Abstract
The risk for thrombosis is increased in patients with hereditary hydrocytosis, an uncommon variant of hereditary stomatocytosis. Erythrocytes from 2 patients with hydrocytosis were studied to gain insight into the mechanism of thrombosis in this disorder. Erythrocytes demonstrated abnormal osmotic scan ektacytometry and decreased erythrocyte filtration rates. There was also a mild increase in adherence of erythrocytes to endothelial monolayers in a micropipette assay. Adhesion of erythrocytes to the subendothelial matrix proteins thrombospondin and laminin, however, was not significantly increased. Percentages of hydrocytosis erythrocytes and reticulocytes with phosphatidylserine exposed on the outer surfaces were increased in both patients compared with healthy controls, indicating altered membrane phospholipid asymmetry. Increased phosphatidylserine exposure accelerating thrombin-forming processes has been proposed as a mechanism for thrombosis in sickle cell disease and β-thalassemia and may play a similar role in hereditary hydrocytosis.
Introduction
The risk for thrombosis is increased in many erythrocyte disorders, including sickle cell disease and β-thalassemia.1,2 Thrombosis is also increased in the hereditary stomatocytosis syndromes, an uncommon, heterogeneous group of disorders characterized by mouth-shaped erythrocyte morphology on peripheral blood smear.3, 4, 5 Erythrocyte membranes of patients with stomatocytosis exhibit abnormal permeability to sodium and potassium, with resultant modification of intracellular water content.4,5
One rare stomatocytosis variant, hydrocytosis, is characterized by a net gain of sodium and potassium that leads to the retention of water, forming swollen, overhydrated erythrocytes. The molecular basis of hydrocytosis is unknown. We studied 2 patients with hydrocytosis, one whose affected father had multiple thromboses and another who has experienced multiple thromboses since age 19.
Study design
Patients
Patient 1 is the 11-year-old son of the proband in the report by Zarkowsky et al.6 Typical red blood cell indices are: hemoglobin level, 8 to 11 g/dL; mean corpuscular volume (MCV), 125 to 140 fL; mean corpuscular hemoglobin concentration (MCHC), 26% to 27%; reticulocyte count, 7% to 25%. Peripheral blood smear shows marked stomatocytosis. Incubated erythrocytes demonstrate increased osmotic fragility. He underwent splenectomy at 5 years of age and has not had any episodes of thrombosis. His father, who underwent splenectomy in the second year of life, had multiple chronic pulmonary thromboemboli that resulted in his death at 34 years of age.
Patient 2 has had severe stomatocytosis with hemolytic anemia and jaundice since birth. He required multiple blood transfusions until age 4 years, when he underwent splenectomy. He then experienced fewer hospital stays for hemolytic crises and was nearly asymptomatic from age 7 to 15. Hemolytic crises recurred during adolescence. At 19 years of age, he had pulmonary embolus and was treated with heparin followed by coumadin. He has experienced additional thrombotic events, including deep vein thrombosis, myocardial infarction at age 29, and pulmonary hypertension attributed to chronic pulmonary emboli. Now 36 years of age, he receives regular transfusions of erythrocytes. Before transfusion therapy, typical red blood cell indices were: hemoglobin level, 11.9 to 12.8 g/dL; MCV, 124 to 136 fL; MCHC, 28% to 29%; reticulocyte count, 26% to 28%. Incubated erythrocyte osmotic fragility was increased. Erythrocyte membranes demonstrate significantly decreased stomatin immunoreactivity.
Materials
Except where noted, whole blood was centrifuged, the plasma and buffy coat were removed, and the remaining erythrocytes were washed 3 times by repeated resuspension and centrifugation at 800g, 600g, and 500g, respectively.7 This washing procedure was shown to efficiently remove platelets and leukocytes, which represented at most 0.05% of the residual cells. To collect a reticulocyte-rich fraction, buffy coat–free erythrocytes were centrifuged at 27 000g for 60 minutes at 30°C.8 The top 5% of cells were harvested, and reticulocytes were counted by the manual method.8 Samples from patient 2 were obtained 4 to 5 weeks after his last transfusion.
Ektacytometry
Packed erythrocytes were suspended in 4% polyvinylpyrrolidone solution and were subjected to increasing osmolality (50-500 mOsm/kg) at constant shear stress or to constant osmolality at increasing shear stress (0-250 dyne/cm2).9 The axial ratio of the deformed cells was designated the deformability index (DI).
Erythrocyte filtration rate
Analysis of the rate of filtration of an erythrocyte suspension through a 4.6-μm diameter nickel mesh filter was conducted by a gravity-based, vertical tube method that measures the rate of passage through the filter as a function of hydrostatic pressure (Tsukusa Sokken, Tokyo, Japan).7 Data shown are means ± SD of 4 to approximately 6 measurements from 2 independent experiments.
Phosphatidylserine exposure
Unfractionated, reticulocyte-rich, and reticulocyte-depleted erythrocytes were labeled with annexin V (Roche, Indianapolis, IN) and were analyzed by flow cytometry using the method of Kuypers et al10 with minor modifications. After incubation at 0.04% hematocrit for 15 minutes with 4 nM fluorescein isothiocyanate (FITC)–labeled annexin V, cells were diluted to 0.01% hematocrit and immediately analyzed by flow cytometry (Coulter XL-MCL, Hialeah, FL). Each sample was assayed in triplicate.
Endothelial adhesion
Adherence of erythrocytes to endothelium was quantified by measuring the shear force required to separate individual cells from cultured human umbilical endothelial cell layers using a micropipette technique.11 Adhesion of erythrocytes to the subendothelial matrix proteins thrombospondin and laminin was quantified as described.12
Results and discussion
Ektacytometry
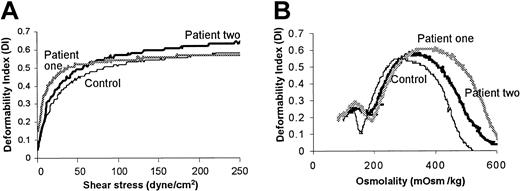
Purified erythrocytes were examined by ektacytometry to obtain information on cell water content, surface area–volume ratio, and membrane deformability.13,14 There was a reproducible but mild increase in erythrocyte deformability as a function of increasing shear stress in both patients compared with controls (Figure 1A).
Evaluation of erythrocytes from patients with hereditary hydrocytosis and from healthy donors by ektacytometry. (A) Whole cell deformability was measured by subjecting isotonic erythrocytes to increasing shear stress. (B) Osmotic deformability was measured by subjecting erythrocytes to moderate shear stress while increasing osmotic pressure. The data shown were highly similar in 2 experiments conducted on the patients' blood collected on 2 separate occasions.
Evaluation of erythrocytes from patients with hereditary hydrocytosis and from healthy donors by ektacytometry. (A) Whole cell deformability was measured by subjecting isotonic erythrocytes to increasing shear stress. (B) Osmotic deformability was measured by subjecting erythrocytes to moderate shear stress while increasing osmotic pressure. The data shown were highly similar in 2 experiments conducted on the patients' blood collected on 2 separate occasions.
Osmotic scan ektacytometry demonstrated that the hypertonic, declining arms of the curves (Figure 1B) were displaced to higher osmolalities. The Omin, a measure of the average surface area–volume ratio of the erythrocyte, was increased in the hydrocytosis cells as was the Ohyper, which reflects the low mean corpuscular hemoglobin concentrations of these erythrocytes.13 The maximum value of the deformability index (DImax), a measure of the maximal deformability at optimal cell hydration, was also slightly increased, suggesting possible expansion of the membrane surface area.
Erythrocyte filterability
Erythrocyte filterability, a direct measure of cellular rheology, was examined on purified erythrocytes. Filtration rates were reduced for both patients, indicating a marked decrease in the filterability of hydrocytosis erythrocytes (Table 1). This is probably because of the swollen nature of the hydrocytosis cells, which could cause them to traverse pores more slowly.7 Alternatively, considering the thrombotic tendencies in patients with stomatocytosis, hydrocytosis erythrocytes may tend to aggregate, which would also lead to the increase in resistance during flow through a 4-μm pore.
Characteristics of stomatocytic erythrocytes
. | Patient 1 . | Control . | Patient 2 . | Control . |
|---|---|---|---|---|
| Filtration rate, % buffer control | 21 ± 12 | 92 ± 9 | 67 ± 5 | 90 ± 2 |
| Erythrocyte adherence | ||||
| Shear force, dyne/cm2 | ||||
| 20 | 10 (2000) | 7 (2000)* | 9 (1000) | 3 (1000) |
| 30 | 6 (2000) | 4 (2000)* | 5 (1000) | 2 (1000) |
| 55 | 3 (2000) | 1 (2000)* | 2 (1000) | 0 (1000) |
| Thrombospondin adhesion erythrocytes/mm2 | <1 | 9† | 80 | 30 |
| Laminin adhesion erythrocytes/mm2 | <1 | 102† | 180 | 150 |
| Annexin V binding, %‡ | ||||
| Unfractionated erythrocytes | 2.34 + 0.12§ | 0.80 + 0.16 | 2.77 + 0.25∥ | 0.94 + 0.08 |
| Reticulocyte enriched¶ | 2.25 + 0.36# | 0.27 + 0.05 | 2.19 + 0.03** | 0.58 + 0.02 |
| Reticulocyte depleted¶ | 0.83 + 0.09** | 0.08 + 0.01 | 1.71 + 0.16†† | 0.47 + 0.01 |
. | Patient 1 . | Control . | Patient 2 . | Control . |
|---|---|---|---|---|
| Filtration rate, % buffer control | 21 ± 12 | 92 ± 9 | 67 ± 5 | 90 ± 2 |
| Erythrocyte adherence | ||||
| Shear force, dyne/cm2 | ||||
| 20 | 10 (2000) | 7 (2000)* | 9 (1000) | 3 (1000) |
| 30 | 6 (2000) | 4 (2000)* | 5 (1000) | 2 (1000) |
| 55 | 3 (2000) | 1 (2000)* | 2 (1000) | 0 (1000) |
| Thrombospondin adhesion erythrocytes/mm2 | <1 | 9† | 80 | 30 |
| Laminin adhesion erythrocytes/mm2 | <1 | 102† | 180 | 150 |
| Annexin V binding, %‡ | ||||
| Unfractionated erythrocytes | 2.34 + 0.12§ | 0.80 + 0.16 | 2.77 + 0.25∥ | 0.94 + 0.08 |
| Reticulocyte enriched¶ | 2.25 + 0.36# | 0.27 + 0.05 | 2.19 + 0.03** | 0.58 + 0.02 |
| Reticulocyte depleted¶ | 0.83 + 0.09** | 0.08 + 0.01 | 1.71 + 0.16†† | 0.47 + 0.01 |
Values for erythrocyte adherence outside the parentheses represent total adherent cells, and inside the parentheses they represent total RBCs observed.
Sickle cell controls (total adherent cells/total RBCs observed): 20 dyne/cm2, 10.9/1000; 30 dyne/cm2, 6.5/1000; 30 dyne/cm2, 9.1/1000.
Sickle cell controls: thrombospondin, 750 erythrocytes/mm2; laminin, 2500 erythrocytes/mm2.
Value for positive control erythrocytes treated with calcium and A23187 was 54% ± 4%.
P = .0002 (unpaired Student t test).
P = .0003.
Manual reticulocyte counts (%). Enriched fraction: patient 1 and control, 24% ± 4% and 1% ± 0.2%; patient 2 and control, 32.3% ± 8.1% and 1.96% ± 0.8%. Depleted fraction: patient 1 and control, 6.5% ± 1.4% and 0%; patient 2 and control, 1.71% ± 0.01% and 0.47% ± 0.01%.
P = .0007.
P = .0001.
P = .0002.
Erythrocyte phosphatidylserine exposure
An increase in phosphatidylserine exposure has been observed in a subpopulation (2%-3%) of erythrocytes from patients with sickle cell disease10,15 and β-thalassemia.16,17 This increase in PS-exposing erythrocytes has been proposed as a mechanism for the prothrombotic state seen in these disorders10,15,17 because PS exposure may lead to the assembly of the contact coagulation factors by accelerating thrombin-forming processes.18
Unfractionated erythrocytes from both hydrocytosis patients demonstrated increased PS exposure (Table 1) in the range observed in erythrocytes of patients with sickle cell disease or β-thalassemia. These values represent the mean ± SD of results from samples obtained on separate dates. Reticulocyte-enriched and reticulocyte-depleted erythrocytes demonstrated significant increases in annexin V binding in both patients (Table 1).
Endothelial adherence
Adherence of hydrocytosis erythrocytes to endothelium was mildly increased in both patients, as determined by a micropipette shear stress technique (Table 1).11 Under low-flow conditions (1 dyne/cm2), there was slightly increased adhesion of erythrocytes from patient 2 to thrombospondin, but it was much less than was observed with sickle erythrocytes.12 Erythrocytes from the patients with hydrocytosis did not demonstrate significantly increased adhesion to laminin.
Increased endothelial adherence of erythrocytes with altered membrane phospholipid asymmetry has been observed.19, 20, 21, 22, 23, 24 Sickle erythrocyte phosphatidylserine exposure strongly correlates with adhesion to resting microvascular endothelial cells,25 in agreement with our observation of mildly increased adhesion of hydrocytosis erythrocytes to endothelium. Phosphatidylserine exposure may also contribute to erythrocyte adhesion to matrix-bound thrombospondin.23 We did not observe a significant increase in thrombospondin binding, probably because of the limited number of patients and differences in experimental conditions. Cells in this earlier report were treated with calcium and A23187,23 elevating the number of PS-exposing erythrocytes from approximately 2% to approximately 5% to 10%. Hydrocytosis erythrocytes were not pretreated before the thrombospondin-binding assay. It is still possible that significantly increased PS exposure leads to increased endothelial adhesion either through thrombospondin or directly to the endothelial cell in vivo.
This very small sample size precludes any definitive conclusions about the mechanisms of thrombosis in hydrocytosis. However, these initial data suggest that increased erythrocyte endothelial adhesion, loss of membrane asymmetry, and exposure of phosphatidylserine may contribute to the thrombosis risk for patients with hydrocytosis.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2001-12-0329.
Supported in part by National Institutes of Health grant HL70981 (C.A.H.) and by the March of Dimes Birth Defects Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal