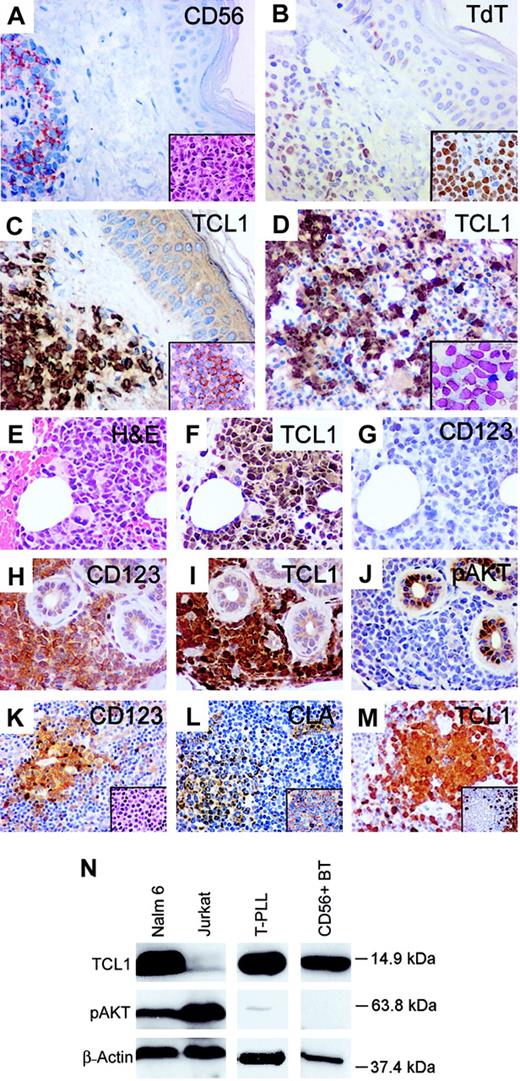
Comparison of immunophenotype of CD4+ CD56+ blastic tumors and plasmacytoid dendritic cells. (A-G) A case of CD4+ CD56+ BT presenting in skin and lymph node that transformed into monocytic AML at 22 months. Tumor cells in skin show uniform expression of CD56 (A), with a lymphoid morphology (inset). Variable TdT expression was seen in the skin tumor (B), with stronger TdT expression seen in tumor cells from an involved lymph node (inset). Tumor cells in skin show strong nuclear and cytoplasmic staining for TCL1 (C) and CD123 (inset), with marrow tumor cells (D inset; Wright-Giemsa stained smear) also positive for TCL1 (D). Bone marrow blasts in the subsequent myelomonocytic leukemic transformation (E) also show strong TCL1 immunostaining (F) but were negative for CD123 (G). (H-J) Another CD4+ CD56+ BT of skin with strong CD123 (H) and TCL1 expression (I) but absence of pAKT immunostaining (J). (K-M) Plasmacytoid dendritic cells (DC2s) in a reactive lymph node (inset K) stain for CD123 (K), CLA (L), CD45RA (inset L), and TCL1 (M) and are negative for CD20 (inset M). (N) Western blot analysis with TCL1 antiserum detects a 14-kDa protein in a B-lymphoblastic cell line (NALM-6), T-PLL carrying a chromosome14 inversion, and tumor cells from a case of CD4+ CD56+ BT. The latter case demonstrated strong immunostaining for TCL1 (I) and absence of pAKT-Ser473 expression (J), both of which were confirmed by Western blot analysis (N). Original magnifications, × 400; insets, × 1000.
Comparison of immunophenotype of CD4+ CD56+ blastic tumors and plasmacytoid dendritic cells. (A-G) A case of CD4+ CD56+ BT presenting in skin and lymph node that transformed into monocytic AML at 22 months. Tumor cells in skin show uniform expression of CD56 (A), with a lymphoid morphology (inset). Variable TdT expression was seen in the skin tumor (B), with stronger TdT expression seen in tumor cells from an involved lymph node (inset). Tumor cells in skin show strong nuclear and cytoplasmic staining for TCL1 (C) and CD123 (inset), with marrow tumor cells (D inset; Wright-Giemsa stained smear) also positive for TCL1 (D). Bone marrow blasts in the subsequent myelomonocytic leukemic transformation (E) also show strong TCL1 immunostaining (F) but were negative for CD123 (G). (H-J) Another CD4+ CD56+ BT of skin with strong CD123 (H) and TCL1 expression (I) but absence of pAKT immunostaining (J). (K-M) Plasmacytoid dendritic cells (DC2s) in a reactive lymph node (inset K) stain for CD123 (K), CLA (L), CD45RA (inset L), and TCL1 (M) and are negative for CD20 (inset M). (N) Western blot analysis with TCL1 antiserum detects a 14-kDa protein in a B-lymphoblastic cell line (NALM-6), T-PLL carrying a chromosome14 inversion, and tumor cells from a case of CD4+ CD56+ BT. The latter case demonstrated strong immunostaining for TCL1 (I) and absence of pAKT-Ser473 expression (J), both of which were confirmed by Western blot analysis (N). Original magnifications, × 400; insets, × 1000.
TCL1 expression as detected by immunostaining and Western blot
Diagnosis . | TCL1+ (%) . |
|---|---|
| CD4+CD56+ blastic tumor | 10/12 (83)* |
| Nasal-type NK-cell lymphoma | 0/15 |
| Aggressive NK-cell leukemia | 0/1 |
| NK-cell large granular lymphocyte leukemia | 0/2 |
| Chronic myelogenous leukemia, 2 chronic phase, 2 T-cell blast crisis | 0/4 |
| Extramedullary myeloid cell tumors with other AML-M4 or M5 immunophenotypes† | 1/14 (7)† |
| Extramedullary myeloid cell tumors with other myeloid immunophenotypes | 0/7 |
| T-cell lymphoblastic lymphoma/leukemia | 1/7 (14)‡ |
| T-prolymphocytic leukemia | 30/40 (75)§ |
| Adult T-cell leukemia/lymphoma | 1/5 (20)† |
| T-cell large granular lymphocyte leukemia | 0/1 |
| Hepatosplenic T-cell lymphoma | 0/2 |
| Enteropathy-type T-cell lymphoma | 0/2 |
| Angioimmunoblastic lymphoma | 0/10 |
| ALCL, including 5 primary cutaneous ALCL | 0/21 |
| Nodal peripheral T-cell lymphoma, unspecified | 0/20 |
| Mycosis fungoides/Sézary syndrome | 0/36§ |
| Subcutaneous panniculitis-like T-cell lymphoma | 0/1 |
| Lymphomatoid papulosis | 0/6 |
| Cutaneous T-cell lymphoma, other types | 0/6 |
| Lymphomatoid granulomatosis | 0/2 |
| Hodgkin lymphoma | 0/10 |
Diagnosis . | TCL1+ (%) . |
|---|---|
| CD4+CD56+ blastic tumor | 10/12 (83)* |
| Nasal-type NK-cell lymphoma | 0/15 |
| Aggressive NK-cell leukemia | 0/1 |
| NK-cell large granular lymphocyte leukemia | 0/2 |
| Chronic myelogenous leukemia, 2 chronic phase, 2 T-cell blast crisis | 0/4 |
| Extramedullary myeloid cell tumors with other AML-M4 or M5 immunophenotypes† | 1/14 (7)† |
| Extramedullary myeloid cell tumors with other myeloid immunophenotypes | 0/7 |
| T-cell lymphoblastic lymphoma/leukemia | 1/7 (14)‡ |
| T-prolymphocytic leukemia | 30/40 (75)§ |
| Adult T-cell leukemia/lymphoma | 1/5 (20)† |
| T-cell large granular lymphocyte leukemia | 0/1 |
| Hepatosplenic T-cell lymphoma | 0/2 |
| Enteropathy-type T-cell lymphoma | 0/2 |
| Angioimmunoblastic lymphoma | 0/10 |
| ALCL, including 5 primary cutaneous ALCL | 0/21 |
| Nodal peripheral T-cell lymphoma, unspecified | 0/20 |
| Mycosis fungoides/Sézary syndrome | 0/36§ |
| Subcutaneous panniculitis-like T-cell lymphoma | 0/1 |
| Lymphomatoid papulosis | 0/6 |
| Cutaneous T-cell lymphoma, other types | 0/6 |
| Lymphomatoid granulomatosis | 0/2 |
| Hodgkin lymphoma | 0/10 |
ALCL indicates anaplastic large cell lymphoma.
Karyotypic analysis of 6 cases revealed 3 diploid karyotypes, 1 case with monosomy 5, another with monosomy 7, and a third with trisomy 8. No karyotypic abnormalities of chromosome 14 were noted. Multiple chromosomal losses were previously found in CD4+CD56+ tumors.7,22
TCL1 staining intensity was weak and variable with only focally nuclear staining. The weakly TCL1+ AML-M5 was CD56+ and negative for CD4 and CD123. Among extramedullary myeloid cell tumors with monocytic differentiation, 3 of 11 cases were CD56+ and all were negative for TdT.
The TCL1+ case of T-cell lymphoblastic lymphoma/leukemia was TdT+ and CD56-.
Immunostaining results were confirmed by TCL1 Western blot analysis in 14 T-PLL and 3 Sézary syndrome cases.
By immunohistochemistry, all cases (11/11 skin, 1/1 lymph node, 5/5 bone marrow) showed membrane expression of CD123/IL-3 receptor α-chain (Figure 1C, inset), which was variable in 4 cases. Of 12 BTs, 10 (83%) showed strong uniform nuclear and cytoplasmic expression for TCL1 (Figure 1C), including skin (10/12, 83%) and bone marrow (4/5, 80%) sites (Figure 1C-D). Western blot analysis of freshly isolated tumor cells from PB in one BT case confirmed TCL1 expression (Figure 1). All 3 cases with subsequent myeloid transformation showed strong TCL1 expression in the myelomonocytic bone marrow blasts (Figure 1E-F), with 1 of 3 tumors no longer expressing CD123 (Figure 1G).
TCL1 is highly expressed in CD123+ lymph node plasmacytoid dendritic cells (DC2s)
We examined TCL1 expression in normal hematopoietic cell subsets, and tumors possibly related to BTs.23,24 In normal spleen (n = 5), tonsil (n = 3), and bone marrow (n = 4), TCL expression was confined to CD20+ B cells and rare cells with dendritic morphology. In reactive lymph nodes (n = 5), aside from B cells the only other TCL1+ cells noted were clusters of plasmacytoid DC2s, which also expressed CD4 (dim), CD45RA (dim), CD68, and CD123. The clusters were variably positive for CLA and negative for CD3, CD20, and TdT (Figure 1K-M, and not shown), based on immunostaining of serial sections.
TCL1 is negative in true NK-cell lymphomas, mature T-cell malignancies, and myeloid leukemias
In a survey of true NK-cell tumors and other CD56+ myeloid neoplasms involving skin and extramedullary sites, as well as chronic myeloproliferative processes, and a variety of T-cell tumors, nearly all tumors were completely negative for TCL1 (Table 1). TCL1+ cases included most cases of T-PLL (30/40, 75%) and a single case each of precursor T-cell lymphoblastic lymphoma/leukemia, AML with monocytic differentiation, and adult T-cell leukemia/lymphoma, with each showing TCL1 staining in a subset of tumor cells (Table 1).
TCL1 expression does not correlate with levels of pAKT
Given that TCL1 has been implicated in vitro in activation of AKT by phosphorylation, we compared the pattern of pAKT with that of TCL1.17-20 Most TCL1+ BT cases (6/9; 66%) were negative for both Ser473 and Thr308 pAKT by immunostaining (Figure 1J). Absence of pAKT was also noted in a case of BT analyzed by Western blot (Figure 1N). Additionally, most cases of T-PLL were negative for pAKT, with low levels of pAKT detected by Western blot in 3 (30%) of 10 TCL1+ T-PLL (Figure 1N) and weak immunostaining noted in 2 (15%) of 13 TCL1+ T-PLL. Variable, often strong, pAKT immunostaining, however, was seen in a variety of TCL1– T-cell lymphomas (44/79, 56%).
In conclusion, BTs and their myelomonocytic transformations highly express TCL1, a feature shared with plasmacytoid dendritic cells (DC2s) but not with myelomonocytic leukemia, true NK-cell tumors, or mature T-cell malignancies, except T-PLL. TCL1 expression in primary BT and T-PLL samples was infrequently associated with pAKT, suggesting other mechanisms of TCL1 action in vivo. CD123 and TCL1 represent useful markers to identify BTs and distinguish them from other CD56+ cutaneous tumors, including extranodal NK-cell lymphomas of nasal-type and myelomonocytic leukemias. Expression of TCL1 demonstrates another immunophenotypic linkage between normal DC2s and the immature blastoid (TdT+), lymphoid-appearing (TdT–), and subsequent monocytic (butyrate esterase+) phases of CD4+ CD56+ blastic tumors.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-10-3297.
Supported by research grant CA16672 awarded by the National Cancer Institute, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal