Abstract
T-cell defects and premature thymic atrophy occur in cancer patients and tumor-bearing animals. We demonstrate that exposure of mice to recombinant vascular endothelial growth factor (VEGF) at concentrations similar to those observed in advanced stage cancer patients reproduces this profound thymic atrophy and is highlighted by a dramatic reduction in CD4+/CD8+ thymocytes. We find that VEGF does not induce thymocyte apoptosis, but instead rapidly decreases the number of the earliest observable progenitors in the thymus. VEGF does not inhibit thymocyte development in fetal thymic organ culture, further suggesting a prethymic effect. We also demonstrate that bone marrow progenitors from animals infused with recombinant VEGF and transferred to irradiated untreated animals recolonize the thymus more efficiently than progenitors from control animals. This suggests that VEGF exposure is associated with an increased population of thymus-committed progenitors in the bone marrow. We hypothesize that pathophysiologically relevant concentrations of VEGF may block the differentiation and/or emigration of these progenitors resulting in the observed thymic atrophy. Removal of VEGF via cessation of infusion or adoptive transfer of progenitors to a congenic host induces a preferential commitment of lymphoid progenitors to the T lineage and results in a restoration of the normal composition and cellularity of the thymus. These data demonstrate that at pathophysiologic concentrations, VEGF interferes with the development of T cells from early hematopoetic progenitor cells and this may contribute to tumor-associated immune deficiencies.
Introduction
Decreased immune function in cancer patients is well established,1 and it is self-evident that clinically apparent tumors avoid effective antitumor immune responses. Tumor-associated immune defects are observed in all immune organs and cell lineages, including the thymus and T cells.2-8 While thymic atrophy accompanies normal aging,9 a high incidence of premature thymic involution is seen in childhood malignancies,10 and this often rebounds after curative treatment. This can be modeled in mice that received transplants of mammary adenocarcinomas, which demonstrate rapid thymic involution associated with depletion and/or alterations of thymocyte subpopulations.11-14 The mechanism of this cancer-associated thymic atrophy, and more generally the factors responsible for lineage commitment, migration to the thymus, and progression through thymocyte developmental checkpoints remain poorly understood.
Interleukin-10 (IL-10), tumor necrosis factor (TNF), transforming growth factor β (TGF-β), and vascular endothelial growth factor (VEGF) are among the known tumor-associated factors that inhibit immune cell function, impairing both effector function and early stages of hematopoiesis. Multiple cell lineages are involved. Serum size fractionation studies have shown thymic atrophy associated with a nonimmunoglobulin serum component with a molecular weight higher than 25 000 Da in mice that received transplants of Lewis lung carcinoma cells.15 Consistent with this finding, VEGF (42 kDa) and its receptors have profound effects on the early development and differentiation of both vascular and hematopoetic progenitors,16 and VEGF receptors are present on some of the earliest hematopoetic progenitor cells (HPCs).17 We have previously demonstrated that VEGF, a factor produced by a large fraction of solid tumors, causes a defect in the functional maturation of dendritic cells (DCs) from early HPCs.18,19
In addition to DC defects, non–tumor-bearing mice treated with a continuous infusion of recombinant VEGF have a decreased number of T cells and a decreased T-cell–to–B-cell ratio in their lymph nodes and spleen,19 and thus VEGF represents a candidate mediator of the tumor-associated thymic defect. This increased B-cell population may be compensatory in nature and suggests that in addition to causing defective developmental progression within the myeloid lineage, lymphoid lineage commitment may be similarly effected by VEGF. In this study we investigate whether pathologically relevant concentrations of VEGF inhibit T-cell development, and we explore the cellular mechanisms involved in this process.
Materials and methods
Animals
Female BALB/c mice (6 to 8 weeks old) were purchased from Harlan (Indianapolis, IN). DO11.10, C57Bl/6, and B6.SJL-PtprcaPep3b/BoyJ were purchased from Jackson Laboratories (Bar Harbor, ME). All animal studies have been approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Antibodies and reagents
VEGF and Flt-1(1-3)immunoglobulin G (IgG) were generous gifts from Genentech (South San Francisco, CA). Murine anti-VEGF neutralizing antibody was purchased from R&D Systems (Minneapolis, MN). The following antibody-producing hybridomas were obtained from American Type Culture Collection (ATCC, Rockville, MD) and used as culture supernatants: anti-CD4 (L3T4, TIB-207), anti-CD8 (Lyt-2.2, TIB-210), and anti–major histocompatibility complex class II (TIB-120). Anti-CD3, -CD4, -CD8, -CD25, -CD34, -CD44, -CD45R/B220, -CD45.1, –c-kit, –IL-7Rα, –Sca-1, and –Thy 1.2 fluorescent antibodies were purchased from BD Pharmingen (San Diego, CA). The D459 tumor cell line is a poorly immunogenic fibrosarcoma described in detail elsewhere.19,20
VEGF administration
VEGF was delivered into mice via Alzet osmotic pumps (Durect, Cupertino, CA) as previously described19 for 7 to 28 days at 50 to 100 ng/h. Control pumps were filled with phosphate-buffered saline (PBS).
Thymocyte isolation and analysis of cell-surface receptors
A single-cell suspension was prepared from thymus by pressing the tissues through 70-μm nylon mesh. For analysis of cell-surface receptors, cells were washed in PBS supplemented with 0.1% fetal calf serum and labeled with appropriate antibodies for 30 minutes at 4°C. Cells were then washed with PBS and analyzed on a dual laser FACScan flow cytometer (Becton Dickinson, Mountain View, CA). In all samples, 10 000 events were acquired and dead cells were excluded by scatter analysis.
Detection of apoptotic cells and cell-cycle profiles
Thymocyte apoptosis was analyzed using the Annexin V–fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Pharmingen) Thymocytes incubated for one hour at 42°C were used as positive controls. Thymocyte apoptosis was confirmed by TUNEL (TdT-mediated dUTP nick-end labeling) staining using the DeadEnd Colorimetric TUNEL System (Promega, Madison, WI).
Cell cycle profiles of thymocytes were determined by staining with FITC–anti–bromo-deoxyuridine (BrdU) and 7-amino-actinomycin D (7-AAD) via the BrdU Flow Kit (BD Pharmingen)
Immunohistochemistry
Mouse thymus, lung, and kidney tissues were harvested without perfusion and fixed in 10% neutral-buffered formalin for 12 hours. The tissues were paraffin-embedded and sectioned (5 μm). Hematoxylin and eosin (H&E; Mayers, Medical Chemical, Torrance, CA), CD31, and Gomori one-step trichrome (blue) were used for general and component staining.
Corticosterone radioimmunoassay
Serum corticosterone levels were assayed by I125 radioimmunoassay (RIA) by the Vanderbilt Mouse Metabolic Physiology Analytical Resources Core using a Rat Corticosterone Coat-a-count RIA Kit (Diagnostic Products, Los Angeles, CA). All samples were collected at the same time of day to minimize diurnal variation in corticosterone levels.
Fetal thymic organ culture (FTOC)
Day-14.5 fetal thymic lobes were cultured on 0.8-μm Isopore membrane filters (Millipore, Carrigtwohill, County Cork, Ireland) resting on top of Dulbecco modified Eagle medium –10% fetal bovine serum (FBS)–saturated 12-mm to 7-mm Gelfoam absorbable gelatin sponges (Pharmacia & Upjohn, Kalamazoo, MI) ± VEGF (5-100 ng/mL) at 7% CO2. Recombinant murine VEGF (R&D Systems) was added to the cultures on days 0 and 2. Recombinant murine IL-7 (R&D Systems) and EGF (a generous gift from Dr Graham Carpenter, Vanderbilt University, Nashville, TN) were used as controls.
T-cell receptor excision circle (TREC) analysis
Signal joint (sj) TREC analysis was performed as described previously.21 Briefly, mouse thymus and spleen were excised from killed animals and weighed. Thymus lobes were snap frozen in a dry ice/ethyl alcohol bath and stored in liquid nitrogen. Genomic DNA samples from whole thymus tissues were prepared by homogenization in 1 mL TRIZOL Reagent (Invitrogen, Carlsbad, CA) using a Tekmar Tissumizer homogenizer (Cincinnati, OH). Red blood cells were removed from total splenocytes by osmotic shock and remaining cells were cryopreserved in 10% dimethyl sulfoxide, 10% FBS, and 80% RPMI 1640 in liquid nitrogen. CD4+ and CD8+ T cells were isolated from cryopreserved splenocytes using magnetic beads (Miltenyi Biotec, Auburn, CA).
Signal joint excision circles containing the T-cell receptor δ (TCRδ) locus formed by the recombination between ψJα and δRec1 were quantitated by real-time quantitative polymerase chain reaction (PCR). Both the mTREC standard used and specific PCR primers/probe have been described in detail previously.21
Adoptive transfer experiments and bone marrow (BM) analysis
Female C57BL/6J (Ly5.2) mice (6- to 8-weeks old) were exposed to 700 cGy of gamma irradiation from a Cesium source and injected with bone marrow from congenic female mice (B6.SJL-PtprcaPep3b/BoyJ [Ly5.1]). Femoral BM cells were depleted of mature cells from donor mice (± VEGF [50 ng/h] for 14 days) by incubation with a cocktail of hybridoma supernatants (TIB 207, TIB 210, and TIB 120), followed by complement incubation (Low-Tox-Guinea Pig Complement; Cedarline, Ontario, Canada) and gradient centrifugation on Lympholyte M (Cedarline). Approximately 1 million lineage+ HPCs were transplanted into irradiated recipients by intravenous tail-vein injection. Mice were maintained on antibiotic water (1:100 dilution of pediatric sulfatrim suspension; Alpharma USPD, Baltimore, MD).
For identification of long-term hematopoietic stem cells (LT-HSCs) and common lymphoid progenitors (CLPs), BM was depleted of CD5+, B220+, TER-119+,Gr-1+, and CD11b+ cells as described in the previous paragraph and stained with appropriate antibodies to identify the following subsets: LT-HSCs (Lin–, CD34–, Thy 1.2lo, Sca-1+, and c-kit+)22,23 and CLPs (Lin–, IL-7Rα+, Sca-1lo, and c-kitlo).24
Results
Antibodies to VEGF reverse tumor-associated thymic atrophy
Dramatic thymic atrophy is known to occur in tumor-bearing animals (Figure 1A), and we hypothesized that VEGF is an important mediator of this effect. In the following studies, mice were injected subcutaneously with approximately 200 000 D459 tumor cells, and thymic weights were measured at 4 weeks. D459 is a poorly immunogenic fibrosarcoma tumor cell line known to induce tumor formation and expression of high levels of VEGF (100-300 pg/mL) when implanted into normal immune-competent balb/c mice.20 We evaluated whether inhibition of VEGF signaling in these animals could reverse this tumor-associated thymic atrophy.
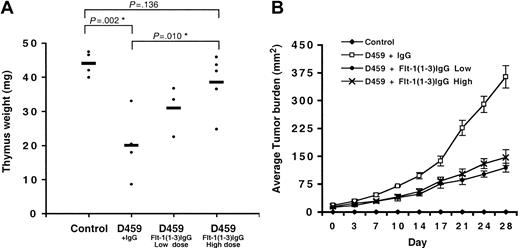
Inhibition of VEGF results in reduced thymic atrophy in tumor-bearing animals. Inhibition of VEGF signaling in tumor-bearing animals by Flt-1(1-3)IgG is able to significantly reverse tumor-associated thymic atrophy. (A) Thymus weight (mg) ± Flt-1(1-3)IgG. Heavy horizontal bars represent mean thymus weight per group of mice. (B) Average tumor burden ± Flt-1(1-3)IgG. Flt-1(1-3)IgG low dose: 10 mg/kg twice weekly; high dose: 15 mg/kg every other day. *Denotes statistical significance. Error bars indicate SE.
Inhibition of VEGF results in reduced thymic atrophy in tumor-bearing animals. Inhibition of VEGF signaling in tumor-bearing animals by Flt-1(1-3)IgG is able to significantly reverse tumor-associated thymic atrophy. (A) Thymus weight (mg) ± Flt-1(1-3)IgG. Heavy horizontal bars represent mean thymus weight per group of mice. (B) Average tumor burden ± Flt-1(1-3)IgG. Flt-1(1-3)IgG low dose: 10 mg/kg twice weekly; high dose: 15 mg/kg every other day. *Denotes statistical significance. Error bars indicate SE.
Flt-1(1-3)IgG fusion protein contains a portion of the vascular endothelial growth factor receptor 1 (VEGFR1, Flt-1) extracellular domain fused to mouse IgG and serves as a potent inhibitor of VEGF signaling in vivo. D459 tumor–bearing mice were given injections of Flt-1(1-3)IgG at either a low dose (10 mg/kg twice weekly) or a high dose (15 mg/kg every other day) for a duration of 4 weeks. Normal mouse IgG was used as a control. As expected, we observed dramatic thymic atrophy in D459 tumor–bearing animals compared with age-matched controls (thymus weight [mg]: control, 44.1 ± 3.6; IgG, 20.1 ± 10.1 [P = .002, n = 4]). Treatment of animals with Flt-1(1-3)IgG substantially corrected the observed thymic atrophy in a dose-dependent manner (Figure 1A) (IgG, 20.1 ± 10.1; low dose, 31.0 ± 7.4 [P = .089]; high dose, 38.6 ± 8.5 [P = .010]), but in both instances, treatment with Flt-1(1-3)IgG resulted in a significant inhibition of tumor growth (Figure 1B) and vascularization (data not shown). We were unable to find a dose that convincingly reversed thymic atrophy without a significant antitumor effect.
These data make it difficult to differentiate between direct effects of VEGF on T-cell development in tumor-bearing mice and secondary effects caused by the resulting reduction in tumor burden. To isolate the effects of VEGF from other potential tumor mediators, we studied the effects of subcutaneous infusion of recombinant VEGF on T-cell development.
Prolonged exposure to recombinant VEGF induces thymic atrophy in vivo
We have previously observed that a continuous infusion of VEGF (50 ng/h) results in a serum concentration of 120 to 160 pg/mL and a decreased percentage of T cells in the spleen and lymph nodes of non–tumor-bearing animals.19 The mean serum VEGF concentration in advanced stage cancer patients is similar at approximately 150 pg/mL.25 To study the specific effects of VEGF on T-cell development, 6- to 8-week-old balb/c female mice were given continuous infusions of recombinant VEGF for 7 to 28 days, and thymic lobes were sectioned and stained with H&E (Figure 2).
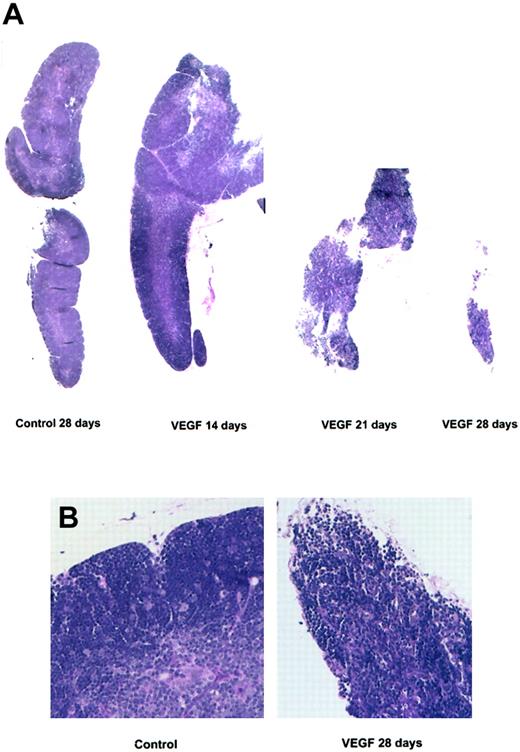
Photomicrograph of thymus sections stained with H&E. Balb/c mice (6 to 8 weeks old) were treated with a continuous infusion of VEGF (50 ng/h). Mice were killed on days 14, 21, and 28. (A) Photomicrographs are representative of 3 or more independent experiments (original magnification, × 20). (B) Control and VEGF-treated thymus sections following 28 days of infusion from panel A at higher magnification (× 400).
Photomicrograph of thymus sections stained with H&E. Balb/c mice (6 to 8 weeks old) were treated with a continuous infusion of VEGF (50 ng/h). Mice were killed on days 14, 21, and 28. (A) Photomicrographs are representative of 3 or more independent experiments (original magnification, × 20). (B) Control and VEGF-treated thymus sections following 28 days of infusion from panel A at higher magnification (× 400).
After 3 to 4 weeks of VEGF treatment, we observed a dramatic reduction in the size of the thymus and a striking decrease in thymocyte cellularity compared with age-matched, PBS-infused controls (Figure 2A). The normally clear distinction between cortical and medullary regions of the thymus was no longer present (Figure 2B), and broad hypocellular areas containing collagen, fibroblasts, and dilated blood vessels separated the remaining follicles. These observations were confirmed by immunohistochemistry using an anti-CD31 antibody to stain for endothelial cells, and Gomori one-step trichrome (blue) staining to highlight collagen (data not shown). The relative percentage of both thymic epithelial cells and vascular structures was increased in VEGF-treated lobes, indicating that cell loss is primarily restricted to the lymphocyte populations.
There was no significant difference in thymus size, structure, or cellularity following infusions of fewer than 14 days or with PBS, suggesting that the observed effects were not due to postsurgical stress. This was confirmed by measuring serum corticosterone levels in mice infused with VEGF for 14, 21, or 28 days. There was no significant elevation in corticosterone levels in any of the animals given VEGF in comparison with PBS-infused or untreated controls (data not shown), and therefore it is unlikely that the observed atrophy is due to chronic stress.
Thymic structure and cellularity rebounds with cessation of VEGF infusion
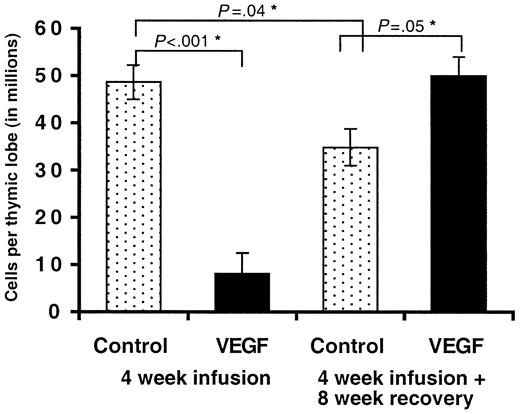
Patients exhibiting thymic atrophy associated with childhood malignancies experience a measurable rebound in thymus size following successful treatment. We were interested in whether chronic exposure to VEGF resulted in permanent damage to the thymus or whether the thymic lobes would return to normal following cessation of infusion. Mice given a continuous infusion of VEGF (50 ng/h) or PBS for 4 weeks were allowed to recover for an additional 8 weeks. We estimated that this time period would be sufficient to allow for any residual VEGF in the pump to be expressed, give sufficient time for unaltered BM progenitors to migrate to and seed the thymus, and provide adequate time for these cells to develop into a normal thymic repertoire. Infusions were initiated in 6- to 8-week-old mice, and therefore these animals were 18- to 20-weeks old at the time of death, compared with 10- to 12-weeks in all other experiments. Interestingly, we observed a small but significant decrease in total thymocyte cellularity in control mice killed 12 weeks after the initiation of infusion (4-week infusion + 8-week recovery) compared with those killed after 4 weeks (Figure 3). (Cells per thymic lobe: control [4 weeks], 48.6 × 106 ± 11.97; control [12 weeks], 34.8 × 106 ± 5.52 [P = .04].) We attribute this decrease to early evidence of age-associated involution. Mice given a 28-day infusion of VEGF and then allowed to recover had thymic lobes indistinguishable from younger (10- to 12-weeks old) PBS-infused animals, by either total cell numbers (Figure 3) (PBS [4 weeks], 48.6 × 106 ± 11.9; VEGF [12 weeks], 49.9 × 106 ± 5.69 [P = .44]) or H&E staining (data not shown) suggesting a compensatory rebound effect in these animals. Both the number and percentage of CD3+, CD4+, and CD8+ T cells in these mice were indistinguishable from controls.
Thymocyte cellularity following inhibition of VEGF. Mice were treated with a continuous infusion of VEGF (50 ng/h) or PBS (controls) for a period of 4 weeks. Some animals were killed at 4 weeks for comparison purposes. Animals were then monitored for an additional 8 weeks. Total thymocytes were counted using a hemocytometer. Mean of 3 independent experiments ± SE shown. *Denotes statistical significance.
Thymocyte cellularity following inhibition of VEGF. Mice were treated with a continuous infusion of VEGF (50 ng/h) or PBS (controls) for a period of 4 weeks. Some animals were killed at 4 weeks for comparison purposes. Animals were then monitored for an additional 8 weeks. Total thymocytes were counted using a hemocytometer. Mean of 3 independent experiments ± SE shown. *Denotes statistical significance.
This indicates that while VEGF infusion results in rapid and dramatic thymic atrophy, it does not cause permanent destruction of the thymic architecture. These data strongly suggest that thymocyte loss is primarily restricted to the renewable lymphocyte component, and we hypothesize that the stromal environment remains relatively unperturbed with VEGF exposure.
Effect of VEGF on thymocyte maturation
While continuous infusion of VEGF results in measurable thymic atrophy and loss of mature T cells, the remaining peripheral T cells proliferate normally in allogeneic mixed lymphocyte reactions (data not shown). A reduced number of peripheral T cells, accompanied by thymic atrophy, is consistent with an inhibition of thymocyte production (shown schematically in Figure 4A). To determine the effects of VEGF on intrathymic T-cell maturation, we isolated thymocytes from mice exposed to VEGF (7-28 days) and stained these cells with antibodies to the T-lineage receptors CD3, CD4, and CD8.
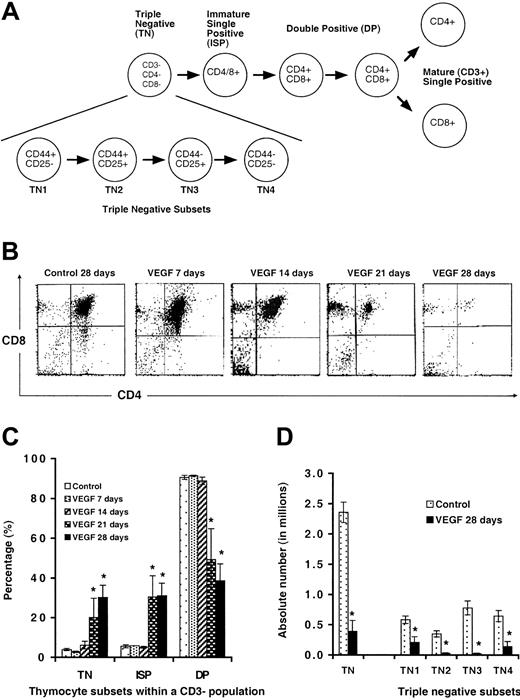
Development of thymocytes from VEGF-treated and tumor-bearing mice. (A) Schematic representation of T-cell development in the thymus. (B) Mice treated with VEGF as described in Figure 1 were killed and thymocytes analyzed for expression of CD4 and CD8 within a gated CD3–/low population. Representative FACS plots are shown. (C) Percentage of thymocyte subsets in mice infused with VEGF (mean ± SE; N > 3). (D) Absolute number of thymocytes within TN subsets (mean ± SE; N > 3). *Denotes statistical significance.
Development of thymocytes from VEGF-treated and tumor-bearing mice. (A) Schematic representation of T-cell development in the thymus. (B) Mice treated with VEGF as described in Figure 1 were killed and thymocytes analyzed for expression of CD4 and CD8 within a gated CD3–/low population. Representative FACS plots are shown. (C) Percentage of thymocyte subsets in mice infused with VEGF (mean ± SE; N > 3). (D) Absolute number of thymocytes within TN subsets (mean ± SE; N > 3). *Denotes statistical significance.
Thymocytes from mice treated with VEGF for 7 or 14 days showed normal distribution of triple-negative (TN; CD3–/low, CD4–, and CD8–), intermediate single-positive (ISP; CD3–/low and either CD4+ or CD8+) and double-positive (DP; CD3–/low, CD4+, and CD8+) thymocytes (Figure 4B). Total thymocytes were significantly decreased by day 21 in VEGF-treated mice. Even though the absolute numbers of all subsets were significantly reduced, the decrease within the DP thymocyte population was proportionately greater (control, 90.5 ± 1.9%; VEGF [21 days], 49.3 ± 20.5% [P < .02]; VEGF [28 days], 38.6 ± 15.9% [P < .001]). The relative percentage of TN thymocytes increased proportionally (control, 3.9 ± 1.1%; VEGF [21 days], 20.1 ± 12.9% [P < .10]; VEGF [28 days], 30.2 ± 11.5% [P < .001]). Both 21- and 28-day mice demonstrated an increase in the percentage of ISP cells compared with controls (control, 5.6 ± 1.4%; VEGF [21 days], 30.6 ± 13.9% [P < .05]; VEGF [28 days], 31.1 ± 11.6% [P < .001]). These data show that in addition to decreased total numbers of thymocytes, there may be defective thymocyte progression during continuous VEGF exposure.
In order to look more closely at the increased percentage of TN thymocytes within the thymus of VEGF-infused animals, we stained cells with antibodies to CD44 and CD25 within the TN population (Figure 4D). The earliest stages of thymocyte development are characterized as TN1-4 by their expression of CD44 and CD25 on the cell surface: TN1, CD44+/CD25–; TN2, CD44+/CD25+; TN3, CD44–/CD25+; and TN4, CD44–/CD25– (Figure 4A).
We demonstrate here that while the overall percentage of TN thymocytes is increased, there is a clear reduction in the absolute number of TN cells across all 4 TN subsets (Figure 4D). Transition from the TN to DP stage is accompanied by a large proliferative burst, and this may explain the seemingly preferential loss of DP cells with VEGF infusion. We hypothesize that any defective progression is likely to be secondary to general cell loss and not a preferential depletion of any particular thymocyte subset.
VEGF effect on apoptosis and cell-cycle profiles of developing thymocytes
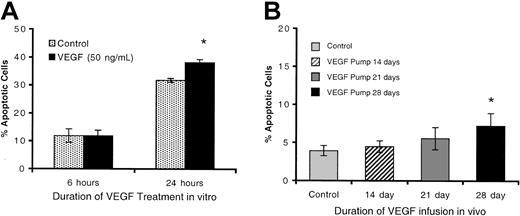
Thymic atrophy may be induced by a variety of mechanisms: decreased influx of thymocyte progenitors, defective maturation of these progenitors once they colonize the thymus, decreased intrathymic proliferation, or increased intrathymic apoptosis. Apoptosis was measured in thymocytes from control mice incubated in vitro in the presence of 100 ng/mL VEGF for 3, 6, or 24 hours by staining with Annexin V and propidium iodide (PI). There was no increase in the percentage of early apoptotic cells (Annexin V+, PI–) after 3 or 6 hours of incubation, but spontaneous apoptosis of thymocytes was minimally increased after 24 hours (control, 31.8 ± 0.7%; VEGF, 38.2 ± 1.0% [P < .05]) (Figure 5A).
Spontaneous apoptosis in VEGF-treated thymocytes. (A) Spontaneous apoptosis of thymocytes cultured ± VEGF (100 ng/mL) in vitro for 3, 6, or 24 hours. Results are presented as the mean percentage of early-apoptotic thymocytes (Annexin V+, PI–) (n = 9). (B) Annexin V staining of thymocytes from mice treated in vivo ± VEGF for 14, 21, and 28 days. Mice treated with VEGF as described in Figure 1 were killed and thymocytes analyzed for early-apoptotic cells via Annexin V staining (PI–) (mean ± SE; N > 3). Control shown in panel B is an average of controls from 0-, 14-, 21-, and 28-day samples. *Denotes statistical significance.
Spontaneous apoptosis in VEGF-treated thymocytes. (A) Spontaneous apoptosis of thymocytes cultured ± VEGF (100 ng/mL) in vitro for 3, 6, or 24 hours. Results are presented as the mean percentage of early-apoptotic thymocytes (Annexin V+, PI–) (n = 9). (B) Annexin V staining of thymocytes from mice treated in vivo ± VEGF for 14, 21, and 28 days. Mice treated with VEGF as described in Figure 1 were killed and thymocytes analyzed for early-apoptotic cells via Annexin V staining (PI–) (mean ± SE; N > 3). Control shown in panel B is an average of controls from 0-, 14-, 21-, and 28-day samples. *Denotes statistical significance.
However, the observation that more than 30% of thymocytes are actively undergoing apoptosis after 24 hours in culture highlights the low survivability of thymocytes in vitro. Treatment with VEGF may affect the survival of these cells by making them more susceptible to other proapoptotic stimuli. No changes in BrdU incorporation or cell-cycle profiles were observed under any of these conditions (data not shown).
We also studied the percentage of apoptotic thymocytes in vivo following chronic VEGF exposure. Thymocytes from animals exposed to VEGF showed a modest increase in early (Annexin V+, PI–) apoptotic events compared with controls, but this trend did not reach statistical significance with fewer than 8 days of VEGF infusion (control, 3.9 ± 2.2%; VEGF [28 days], 7.2 ± 4.0% [P < .05]) (Figure 5B). Following 28 days of infusion, thymus lobes have generally undergone a nearly complete atrophy, secondary structure is disrupted, and thymocyte numbers are reduced 10- to 20-fold. At this point, we expect that the thymus microenvironment is sufficiently altered as to render it unlikely to be capable of supporting normal thymocyte development. The modest increase in apoptosis observed may reflect this hypothesis. It is interesting to note that none of the thymus lobes that we observed in intermediate stages of thymic atrophy (generally following 21 days of infusion, and considered to be actively atrophying) displayed any increased proportion of thymocytes currently undergoing apoptosis. Furthermore, no significant increase in apoptotic cells was observed by TUNEL analysis of thymus sections from mice treated with VEGF for 7, 14, 21, or 28 days (data not shown). The low level of apoptosis and the slow dynamics of this process observed both in vitro and in vivo argue against a direct induction of thymocyte apoptosis by VEGF.
Effects of VEGF on TCR rearrangement, differentiation of residual thymocytes, and contribution of recent thymic emigrants to the periphery
We have shown that chronic exposure to VEGF at pathologic concentrations results in an overall decrease in absolute cell number across all thymocytes subsets, but we considered the hypothesis that thymic atrophy may be a result of defective maturation of T-cell progenitors once they colonize the thymus.
Since progression of TN cells to the DP stage is dependent on successful TCRβ rearrangement, the seemingly preferential loss of DP thymocytes suggests that VEGF may inhibit T-cell development by interfering with the ability of thymocytes to produce functional rearrangements of T-cell–receptor (TCR) genes. DO11.10 mice contain a transgene expressing a functionally rearranged TCR specific for the ovalbumin peptide, and therefore thymocytes in these mice do not need to rearrange endogenous TCRβ genes to proceed through intrathymic selection checkpoints. If defective TCRβ rearrangement is the primary mechanism of VEGF-induced thymic atrophy, thymocytes from DO11.10 mice infused with VEGF should develop normally.
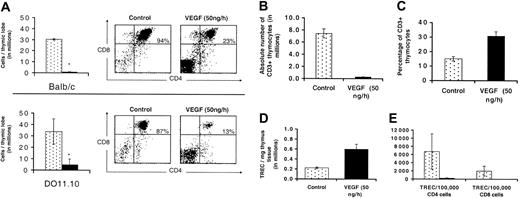
To test this hypothesis, DO11.10 and balb/c mice were given a continuous infusion of VEGF for 28 days and analyzed for thymocyte cellularity and developmental progression. VEGF infusion produced thymic atrophy, a severe loss of total thymocytes, and altered CD3/CD4/CD8 profiles in DO11.10 mice that were indistinguishable from the effects observed in balb/c mice (Figure 6A).
Effects of VEGF on TCR rearrangement, thymopoeisis, and contribution of recent thymic emigrants to the periphery. Balb/c mice and D011.10 TCR transgenic mice were treated with a continuous infusion of VEGF (50 ng/h) for 28 days. Thymic lobes were removed and total thymocytes counted. (A) Mean number of cells per thymic lobe (n = 3, ± SE, left panels). Thymocytes were also stained with antibodies to CD4 and CD8 within a CD3–/low population and representative FACS plots are shown (right panels). * denotes statistical significance (P < .05). (B) Absolute number of CD3+ thymocytes per thymic lobe in balb/c mice. (C) Percentage of CD3+ thymocytes in balb/c mice. (D) Number of TRECs/mg of thymus tissue. (E) Contribution of recent thymic emigrants to the peripheral T-cell pool was also investigated. CD4+ and CD8+ T cells were purified from total splenocytes via magnetic bead separation, and the number of TRECs/100 000 splenic T cells is shown (n = 3, ± SE).
Effects of VEGF on TCR rearrangement, thymopoeisis, and contribution of recent thymic emigrants to the periphery. Balb/c mice and D011.10 TCR transgenic mice were treated with a continuous infusion of VEGF (50 ng/h) for 28 days. Thymic lobes were removed and total thymocytes counted. (A) Mean number of cells per thymic lobe (n = 3, ± SE, left panels). Thymocytes were also stained with antibodies to CD4 and CD8 within a CD3–/low population and representative FACS plots are shown (right panels). * denotes statistical significance (P < .05). (B) Absolute number of CD3+ thymocytes per thymic lobe in balb/c mice. (C) Percentage of CD3+ thymocytes in balb/c mice. (D) Number of TRECs/mg of thymus tissue. (E) Contribution of recent thymic emigrants to the peripheral T-cell pool was also investigated. CD4+ and CD8+ T cells were purified from total splenocytes via magnetic bead separation, and the number of TRECs/100 000 splenic T cells is shown (n = 3, ± SE).
Successful TCRβ rearrangement does not rescue the VEGF phenotype and is therefore not a primary defect associated with VEGF infusion.
We looked at several additional parameters to measure the ability of thymocytes to progress to late stages of development. First, we evaluated the expression of CD3 on the cell surface by fluorescence activated cell sorting (FACS) analysis. While the absolute number of CD3high cells is reduced considerably (Figure 6B), the percentage of these cells was actually increased approximately 2-fold (Figure 6C). This observation strengthened our hypothesis that cells remaining in the thymus continue to progress through later stages of T-cell development.
Next, we evaluated the ability of these cells to rearrange their TCRα locus. This stage of development follows both TCRβ rearrangement and expression of CD3 on the cell surface. Germline rearrangement of the TCRα genes results in the excision of intermediary DNA sequences that code for the TCRδ chain. These DNA fragments circularize, forming what is referred to as signal joint TCR delta (sjTCRδ) excision circles (mTRECs). These nonreplicated episomal DNA circles remain present in T cells through a limited number of cell divisions, and their presence can be measured via real-time quantitative PCR.21
The number of mTREC/mg of thymus tissue is considered a good measure of thymopoeisis and reflects the changing thymocyte population as it progresses to later stages of development. By studying the presence of mTRECs in the thymus, we hoped to gain insight into whether residual cells present in the thymus after prolonged VEGF exposure are actually developmentally impaired or are, as we hypothesized, progressing normally through recognizable stages of development.
In order to measure thymopoeisis associated with TCR rearrangement, thymus lobes were removed from mice treated with VEGF for 28 days. We observed that the number of molecules of mTREC/mg thymus was significantly increased following 28 days of VEGF infusion (control, 2.21 × 105 ± 0.24; VEGF, 5.97 × 105 ± 1.76 [P = .032]) (Figure 6D). This correlates nicely with the increased percentage of CD3+ cells that we observe in the thymus (Figure 6C) and illustrates that despite dramatic thymic atrophy and overall cell loss, residual cells in the thymus continue to progress through later stages of development. Increased mTREC/mg of thymus tissue and the failure of successful TCRβ rearrangement (D011.10 mice) to correct VEGF-induced thymic atrophy confirm that VEGF does not directly interfere with TCR rearrangement. Additionally, these data also provide compelling evidence that early T-cell development in the thymus (up to and including these checkpoints) is not directly impaired by VEGF.
As mentioned previously, thymic atrophy is accompanied by a decreased number of T cells in the peripheral lymphoid organs, and yet this remaining population is functionally competent. Next, we were interested in the capacity of the atrophying thymus to export naive T cells to the periphery following chronic VEGF infusion.
CD4+ or CD8+ T cells were purified from total splenocytes, and the number of mTREC/100 000 splenic T cells was measured. Following 28 days of VEGF infusion, both mTREC/100 000 CD4+ and mTREC/100 000 CD8+ T cells were measurably decreased (P = .068 and P = .058, respectively) (Figure 6E). Based on these data, we calculated that the contribution of recent thymic emigrants to the splenic T-cell pool is less than 1% with prolonged VEGF infusion, compared with approximately 4% to 5% in controls. This is likely to be an underestimate, but clearly demonstrates a significant reduction in T-cell production by the thymus. Taken together, these data demonstrate that while development of residual thymocytes appears to be progressing normally, thymic atrophy and the overall loss of thymocyte cellularity following VEGF infusion results in a virtually complete inhibition of T-cell production by the thymus. The result is a negligible contribution of recent thymic emigrants to the periphery and the potential for progressive immune failure with sustained exposure to VEGF.
FTOC
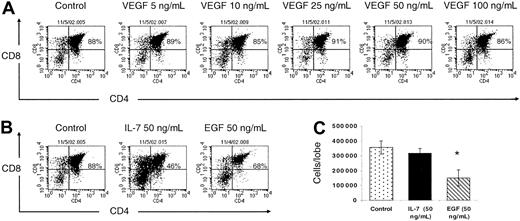
At this point, our data fail to demonstrate a direct effect of VEGF on thymocyte survival, proliferation, or progression through various developmental checkpoints. To confirm this observation, we studied the effects of VEGF on T-cell development in FTOC. FTOC allows us the ability to study a single wave of thymocyte development in vitro. Following 5 days in FTOC, the majority of the thymocytes have reached the DP stage. Since the most profound cell loss associated with VEGF infusion is seen within this subset, we felt that this time point would most accurately reflect the phenotype we have observed in vivo. Thymic lobes were harvested from day-14.5 embryos and cultured for 5 days ± VEGF (5-100 ng/mL). Thymocytes were isolated and analyzed for CD3, CD4, and CD8 expression. We observed efficient T-cell maturation in FTOC regardless of VEGF exposure, and there was no discernable difference between VEGF-treated or -untreated thymic lobes with regard to either total thymocytes harvested (data not shown) or CD4/CD8 FACS profiles (Figure 7A).
VEGF does not inhibit thymocyte development in FTOC. (A) Day-14.5 fetal thymic lobes were cultured ± VEGF (5-100 ng/mL). On day 5, lobes were isolated, a single-cell suspension prepared, and thymocytes were counted and analyzed for CD4 and CD8 expression. Representative data from 5 independent experiments are shown. rmIL-7 (50 ng/mL) and recombinant murine epidermal growth factor (rmEGF) (50 ng/mL) were used as positive controls. (B) Percentage CD4+ and CD8+ cells with IL-7 and EGF treatment. (C) Mean number of cells per thymic lobe ± IL-7 and EGF (n = 3, ± SE). *Denotes statistical significance.
VEGF does not inhibit thymocyte development in FTOC. (A) Day-14.5 fetal thymic lobes were cultured ± VEGF (5-100 ng/mL). On day 5, lobes were isolated, a single-cell suspension prepared, and thymocytes were counted and analyzed for CD4 and CD8 expression. Representative data from 5 independent experiments are shown. rmIL-7 (50 ng/mL) and recombinant murine epidermal growth factor (rmEGF) (50 ng/mL) were used as positive controls. (B) Percentage CD4+ and CD8+ cells with IL-7 and EGF treatment. (C) Mean number of cells per thymic lobe ± IL-7 and EGF (n = 3, ± SE). *Denotes statistical significance.
Additionally, we used 2 positive controls in these studies: IL-7 (50 ng/mL) and EGF (50 ng/mL). Both have been shown to disrupt early stages of normal T-cell development in FTOC. IL-7 has been shown to directly inhibit pre–T-cell differentiation via signaling through the pre-TCR on the surface of thymocytes.26 EGF disrupts fetal stroma growth in the thymus and inhibits progression of fetal thymocytes from the TN to DP stage II.27 We confirm that both IL-7 and EGF inhibit early thymocyte development as evidenced by decreased percentages of DP cells recovered from the thymus lobes (Figure 7B). Furthermore, addition of EGF results in a significantly reduced number of cells recovered from FTOC (Figure 7C).
Despite potential differences between fetal and adult thymocytes and thymic epithelium, we conclude from these data that VEGF does not inhibit T-cell development by direct action on T lymphocyte differentiation in the thymus. In addition, these data argue against the hypothesis that VEGF may inhibit thymocyte development by primarily disrupting the thymic microenvironment, as fetal thymic lobes exposed to high levels of VEGF appear to support normal T-cell development.
Effects of VEGF on lymphoid progenitors in the BM
Finally, we considered the possibility that VEGF may induce thymic atrophy extrathymically by inhibiting the differentiation and commitment of HPCs to the T-cell lineage or blocking the migration of committed T-cell progenitors to the thymus. Interestingly, we observe a significant increase in B cells in the BM of VEGF-infused animals (B220+ cells: control, 13.5% ± 2.8%; VEGF, 19.4% ± 2.5% [P < .05, n = 5]; data not shown). This observation mirrors the decreased T-cell–to–B-cell ratio observed in the periphery of VEGF-treated mice19 and strongly supports the hypothesis that effects of VEGF on lymphoid development are likely to occur early in differentiation. Unlike B-cell progenitors in the BM, no distinct cell-surface marker phenotype identifies a corresponding thymic precursor population.
To directly investigate the effects of VEGF on thymus-directed progenitors in the BM, we used congenic strains of mice to study the ability of VEGF-treated HPCs to reconstitute the thymus of an irradiated host. B6.SJL mice were treated with a continuous infusion of VEGF for 14 days. HPCs were then purified from the BM of control and VEGF-treated animals and injected intravenously into irradiated (700 cGy) untreated C57Bl/6 hosts. Mice were killed 2 to 5 weeks after transplantation and analyzed for the ability of donor (Ly5.1) cells to reconstitute the thymus of irradiated recipient mice (Ly5.2).
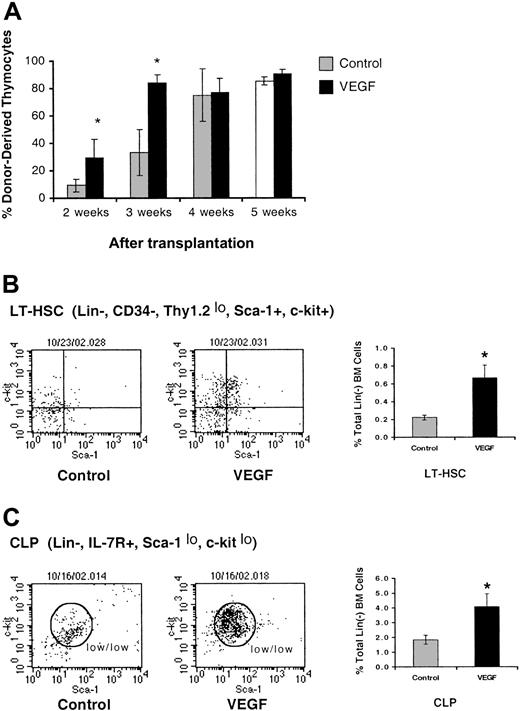
Lineage– HPCs isolated from VEGF-treated mice demonstrate a dramatically enhanced ability to recolonize the thymus of irradiated C57Bl/6 mice (Figure 8A).
VEGF affects the ability of lineage– BM progenitor cells to reconstitute irradiated congenic mice. (A) Mice were sublethally irradiated (700 cGy) and then given intravenous injections of 1 × 106 Lin– BM progenitor cells isolated from mice treated with VEGF (50 ng/h) in vivo for 14 days. Mice were killed 2 to 5 weeks after transplantation and analyzed for the ability of donor BM to reconstitute the host. VEGF infusion results in an accumulation of LT-HSCs (B) and CLPs (C) in the marrow. Representative FACS plots are shown. Graphs represent the percent of LT-HSCs and the percent of CLPs within the total lineage– BM cell population (mean ± SE; n = 4). *Denotes statistical significance.
VEGF affects the ability of lineage– BM progenitor cells to reconstitute irradiated congenic mice. (A) Mice were sublethally irradiated (700 cGy) and then given intravenous injections of 1 × 106 Lin– BM progenitor cells isolated from mice treated with VEGF (50 ng/h) in vivo for 14 days. Mice were killed 2 to 5 weeks after transplantation and analyzed for the ability of donor BM to reconstitute the host. VEGF infusion results in an accumulation of LT-HSCs (B) and CLPs (C) in the marrow. Representative FACS plots are shown. Graphs represent the percent of LT-HSCs and the percent of CLPs within the total lineage– BM cell population (mean ± SE; n = 4). *Denotes statistical significance.
At 2 weeks after transplantation, the percentage of donor-derived cells in the thymus is approximately 3-fold higher in animals given VEGF-treated BM cells (control, 9.5 ± 4.83%; VEGF, 29.7 ± 13.59% [P < .05]). Similarly, at 3 weeks after transplantation, there is a 2.5-fold higher percentage of donor-derived cells in the thymus with VEGF treatment (control, 33.5 ± 16.79%; VEGF, 83.6 ± 5.83% [P < .05]). VEGF treatment of HPCs did not affect the percentage of donor-derived cells in the thymus after 4 weeks, as the thymus approaches normal cellularity. Both VEGF-exposed and untreated HPCs were capable of efficient progression to later stages of thymocyte development, as normal percentages of donor-derived TN and DP thymocytes were present in all reconstituted animals (data not shown). This confirms an enhanced population of functional thymic precursors in the BM of animals infused with VEGF.
We also demonstrate that prolonged infusion of VEGF (> 21 days) results in the accumulation of both long-term hematopoietic stem cells (LT-HSCs)22,23 (Figure 8B) and common lymphoid progenitors (CLPs)24 (Figure 8C) in the BM. This observation likely explains the enhanced colonization of the thymus by BM isolated from mice given VEGF infusions, but unfortunately cannot confirm the presence or absence of a distinct thymus-directed precursor in the marrow. What is clear, however, is that the more rapid reconstitution of the recipient thymus by VEGF-treated progenitors demonstrates an increased population of functional thymic precursors, and the accumulation of LT-HSCs and CLPs confirms that VEGF plays an important role in early lymphoid differentiation. Future experiments will seek to understand the inability of these progenitors to colonize the thymus in the presence of systemic VEGF.
Discussion
In order for a cancer to become clinically apparent, it must avoid immune recognition and elimination. Disorders of immune cells and immune responses in cancer patients are frequently observed and poorly understood. We have shown previously that VEGF, a factor produced in abundance by most solid tumors and essential for the formation of the tumor neovasculature, affects the myeloid lineage and specifically, dendritic cell maturation and function. In this study, we demonstrate that continuous administration of recombinant VEGF mimics the profound thymic atrophy observed in tumor-bearing mice and inhibits the production of T cells. We show that VEGF acts on thymic progenitors rather than directly on the thymus itself and propose that exposure to supraphysiologic levels of VEGF results in defective seeding of the thymus by BM-derived progenitors. These earliest thymocytes fail to replace maturing T cells emigrating to the periphery, and a depletion of total thymocytes results. We hypothesize that with continued chronic VEGF exposure (as observed in cancer patients and tumor-bearing animals) normal immune function would continue to progressively deteriorate as the thymus failed to replenish the peripheral T-cell pool.
We also demonstrate that lineage– cells from the BM of VEGF-treated mice, when transferred into an irradiated host, reconstitute the thymus 2.5- to 3-fold more efficiently than control cells. This indicates an increased number of functional thymus-directed progenitors in the marrow of VEGF-treated animals, and we have confirmed that VEGF infusion results in an accumulation of both LT-HSCs and CLPs in the BM. We hypothesize that VEGF induces a block in the differentiation of thymic precursors from lymphoid progenitors, resulting in the accumulation of cells upstream from this block. This failure to differentiate into committed T-cell progenitors likely results in a default commitment of CLPs to the B lineage as evidenced by the increased percentage of B lymphocytes in the BM, lymph nodes, and spleen of VEGF-treated animals. When cells are removed from this VEGF block, the result may be a preferential commitment to the T lineage resulting in the enhanced thymic recolonization seen following cessation of VEGF infusion and adoptive transfer of VEGF-treated marrow into normal recipient animals.
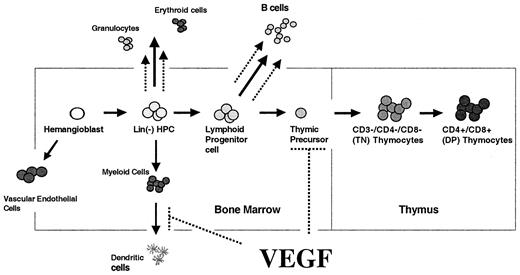
Figure 9 schematically represents our current model of the effects of pathophysiologic levels of VEGF on the major hematopoetic lineages, with increased neutrophil, erythroid, and B-cell lineages and blocked DC and T-cell differentiation.
Model of our current understanding of VEGF effects on hematopoiesis. We have shown previously that pathologic cells of VEGF inhibit dendritic cell development and enhance production of neutrophils, erythroid progenitors, and B cells. In this study, we demonstrate that VEGF also inhibits T-cell development via effects on T-cell precursor cells in the BM. Effects of VEGF indicated by dashed lines.
Model of our current understanding of VEGF effects on hematopoiesis. We have shown previously that pathologic cells of VEGF inhibit dendritic cell development and enhance production of neutrophils, erythroid progenitors, and B cells. In this study, we demonstrate that VEGF also inhibits T-cell development via effects on T-cell precursor cells in the BM. Effects of VEGF indicated by dashed lines.
This proposed model of altered CLP differentiation also explains the delayed onset of thymic atrophy. The failure of CLPs to differentiate into thymic precursors would be apparent only as progenitors failed to replace the more differentiated (and unaffected) thymocytes as they progress through positive and negative selection and emigrate from the thymus. It has recently been observed that migration of progenitors from the BM to the thymus may be a gated phenomenon.28 This helps explain the delayed onset of atrophy in our model and also potentially explains the rapid progression of thymic atrophy between 21 and 28 days of VEGF exposure.
A direct effect of VEGF on lymphocyte differentiation from hematopoetic progenitors likely has its roots in embryonic development. Both hematopoetic progenitor cells and vascular endothelial progenitor cells are derived from a common embryonic origin, and a significant body of work has been performed in search of a common precursor for both of these lineages, termed the hemangioblast. Furthermore, an essential role for VEGF in hematopoetic development has been established, and both VEGFR1 and VEGFR2 have been implicated as important markers of hematopoietic stem cells.17,29 The essential role of VEGF in hematopoietic differentiation is underscored by the fact that heterozygous knock outs of VEGF are embryonic lethal and have defective blood island formation.16 We show that VEGF dependence persists in postnatal BM differentiation, and acquired pathologic levels of VEGF may have profound effects on lymphoid as well as myeloid lineage cells. These effects may partially explain cancer-associated defects in cell-mediated immunity and contribute to the ability of tumors to evade normal immune surveillance.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-07-1956.
Supported in part by National Institutes of Health (NIH) grant CA76321 to D.P.C., and NATO grant LST.CLG.975197 to D.I.G and E.K.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Genentech for their continual support of our studies; Dr Roy Jensen and Dr John Cousar for review of histology sections; Donna Hicks and Dr Greg Hanley for assistance with intravenous injections; and Dr Mark Boothby and Dr Gene Oltz for overall assistance and advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal