Abstract
Gemtuzumab ozogamicin (GO) is a humanized anti-CD33 antibody conjugated to the anticancer agent calicheamicin, approved for the treatment of CD33+-relapsed acute myeloid leukemia. We have investigated the effects of GO on 4 human myeloid leukemia lines of different French-American-British (FAB) types (KG-1, THP-1, HL-60, and NB-4), observing 3 different types of response. Exposure to GO (10-1000 ng/mL) induced G2 arrest (up to 80% of the cells) followed by apoptosis (45% of the cells) in HL-60 and NB-4 cells. By contrast, in THP-1 cells we observed a strong G2 arrest (up to 75% of the cells) with little apoptosis. Finally, the KG-1 line was completely resistant to the same concentrations of GO. These different responses did not correlate with the levels of expression of either CD33 or multiple-drug resistance proteins, although the higher cyclosporin A (CsA)–inhibitable efflux activity of KG-1 cells may play a role in the resistance of this line to the drug. We could show that Chk1 and Chk2 phosphorylation, but not p53 or p21 expression, correlated with G2 arrest, implicating the ataxia-telangiectasia mutated/ataxia-telangiectasia related (ATM/ATR)–Chk1/Chk2 pathway in the cell cycle response to GO. However, apoptosis was associated with caspase 3 activation. Freshly isolated acute myeloid leukemia (AML) cells showed patterns of response to GO in vitro similar to those observed with the cell lines, including phosphorylation of Chk2 and caspase 3 activation. Our results suggest that the different molecular pathways induced by the drug in vitro may reflect, at least in part, the variable response to GO obtained in vivo.
Introduction
The development of therapeutic monoclonal antibodies (mAbs) holds enormous potential for the treatment of hematologic malignancies.1, 2, 3, 4 Current conventional treatments for patients with acute myeloid leukemia (AML) result in a high percentage of clinical response in most patients. However, a large subset of patients still remain refractory to primary therapies or relapse later.5,6 Treatment of AML is especially difficult in those older than 60 years (which represents most patients), as their ability to withstand the intensity of chemotherapy is reduced, and unfavorable cytogenetic and morphologic factors are frequent.7
Gemtuzumab ozogamicin (GO, Mylotarg) is the first example of antibody-targeted chemotherapy for the treatment of AML.8,9 The recently reported combined results of 3 multicenter phase 2 trials of GO show that overall 30% of patients with recurrent AML achieve remission with relatively low toxicity.9,10 Despite these promising clinical results there is still heterogeneity of response among different patients, presumably reflecting at least in part different molecular responses.
GO is composed of a humanized immunoglobulin G4 (IgG4) monoclonal antibody (hP67.6) linked to a calicheamicin derivative (N-acetyl γ1 calicheamicin).11,12 This molecule is a member of the enediyne family of antitumor antibiotics that is roughly 1000-fold more cytotoxic than clinically used anticancer agents.13 GO targets CD33, an appealing target for therapies because it is expressed in approximately 90% of cases of AML and is absent from healthy hematopoietic stem cells and nonhematopoietic tissues. GO is rapidly internalized after binding to its target, followed by release of the calicheamicin derivative.14 Intracellular conversion of the drug to a highly reactive diradical species leads to double-stranded DNA breaks.13
When exposed to DNA-damaging agents, cells undergo cell cycle arrest to allow DNA repair and/or apoptosis. Cell cycle arrest occurs through the activation of signal transduction pathways called checkpoints.15,16 Checkpoints arrest cells in the G1/S phase to prevent replication of damaged DNA or in the G2/M phase to prevent the segregation of damaged chromosomes during mitosis. The proportion of cells that arrest at G1/S or G2/M depends on cell type, growth conditions, and the checkpoint controls active in the cells.17
Here we describe the response to GO in vitro of acute myeloid leukemia cells and individuate some of the molecular pathways that are triggered by GO.
Materials and methods
Cell culture
The myeloid leukemia lines HL-60, THP-1, NB-4, and KG-1 and the B-lymphoid line MEC-218 were grown in RPMI 1640 medium (Seromed, Berlin, Germany) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT), glutamine (Gibco-BRL, Paisley, Scotland), and penicillin/streptomycin. GO (Mylotarg) and the unconjugated parent antibody hP67.6 were a kind gift from Wyeth-Ayerst Research (Pearl River, NY). They were dissolved in phosphate-buffered saline (PBS) at 1 mg/mL, divided into aliquots, and stored frozen at –80°C. Peripheral blood mononuclear cells from patients with AML at diagnosis or at relapse were separated by Ficoll-Hypaque centrifugation and used immediately. Leukemic blasts were 80% to 100%.
Proliferation assays
Cell lines were plated at 5 × 105/mL in the presence of increasing concentrations of GO. Primary AML cells were plated at 106/mL in RPMI 1640 medium supplemented with 10% FCS, 50 ng/mL recombinant human interleukin 3 (rhIL-3; Behring, Germany) and 10 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; Shering-Plough, Milan, Italy). After 48 hours of culture, cells were pulsed for 16 hours with 0.00185 MBq (0.5 μCi) 3H-thymidine and were harvested in a cell harvester (Tomtec, Hamden, CT).
Viability assays
Viability assays were performed as described.19 Cell lines were plated at 2 × 104/well in 96-well plates. Primary AML cells were plated at 5 × 104/well in the presence of rhIL-3 and rhGM-CSF as described in “Proliferation assays.” After 48 hours of culture, 1/10 volume of Alamar blue solution was added (Biosource International, Camarillo, CA). Incubation was carried on overnight at 37°C, and the plates were read in a fluorometer (Cytofluor 2300; Millipore, Bedford, MA) with excitation at 530 nm and emission at 590 nm. Triton X-100 (0.25%; Sigma, Steinheim, Germany) was added to the set of wells used to set up the background fluorescence (all cells lysed).
Cell cycle analysis
Monoparametric cell cycle analysis on ethanol-fixed cells was performed as described.20 The Baisch method was used to assess the cell cycle phase distribution. For cyclin B1 staining, ethanol-fixed cells were permeabilized with 0.25% Triton X-100 (Sigma) for 5 minutes on ice, cells were incubated with 2.5 μg/mL antihuman cyclin B1 antibody (clone 7A9; Novocastra Laboratories, Newcastle, United Kingdom). Subsequently, cells were probed with fluorescein isothiocyanate (FITC)–conjugated goat antimouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA) before standard propidium iodide staining and fluorescence-activated cell sorter (FACS) analysis.
Western blots
Cells were lysed in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, 250 mM NaCl, 0.1% NP40, 5 mM EDTA (ethylenediaminetetraacetic acid), protease inhibitors (Roche Diagnostics, Monza, Italy), and 10 mM sodium orthovanadate. Proteins (60-100 μg) were separated on 10% to 15% sodium dodecyl sulfate (SDS)–polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). membranes were blocked with 5% skimmed milk, incubated with the primary antibodies at 4°C overnight in TBS-T (10 mM Tris-HCl, pH8, 150 mM NaCl, 0.05% Tween 20), and followed by a 2-hour incubation with horseradish peroxidase–conjugated secondary antibodies. Signals were detected using the electrogenerated chemiluminescence (ECL) kit (Amersham, Little Chalfont, United Kingdom). Primary antibodies were p53, p21, Chk1, Chk2, β-actin, and procaspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA; 1 μg/mL in 5% skimmed milk); phospho-Chk1–specific antibody (Cell Signaling Technology, Beverly, MA; 1:100 in 5% bovine serum albumin [BSA]).
Immunofluorescence
Cells were stained with an FITC-conjugated anti-CD33 mAb (clone P67.6; Becton Dickinson) according to standard procedures. For detection of surface P-glycoprotein (Pgp), indirect immunofluorescence was performed using a mouse anti-Pgp mAb (clone MRK16; Kamiya Biomedical, Seattle, WA). For the detection of intracellular Pgp (mAb JSB-1), multidrug resistance protein 1 (MRP1) and lung resistance protein (LRP) cells were fixed and permeabilized using the Fix and Perm Kit (Caltag, Burlingame, CA). Cells were then incubated with the JSB-1, anti-MRP1, and anti-LRP mAbs, respectively (Kamiya Biomedical Company), washed, and stained with FITC-conjugated secondary antibody (Becton Dickinson). All samples were analyzed on a FACscan flow cytometer (Becton Dickinson).
Dye efflux
Cells (5 × 105) were loaded with 10 ng/mL DiOC2 (diethyloxadicarbocyanine iodide; Sigma) for 30 minutes at 37°C. After washing, one aliquot was transferred to 4°C for subsequent analysis or resuspended in fresh medium in the presence or absence of 2 μg/mL cyclosporin A (CsA) and returned to 37°C for 90 minutes to allow active efflux. All samples were analyzed on a FACscan flow cytometer using CellQuest software (Becton Dickinson). Dye efflux was measured using a modification of Kolmogorov-Smirnov (KS) statistics, denoted D, which measures the difference between 2 distribution functions and generates a value ranging from 0 to 1.0.21,22 Dim efflux was defined as 0.20 ≤ D < 0.25, moderate efflux as 0.25 ≤ D < 0.40, and strong efflux as D ≥ 0.40, according to Leith et al.22 For CsA-inhibited efflux, the difference between samples incubated at 37°C in the presence or absence of CsA was analyzed with KS statistics. A significant difference was defined as D > 0.15.22
Apoptosis assays
For DNA fragmentation, cells were lysed in 1 M Tris-HCl pH 8.5, 5 mM EDTA, 0.2% SDS, 0.2 M NaCl, and 700 μg/mL proteinase K overnight at 37°C. After RNase A treatment (10 mg/mL), DNA was extracted with phenol/chloroform, precipitated, and analyzed in a 2% agarose gel.
Apoptosis was measured using the in situ cell death detection assay kit (Roche Diagnostics, Milan, Italy) following the manufacturer's instructions. Analysis was performed on a FACstar Plus cytometer (Becton Dickinson).
Results
AML lines show different patterns of response to GO in vitro
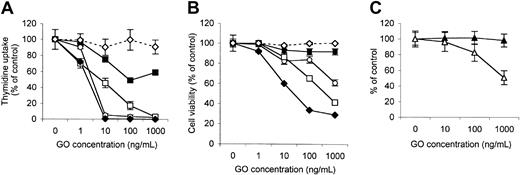
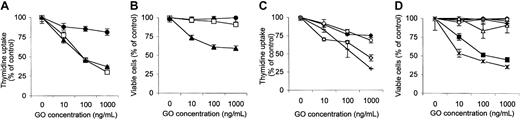
To study the mechanism of action of GO in vitro, we have tested the effect of increasing doses of this drug on the proliferation of 4 different established AML cell lines, using the 3H-thymidine uptake assay. As shown in Figure 1A, the different cell lines showed a widely variable response to GO. Three lines (HL-60, THP-1, and NB-4) were very sensitive to GO, showing a concentration that inhibits 50% (IC50) of 2 to 6 ng/mL GO. The KG-1 line was, on the contrary, much more resistant with an IC50 of more than 1000 ng/mL. Growth inhibition was specific for the conjugated antibody, because the unconjugated anti-CD33 parent antibody hP67.6 had no significant effect on any of the cell lines even at the highest concentration. The results obtained with HL-60 cells are shown in Figure 1A as an example. Similarly, the effect of GO on the proliferation of a CD33– cell line was also investigated. Proliferation of the B-cell line MEC-2 was partially inhibited by GO only at high GO concentrations, with an IC50 of 1000 ng/mL (Figure 1C). Similar results were obtained with other cell lines such as the T-cell line CEM (data not shown). These results show that the effects of GO observed on CD33+ cell lines at low concentrations (1-100 ng/mL) are specific for CD33+ cells, but that at high GO concentrations calicheamicin may enter cells through mechanisms independent from CD33.
Response of different leukemic cell lines to GO in vitro. (A-B) Exponentially growing AML cell lines were plated in the presence or absence of increasing concentrations of GO or of the unconjugated parent antibody hP67.6. 3H thymidine uptake (A) and cell viability (B) were measured at 48 to 60 hours. THP-1 ± GO is indicated by ○; NB-4 ± GO, □; KG-1 ± GO, ▪; HL-60 ± GO, ♦; HL-60 ± hP67.6, ⋄. (C) The B-cell line MEC-2 was also tested for 3H-thymidine uptake (▵) and cell viability (▴) in the same experimental conditions. The results are the mean and standard deviations of triplicate wells and are representative of at least 3 independent experiments.
Response of different leukemic cell lines to GO in vitro. (A-B) Exponentially growing AML cell lines were plated in the presence or absence of increasing concentrations of GO or of the unconjugated parent antibody hP67.6. 3H thymidine uptake (A) and cell viability (B) were measured at 48 to 60 hours. THP-1 ± GO is indicated by ○; NB-4 ± GO, □; KG-1 ± GO, ▪; HL-60 ± GO, ♦; HL-60 ± hP67.6, ⋄. (C) The B-cell line MEC-2 was also tested for 3H-thymidine uptake (▵) and cell viability (▴) in the same experimental conditions. The results are the mean and standard deviations of triplicate wells and are representative of at least 3 independent experiments.
We also assessed the effect of GO on cell viability using the Alamar blue assay.19,23 As shown in Figure 1B, the HL-60 and NB-4 cell lines showed the most evident loss in the percentage of viable cell with an IC50 of 22 and 100 ng/mL, respectively. The THP-1 cell line, however, showed only a weak decrease in viable cells relative to control, reaching 40% at the highest dose of GO (1000 ng/mL), despite a very strong block in thymidine uptake (Figure 1A). Finally, the KG-1 cells were again most resistant to GO, with a less than 10% decrease in viable cells at the highest GO concentration. The parent unconjugated antibody hP67.6 had no significant effect on the viability of any of the cell lines even at 1000 ng/mL. The results obtained with the HL-60 cell line are shown in Figure 1B. Finally, GO did not affect cell viability of the CD33– cell line MEC-2 up to 1000 ng/mL (Figure 1C).
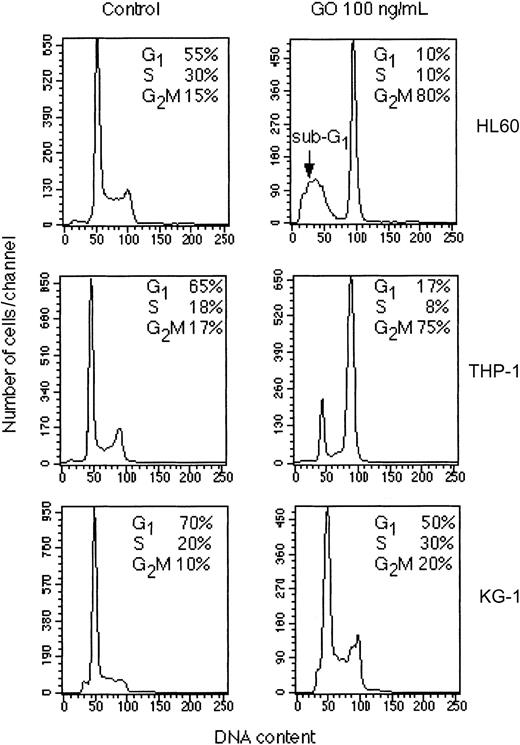
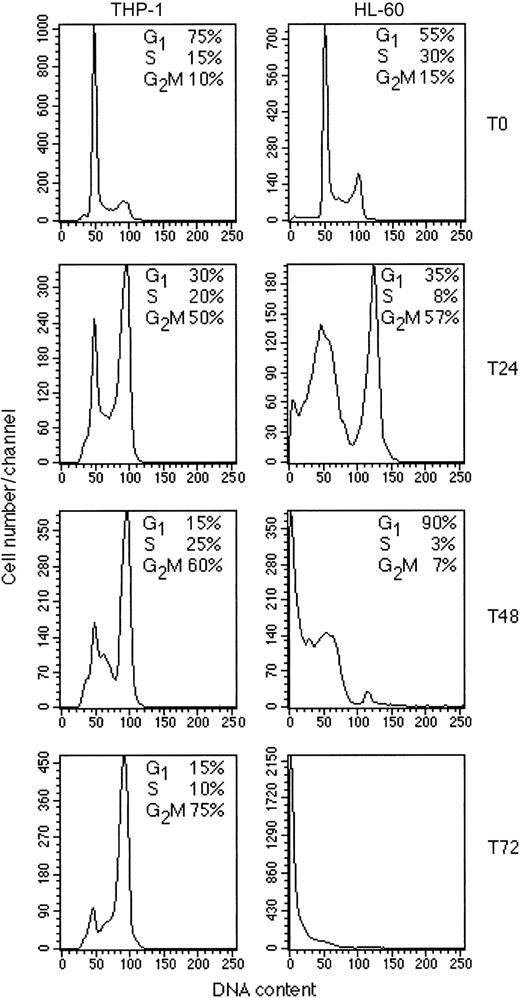
To determine whether the effects observed were due to a cell cycle block or to the induction of cell death, we performed cell cycle analysis of all cell lines cultured for 48 hours in the presence or absence of GO. As shown in Figure 2, 100 ng/mL GO induced different responses in the 4 cell lines. HL-60 cells showed a strong block in the G2/M phase of the cell cycle (reaching 80% compared with 15% in the untreated controls), as well as the appearance of a marked sub-G1 peak because of the presence of many dead cells (Figure 2). The same pattern was observed with NB-4 cells (data not shown). THP-1 cells, however, showed a strong block in G2/M, with 75% of the cells arrested at this stage, but in the absence of a significant cell death as shown by lack of cells with sub-G1 DNA content (Figure 2). Microscopic examination of THP-1 cells confirmed the presence of large (G2/M arrested) cells but few dead cells (data not shown). Finally, the KG-1 cell line was very resistant to the same concentration of GO, with only a small increase in G2/M phase cells in the treated cells (20%) relative to the control (10%) and no significant increase in sub-G1 cells (Figure 2). Similar results were obtained at 300 ng/mL GO, which was highly cytotoxic for both HL-60 and NB-4 cells, whereas they induced only G2/M arrest in THP-1 cells without significant cell death and had little effect on KG-1 cells (data not shown). A time course of the response of THP-1 and HL-60 cells to 300 ng/mL GO is shown in Figure 3. At 24 hours a strong increase in G2/M cells can be observed in both cell lines, but this increase is rapidly followed by cell death in HL-60 cells but not THP-1 cells.
Cell cycle analysis of selected leukemic lines. The indicated cell lines were cultured for 48 hours in the presence or absence of 100 ng/mL GO. Cells were then fixed in 70% ethanol and stained with propidium iodide for cell cycle analysis. The calculated percentages of cells in the G1, S, and G2/M phases of the cell cycle are indicated in each panel. The results are representative of at least 2 independent experiments.
Cell cycle analysis of selected leukemic lines. The indicated cell lines were cultured for 48 hours in the presence or absence of 100 ng/mL GO. Cells were then fixed in 70% ethanol and stained with propidium iodide for cell cycle analysis. The calculated percentages of cells in the G1, S, and G2/M phases of the cell cycle are indicated in each panel. The results are representative of at least 2 independent experiments.
Time course of response of THP-1 and HL-60 cells to GO. The THP-1 and HL-60 cell lines were cultured in the presence of 300 ng/mL, and cells were fixed at the beginning of culture (T0) or after 24 hours (T24), 48 hours (T48), or 72 hours (T72). Cells were then stained with propidium iodide (PI) and analyzed for cell cycle. The results are representative of at least 2 independent experiments.
Time course of response of THP-1 and HL-60 cells to GO. The THP-1 and HL-60 cell lines were cultured in the presence of 300 ng/mL, and cells were fixed at the beginning of culture (T0) or after 24 hours (T24), 48 hours (T48), or 72 hours (T72). Cells were then stained with propidium iodide (PI) and analyzed for cell cycle. The results are representative of at least 2 independent experiments.
To exclude that the cell lines differed only in the kinetics of the apoptotic response, they were treated with up to 1000 ng/mL GO and analyzed after 4 and 7 days of culture, feeding cells with fresh medium on day 4. The results show that even at later times, only limited cell death could be observed for the THP-1 and KG-1 cell lines, in the presence of a marked block in G2/M in THP-1 but not KG-1 (data not shown). HL-60 and NB-4 cells were mostly all dead at the same time points.
Altogether, these data demonstrate that AML cell lines show 3 different types of response to GO: resistance (KG-1), arrest in G2/M with little cytotoxicity (THP-1), or block in G2/M phase followed by rapid cell death (HL-60 and NB-4).
Role of CD33 and MDR proteins
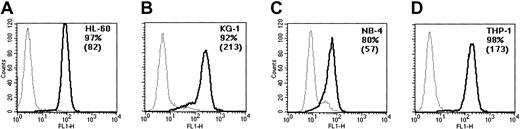
The differential susceptibility of the cell lines to GO could have been due to lower levels of CD33 expression, leading to poor binding and entry of the drug. We, therefore, determined CD33 expression levels by standard immunofluorescence on all lines in parallel. As shown in Figure 4, all 4 cell lines expressed the CD33 antigen on more than 80% of the cells. Fluorescence intensity varied from 57 to 213 mean fluorescence intensity (MFI) but did not correlate with susceptibility to GO. For example, the resistant KG-1 cell line showed an MFI of 213 compared with only 82 for the sensitive HL-60 cells.
CD33 expression in the cell lines. The indicated cell lines were stained with FITC-labeled anti-CD33 antibody (thick lines), or control antibody (thin lines), and analyzed by flow cytometry. The percentage of positive cells (after substraction of background) and mean fluorescence intensities (in parentheses) are indicated in each panel. (A) HL-60 line. (B) KG-1 line. (C) NB-4 line. (D) THP-1 line.
CD33 expression in the cell lines. The indicated cell lines were stained with FITC-labeled anti-CD33 antibody (thick lines), or control antibody (thin lines), and analyzed by flow cytometry. The percentage of positive cells (after substraction of background) and mean fluorescence intensities (in parentheses) are indicated in each panel. (A) HL-60 line. (B) KG-1 line. (C) NB-4 line. (D) THP-1 line.
MDR1 protein (Pgp) is an adenosine triphosphate (ATP)–dependent transporter strongly implicated in resistance to a range of cytotoxic drugs, including GO.24, 25, 26 We have, therefore, investigated cell surface as well as intracellular MDR1 expression in the 4 cell lines. As shown in Table 1, cell surface MDR1 expression, detected with the MRK16 antibody, was highest in the THP-1 and NB-4 lines and weaker in the HL-60 and KG-1 lines. Detection of an intracellular epitope of MDR1 using the JSB1 antibody showed a similar, although not identical, pattern of expression. Neither surface nor intracellular expression of MDR1 protein correlated with resistance to GO, because the resistant cell line KG-1 expressed intermediate to low levels, and the highest levels of MDR1 were found in the THP-1 cell line that was strongly blocked by GO even at low concentrations.
Expression and function of MDR proteins in leukemic cell lines
. | % MDR1+ cells* . | . | . | . | DiOC2 efflux . | . | ||
|---|---|---|---|---|---|---|---|---|
| Cell line . | MRK16 . | JSB1 . | % MRP1+ cells* . | % LRP+ cells* . | D efflux† . | D ± CsA‡ . | ||
| HL-60 | 7 | 1 | 0 | 0 | 0.28 (mod) | 0.06 | ||
| THP-1 | 69 | 48 | 70 | 22 | 0.31 (mod) | 0.05 | ||
| NB-4 | 40 | 1 | 0 | 0 | 0.17 (neg) | 0.04 | ||
| KG-1 | 11 | 26 | 0 | 9 | 0.43 (strong) | 0.33 | ||
. | % MDR1+ cells* . | . | . | . | DiOC2 efflux . | . | ||
|---|---|---|---|---|---|---|---|---|
| Cell line . | MRK16 . | JSB1 . | % MRP1+ cells* . | % LRP+ cells* . | D efflux† . | D ± CsA‡ . | ||
| HL-60 | 7 | 1 | 0 | 0 | 0.28 (mod) | 0.06 | ||
| THP-1 | 69 | 48 | 70 | 22 | 0.31 (mod) | 0.05 | ||
| NB-4 | 40 | 1 | 0 | 0 | 0.17 (neg) | 0.04 | ||
| KG-1 | 11 | 26 | 0 | 9 | 0.43 (strong) | 0.33 | ||
Mod indicates moderate; and neg, negative.
Surface MDR1 (MRK16) and intracellular MDR1 (JSB1), MRP1, and LRP expression were measured by indirect immunofluorescence.
Difference values (D; range, 0-1.0) as measured by Kolmogorov-Smirnov (KS) statistics for samples loaded with DiOC2 and allowed to efflux the drug for 90 minutes at 37°C. D value definition for efflux is as follows: weak, 0.20 ≤ D < 0.25; moderate, 0.25 ≤ D < 40; strong, D ≥ 0.40.21
Difference values by KS statistics for efflux in presence versus absence of CsA. D > 0.15 was defined as significant.21
We also investigated expression of 2 other multidrug-resistance proteins implicated in resistance of leukemic cells to cytotoxic drugs, the MRP1 protein and LRP.22,27 Both MRP1 and LRP proteins were expressed at the highest levels in THP-1 cells (70% and 22%, respectively), whereas only low expression of LRP (9%) could be detected in KG-1 cells. Finally, HL-60 and NB-4 cells were negative for both proteins (Table 1). Thus, resistance to GO did not correlate with MRP/LRP expression.
It is known that efflux pump function does not strictly correlate with the expression levels of the different MDR proteins. We have, therefore, also analyzed the pump activity of the different cell lines using the fluorescent dye DiOC2 in the presence or absence of the MDR inhibitor cyclosporin A. As shown in Table 1, the cell lines varied in their capacity to extrude the dye, with KG-1 cells being the most and NB-4 cells the least efficient (D values, 0.43 and 0.17, respectively). HL-60 and THP-1 cells showed a similar and moderate pump activity (D values, 0.28 and 0.31, respectively). Only the efflux of KG-1 cells was significantly inhibited by CsA (Table 1).
These data confirm that MDR protein expression does not correlate with efflux activity and suggest that higher pump activity may explain at least in part the resistance of KG-1 cells to GO but not the lack of apoptosis of THP-1 relative to HL-60 and NB-4 cells.
GO induces apoptosis in HL-60 cells through caspase 3 activation
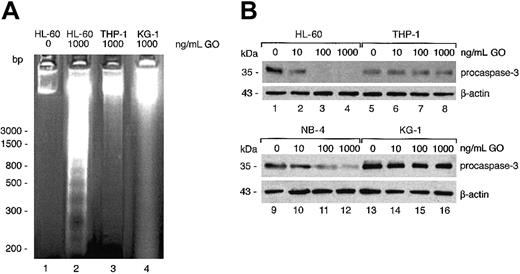
To determine whether GO induces apoptosis rather than necrosis in susceptible cells, a standard DNA ladder assay was performed. A DNA ladder was indeed observed following treatment of HL-60 but not THP-1 or KG-1 cells with up to 1000 ng/mL GO (Figure 5A). A TUNEL assay and FACS analysis confirmed the induction of strong apoptosis in HL-60 cells (up to 45%) but much less so in THP-1 cells (up to 14% at the highest GO concentration) (Table 2).
GO induces apoptosis and caspase 3 activation in sensitive cell lines. (A) The indicated cell lines were cultured in the presence or absence of 1000 ng/mL GO for 24 hours. DNA was then extracted and 1 μg run in a 2% agarose gel (lanes 2-4). As control, 100 ng DNA extracted from untreated HL-60 cells was also analyzed (lane 1). (B) The indicated cell lines were incubated with increasing concentrations of GO for 24 hours. Cytoplasmic extracts (50 μg) were analyzed by Western blotting with a procaspase 3–specific antibody, followed by a β-actin–specific antibody. The data are representative of at least 2 independent experiments. bp indicates base pair.
GO induces apoptosis and caspase 3 activation in sensitive cell lines. (A) The indicated cell lines were cultured in the presence or absence of 1000 ng/mL GO for 24 hours. DNA was then extracted and 1 μg run in a 2% agarose gel (lanes 2-4). As control, 100 ng DNA extracted from untreated HL-60 cells was also analyzed (lane 1). (B) The indicated cell lines were incubated with increasing concentrations of GO for 24 hours. Cytoplasmic extracts (50 μg) were analyzed by Western blotting with a procaspase 3–specific antibody, followed by a β-actin–specific antibody. The data are representative of at least 2 independent experiments. bp indicates base pair.
GO-induced apoptosis
. | % apoptotic cells . | . | |
|---|---|---|---|
| GO ng/mL . | HL-60 . | THP-1 . | |
| 10 | 7 | 2 | |
| 100 | 35 | 11 | |
| 1000 | 45 | 14 | |
. | % apoptotic cells . | . | |
|---|---|---|---|
| GO ng/mL . | HL-60 . | THP-1 . | |
| 10 | 7 | 2 | |
| 100 | 35 | 11 | |
| 1000 | 45 | 14 | |
Apoptosis was measured with the TUNEL assay and FACS analysis.
Because caspase 3 activation is central to several apoptotic pathways,28 this protein was analyzed in the same cell lines. Cells were cultured for 24 hours in the presence or absence of GO, and Western blots were performed. As shown in Figure 5B, the 35-kDa procaspase 3 band completely disappeared in HL-60 cells treated with as little as 100 ng/mL GO, demonstrating protease digestion to caspase 3 in these cells. Similar results were obtained with NB-4 cells. On the contrary, no significant procaspase 3 degradation could be observed in THP-1 or KG-1 cells even after treatment with 1000 ng/mL GO. Analysis of β-actin on the same blot confirmed the presence of equivalent amounts of protein in each lane (Figure 5B).
These data demonstrate that GO induces apoptosis of HL-60 and NB-4 cells through caspase 3 activation and that the other cell lines such as THP-1 are resistant to the proapoptotic activity of GO.
GO induces G2 arrest and Chk1 and Chk2 phosphorylation
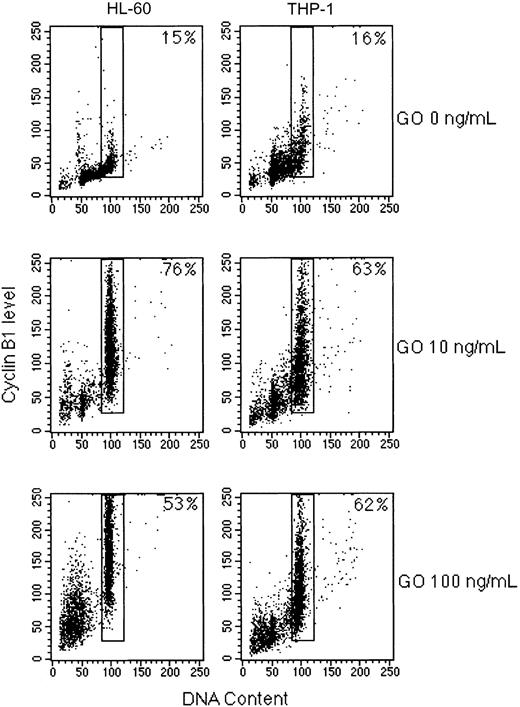
Analysis of DNA content described in “GO induces apoptosis in HL-60 cells through caspase 3 activation” had shown that GO induces arrest in the G2/M phase in susceptible cell lines. To determine whether the block is in G2 or M, we analyzed cyclin B1 expression, because cyclin B1 is induced in the G2 phase of the cell cycle and is degraded in the M phase.29 Cyclin B1 was strongly induced by 10 ng/mL GO (from 15%-16% to 63%-76%) in both HL-60 and THP-1 cells (Figure 6). As expected, cyclin B1–positive cells were those with 4N DNA content, ie, cells in the G2 phase of the cell cycle. These data demonstrate that GO induces a block in the G2 phase in susceptible cell lines.
GO induces cyclin B1 expression in sensitive cell lines. The indicated cell lines were cultured for 48 hours in the presence of 0, 10, or 100 ng/mL GO, fixed, and stained with a FITC-labeled anti–cyclin B1 antibody and propidium iodide. The percentage of cyclin B1–positive G2 cells is indicated in each panel.
GO induces cyclin B1 expression in sensitive cell lines. The indicated cell lines were cultured for 48 hours in the presence of 0, 10, or 100 ng/mL GO, fixed, and stained with a FITC-labeled anti–cyclin B1 antibody and propidium iodide. The percentage of cyclin B1–positive G2 cells is indicated in each panel.
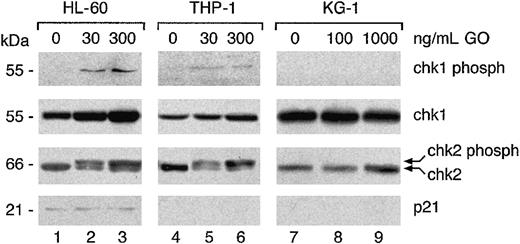
Two different pathways have been implicated in the G2 checkpoint following DNA damage, the Chk1/Chk2 pathway and the p53/p21 pathway.15, 16, 17 Both pathways were, therefore, investigated by Western blotting. As shown in Figure 7, 30 ng/mL GO is sufficient to induce phosphorylation of Chk1 at 24 hours in both HL-60 and THP-1 cells, detected with antibodies specific for the Chk1 phosphorylated on Ser345 (Figure 7). The sensitive NB-4 cells showed Chk1 phosphorylation in response to GO (data not shown). On the contrary, Chk1 phosphorylation could not be detected in the resistant KG-1 cell line, even in the presence of 1000 ng/mL GO (Figure 7). That all cells expressed Chk1 protein was demonstrated by probing the same blot with an anti-Chk1 antibody that detects all forms of the protein (Figure 7). Indeed, all cell lines expressed Chk1 in the presence or absence of GO. Interestingly, total Chk1 protein was induced by GO in HL-60 and THP-1 cells but not in KG-1 cells (Figure 7). This finding is in agreement with the cell cycle data, because chk1 protein expression is known to be up-regulated during the S, G2/M phases of the cell cycle.30,31
Chk1 and Chk2 phosphorylation correlates with G2arrest. The indicated cell lines were cultured for 24 hours in the presence or absence of the indicated concentrations of GO. Cytoplasmic extracts (60 μg) were analyzed by Western blotting using antibodies specific for Chk1 protein, Chk1 phosphorylated on Ser345, Chk2, and p21. The results are representative of at least 2 independent experiments.
Chk1 and Chk2 phosphorylation correlates with G2arrest. The indicated cell lines were cultured for 24 hours in the presence or absence of the indicated concentrations of GO. Cytoplasmic extracts (60 μg) were analyzed by Western blotting using antibodies specific for Chk1 protein, Chk1 phosphorylated on Ser345, Chk2, and p21. The results are representative of at least 2 independent experiments.
Chk2 phosphorylation was also investigated using a Chk2-specific antibody. As shown in Figure 7, GO induced phosphorylation in the HL-60 and THP-1 cell lines already at 30 ng/mL, as shown by the appearance of a second slower migrating band following GO treatment (Figure 7). That this band represented phosphorylated Chk2 was confirmed by probing the same extracts with an antibody specific for Chk2 phosphorylated on Thr68 (data not shown). Chk2 was also phosphorylated by GO in the NB-4 cell line (data not shown). By contrast, GO failed to induce significant phosphorylation of Chk2 in KG-1 cells even at 1000 ng/mL (Figure 7). Western analysis of cell extracts obtained after 48 hours of GO treatment showed that the phosphorylation pattern of Chk1 and Chk2 was identical to that observed at 24 hours (data not shown), demonstrating that this modification was stably induced by GO in all sensitive cell lines.
Expression of p53 was also analyzed following GO treatment. p53 could not be detected in any of the lines, whereas it was present in a positive control run in parallel (data not shown). This finding is in agreement with previous reports.32 Finally, analysis of p21 showed that the protein is detectable in HL-60 cells but not in THP-1 or KG-1 cells, but in any case its expression is not modified by GO treatment (Figure 7).
Altogether, these data suggest that GO induces a G2 block in susceptible cell lines already at low concentrations (10-30 ng/mL) and that this block correlates with Chk1 and Chk2 phosphorylation and up-regulation of cyclin B1 but not with p53 or p21 induction.
Response of primary AML cells
To analyze the response to GO of freshly isolated leukemic cases in vitro, we performed proliferation and viability assays on 10 cases of AML (3 M1, 5 M2, 1 M4, and 1 M5, according to the French-American-British [FAB] classification). In all cases cells were at least 80% positive for CD33 except for patients (pts) 9 and 3 who had 73% and 71% CD33+ cells, respectively (Table 3). MFI for CD33 varied from 41 to 437. All samples were treated with increasing concentrations of GO in the presence of IL-3 and GM-CSF to increase their viability and/or to induce some proliferation in vitro.
CD33 expression and MDR function in leukemic samples
. | . | DiOC2 efflux* . | . | CD33† . | . | ||
|---|---|---|---|---|---|---|---|
| Patient . | Leukemia type (FAB) . | D efflux . | D ± CsA . | % positive cells . | MFI‡ . | ||
| 1 | AML (M1) | 0.57 | 0.17 | 94 | 312 | ||
| 4 | AML (M1) | 0.58 | 0.29 | 93 | 437 | ||
| 8 | AML (M1) | 0.48 | 0.22 | 94 | 63 | ||
| 2 | AML (M2) | 0.58 | 0.13 | 94 | 41 | ||
| 5 | AML (M2) | 0.63 | 0.64 | 80 | 103 | ||
| 6 | AML (M2) | ND | ND | 85 | 80 | ||
| 7 | AML (M2) | 0.56 | 0.31 | 99 | 434 | ||
| 10 | AML (M2) | 0.16 | 0.12 | 89 | 43 | ||
| 3 | AML (M4) | ND | ND | 71 | 100 | ||
| 9 | AML (M5) | 0.84 | 0.71 | 73 | 185 | ||
. | . | DiOC2 efflux* . | . | CD33† . | . | ||
|---|---|---|---|---|---|---|---|
| Patient . | Leukemia type (FAB) . | D efflux . | D ± CsA . | % positive cells . | MFI‡ . | ||
| 1 | AML (M1) | 0.57 | 0.17 | 94 | 312 | ||
| 4 | AML (M1) | 0.58 | 0.29 | 93 | 437 | ||
| 8 | AML (M1) | 0.48 | 0.22 | 94 | 63 | ||
| 2 | AML (M2) | 0.58 | 0.13 | 94 | 41 | ||
| 5 | AML (M2) | 0.63 | 0.64 | 80 | 103 | ||
| 6 | AML (M2) | ND | ND | 85 | 80 | ||
| 7 | AML (M2) | 0.56 | 0.31 | 99 | 434 | ||
| 10 | AML (M2) | 0.16 | 0.12 | 89 | 43 | ||
| 3 | AML (M4) | ND | ND | 71 | 100 | ||
| 9 | AML (M5) | 0.84 | 0.71 | 73 | 185 | ||
ND indicates not done.
DiOC2 efflux activity and the effect of CsA on efflux are expressed as D values as described in Table 1.
Percentage of CD33 was measured by direct immunofluorescence.
MFI indicates mean fluorescence intensity.
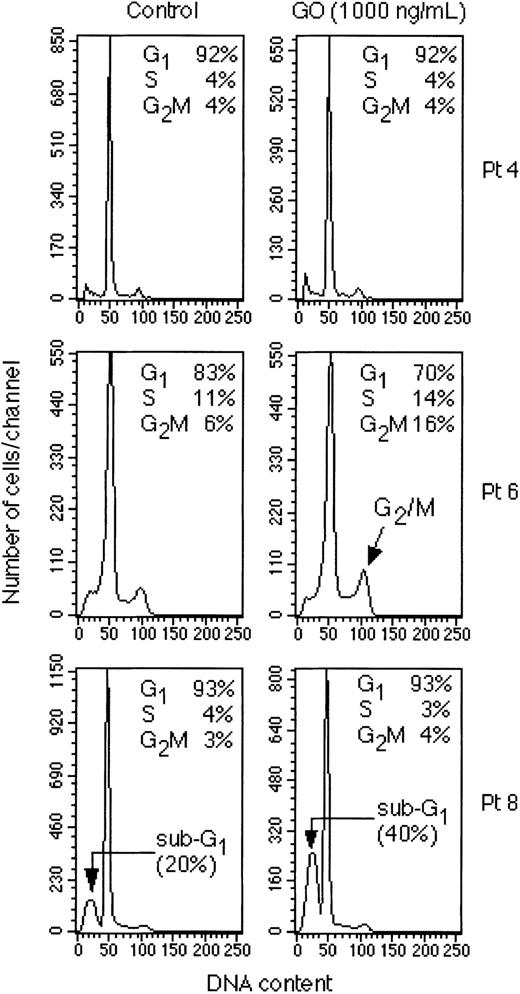
The dose-response curves of thymidine uptake and viability assays of 3 representative cases (Figure 8A-B, respectively) demonstrate that also freshly isolated AML cells show different responses to GO in vitro. For example, patient 4 was resistant to even high concentrations of GO with only a 15% decrease in thymidine uptake and no decrease in cell viability at 1000 ng/mL GO. Patient 8 was among the most sensitive cases with 60% inhibition of thymidine uptake and 40% decrease in viable cells at 1000 ng/mL GO. Finally, patient 6 showed a pattern similar to that of THP-1 cells with a strong inhibition of thymidine uptake (> 65%) with little loss of cell viability (< 10%) (Figure 8A-B), suggesting a block in G2 with little induction of apoptosis. The results of the thymidine uptake and viability assays for the other AML cases tested are shown in Figure 8C-D, although 3 cases were not tested for thymidine uptake because too few cells were available. The results of the other cases confirm that a range of responses is observed. Considering all results, 3 of 9 patients appeared to fall in the resistance category, because these patients showed less than a 25% decrease in either thymidine uptake or viability (pts 4, 7, and 3). Three of 10 patients were likely to be mostly blocked in cell cycle with little apoptosis, because they showed a strong inhibition of thymidine uptake and little decrease in viability (pts 5, 6, and 10). Finally, 3 of 10 patients showed a strong decrease in cell viability in the presence of GO, suggesting induction of apoptosis (pts 1, 2, and 8). One patient (pt 9) could not be categorized, because only the viability assay was performed (Figure 8).
Response of freshly isolated AML cells to GO in vitro Peripheral blood mononuclear cells isolated from patients with AML were cultured in vitro in the presence of IL-3 and GM-CSF and in the presence of increasing concentrations of GO. 3H-thymidine uptake and cell viability were measured at 48 to 60 hours. The results are expressed as percentage of the untreated controls. (A) Dose-response curve of thymidine uptake of patients 4 (•), 6 (□), and 8 (▴). (B) Dose-response curve of cell viability assay of patients 4(•), 6 (□), and 8 (▴). (C) Dose-response curve of thymidine uptake of patients 3, 5, 7, and 10. (D) Dose-response curve of cell viability assay of patients 1, 2, 3, 5, 7, 9, and 10. Pt 3 is represented by ♦; pt5, ⋄; pt2, ▪; pt9, ▵; pt7, ○; pt 1, *; and pt 10, +.
Response of freshly isolated AML cells to GO in vitro Peripheral blood mononuclear cells isolated from patients with AML were cultured in vitro in the presence of IL-3 and GM-CSF and in the presence of increasing concentrations of GO. 3H-thymidine uptake and cell viability were measured at 48 to 60 hours. The results are expressed as percentage of the untreated controls. (A) Dose-response curve of thymidine uptake of patients 4 (•), 6 (□), and 8 (▴). (B) Dose-response curve of cell viability assay of patients 4(•), 6 (□), and 8 (▴). (C) Dose-response curve of thymidine uptake of patients 3, 5, 7, and 10. (D) Dose-response curve of cell viability assay of patients 1, 2, 3, 5, 7, 9, and 10. Pt 3 is represented by ♦; pt5, ⋄; pt2, ▪; pt9, ▵; pt7, ○; pt 1, *; and pt 10, +.
MDR pump function could be measured with DiOC2 in 8 of 10 AML cases, and the results are shown in Table 3. Seven of 8 patients showed high pump activity, with a D value ranging from 0.48 to 0.83. Only one patient (pt 10) showed little activity (D = 0.16). CsA partially inhibited pump activity in 3 cases (pts 4, 7, and 8) and fully inhibited it in 2 cases (pts 5 and 9). There was no clear correlation between GO resistance and pump activity (Table 3).
We next performed cell cycle analysis of the 3 representative cases for which a sufficient number of cells were available. The results confirmed the hypothesis made on the basis of the proliferation and viability assays. Patient 4 did not show any measurable block in G2 or any increase in apoptotic cells after 48 hours of treatment with GO, suggesting strong resistance to the drug (Figure 9). Patient 6 showed a significant increase in cells in the G2/M phases of the cell cycle but no significant increase in sub-G1 cells, suggesting G2 arrest without apoptosis (Figure 9). Finally, patient 8 showed a strong increase in sub-G1 cells without a detectable block in G2/M, confirming that these cells go into apoptosis in response to GO (Figure 9).
Cell cycle analysis of freshly isolated AML cells. Peripheral blood mononuclear cells from the indicated patients were cultured for 48 hours in the presence of IL-3 and GM-CSF and in the presence or absence of 1000 ng/mL GO. Cell cycle analysis was then performed. The percentages of cells in the sub-G1, G0 /G1, S, and G2/M phases of the cell cycle are indicated in each panel.
Cell cycle analysis of freshly isolated AML cells. Peripheral blood mononuclear cells from the indicated patients were cultured for 48 hours in the presence of IL-3 and GM-CSF and in the presence or absence of 1000 ng/mL GO. Cell cycle analysis was then performed. The percentages of cells in the sub-G1, G0 /G1, S, and G2/M phases of the cell cycle are indicated in each panel.
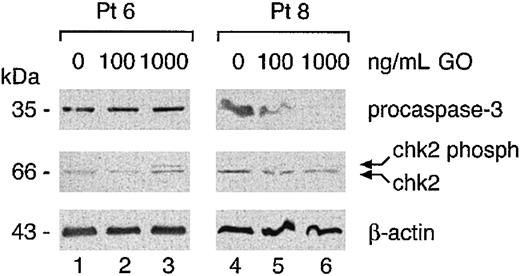
Finally, we determined whether the molecular pathways triggered by GO in cell lines were also operative in patients. Western blot analysis of caspase 3 and Chk2 was performed on lysates from the 2 sensitive patients (pts 6 and 8) treated with 100 to 1000 ng/mL GO. As expected, patient 8, who went into apoptosis in the presence of GO, showed procaspase 3 degradation (Figure 10 lanes 4-6). On the contrary, patient 6, who responded with a block in G2 without apoptosis, showed an increase in Chk2 phosphorylation but no procaspase 3 degradation (Figure 10, lanes 1-3). Analysis of 2 resistant cases showed no Chk2 phosphorylation or procaspase 3 degradation (pts 4 and 7, data not shown).
Chk2 and caspase 3 activation in freshly isolated AML samples. Cells isolated from the indicated patients were cultured for 48 hours with 100 to 1000 ng/mL GO. Cytoplasmic extracts (60 μg) were analyzed in Western blot with the indicated antibodies.
Chk2 and caspase 3 activation in freshly isolated AML samples. Cells isolated from the indicated patients were cultured for 48 hours with 100 to 1000 ng/mL GO. Cytoplasmic extracts (60 μg) were analyzed in Western blot with the indicated antibodies.
We conclude that freshly isolated AML cells may respond to GO either by an arrest in G2 or by undergoing apoptosis or may be resistant to even high doses of this compound. Block in G2 is accompanied by Chk2 phosphorylation and apoptosis by caspase 3 activation.
Discussion
In this report we show that the response of AML cell lines to GO fall into 3 groups: cells that become blocked in the G2 phase of cell cycle but do not go into apoptosis (THP-1), those that are blocked in G2 but rapidly go into apoptosis (HL-60 and NB-4), and, finally, cells resistant to even high doses of GO (KG-1). Furthermore, we show that apoptosis involves caspase 3 activation and that G2 arrest correlates with phosphorylation of Chk1 and Chk2 proteins but not with p53 and p21 induction. Interestingly, a study of 10 samples from patients with AML suggests that a similar pattern of response to GO also occurs in freshly isolated AML cells, including Chk2 and caspase 3 activation.
The data presented show that widely different biologic responses to GO can take place at least in vitro, including resistance. Resistance was not due to lack of binding of GO to the resistant line (KG-1), because it expressed the highest levels of CD33, and GO binding to the cells was demonstrated in competition experiments with FITC-labeled anti-CD33 antibody (clone P67.6; J.G., unpublished observations). Surprisingly, there was an inverse correlation between CD33 expression levels and sensitivity to GO in the cell lines. A significant correlation cannot be made on only 4 cell lines (it may be just casual). In fact, the correlation does not hold when patients' samples are considered, suggesting that no such correlation exists. GO has been shown by others to be internalized following binding to cell lines or freshly isolated cells.14 Although we cannot exclude differences in GO endocytosis between cell lines, the fact that all lines bound to GO efficiently makes this possibility less likely.
One other possible mechanism of resistance is the activity of MDR proteins.26,27,33 Indeed, measurement of pump function using DiOC2 has shown that the KG-1 line has the highest pump activity. Thus, it is possible that the resistance of KG-1 cells to GO is at least in part due to the increased capacity of these cells to extrude calicheamicin. Interestingly, pump activity and its modulation by the MDR1 inhibitor CsA did not correlate with expression of the MDR proteins MDR1/Pgp, MRP1, and LRP in the different cell lines.24,25,34 Indeed, the sensitive THP-1 cell line expressed the highest levels of MDR1, MRP1, as well as LRP proteins, and showed intermediate pump activity, whereas the resistant cell line KG-1 showed low levels of MDR1 and LRP and no MRP1 but highest pump function. These data are in agreement with previous reports showing a lack of correlation between efflux and MDR protein expression in AML cell lines as well as freshly isolated samples.22,35
Although higher pump activity may explain at least in part the resistance of the KG-1 cell line, it does not explain the differential response of THP-1 cells compared with HL-60 or NB-4 cells. All 3 lines respond to GO by undergoing G2 arrest with an IC50 in all cases of 3 to 6 ng/mL. This finding suggests that GO enters into all 3 cell lines with similar efficiency. However, only in the case of HL-60 and NB-4 cells this entrance is followed by apoptosis at doses of 10 to 100 ng/mL. THP-1 cells on the contrary are still resistant to apoptosis even at high GO concentrations (1000 ng/mL). At this high concentration, GO affected growth also of a CD33– cell line, presumably because in these conditions free calicheamicin enters cells through CD33-independent mechanisms. Thus, it is unlikely that the resistance of THP-1 cells to apoptosis is due to lack of entry of the drug but rather to factors intrinsic to the cells. These factors still remain to be identified.
Cell cycle analysis showed that sensitive cell lines were blocked in the G2/M phase of the cell cycle, in agreement with a previous report.33 That the block occurred in G2 was demonstrated here by the increased expression of cyclin B1 in the presence of GO, because cyclin B1 is rapidly degraded during the M phase of the cycle.29 After CD33/GO internalization, calicheamicin is thought to be released from the antibody moiety in endosomes/lysosomes11 and causes DNA double-strand breaks.13 Two major pathways are known to induce G2 arrest after DNA damage. One involves the ataxia-telangiectasia mutated/ataxia-telangiectasia related (ATM/ATR)–Chk1/Chk2 kinases30 and the other the p53/p21 proteins.17,36 We demonstrated here that the former and not the latter pathway is involved in GO-induced cell cycle arrest. Indeed, GO induced Chk1 and Chk2 phosphorylation on Ser345 and Thr68, respectively, and Chk1 and Chk2 phosphorylation correlated with the block in G2. Following DNA damage by a variety of agents, DNA sensors that may include the ATM and ATR kinases become activated.15 These proteins in turn phosphorylate Chk1 and Chk2, and these 2 serine kinases then induce a G2 block by inactivating Cdc25.15,37,38 The respective role of the 2 Chk proteins may depend on cell type and has not been fully defined. To our knowledge, this is the first demonstration of activation of Chk1 and Chk2 by GO. p53 is the other major player in both G2 and G1 arrest following DNA damage, in particular through induction of p21, but lack of expression of p53 and of p21 induction in all cell lines analyzed excluded a role for these proteins in GO-mediated G2 arrest in these cells.
The AML cell lines also showed a differential response to GO in terms of apoptosis. Cell death was associated with caspase 3 activation, which is an executioner caspase involved in both the intrinsic and extrinsic pathways of apoptosis.28 DNA damage can activate apoptosis through several different pathways: ATM/ATR and DNA-dependent protein kinase activate p53 that in turn induces apoptosis through regulation of bcl-2 family proteins.39,40 Because none of the cell lines express p53, alternative mechanisms of apoptosis were clearly induced by GO in HL-60 and NB-4 cells. p53-independent mechanisms of cell death induction following DNA damage are less well understood, although 2 novel pathways have recently been proposed: one suggests that the chk2 kinase phosphorylates the promyelocytic leukemia (PML) protein that in turn induces apoptosis by a yet undefined but p53-independent mechanism.41 The other suggests that DNA damage down-regulates Bcl-XL activity through deamidation,42 leading to apoptosis in cells deficient in p53 and retinoblastoma protein. Thus, it is possible that GO induces one or both of these p53-independent pathways in HL-60 and/or NB-4 cells. However, the resistance of THP-1 cell line to apoptosis may be due to higher expression of antiapoptotic proteins or lower expression of proapoptotic proteins of the bcl2 family, which are known to be crucial players in the regulation of programmed cell death following DNA damage in leukemic and other cell types.28,39,43
The results obtained on 10 freshly isolated AML samples suggested that similar heterogeneity of response occurred, including resistance, G2 arrest, and induction of apoptosis. Furthermore, Western blot analysis showed that Chk2 phosphorylation or caspase 3 activation could be detected in samples showing a block in G2 and apoptosis, respectively. This finding demonstrates that the molecular pathways observed in AML lines are also triggered in at least some primary AML cells. Resistance did not correlate with pump activity, as measured with DiOC2 in 8 of 10 samples, suggesting that factors determining resistance in primary AML are complex and still remain to be fully defined.
The extent of G2 arrest was significantly lower in the patient sample (pt 6) than in the cell lines. This finding is likely due to the low proliferation rate of the freshly isolated leukemic cells relative to the cell lines rather than to heterogeneity of the leukemic cells. Indeed, the observation that 30% of Chk2 became phosphorylated in response to GO suggests that a significant proportion of cells responded to GO.
The AML sample undergoing apoptosis (pt 8) apparently did not show a block in G2, unlike the HL-60 and NB-4 cell lines that underwent both G2 arrest and apoptosis. Two explanations are possible for this difference. The first is that a block in G2 has been missed in the patient sample because cell cycle analysis was performed at 48 hours. Indeed, in the lines, the G2 block was found to precede apoptosis and arrested cells died rapidly (Figure 3). Second, it is possible that some AML cells are sensitive to GO but do not arrest in G2. Indeed, study of the cell lines has allowed us to analyze some, but not necessarily all, pathways activated in response to GO. In particular, it is worth noting that all 4 AML cell lines analyzed here lacked p53, whereas p53 mutations occur in 5% to 15% of AML cases. Because p53 is involved in both G2 and G1 arrest as well as in apoptosis, the presence of p53 may also affect the response to GO in AML cells expressing wild-type p53.
Knowledge of the intracellular signaling pathways induced by GO should allow for the design of novel strategies to improve GO efficacy in vivo.15,17 For example, previous studies suggest that inhibiting the ATM/ATR kinases sensitizes p53-deficient cells to killing by DNA-damaging agents.38,44,45 Such strategies should also be investigated with GO.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-07-2311.
Supported in part by the Italian Ministry for University and Research (project FIRB no. RBAU01J2ER and RBAU01H8SX), the Associazione Italiana Ricerca sul Cancro (AIRC), and the Associazione Paolo Belli-Lotta alla Leucemia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Wyeth-Ayerst Research, Pearl River, NY, for kindly providing gemtuzumab ozogamicin as well as the control hP67.6 antibody; Dr M. Mecchia and Dr G. Riggi, Oncology Medical Department, Wyeth Italy, for their support; Dr M. Broggini, Istituto Mario Negri, Italy, for providing us with several antibodies; and Gian-Maria Borleri for technical help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal