Abstract
Evaluation of myocardial iron during iron chelation therapy is not feasible by repeated endomyocardial biopsies owing to the heterogeneity of iron distribution and the risk of complications. Recently, we described a noninvasive method based on magnetic resonance imaging. Here, the method was used for repeated estimation of the myocardial iron content during iron chelation with deferrioxamine in 14 adult nonthalassemic patients with transfusional iron overload. We investigated the repeatability of the method and the relationship between the myocardial iron estimates and iron status. The repeatability coefficient (2sD) was 2.8 μmol/g in the controls (day-to-day) and 4.0 μmol/g in the patients (within-day). Myocardial iron estimates were elevated in 10 of all 14 patients at first examination, but normalized in 6 patients after 6 to 18 months of treatment. If liver iron declined below 350 μmol/g all but one of the myocardial iron estimates were normal or nearly normal. At start (R2 = 0.69, P = .0014) and still after 6 months of iron chelation (R2 = 0.76, P = .001), the estimates were significantly and more closely related to the urinary iron excretion than to liver iron or serum ferritin levels. In conclusion, our preliminary data, which may only pertain to patients with acquired anemias, suggest the existence of a critical liver iron concentration, above which elevated myocardial iron is present, but its extent seems related to the size of the chelatable iron pool, as reflected by the urinary iron excretion. This further supports the concept of the labile iron pool as the compartment directly involved in transfusional iron toxicity.
Introduction
Cardiac disease caused by deposition of transfusional iron within the myocardium is the most harmful manifestation of transfusional iron overload and the leading cause of death in β-thalassemia major.1 Evaluation of myocardial iron based on myocardial tissue iron concentration measurements in endomyocardial biopsies is unreliable because myocardial tissue iron is not homogeneously distributed within the myocardium.2, 3, 4 Moreover, endomyocardial biopsies cannot be taken repeatedly during follow-up because of the risk of complications.
The paramagnetic properties of tissue iron allow the tissue iron concentration to be evaluated noninvasively. Clinically useful estimates of the hepatic iron content have been obtained by a superconducting quantum interference device (SQUID)5,6 and by magnetic resonance imaging (MRI).7, 8, 9, 10, 11, 12, 13 Estimation of myocardial iron by the SQUID has so far not been reported, but a few studies have explored the use of MRI for this purpose.14, 15, 16, 17, 18 Except for 2 studies,14,18 all studies demonstrated a significant correlation between the applied myocardial MRI index and the serum ferritin concentration by heart-to-muscle signal intensity ratio (SIR) measurements obtained from spin-echo images,15,17 by T2 relaxometry,15,16 or by magnetization transfer ratio measurements.16 Anderson et al18 did not find this relationship, but demonstrated an interesting, strong relationship between declining myocardial T2-star (T2*) and impaired ventricular function by use of gradient-echo imaging. A limitation of all these studies is the lack of a definitive prediction of the myocardial iron concentration from the applied myocardial MRI iron indexes, because no validation is performed with endomyocardial tissue. Nonetheless, the demonstrated significant correlations in the literature between the myocardial MRI indexes and the serum ferritin concentration and the ventricular function strongly suggest an empirical value of MRI for the evaluation of myocardial iron. Moreover, validation data from liver biopsies, supplied in the study of Anderson et al18 and in our own,11 show a direct reflection of the tissue iron concentration by the applied MRI index.
The usefulness of MRI in evaluating myocardial iron would be further supported by the demonstration of normalizing MRI-based myocardial iron estimates collected repeatedly during iron chelation in patients with transfusional iron overload. The aim of the present study was therefore to evaluate myocardial iron repeatedly during iron chelation with deferrioxamine (DFO) in adult patients with transfusional iron overload caused by acquired anemias. We also investigated the relationship of myocardial iron estimates to the iron status, including the liver iron concentration, the serum ferritin concentration, and the urinary iron excretion, and studied the repeatability of the MRI-based myocardial iron estimates in patients with transfusional iron overload and in healthy controls.
Patients, materials, and methods
Patients
The present study, approved by the local ethics committee, is based on 2 groups of patients. One group consisted of 20 patients who had given written, informed consent to participate in the investigation of the repeatability of the MRI-based estimation of the myocardial iron concentration. Twelve of these patients had transfusional iron overload caused by hematologic malignancies and in 8 patients by hereditary hemochromatosis. Nine of the patients with transfusional iron overload were undergoing iron chelation with DFO. The mean liver iron concentration in all 20 patients was 283 ± 170 μmol/g (range, 15-483 μmol/g). This part of the study also included 11 healthy controls. The present study also included 14 consecutive patients with transfusional iron overload who had given written, informed consent to participate in the evaluation of the consequences of transfusional iron overload during iron chelation. This evaluation included estimations of the liver iron concentration and the myocardial iron concentration by MRI, multigated (MUGA) scans, and serial indices of iron status and routine liver tests. After the inclusion of 11 patients, we published follow-up data on the efficacy of DFO treatment evaluated by repeated MRI-based estimation of the liver iron concentration and the serum ferritin19 and on left ventricular ejection fraction (LVEF) evaluated by MUGA scan.20 One of the patients was excluded from the present study owing to variable compliance, and 4 new patients were included later. Hence, the present study includes 14 previously untreated patients with transfusional iron overload followed from 12 to 48 months during DFO treatment. Patient 9 had already been on iron chelation for 9 months when the first MRI examination was performed. For patient 8 no relevant slices of the heart were available at baseline and no MRI examination had been performed after 3 months of DFO treatment. Table 1 summarizes the clinical characteristics of the patients.
Clinical characteristics of patients at start of iron chelation with DFO
Patient no. . | Diagnosis . | Age, y . | Liver iron level, μmol/g . | Ferritin level, μg/L . | TfS, % . | Transfused blood, U* . | Blood needs, U/mo . | Myocardial iron value, μmol/g . | Urinary iron level, mg/24 h . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MDS, RARS | 58 | 501 | 3600 | 88 | 132 | 8.1 | 12.8 | 53.6 |
| 2 | MDS, RA | 67 | 521 | 4340 | 79 | 77 | 3.8 | 8.5 | 20.7 |
| 3 | MDS, RAEB | 63 | 558 | 1780 | 94 | 116 | 4.7 | 8.0 | 24.2 |
| 4 | MDS, RA + ERD | 47 | 403 | 2510 | 75 | 105 | 4.8 | 6.3 | 12.3 |
| 5 | MDS, RA | 64 | 540 | 5770 | 83 | 88 | 5.7 | 16.4 | 32.3 |
| 6 | MDS, RA | 46 | 558 | 3800 | 88 | 75 | 3.2 | 1.6 | 19.9 |
| 7 | MDS, RARS | 69 | 491 | 4790 | 102 | 90 | 4.0 | 12.8 | 35.2 |
| 8 | MDS, RA | 41 | 623 | 8715 | 100 | 254 | 2.9 | 21.2 | 43.5 |
| 9 | DBS | 37 | 388 | 2380 | 108 | 420 | 3.1 | 9.9 | 17.5 |
| 10 | MDS, RAEB-t | 65 | 626 | 7370 | 100 | 207 | 6.6 | 19.5 | 36.6 |
| 11 | CH | 41 | 479 | 1280 | 66 | 70 | 1.0 | 1.5 | 11.9 |
| 12 | MDS RA | 32 | 431 | 4000 | 107 | 84 | 6.0 | 10.1 | ND |
| 13 | AML CR | 34 | 468 | 2200 | 62 | 109 | 3.0 | 3.6 | 11.7 |
| 14 | MDS RA | 32 | 405 | 1740 | 84 | 44 | 3.0 | 1.3 | 9.9 |
Patient no. . | Diagnosis . | Age, y . | Liver iron level, μmol/g . | Ferritin level, μg/L . | TfS, % . | Transfused blood, U* . | Blood needs, U/mo . | Myocardial iron value, μmol/g . | Urinary iron level, mg/24 h . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MDS, RARS | 58 | 501 | 3600 | 88 | 132 | 8.1 | 12.8 | 53.6 |
| 2 | MDS, RA | 67 | 521 | 4340 | 79 | 77 | 3.8 | 8.5 | 20.7 |
| 3 | MDS, RAEB | 63 | 558 | 1780 | 94 | 116 | 4.7 | 8.0 | 24.2 |
| 4 | MDS, RA + ERD | 47 | 403 | 2510 | 75 | 105 | 4.8 | 6.3 | 12.3 |
| 5 | MDS, RA | 64 | 540 | 5770 | 83 | 88 | 5.7 | 16.4 | 32.3 |
| 6 | MDS, RA | 46 | 558 | 3800 | 88 | 75 | 3.2 | 1.6 | 19.9 |
| 7 | MDS, RARS | 69 | 491 | 4790 | 102 | 90 | 4.0 | 12.8 | 35.2 |
| 8 | MDS, RA | 41 | 623 | 8715 | 100 | 254 | 2.9 | 21.2 | 43.5 |
| 9 | DBS | 37 | 388 | 2380 | 108 | 420 | 3.1 | 9.9 | 17.5 |
| 10 | MDS, RAEB-t | 65 | 626 | 7370 | 100 | 207 | 6.6 | 19.5 | 36.6 |
| 11 | CH | 41 | 479 | 1280 | 66 | 70 | 1.0 | 1.5 | 11.9 |
| 12 | MDS RA | 32 | 431 | 4000 | 107 | 84 | 6.0 | 10.1 | ND |
| 13 | AML CR | 34 | 468 | 2200 | 62 | 109 | 3.0 | 3.6 | 11.7 |
| 14 | MDS RA | 32 | 405 | 1740 | 84 | 44 | 3.0 | 1.3 | 9.9 |
In patient 9 the first MRI examination was performed after 9 months of iron chelation. In patient 8 the first estimation of myocardial iron was performed after 6 months of iron chelation. Normal range (mean ± 2 SD) for MRI liver iron is 1 to 15 μmol/g and for MRI myocardial iron, 0.1 to 5.7 μmol/g. Urinary iron excretion was measured at start of DFO treatment by use of DFO dosages as indicated in Figure 4. MDS indicates myelodysplastic syndrome; RARS, refractory anemia with ringed sideroblasts; RA, refractory anemia; RAEB, refractory anemia with excess of blasts; ERD, end-stage renal disease; DBS, Diamond-Blackfan syndrome; RAEB-t, refractory anemia in transformation to AML; CH, chronic hemolysis of unknown etiology; ND, not determined; AML (CR), acute myeloid leukemia in complete remission; and TfS, iron saturation of serum transferrin.
Number of transfused blood units.
Iron chelation therapy
DFO was administered subcutaneously in the abdominal wall by 12-hour infusions 5 days a week by means of a computer-assisted infusion pump (Deltec, CADD II, SIMS Deltec, St Paul, MN) in 4 patients and in 10 patients by subcutaneous bolus injection twice daily.19 Daily DFO doses are shown in Figure 4. Vitamin C supplementation (200 mg once a day) was started 6 to 18 months after start of DFO therapy.
Measurement of iron parameters in serum
The serum ferritin concentration was assessed by an enzyme-immunometric assay (Amerlite, Ferritin assay, monoclonal, Amersham, Cardiff, United Kingdom). The reference range was 10 to 120 μg/L for women and 15 to 300 μg/L for men. The serum transferrin (Tf) concentration (normal range, 23-40 μM) was determined by nephelometric measurement of immune complexes using rabbit antibodies against human Tf. The serum iron concentration (reference range, 11-31 μM) was measured by a spectrophotometric method.
Measurement of urinary iron excretion
At the beginning of treatment the DFO dose response was studied in 13 patients (except patient 12). Increasing doses of DFO (from 1 to 4 g) were given by 12-hour subcutaneous infusion on successive days with 24-hour collection of urine from the beginning of each infusion.21 In patient 7 iron excretion was only measured after 2 g DFO, and in patient 8 not after 4 g. The iron excretion was determined by atomic absorption spectrophotometry.22 Follow-up values represent means of three 24-hour collections performed on 3 successive days. The iron excretion follow-up was not performed in patients 7, 12, and 14.
Estimation of liver iron concentration
MRI studies were performed by using a Gyroscan, S 15-HP (Philips, Best, The Netherlands) operating at 1.5 T. The images were obtained using the following electrocardiographic (ECG)–gated spin-echo sequence: echo time 25 ms, repetition time equal to the heart rate (500-960 ms). The field of view was 450 mm2 matrix (256 × 256 pixels). Oblique images (15-25 slices, with a slice thickness of 9 mm) were recorded, visualizing the right and left ventricle, right liver lobe, and posterior vertebral muscles in the same slice.14 The liver-to-muscle SIR was calculated and corrected for variable repetition time as described earlier.11 Validation experiments11 have shown a close linear inverse and semilogarithmic relationship (R2 = 0.98, P < .0001) between the liver-to-muscle SIR and the liver iron concentration determined by chemical analysis of liver biopsies within the range 5 to 650 μmol/g (dry weight). The established normal range for the liver iron concentration is 1 to 15 (mean ± 2 SD) μmol/g. The day-to-day variation was 2.9 ± 2.7 μmol/g.
Estimation of the myocardial iron concentration
We have recently described an image analysis for estimating myocardial iron using the aforementioned oblique MRI recordings, simultaneously visualizing the heart, liver, and skeletal muscle.17 In short, first we enumerated the slices based on recognition of a key slice. Then the region of interest (ROI) was positioned within the lateral wall of the left ventricle and within the skeletal muscle (internal standard). Fat was excluded from the skeletal muscle by the positioning of the ROI and in the present study further by excluding SI values outside ± 1 SD around the mean within the SI histogram. The SIRs between the myocardial wall (lateral wall of left ventricle) and skeletal muscle (left paraspinous muscles and a section of latissimus dorsi muscle) were calculated. To make the interpretation of the SIRs easier, we converted them into tissue iron concentration values using a modified calibration curve from our liver model.11,17 Owing to the previously described variation in the SI values between slices, the data in the present study are given as a mean of 3 appropriate adjacent slices (slice 1, 2, 3).17 The normal range (mean ± 2 SD) of the estimated myocardial iron concentration established in 15 healthy controls was 0.5 to 5.7 μmol/g dry weight.
Data analysis
Data are given as percentages or as means ± SD for continuous variables with symmetric distributions if not otherwise indicated. Data with skewed distributions are given as median. Linear relationships between variables were investigated by linear regression analysis (least square method). If appropriate, logarithmic transformation of the data was performed before analysis. The quality of the regression model was assessed by calculation of the coefficient of determination (R2) and F-statistics. The patterns of relationships between variables monitored during iron chelation (including repeated measurements on the same individual) were investigated by a locally weighted scatter plot smoother (lowess), producing lowess curves based on least-squares fits. Multiple comparisons of means between subgroups of patients were performed by the Scheffé F test. All statistical tests were 2-tailed and were done on Stat View 5.0 software for Macintosh (1992-1998, SAS Institute, Cary, NC). A significance level of P < .05 was used.
Results
Repeatability of the myocardial iron concentration estimates
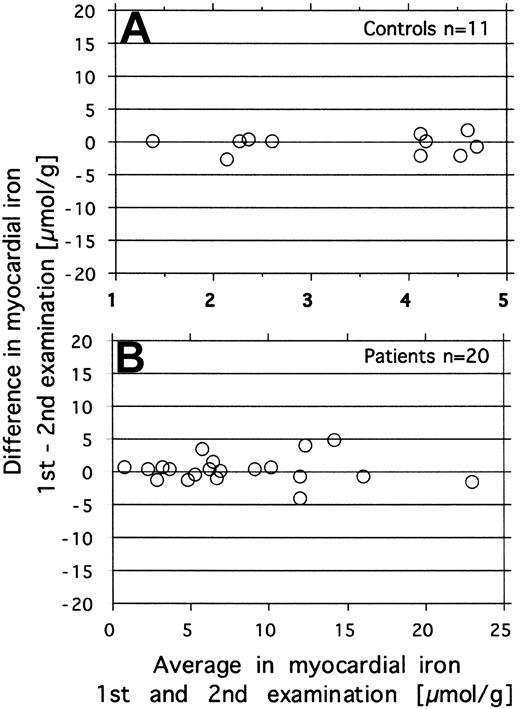
Repeatability was investigated by repeated estimation of the myocardial iron concentration in 20 patients with various amounts of iron overload and in 11 healthy controls. The paired data for each subject are displayed in Figure 1, showing the difference against the mean for each pair of measurements according to Bland and Altman.23 In the patients the MRI was repeated within the same day, in controls on different days (within 1 month). The repeatability coefficient (2sD)23 for the difference between 2 measurements was 2.8 μmol/g in the controls and 4.0 μmol/g in the patients.
Investigation of repeatability of the MRI-derived estimates of myocardial iron. Repeated measurements were done in 11 controls (day-to-day variation, top graph) and in 20 patients (within-day variation, bottom graph). The figure shows the difference against the mean for each pair of measurements.
Investigation of repeatability of the MRI-derived estimates of myocardial iron. Repeated measurements were done in 11 controls (day-to-day variation, top graph) and in 20 patients (within-day variation, bottom graph). The figure shows the difference against the mean for each pair of measurements.
Myocardial iron estimates and iron status at start of treatment
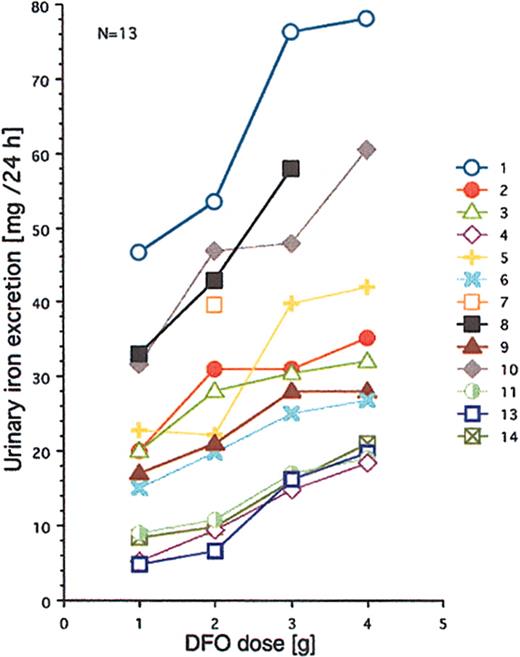
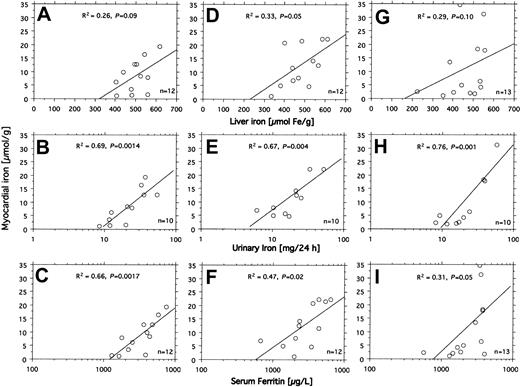
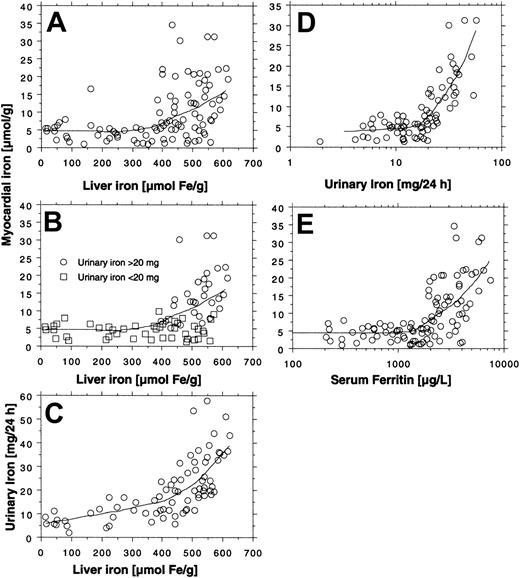
All 14 patients had heavy transfusional iron overload at start of treatment (Table 1). Twelve of these patients had been followed by repeated MRI-based estimation of the myocardial iron concentration from the start of iron chelation. Eight of them had an elevated myocardial iron estimate (range, 6.3-21.2 μmol/g). At start of iron chelation the DFO dose response was studied in 13 patients (Figure 2). After increasing the DFO dose, a similar increase in urinary iron excretion was seen in most patients, but the level of the curve differed considerably between patients. Figure 3A-C shows that the myocardial iron estimates at treatment start (t = 0) are closely related to the urinary iron excretion (R2 = 0.69, P = .0014) and to the serum ferritin concentration (R2 = 0.66, P = .0017), but not to the liver iron concentration and not to the number of blood units given or the iron saturation of transferrin (data not shown).
Relationship between DFO dose and urinary iron excretion. DFO was administered on 4 successive days by 12-hour subcutaneous infusion (pump). Urinary iron excretions are 24-hour collections. Numbers identify the patients. For more details, see “Patients, materials, and methods.”
Relationship between DFO dose and urinary iron excretion. DFO was administered on 4 successive days by 12-hour subcutaneous infusion (pump). Urinary iron excretions are 24-hour collections. Numbers identify the patients. For more details, see “Patients, materials, and methods.”
Relationship between indices of iron status and myocardial iron estimates. The relationships between liver iron concentration, urinary iron excretion, serum ferritin concentration, and the myocardial iron estimates were examined by linear regression analysis in 14 patients with transfusional iron overload investigated by MRI at start (A-C), after 3 months (D-F), and after 6 months (G-I) of DFO treatment. Variation in number of examinations in the panels is due to absent (patient 12) or incomplete (patients 7 and 14) urinary iron determination and incomplete MRI follow-up in patients 8 and 9. For details, see “Patients, materials, and methods.” Diagonal lines indicate linear regression lines.
Relationship between indices of iron status and myocardial iron estimates. The relationships between liver iron concentration, urinary iron excretion, serum ferritin concentration, and the myocardial iron estimates were examined by linear regression analysis in 14 patients with transfusional iron overload investigated by MRI at start (A-C), after 3 months (D-F), and after 6 months (G-I) of DFO treatment. Variation in number of examinations in the panels is due to absent (patient 12) or incomplete (patients 7 and 14) urinary iron determination and incomplete MRI follow-up in patients 8 and 9. For details, see “Patients, materials, and methods.” Diagonal lines indicate linear regression lines.
Changes in the myocardial iron estimates during iron chelation
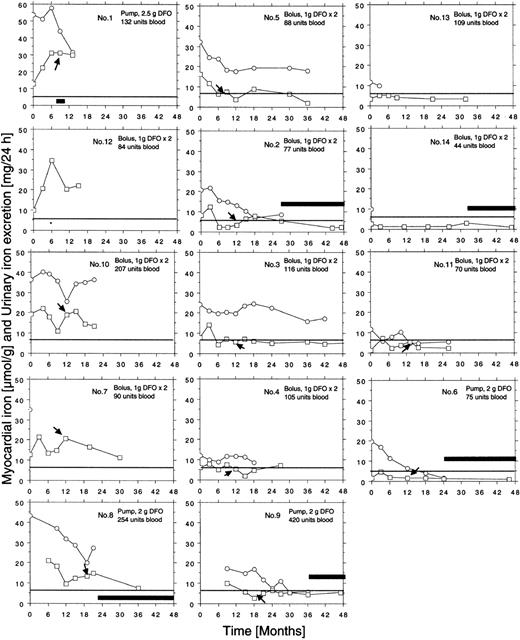
Individual curves of the myocardial iron estimates for all 14 patients are given in Figure 4. Ten of the 14 patients had elevated myocardial iron estimates at first MRI examination. In 6 patients, the estimates normalized within 6 to 18 months of treatment. The 12 patients followed from the onset of iron chelation show a common pattern in that all, except patients 5 and 14, had increased myocardial iron estimates after 3 months compared with baseline. This 3-month value was clearly outside the normal range in 8 patients, but after 6 months estimates were normal or nearly normal in 8 of the 12 patients and remained so. In 5 patients (nos. 2, 4, 5, 9, 11), an intermittent increase of the myocardial iron concentration up to or slightly above the upper limit of the normal range was seen later on. Five patients (nos. 1, 7, 8, 10, 12) showed persistently, severely elevated myocardial iron estimates. We could distinguish 3 groups of patients: (1) group A (patients 1, 7, 8, 10, 12) with persistently elevated myocardial iron estimates, (2) group B (patients 2, 3, 4, 5, 9) with clearly elevated values only at the initial stage, and (3) group C (patients 6, 11, 13, 14) with normal values throughout the entire follow-up period. Analysis of the DFO dose-response curves (Figure 2) revealed that patients in group A had the largest urinary iron excretion after infusion of 2 g DFO (> 35 mg/24 h). Within group B the iron excretion range ranged from 20 to 35 mg/24 h (patient 4 with chronic renal disease excluded), and group C patients had an iron excretion less than 20 mg/24 h. Accordingly, the mean urinary iron excretion was significantly larger in group A (62.6 ± 22.1 mg/24 h) than in group B (20.7 ± 7.2 mg/24 h, P = .003) and group C (12.4 ± 6.8 mg/24 h, P = .002, Scheffé F test), but the mean serum ferritin concentration, mean liver iron concentration, or mean number of blood units given was not significantly different.
Relationship between iron status and myocardial iron during iron chelation
Figure 3D-I shows how the myocardial iron estimates are related to the urinary iron excretion, the serum ferritin concentration, and the liver iron concentration after 3 and 6 months of DFO treatment. The estimates were most closely related to the urinary iron excretion both after 3 months (R2 = 0.67, P = .004) and after 6 months (R2 = 0.76, P < .001). Despite this close relationship myocardial iron and urinary iron excretion only moved in parallel in 3 of 8 evaluable individual patients during the first 3 months of iron chelation treatment, but during the following 3 months of treatment, this occurred in 7 patients of 8 evaluable patients (Figure 4). Parallel movement of both variables tended to be more frequent at urinary iron excretions more than 20 mg/24 h, but at lower iron excretions, especially later during DFO treatment, even antagonistic movements were observed. We then studied the pattern of the overall relationship within the follow-up data obtained between the myocardial iron estimates and the corresponding urinary iron determinations (data from Figure 4), the liver iron concentrations, and serum ferritin measurements by lowess regression analysis (Figure 5). We found that all but one of the myocardial iron estimates were normal or nearly normal (≤ 8 μmol/g) in all patients when the liver iron concentration had declined below around 350 μmol/g (Figure 5A). Elevated myocardial iron estimates were only seen above this liver iron level at which, however, the lowess curve does not suggest a proportional increase of myocardial with liver iron. Such proportionality was, however, observed for myocardial iron estimates and the log-transformed urinary iron excretion (Figure D). The lowess curve strongly suggested a proportional increase of myocardial iron for a urinary iron excretion exceeding around 20 mg/24 h, but below this value all myocardial iron estimates were 8 μmol/g or less. Figure 5C suggests that the urinary iron excretion also increases proportionally with the liver iron concentration at values exceeding around 400 μmol/g. The myocardial iron estimates may be 8 μmol/g or less even if the liver iron concentration exceeds 350 μmol/g (Figure 5B), but myocardial iron estimates more than 8 μmol/g are only seen if the corresponding urinary iron excretion exceeds 20 mg/24 h. Figure 5E displays the relationship between serum ferritin and the myocardial iron estimates. All estimates were 8 μmol/g or less if the serum ferritin was 1800 μg/L or less.
Individual follow-up data on estimates of myocardial iron concentration and urinary iron excretion collected during iron chelation in 14 patients with transfusional iron overload. Each patient is given identification number, route of administration, dose of DFO, and number of blood units received at start of treatment. ○ indicates urinary iron; □, myocardial iron. The horizontal line represents the upper limit of reference range (5.7 μmol/g). Arrows indicate start of vitamin C supplementation. * indicates increase of DFO dose to 1.5 g twice daily in patient 12. Clinical patient data are given in Table 1. Broad, horizontal bars reflect periods of time off DFO treatment. The iron excretion follow-up was not performed in patients 7, 12, and 14, and was incomplete in patients 4 and 13.
Individual follow-up data on estimates of myocardial iron concentration and urinary iron excretion collected during iron chelation in 14 patients with transfusional iron overload. Each patient is given identification number, route of administration, dose of DFO, and number of blood units received at start of treatment. ○ indicates urinary iron; □, myocardial iron. The horizontal line represents the upper limit of reference range (5.7 μmol/g). Arrows indicate start of vitamin C supplementation. * indicates increase of DFO dose to 1.5 g twice daily in patient 12. Clinical patient data are given in Table 1. Broad, horizontal bars reflect periods of time off DFO treatment. The iron excretion follow-up was not performed in patients 7, 12, and 14, and was incomplete in patients 4 and 13.
Overall relationship between estimate of myocardial iron concentration and indices of iron status in 14 patients with transfusional iron overload during iron chelation. Myocardial iron estimates are obtained from Figure 4. The relationship between the myocardial iron estimates and the urinary iron excretion, the liver iron concentration, and the serum ferritin concentration was investigated by lowess regression analysis (tension 66). Panels A and E are based on 99 MRI examinations (5-10 examinations in each patient), and panels B-D on 76 examinations (no follow-up of urinary iron in patients 7, 12, and 14).
Overall relationship between estimate of myocardial iron concentration and indices of iron status in 14 patients with transfusional iron overload during iron chelation. Myocardial iron estimates are obtained from Figure 4. The relationship between the myocardial iron estimates and the urinary iron excretion, the liver iron concentration, and the serum ferritin concentration was investigated by lowess regression analysis (tension 66). Panels A and E are based on 99 MRI examinations (5-10 examinations in each patient), and panels B-D on 76 examinations (no follow-up of urinary iron in patients 7, 12, and 14).
Cardiac disease during iron chelation
All patients were without clinical signs or symptoms suggestive of cardiac disease during the whole follow-up period, except patients 1 and 12, who had persistent elevation of the myocardial iron estimates. Patient 1 had ischemic heart disease, successfully treated by coronary bypass surgery about 2 years before start of iron chelation. He had the largest hemoglobin requirement of all patients (8 U/mo), and had received 132 U blood on starting iron chelation treatment. Although he achieved negative iron balance (declining liver iron concentration) he developed cardiac failure after 11 months of DFO treatment. Despite anticongestive treatment, he died a few months after leaving the study. Patient 12 had preexisting alcoholic liver disease with biopsy-verified micronodular cirrhosis. Cardiac evaluation (including echocardiography) was normal before start of iron chelation. He also had a large hemoglobin requirement (6 U/mo) and had received 84 U blood on starting iron chelation. Although this patient achieved negative iron balance he began to have dizzy spells after 15 months of DFO treatment. Eleven months later he presented with severe congestive heart failure. The myocardial iron level was still severely elevated, and endomyocardial biopsies from the right ventricle revealed extensive deposition of iron pigment. The congestive heart failure reversed within weeks on inotropic and antiarrhythmic medication. The patient remained without cardiac complaints, and at the last follow-up after 4 years of DFO treatment, left ventricular function was normal.
Discussion
Methodologic aspects
The present study is the first to report multiple repeated myocardial iron estimates in patients with transfusional iron overload while on iron chelation therapy. Such data can only be obtained by a noninvasive technique. Presently, only MRI techniques offer this potential, but data are still sparse. Available data include a few congress abstracts24,25 and case reports.26,27 Changes in MRI-derived indices described in these reports indicate a reduction of the myocardial iron concentration during iron chelation and suggest that MRI may be useful for follow-up of myocardial iron. Supporting these preliminary findings, the present study suggests elevated myocardial iron before start of iron chelation in 10 of 14 patients and normalization of iron during chelation in 6 patients. We found our method sufficiently sensitive and repeatable to allow discrimination of changes during iron chelation that seem to be clinically relevant. The difference between 2 estimates must exceed ± 4 μmol/g in patients and ± 2.8 μmol/g in the controls to be significant. The lower repeatability in patients compared with controls may be because cardiac iron deposits are patchy.2,4,28 Repeatability may possibly be increased further by enhancing the image quality, which would improve recognition of the relevant slices. Our earlier observations17 have established that slice recognition is crucial because of the variation in signal intensity between slices. Thus, we previously reported iron estimates within cranial slices of the lateral wall of the left ventricle to be significantly higher than estimates obtained for the caudal slices,17 representing one reason for the variation in signal intensity between slices. We therefore used the average of myocardial iron estimates of 3 appropriate, adjacent slices in the present study and will do so in future studies. Our MRI method for the estimation of myocardial iron is based on the calculation of the SIR between the myocardial wall and skeletal muscle obtained from MRI scans recorded by the use of a spin-echo sequence. Other techniques may also be useful, for instance the use of a gradient-echo imaging and the calculation of T2* as described in the recent study of Anderson et al.18 Gradient-echo imaging has been used by other groups for assessing the liver iron by calculating the SIR.12,29,30 In the latter 2 studies a strong, significant overall relationship was demonstrated between the SIR and the chemically determined liver iron concentration. In the study of Bonkovsky et al30 a coefficient of determination (R2)of 0.88 was found, comparable with the results of Anderson et al18 (R2 = 0.87). The correlation was even stronger in the study of Angelucci et al (R2 = 0.98),12 which is comparable with the characteristics of our own method (R2 = 0.98).11 One advantage of the gradient-echo method used by Anderson and colleagues in comparison with the spin-echo method used in our study is a shorter total imaging time, allowing the acquisition of an image in one breath-hold, reducing possible movement artifacts. Moreover, gradient-echo imaging may be more sensitive than spin-echo imaging with comparable time parameters to the presence of iron, such as paramagnetic ferritin and hemosiderin particles,31 and may therefore be especially useful for low-degree iron overload as encountered within the myocardial wall. However, one problem with the T2* measurements by gradient-echo imaging in the study of Anderson and colleagues,18 as also acknowledged by the authors themselves, is the possible dependence of T2* on machine and sequence parameters. This is the reason that we use SIR measurements with skeletal muscle as an internal reference. Nevertheless, the approach to gradient-echo imaging by Anderson et al18 is interesting, and we are planning to compare their method with our own. In a subsequent study the authors used the myocardial T2* measurement successfully for comparison of the effect of oral deferiprone and subcutaneous deferrioxamine.32 Their study illustrates the potential significance of MRI-based methods in the future for the clinical evaluation of different chelation strategies.
It should still be kept in mind that our method, as well as the MRI methods in the aforementioned studies in the literature, is not directly validated with cardiac tissue. Moreover, the conversion of the heart-to-muscle SIR into myocardial tissue iron concentrations in the present study is based on the calibration curve from our MRI method for quantification of the liver iron concentration,11 which nonetheless is calculated by use of the same MRI recordings. Moreover, MRI measures tissue iron indirectly, by detecting the effect of ferritin and hemosiderin molecules on relaxation processes of protons in tissue water,31 which may be influenced by a variety of factors potentially different in myocardial and hepatic tissue. For example, tissue water may not have identical properties in both tissues. The myocardial iron concentration estimates may therefore not be directly comparable with those in other studies. This limitation should, however, be seen in the light of the problems encountered when trying to calibrate a noninvasive method for determination of the myocardial iron concentration. A large sampling error has to be expected because of the inhomogeneous distribution of iron within the myocardium as have problems in keeping the biopsy site constant. Moreover, only a restricted area of the myocardial wall is accessible by the bioptome.33 These restrictions may exclude a reliable comparison between the MRI-based myocardial iron estimates and chemically determined iron concentrations based on endomyocardial biopsies. Nonetheless, the demonstrated normalization of the myocardial iron estimates in several of our patients during iron chelation, and the close relationship between urinary iron excretion and myocardial iron estimates represent further indirect evidence for the empirical value of our MRI-based myocardial iron estimates.
Relationship between the myocardial iron estimate and urinary iron excretion
The most unexpected finding in the present study is the very close relationship between the myocardial iron estimates and the urinary iron excretions. This close relationship existed not only before but also during iron chelation. The myocardial iron estimates increased steeply and proportionally with the log-transformed urinary iron excretion when exceeding around 20 mg/24 h. This amount of excreted urinary iron was only exceeded if the liver iron concentration exceeded around 350 μmol/g, suggesting that the chelatable iron pool, as reflected by the urinary iron excretion, is only allowed to expand to this critical size when the liver iron concentration exceeds the critical level. This observation also suggests that iron is only deposited within the myocardium if the chelatable iron pool exceeds a critical size. Recently, we published data on the relationship between the extent of hepatocellular injury as evaluated by the serum aminotransferase activity and the urinary iron excretion and the liver iron concentration prior to and during iron chelation.34 The data showed that aminotransferase levels were only elevated if the liver iron concentration exceeded 300 to 400 μmol/g and, moreover, the aminotransferase levels also increased proportionally with the urinary iron excretion for iron excretions exceeding 15 mg/24 h. All together, the findings in that study and the present study suggest that the size of the chelatable, labile iron pool, as reflected by the urinary iron excretion, is pivotal for development of myocardial siderosis and for liver dysfunction caused by transfusional iron. Experimental and clinical observations indicate that iron excreted in the urine is mainly derived from the reticuloendothelial cells catabolizing the hemoglobin in nonviable erythrocytes.35 Most catabolic iron is recycled to the plasma transferrin, but if the transferrin is already saturated then increased amounts of non–transferrin-bound plasma iron (NTPI) may appear in the circulation, accessible for iron chelation.36 NTPI is toxic by promoting the formation of free hydroxyl radicals accelerating the peroxidation of membrane lipids.37 In myocyte cultures it has been shown that NTPI uptake is up-regulated by iron loading of the myocytes,38 and the rate of NTPI uptake is more than 200 times the rate of iron uptake from transferrin iron.39 This transferrin-independent uptake of iron40 may be the underlying mechanism that explains the close relationship between the myocardial iron estimates and the urinary iron excretion demonstrated in our study.
Porter et al41 recently reported data suggesting that the NTPI clearance achieved by DFO is of short duration. Thus, the NTPI reappeared within hours after stopping DFO infusion and in several patients the NTPI concentration was higher than the baseline level 12 hours after the infusion was stopped. Because our patients were on intermittent treatment by either 12-hour infusions or bolus injections twice daily, this rebound effect of NTPI to the plasma may possibly explain our unexpected finding of increased myocardial iron estimates compared with baseline values, most pronounced after 3 months of iron chelation treatment. This potential reappearance of NTPI after stopping DFO infusion may give continuous 24-hour DFO infusions an advantage over discontinuous therapy.42 Another possible explanation for the increased 3-month myocardial iron estimates is that ferrioxamine may contribute to the MRI signal. Relaxation measurements (T2*) in aqueous ferrioxamine solutions up to 10 μM appear do not appear to support this hypothesis,43 but further research is required.
The demonstrated dependence of the urinary iron excretion on a critical liver iron concentration suggests that the liver is responsible for the regulation of the size of the chelatable iron pool. The size of the cytosolic, labile iron pool, classically referred to as the chelatable iron pool,44 is controlled by efficient regulatory mechanisms, but massive iron loading may result in an uncontrollable expansion of the pool, which fails to be matched by the sequestering capacity of cellular ferritin.45 Hepatic iron concentrations exceeding the critical level around 350 μmol/g may reflect the iron load within the hepatocyte that exceeds the iron sequestration capacity of the described regulatory system, causing expansion of the chelatable iron pool and increase in myocardial iron.
Prediction of the myocardial iron concentration
A thorough evaluation of the usefulness of the applied indices of iron status in predicting myocardial iron could not be performed on the basis of the present data because they contain repeated measures on the same individual and because the myocardial iron concentration has not been determined chemically from endomyocardial biopsies. However, for the reasons mentioned, this may not be a reliable reference method at all. In the absence of a reliable reference method, we used the MRI-based myocardial estimate as a reference. The results suggest that a rough prediction of the myocardial iron concentration may be obtained by measuring the serum ferritin concentration or the urinary iron excretion in response to DFO at start of treatment. Our results also suggest that the later course of myocardial iron concentration during chelation is not likely to be predictable either by the initial myocardial iron estimates or initial serum ferritin concentrations, but may be predictable by measurement of the urinary iron excretion at start of DFO treatment.
Follow-up data collected during iron chelation showed a close nonlinear overall relationship between the myocardial iron estimates and the urinary iron excretion. This relationship was characterized by a proportional increase of myocardial iron with urinary iron for iron excretions greater than around 20 mg/24 h. Only at larger iron excretions, possibly reflecting an uncontrolled expansion of the labile, chelatable iron pool, may the determination of the urinary iron concentration be helpful for estimating the extent of myocardial siderosis. Urinary iron excretions of 20 mg/24 h or less identified all myocardial iron estimates of 8 μmol/g or less, suggesting that this threshold iron excretion may be useful for identifying patients at risk of developing cardiac disease during iron chelation. Similarly, ferritin levels of 1800 μg/L or less identified all, and a liver iron concentration less than 350 μmol/g all but one myocardial iron estimates of 8 μmol/g or less. These threshold values may be useful surrogate markers for the efficacy of myocardial iron chelation. The demonstrated ferritin threshold may explain why ferritin levels reflect cardiac disease-free survival.46,47 For example in the retrospective study of Olivieri et al46 on patients with β-thalassemia receiving DFO treatment, it was found that if less than 33% of the serum ferritin values collected during iron chelation treatment exceeded 2500 μg/L then the estimated rate of survival without cardiac disease was 100% after 10 years, but in patients exceeding this ferritin threshold the corresponding survival rate was only 48%. This reflection of disease-free survival by ferritin levels may be possible because ferritin levels more than 1800 μg/L predominantly reflect the extent of hepatocellular injury,34 owing to an expanded chelatable iron pool, causing a proportional increase in myocardial iron along with increasing ferritin levels. A higher ferritin threshold for developing cardiac disease in the study of Olivieri et al46 than the ferritin threshold for increased myocardial iron in our own patients (most of them without cardiac disease) may suggest that slightly or moderately increased myocardial iron concentrations do not cause cardiac disease, as long as a certain myocardial threshold iron concentrations is not exceeded.
Another attempt to identify threshold values of markers of iron status for myocardial siderosis causing myocardial disease was made by Brittenham et al.48 They studied the relationship between the ratio of the total transfusional iron load to the cumulative use of DFO in patients with thalassemia major. They found a threshold ratio at 0.6 mmol iron per gram DFO referred to a liver iron concentration at 80 μmol/g wet weight (about 270 μmol/g dry weight). Higher concentrations were associated with increased risk of cardiac disease and early death. This threshold liver iron concentration is lower than the threshold in our patients with acquired bone marrow disorders (350 μmol/g dry weight) identifying all but one myocardial estimates less than or equal to 8 μmol/g, suggesting that the critical liver iron concentration for developing cardiac disease may differ in different conditions with transfusion-dependent anemias. The different thresholds may be caused by differences in erythroid activity and in extent of ineffective erythropoiesis, known to influence the amount of urinary iron excreted,49 which may reflect a corresponding change in the size of the labile iron pool. Therefore, until a greater variety of patients with different types of bone marrow diseases causing transfusional iron overload has been studied, our results only pertain to patients with acquired bone marrow disorders.
Recently, Anderson et al demonstrated decreased myocardial T2* values32 (suggesting elevated myocardial iron content) in several well-chelated patients with β-thalassemia with liver iron concentrations well below the threshold concentration found in our study. The authors did not convert the T2* measurements to myocardial tissue iron concentrations. Therefore, it is not possible to compare myocardial iron estimates between the studies. However, differences in the size of the chelatable iron pool, caused by differences in erythropoiesis between thalassemia patients and our patients with acquired refractory anemias is one possible explanation for potential differences in myocardial iron estimates. Another explanation might be that the patients with decreased myocardial T2* levels had liver disease owing to hepatotropic virus infections. As we also have proposed earlier, the threshold liver iron concentration for developing myocardial siderosis may possibly be lowered by liver disease.34 Although the frequency of antihepatitis C virus-positive (HCV+) patients is not given in the study of Anderson et al,32 it is known that HCV infection is still a recognized clinical problem in patients in the United Kingdom, approximately 25% of the thalassemia patients being infected with HCV.50 Only one of our patients was HCV+. Liver disease as a potential factor for development of myocardial siderosis has been suggested earlier.51 Finally, the method used by Anderson et al32 and our own method may have different sensitivities for different forms of myocardial iron (ferritin, hemosiderin, low-molecular-weight iron), and also for the presence of fibrosis.
Contrary to other studies15,16 including the present study, Anderson et al32 did not find a significant correlation between their myocardial iron index (T2*) and the serum ferritin concentration. One likely explanation is that the relation does not exist over the whole range of ferritin values. In our own study patients were followed from start of treatment when ferritin levels were high, and a close relation was only seen for serum ferritin levels more than 1800 μg/L. In the study of Anderson et al32 DFO treatment had been started at a mean of 18.3 years before doing the MRI. Accordingly, the ferritin levels were markedly lower (1250 ± 508 μg/L). Significant relations between ferritin levels and MRI-derived myocardial iron indexes was also demonstrated by Mavrogeni et al15 and Papanikolaou et al.16 Both studies also included patients with high ferritin levels.
In conclusion, our data represent further indirect evidence that myocardial iron may be estimated by our MRI method. The repeatability of the obtained estimates is sufficient to detect significant changes of potential clinical interest. Our results seem to highlight the importance of starting iron chelation early, before the critical liver iron concentration is exceeded. Our findings further support the concept of the labile iron pool as the compartment directly involved in iron toxicity in transfusional iron overload. Owing to the limitations of our method and the small number of patients and the restriction to acquired anemias, our results are preliminary. Further studies are required before our results may serve as clinical guidelines.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-09-2754.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal