Abstract
Human somatic cells have a limited life span in vitro. Upon aging and with each cell division, shortening of telomeres occurs, which eventually will lead to cell cycle arrest. Ectopic hTERT expression has been shown to extend the life span of human T cells by preventing this telomere erosion. In the present study, we have shown that ectopic hTERT expression extends the life span of CD4+ T helper type 1 or 2 and regulatory T-cell clones and affected neither the in vitro cytokine production profile nor their specificity for antigen. In mixed cell cultures, ectopic hTERT-expressing clones were found to expand in greater numbers than untransduced cells of the same replicative age. This ectopic hTERT-induced growth advantage was not due to an enhanced cell division rate or number of divisions following T-cell receptor–mediated activation, as determined in carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeling experiments. Moreover, the susceptibility to activation-induced cell death of both cell types was similar. However, cultures of resting hTERT-transduced T cells contained higher frequencies of Bcl-2–expressing cells and lower active caspase-3–expressing cells, compared with wild-type cells. Furthermore, hTERT-transduced cells were more resistant to oxidative stress, which causes preferential DNA damage in telomeres. Taken together, these results show that ectopic hTERT expression not only protects proliferating T cells from replicative senescence but also confers resistance to apoptosis induced by oxidative stress.
Introduction
Human T lymphocytes display a limited life span when cultured in vitro.1 Once antigen-specific T-cell clones have been established, they can usually not be expanded beyond another 20 population doublings (PDs).2,3 Moreover, subcloning of human T lymphocytes is highly inefficient, and subclones that are derived have little replicative potential. This limited life span of human T cells is a drawback for the application of T cells as a source of effector cells in therapeutic settings.
Human somatic cells can proliferate until a critical point is reached, at which the cells stop dividing and become senescent. Progressive telomere shortening upon proliferation limits the life span of somatic cells.4,5 This telomere erosion can be prevented in human cells by expression of the catalytic subunit telomerase reverse transcriptase (hTERT) of the telomerase complex, which elongates short telomeres and thereby prevents replicative senescence.6,7 In proliferating cells, telomere shortening occurs at each cell division due to incomplete chromosomal replication. Furthermore, telomere shortening may also occur in nonproliferating cells by DNA damage in telomeres that is induced by radiation or oxidative stress. Oxidative stress is the formation of hydroxyl radicals in cells, which causes DNA damage by activating S1 nucleases acting at cleavage sites that are frequently found in telomeric DNA sequences.8,9 In hTERT-transduced fibroblasts, it has been shown that ectopic hTERT expression resulted in a decreased susceptibility of the cells to oxidative stress by enhancing DNA repair at the telomeres.10 It is, however, unknown whether hTERT confers enhanced resistance to oxidative stress in human T cells.
Human T cells express hTERT endogenously upon activation. We have shown recently that inhibition of endogenous telomerase activity by expressing a dominant-negative mutant of the hTERT gene shortens the life span of both human CD8+ and CD4+ T cells, which was associated with cytogenetic abnormalities, indicating that endogenous hTERT contributes to the replicative life span of CD4+ and CD8+ T cells (A. Röth, H.Y., J.P., E.A. Chavez, M. Schertzer, P.M. Lansdorp, H.S., R.M.L., manuscript submitted, March 2003). Thus, endogenous telomerase activity is essential in preserving cytogenetic stability and telomere function in human T cells. Endogenous hTERT, however, does not prevent telomere end loss in the T cells,11,12 and aged T cells show a decreased average telomere length, compared with T cells from young donors.13, 14, 15, 16
Changes in level of hTERT expression upon activation in aging T cells may be one of the reasons for the inability of endogenous telomerase to prevent progressive telomere erosion in T cells, since we found that, in contrast to young cells, long-term cultured T cells do not express endogenous hTERT upon activation (Röth et al, manuscript submitted). The magnitude of hTERT expression that is induced upon activation may decrease during aging of T cells, resulting in progressive telomere erosion toward the end of their life span. Ectopic hTERT expression provides a continuously high level of telomerase activity that overcomes the decrease in endogenous hTERT expression in human T cells during aging and was found to extend the life span of human CD8+ T cells without loss of functionality.17
In the present study, we show that ectopic hTERT expression extends the life span of a series of human CD4+ Th1, Th2, or regulatory T-cell clones without loss of function. Intriguingly, both the hTERT-transduced CD8+ T cells characterized in our previous study and the CD4+ T-cell clones studied here showed a greater expansion in cell numbers than control-transduced cells of the same replicative age. The results described here indicate that ectopic hTERT expression extends the replicative capacity of human T cells upon activation and prolongs survival by protecting against oxidative stress–induced DNA damage and apoptosis.
Materials and methods
Retroviral constructs
The hTERT retroviral construct hTERT-GFP was generated by insertion of hTERT cDNA into the polylinker of LZRS linker–internal ribosomal entry site (IRES)–green fluorescent protein (GFP), as described.17 Transduced cells with this retrovirus can be detected in bulk cultures by the expression of GFP. Amphotropic retrovirus was made by transfection of the retroviral construct into ΦNX-A cells.18 The hTERT construct, used by Migliaccio et al (hTERT-puro19 ) and kindly provided by Dr P. Romero (Ludwig Institute for Cancer Research, Lausanne, Switzerland), consists of the hTERT cDNA sequence inserted into the mouse stem cell virus (MSCV) pac vector.20 Retrovirus made with this construct contains the puromycin resistance gene for selection of transduced cells.
Human T-cell clones
The following T-cell clones were used in this study: The CD4+ Th1 clones CBXI-2.12 and NAD.E178; the Th1/Th0-like clones MoT-72, MoT-81, MoT-98; the Th2 clones PEN J6, PEN J50, NP-12, NP-44, BOY JF157; and the regulatory T-cell clones BOY JF161 and BOY JF180. The peripheral blood–derived T-cell clones MoT-72, MoT-81, and MoT-98 were specific for the tetanus toxoid–derived peptides 941-960 and p16-35, respectively. The T-cell clones NAD.E178, BOY JF157, BOY JF161, and BOY JF180 were obtained from limiting dilution cultures of CD4+ T cells isolated from a skin biopsy after cutaneous sensibilization and challenge with the hapten 2,4-dinitrochlorobenzene (DNCB).21 Clones PEN J6, PEN J50 and NP-12, NP-4422 were obtained from a skin biopsy or from peripheral blood lymphocytes, respectively, of patients allergic to the house dust mite allergen Dermatophagoides pteronysissinus. Clone CBXI-2.12 was isolated from cord blood and was cultured under Th1-polarizing conditions.23 The CD8+ T-cell clone AKR103 recognizes the melanoma antigen recognized by T cells-1 (MART-1) peptide 26-35 presented by HLA-A2 molecules. Clone AKR103 was obtained by stimulating CD8+ T cells from a melanoma patient with the autologous tumor cells expressing the B7 molecule and subsequent cloning by limiting dilution.
Transduction and T-cell culture
Two days prior to transduction, 3 × 105 T cells were stimulated with 106 irradiated (40 Gy) allogeneic PBMC, 105 irradiated (80 Gy) cells of EBV-transformed B cell line JY in the presence of 20 U/mL recombinant human interleukin-2 (rhIL-2) (Proleukin, Chiron, Amsterdam, The Netherlands) and 100 ng/mL PHA (HA16, Murex, Dartford, United Kingdom). T cells were transduced with retroviral supernatant using 24 well plates coated with fibronectin as described24 and cultured by weekly or biweekly stimulation with irradiated peripheral blood mononuclear cells (PBMCs), irradiated JY cells, phytohemagglutinin (PHA), and rhIL-2 in Yssels medium, supplemented with 1% human serum, as described.25 Cells transduced with hTERT-puro retrovirus were selected for hTERT expression by culturing in 1 μg/mL puromycin for a week. All cultures were maintained under equal conditions, and the number of population doublings was calculated 7 days after stimulation. The increase in percentage of transduced cells in cultures transduced with TERT-GFP was measured weekly by flow cytometry.
Cytokine production
Cells were activated with plate-bound anti-CD3 mAb SPV-T3b26 and anti-CD28 mAb B-T3 (a kind gift of Dr John Wijdenes, Diaclone, Besançon, France) for 24 hours, and the production of IL-4, IL-5, IL-10, and interferonγ (IFNγ) was determined by cytokine-specific enzyme-linked immunosorbent assay (ELISA) in culture supernatants, as described.21 Assays were performed in triplicate. Antigen-specific production of IFNγ by T-cell clones MoT-81 and MoT-98, capable of autopresentation of peptides, was carried out using tetanus toxin–derived peptide 16-35, or p941-960 at concentrations ranging from 5 to 50 ng/mL, in the absence of antigen-presenting cells.
TRAP assay
Telomerase activity was determined in cell lysates of hTERT-transduced and untransduced T cells, using the telomerase repeat amplification protocol (TRAP) assay, according to the manufacturer's protocol (TRAPeze Telomerase detection kit, Intergen, Oxford, United Kingdom). Lysates obtained from 105 cells were tested undiluted and in 5- and 25-fold dilutions. Control samples, using undiluted lysate, were heat inactivated at 85°C for 10 minutes. Amplified substrate bands were visualized on a 15% polyacrylamide (PAA) gel stained with CYBR green (Molecular Probes, Leiden, The Netherlands).
Cell division analysis
To analyze the number of cell divisions, 2 × 106 cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) as described.27 CFSE-labeled T cells were stimulated with the feeder cell mixture (irradiated allogeneic PBMCs and JY cells) and PHA or cultured without stimulation in the presence of rhIL-2. The CFSE content of the T cells in combination with a staining with allophycocyanine (APC)–conjugated anti-CD4 antibody (DAKO Diagnostics, Glostrup, Denmark) or APC-conjugated anti-CD8 antibody (Caltag Laboratories, Burlingame, CA) was analyzed by flow cytometry at various time points between day 2 and day 15 after stimulation.
Induction of apoptosis
Cells that were cultured in rhIL-2 for 2 weeks after the last stimulation were washed and depleted of dead cells on a Ficoll Hypaque (Pharmacia, Uppsala, Sweden) gradient. For the induction apoptosis by hydroxyl radicals, cells were incubated with H2O2 at concentrations ranging from 0.25 to 1 mM in medium containing 20 U/mL rhIL-2 and 0.05 mM FeSO4. After 5 hours, 24 hours, or 48 hours of incubation, cells were analyzed for the presence of apoptotic cells. Treatment with dexamethasone (Sigma, Zwijndrecht, The Netherlands) was performed at 10–6 M concentration and incubated for 24 hours or 48 hours. For analysis of activation-induced cell death (AICD), 5 × 105 T cells were stimulated with 1.25 μL anti-CD3 and anti-CD28 mAb-coated beads (Dynabeads CD3/CD28 T-cell expander, Dynal, Oslo, Norway) and 20 U/mL rhIL-2.
Detection of apoptotic cells by flow cytometry
T cells were washed and resuspended in annexin V binding buffer and incubated with phycoerythrin (PE)–conjugated annexin V (BD Pharmingen, Heidelberg, Germany) and 7-AAD (VIA-PROBE, BD Pharmingen) for 20 minutes in the dark. Cells were diluted 4-fold with binding buffer and analyzed on a FACScalibur (Becton Dickinson, San Jose, CA). Expression of Bcl-2 or active caspase-3 expression was detected by intracellular staining: cells were fixed and permeabilized in Cytofix/Cytoperm solution, according to the manufacturer's protocol, washed, and incubated with fluorescein isothiocyanate (FITC)–conjugated anti–Bcl-2 antibody (DAKO Diagnostics) and PE-conjugated antiactive caspase-3 antibody (BD Pharmingen).
Results
hTERT expression extends the life span of human CD4+ helper or regulatory T-cell clones
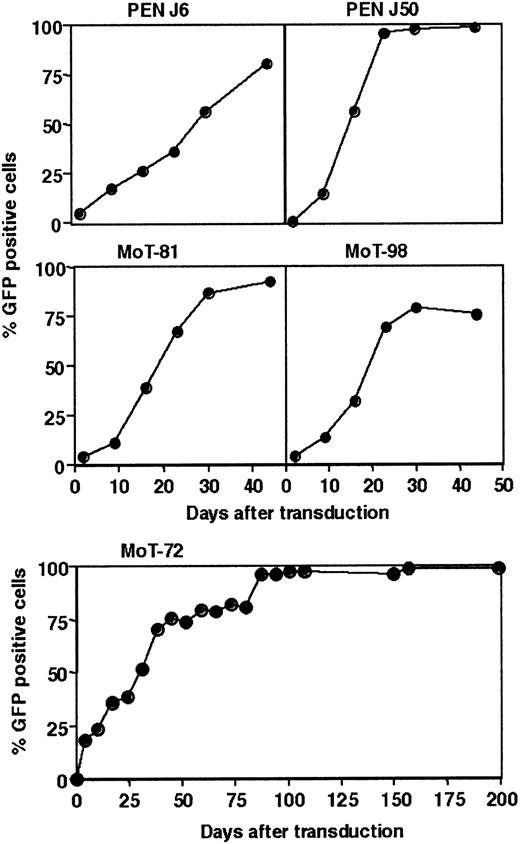
To investigate the capacity of hTERT to extend the life span of human CD4+ T cells, 12 human CD4+ T-cell clones, representing functionally different T-lymphocyte subpopulations,21, 22, 23 were transduced with a hTERT-IRES-GFP gene–containing retrovirus (hTERT-GFP, Table 1). Ectopic expression of hTERT resulted in an increased telomerase activity in hTERT-transduced cells, compared with untransduced cells in TRAP assays (data not shown). In all 12 T-cell clones, the percentage of hTERT-GFP–positive cells increased over time during the culture, resulting eventually in the disappearance of untransduced cells from the hTERT-GFP transduced cultures, which is shown for 5 clones in Figure 1. This accumulation of hTERT-transduced cells in mixed cultures of hTERT-transduced and untransduced cells indicates that ectopic hTERT expression confers an expansion advantage, compared with the untransduced cells.
Life span extension of CD4+ T-cell clones by hTERT transduction
CD4+ T-cell clone . | Subpopulation . | Replicative age,* PD . | Life span,† PD . | No. of experiments . |
|---|---|---|---|---|
| MoT-72 | Th1/Th0 | 30 | 42 | 3‡ |
| MoT-72 | Th1/Th0 | 34 | 42 | 2‡ |
| MoT-81 | Th1/Th0 | 31 | 43 | 2‡ |
| MoT-89 | Th1/Th0 | 28 | 38 | 1 |
| CBXL-2.12 | Th1 | 36 | NE | 1 |
| NAD.E178 | Th1 | 36 | NE | 1 |
| PEN J6 | Th2 | 41 | 56 | 1 |
| PEN J50 | Th2 | 42 | 55 | 1 |
| NP-12 | Th2 | 46 | 47 | 1 |
| NP-44 | Th2 | 34 | 36 | 1 |
| BOY JF157 | Th2 | 36 | NE | 2‡ |
| BOY JF161 | Tr | 36 | NE | 1 |
| BOY JF180 | Tr | 36 | NE | 1 |
CD4+ T-cell clone . | Subpopulation . | Replicative age,* PD . | Life span,† PD . | No. of experiments . |
|---|---|---|---|---|
| MoT-72 | Th1/Th0 | 30 | 42 | 3‡ |
| MoT-72 | Th1/Th0 | 34 | 42 | 2‡ |
| MoT-81 | Th1/Th0 | 31 | 43 | 2‡ |
| MoT-89 | Th1/Th0 | 28 | 38 | 1 |
| CBXL-2.12 | Th1 | 36 | NE | 1 |
| NAD.E178 | Th1 | 36 | NE | 1 |
| PEN J6 | Th2 | 41 | 56 | 1 |
| PEN J50 | Th2 | 42 | 55 | 1 |
| NP-12 | Th2 | 46 | 47 | 1 |
| NP-44 | Th2 | 34 | 36 | 1 |
| BOY JF157 | Th2 | 36 | NE | 2‡ |
| BOY JF161 | Tr | 36 | NE | 1 |
| BOY JF180 | Tr | 36 | NE | 1 |
NE indicates not exactly determined in PD.
Replicative age in population doublings (PD) of the T cells at the time of hTERT transduction. The T cells had undergone approximately 20 PDs during cloning to establish a clonal population.
Life span of the untransduced clones in PD.
Life span extension by hTERT was successful in all experiments.
Ectopic expression of hTERT in human CD4+T-cell clones. Twelve CD4+ T-cell clones were transduced with hTERT-GFP, and the percentage of hTERT-GFP–expressing cells was monitored weekly by flow cytometry. Curves show the accumulation of hTERT-GFP–positive cells in the hTERT-transduced T-cell cultures of clones PEN J6, PEN J50, MoT-81, MoT-98, and MoT-72. Similar results were obtained upon hTERT transduction of the other clones mentioned in Table 1.
Ectopic expression of hTERT in human CD4+T-cell clones. Twelve CD4+ T-cell clones were transduced with hTERT-GFP, and the percentage of hTERT-GFP–expressing cells was monitored weekly by flow cytometry. Curves show the accumulation of hTERT-GFP–positive cells in the hTERT-transduced T-cell cultures of clones PEN J6, PEN J50, MoT-81, MoT-98, and MoT-72. Similar results were obtained upon hTERT transduction of the other clones mentioned in Table 1.
All ectopic hTERT-expressing T-cell clones and their nontransduced parental counterparts were cultured in parallel to directly compare their growth rate and life span. Of the 12 CD4+ T-cell clones, 10 were stably growing after ectopic hTERT expression and could be cultured far beyond the life span of the untransduced cells. In Figure 2 a representative example of the cumulative number of population doublings (PD) in time of clone MoT-72 is shown. The hTERT-transduced MoT-72 cells were stably growing for more than 450 days (Figure 2) and continued to grow without any decrease in proliferative capacity, whereas control GFP-transduced MoT-72 cells or untransduced MoT-72 ceased proliferation after approximately 39 or 42 PD, respectively, leading to death of the culture after approximately 100 days (Figure 2). hTERT transduction extended the life span of MoT-72, MoT-81, and BOY JF157 cells in multiple experiments at different ages ranging from 30 to 36 PD and successfully extended the life span of 8 other T-cell clones in single experiments (Table 1). hTERT transduction did not prolong the life span of Th2 clones NP-12 and NP-44. The reason for this is presently unknown. The results show that ectopic hTERT expression greatly extended the life span of human T-cell clones beyond more than 2 times the life span of untransduced cells.
Long-term growth of hTERT-transduced CD4+T cells compared with untransduced cells. The 12 hTERT-transduced clones were cultured in parallel with the untransduced clones, and the life span extension by hTERT was determined by the growth of the cultures far beyond the life span of the untransduced cells. The graph shows a representative example of the cumulative number of population doublings of hTERT-GFP–transduced CD4+ T-cell clone MoT-72 (□) and control GFP-transduced MoT-72 cells (•) during long-term culture. Transduction of clone MoT-72 was performed at the replicative age of 30 PDs (Table 1), and the graph shows the cumulative number of PDs of the cells during subsequent culture. At day 129 a new culture of the untransduced clone MoT-72 (▵) was started and cultured in parallel. Both GFP-transduced and untransduced T-cell cultures died after 100 days of culture, during which the cultures had reached approximately 39 or 42 PDs, respectively, whereas the hTERT-transduced cells continued to grow to more than 105 PDs. The graph is representative of 3 independent experiments performed with MoT-72 cells at approximately 30 PDs. Similar results were obtained in experiments of MoT-72 cells at approximately 34 PDs and in experiments of the 9 other T-cell clones, summarized in Table 1. † indicates death of the culture.
Long-term growth of hTERT-transduced CD4+T cells compared with untransduced cells. The 12 hTERT-transduced clones were cultured in parallel with the untransduced clones, and the life span extension by hTERT was determined by the growth of the cultures far beyond the life span of the untransduced cells. The graph shows a representative example of the cumulative number of population doublings of hTERT-GFP–transduced CD4+ T-cell clone MoT-72 (□) and control GFP-transduced MoT-72 cells (•) during long-term culture. Transduction of clone MoT-72 was performed at the replicative age of 30 PDs (Table 1), and the graph shows the cumulative number of PDs of the cells during subsequent culture. At day 129 a new culture of the untransduced clone MoT-72 (▵) was started and cultured in parallel. Both GFP-transduced and untransduced T-cell cultures died after 100 days of culture, during which the cultures had reached approximately 39 or 42 PDs, respectively, whereas the hTERT-transduced cells continued to grow to more than 105 PDs. The graph is representative of 3 independent experiments performed with MoT-72 cells at approximately 30 PDs. Similar results were obtained in experiments of MoT-72 cells at approximately 34 PDs and in experiments of the 9 other T-cell clones, summarized in Table 1. † indicates death of the culture.
Life span extension of CD4+ helper or regulatory T-cell clones by hTERT does not alter antigen specificity or phenotype
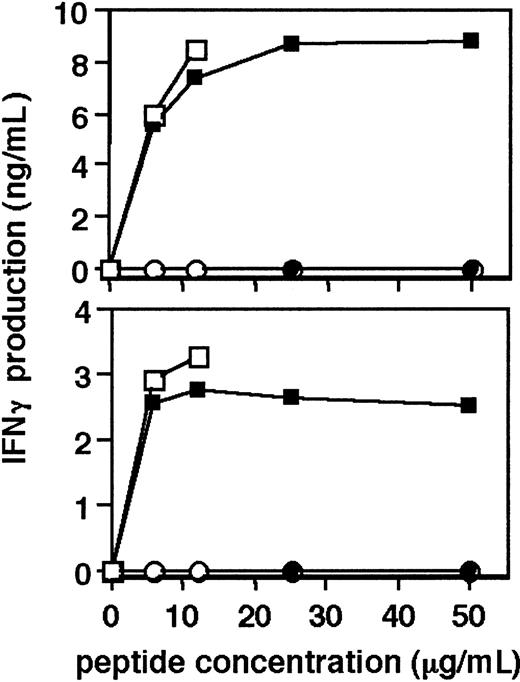
Since long-term culture of human T cells bears the risk of selection of an altered phenotype of the cells and loss of antigen specificity, we tested the specific antigen reactivity of hTERT-transduced CD4+ T-cell clones. The recognition of specific tetanus toxoid peptide 16-35 by untransduced cells and hTERT-transduced cells of T-cell clones MoT-81 and MoT-98, which was determined by the level of IFNγ production, is shown in Figure 3. No production of IFNγ by the cells stimulated with irrelevant tetanus peptide 941-960 was observed, whereas both hTERT-transduced cells and their untransduced counterparts produced IFNγ following stimulation with the 16-35 peptide, indicating that hTERT transduction and subsequent long-term culture had not affected the antigen specificity.
Antigen-specific activation of hTERT-transduced CD4+T cells. Top graph shows the production of IFNγ by hTERT-GFP–transduced (□) and untransduced CD4+ T-cell clone MoT-81 (▪), stimulated for 24 hours with tetanus toxoid peptide 16-35 or control tetanus toxoid peptide 941-960 (○ and •, respectively) at the indicated concentrations. Bottom graph shows the stimulation of hTERT-GFP transduced (□) and untransduced CD4+ T-cell clone MoT-98 (▪) with tetanus toxoid peptide 16-35 or control tetanus toxoid peptide 941-960 (○ and •, respectively). ELISA assays were performed on supernatants of triplicate cultures. Graphs show a representative example of 2 experiments.
Antigen-specific activation of hTERT-transduced CD4+T cells. Top graph shows the production of IFNγ by hTERT-GFP–transduced (□) and untransduced CD4+ T-cell clone MoT-81 (▪), stimulated for 24 hours with tetanus toxoid peptide 16-35 or control tetanus toxoid peptide 941-960 (○ and •, respectively) at the indicated concentrations. Bottom graph shows the stimulation of hTERT-GFP transduced (□) and untransduced CD4+ T-cell clone MoT-98 (▪) with tetanus toxoid peptide 16-35 or control tetanus toxoid peptide 941-960 (○ and •, respectively). ELISA assays were performed on supernatants of triplicate cultures. Graphs show a representative example of 2 experiments.
Untransduced cells and hTERT-transduced cells of different CD4+ T-cell subpopulations were compared for the cytokine profile that is produced upon activation. In Th1 clones NAD.E178 and CBXI-2.12, no differences in cytokine production were found between hTERT-transduced and untransduced cells (Table 2). The cytokine profile of the regulatory T-cell clone BOY JF161, producing IL-5, IL-10, IFNγ, but no IL-4, remained unaltered after ectopic hTERT expression. In Th2 clone BOYJF 157, levels of cytokine production varied during the culture in both untransduced and hTERT-transduced cultures. The data are therefore presented as a range of cytokine levels during the culture. hTERT-transduced cells of Th2 clone BOYJF 157 predominantly produced IL-4, IL-5, and IL-10, which was comparable to the production profile of the untransduced cells. Acquisition of IFNγ production by Th2 polarized T cells was occasionally observed in both untransduced and hTERT-transduced cultures and may be due to the culture conditions.21 Taken together, these results indicate that expression of hTERT extends the life span of CD4+ T cells without altering the phenotype and cytokine production profile of these cells.
hTERT-GFP transduction does not alter the cytokine production profile of CD4+ T-cell clones
T-cell clones . | Subpopulation . | IL-4 . | IL-5 . | IL-10 . | IFNγ . |
|---|---|---|---|---|---|
| NAD.E178 | Th1 | <0.05* | <0.05 | <0.05 | 9.7 |
| NAD.E178TERT | Th1 | <0.05 | <0.05 | <0.05 | 6.5 |
| CBXI-2. 12 | Th1 | 0.1 | <0.05 | 1.2 | 8.9 |
| CBXI-2. 12TERT | Th1 | <0.05 | <0.05 | 0.8 | 6.1 |
| BOY JF157 | Th2 | 1-11† | 76-105 | 0.3-9.7 | 0.05-0.1 |
| BOY JF157TERT | Th2 | 7-30 | 51-363 | 1-6.7 | 0.7-2.7 |
| BOY JF161 | Tr | 0.02 | 88 | 3.5 | 166 |
| BOY JF161TERT | Tr | 0.15 | 81 | 0.04 | 156 |
T-cell clones . | Subpopulation . | IL-4 . | IL-5 . | IL-10 . | IFNγ . |
|---|---|---|---|---|---|
| NAD.E178 | Th1 | <0.05* | <0.05 | <0.05 | 9.7 |
| NAD.E178TERT | Th1 | <0.05 | <0.05 | <0.05 | 6.5 |
| CBXI-2. 12 | Th1 | 0.1 | <0.05 | 1.2 | 8.9 |
| CBXI-2. 12TERT | Th1 | <0.05 | <0.05 | 0.8 | 6.1 |
| BOY JF157 | Th2 | 1-11† | 76-105 | 0.3-9.7 | 0.05-0.1 |
| BOY JF157TERT | Th2 | 7-30 | 51-363 | 1-6.7 | 0.7-2.7 |
| BOY JF161 | Tr | 0.02 | 88 | 3.5 | 166 |
| BOY JF161TERT | Tr | 0.15 | 81 | 0.04 | 156 |
CD4+ T-cell clones derived from the different subpopulations Th0, Th1, Th2, and Tr were transduced with hTERT-GFP, and the production of cytokines, compared with untransduced cells, upon stimulation with anti-CD3 and anti-CD28 antibodies was determined by ELISA.
Average cytokine production (ng per mL) as measured in triplicate by ELISA.
Ranges of values indicate the range of average cytokine production (ng per mL) detected in 3 different experiments during the culture.
Ectopic hTERT expression enhances neither the proliferation of T cells in response to stimulation nor their susceptibility to activation-induced cell death
In mixed cultures of hTERT-transduced and untransduced T cells, we observed that hTERT-GFP–transduced cells gradually increased over time, eventually overgrowing the untransduced cells. Apparently, the hTERT-transduced cells were already growing or surviving better than the untransduced cells in the culture, prior to the effect of aging of the untransduced cells. This suggests that ectopic hTERT expression not only prevents aging of T cells but may also confer a growth or survival advantage immediately following transduction. We analyzed a possible growth advantage as a result of hTERT transduction using cell cycle analysis and CFSE labeling of the cells. For these experiments, we transduced the T cells with a retroviral construct encoding hTERT under the control of the MSCV promoter, combined with the puromycin resistance gene for selection of transduced cells (hTERT-puro19 ), instead of the GFP gene as a marker. In these transduction experiments, the hTERT-puro and the hTERT-GFP constructs were equally able to extend the life span of human CD4+ and CD8+ T-cell clones (data not shown).
Cell cycle analysis indicated that the percentage of cycling cells 2 days after TCR-mediated activation was comparable between hTERT-transduced T cells and untransduced T cells, which was observed in both the CD4+ T-cell clone MoT-72 as well as in the CD8+ T-cell clone AKR103 (data not shown). To determine whether hTERT-transduced cells divided more frequently than their untransduced counterparts, the cells were labeled with CFSE at biweekly intervals during the culture, and the number of cell divisions upon stimulation was analyzed by flow cytometry. As is shown in Figure 4A (left panel), 66% of the MoT-72 CD4+ T cells were activated upon stimulation with allogeneic PBMC and PHA and showed a mean decrease in CFSE content from 901 to 83 fluorescent units, which can be calculated to represent an average of 3.4 cell divisions. Also in the hTERT-transduced MoT-72 cell culture, 66% of the cells were activated, having undergone an average of 3.5 cell divisions, indicating that the hTERT-transduced cells proliferated at an equal rate to proliferation of untransduced cells and that hTERT expression did not enhance the proliferative response of human T cells to stimulation. At various time points during the proliferative period of the untransduced cells, the proliferative response of hTERT-transduced cells and untransduced cells to stimulation remained equal. Likewise, ectopic hTERT expression did not enhance proliferation of the CD8+ T-cell clone AKR103 (Figure 4A, right panel).
hTERT-transduced CD4 or CD8+T cells grow equally to untransduced cells. (A) Cultures of untransduced or hTERT–puro-transduced cells of CD4+ T-cell clone MoT-72 or CD8+ T-cell clone AKR103 were labeled with CFSE and stimulated with feeder cells and PHA. The fraction of dividing cells and the number of cell divisions in each culture was calculated from the mean decrease in CFSE fluorescence intensity at day 8 (MoT-72) or at day 7 (AKR103). MoT-72: 64% proliferating cells with a mean decrease in CFSE fluorescence from 586 (unstimulated cells) to 50, which corresponds to an average of 3.5 cell divisions. MoT-72-hTERT: 63% proliferating cells with a mean decrease in CFSE from 569 to 77, corresponding to 2.9 cell divisions. AKR103: 98% proliferating cells with a mean decrease in CFSE from 610 to 23, which represents 4.7 cell divisions. AKR103-hTERT: 91% proliferating cells with a mean decrease in CFSE from 459 to 21 (= 4.5 cell divisions). The proliferation of the T cells upon stimulation was identical between hTERT-transduced and untransduced cells at 3 different time points measured at biweekly intervals during the culture. The graphs are representative of the 3 independent experiments. (B) Activation-induced cell death. MoT-72 cells and hTERT–puro-transduced MoT-72 cells were stimulated with anti-CD4– and anti-CD28–coated beads and IL-2 (bottom panels) or cultured in IL-2 alone (top panels). The presence of apoptotic cells detected by annexin V binding was analyzed by flow cytometry on day 2. Numbers in the graphs indicate the percentage of annexin V binding cells (7-AAD–positive cells were excluded).
hTERT-transduced CD4 or CD8+T cells grow equally to untransduced cells. (A) Cultures of untransduced or hTERT–puro-transduced cells of CD4+ T-cell clone MoT-72 or CD8+ T-cell clone AKR103 were labeled with CFSE and stimulated with feeder cells and PHA. The fraction of dividing cells and the number of cell divisions in each culture was calculated from the mean decrease in CFSE fluorescence intensity at day 8 (MoT-72) or at day 7 (AKR103). MoT-72: 64% proliferating cells with a mean decrease in CFSE fluorescence from 586 (unstimulated cells) to 50, which corresponds to an average of 3.5 cell divisions. MoT-72-hTERT: 63% proliferating cells with a mean decrease in CFSE from 569 to 77, corresponding to 2.9 cell divisions. AKR103: 98% proliferating cells with a mean decrease in CFSE from 610 to 23, which represents 4.7 cell divisions. AKR103-hTERT: 91% proliferating cells with a mean decrease in CFSE from 459 to 21 (= 4.5 cell divisions). The proliferation of the T cells upon stimulation was identical between hTERT-transduced and untransduced cells at 3 different time points measured at biweekly intervals during the culture. The graphs are representative of the 3 independent experiments. (B) Activation-induced cell death. MoT-72 cells and hTERT–puro-transduced MoT-72 cells were stimulated with anti-CD4– and anti-CD28–coated beads and IL-2 (bottom panels) or cultured in IL-2 alone (top panels). The presence of apoptotic cells detected by annexin V binding was analyzed by flow cytometry on day 2. Numbers in the graphs indicate the percentage of annexin V binding cells (7-AAD–positive cells were excluded).
Interestingly, the yield of both hTERT-transduced and untransduced T-cell cultures was lower than what was to be expected, based on the number of cell divisions, suggesting the occurrence of an elevated degree of cell death during activation and proliferation. We determined the level of AICD by the presence of annexin V binding cells at day 2 after activation. As is shown in Figure 4B, more than 40% cell death was seen in both hTERT-transduced cells and untransduced cells 2 days after activation, which indicates that AICD occurred in hTERT-transduced cells to the same extent as in their untransduced counterparts. These results suggest that ectopic hTERT expression did not change the susceptibility of human T cells to AICD.
hTERT-transduced CD8+ or CD4+ T cells are more resistant to cell death in the absence of antigenic stimulation
The accumulation of hTERT-transduced cells in mixed cultures of transduced and untransduced cells might result from an enhanced survival of hTERT-transduced T cells independently of proliferation, thereby increasing the pool of these cells in culture. During culture, cells may die from apoptosis that is caused by DNA damage by DNA damaging agents or oxidative stress. The effect of ectopic hTERT expression on the susceptibility to these influences can best be determined in a culture of nonproliferating cells. To this end, we investigated the difference in survival between hTERT-transduced and untransduced T cells in a resting state, when cultured without stimulation for 100 days in medium containing rhIL-2 (Figure 5). Proliferation of the T-cell clones had ceased 7 to 11 days after the last stimulation, and the cells did not divide when cultured further in medium with rhIL-2. Under these conditions, untransduced CD4+ T cells of clone MoT-72 can be kept alive in a resting state for 4 to 5 weeks, during which the number of viable cells decreased over time (Figure 5, top panel), and eventually all cells died after around 10 weeks. In contrast, the culture of hTERT-transduced MoT-72 T cells showed a slower decrease in the number of living cells. After the initial drop in cell number during the first 3 weeks of culture, approximately half of the original number of hTERT-transduced MoT-72 cells remained alive for another month, compared with only 10% of the untransduced MoT-72 cells. Likewise, hTERT-transduced AKR103 CD8+ T cells survived significantly longer in the absence of stimulation than untransduced cells (Figure 5, bottom panel). In hTERT-transduced cultures of clone AKR103, more than 60% of the cells were still alive at the time their untransduced counterparts had died.
Cell death in hTERT-transduced T-cell cultures in the absence of stimulation. CD4+ T-cell clone MoT-72 cells (top panel) and CD8+ T-cell clone AKR103 (bottom panel) transduced with hTERT–puro-transduced (•) or untransduced (○), respectively, were seeded at 1 × 105 per mL 12 days after the last stimulation and cultured in medium containing IL-2 without stimulation for 100 days. Viable cells were counted weekly by trypan blue exclusion. † indicates death of the culture.
Cell death in hTERT-transduced T-cell cultures in the absence of stimulation. CD4+ T-cell clone MoT-72 cells (top panel) and CD8+ T-cell clone AKR103 (bottom panel) transduced with hTERT–puro-transduced (•) or untransduced (○), respectively, were seeded at 1 × 105 per mL 12 days after the last stimulation and cultured in medium containing IL-2 without stimulation for 100 days. Viable cells were counted weekly by trypan blue exclusion. † indicates death of the culture.
After 100 days of continuous culture in the presence of rhIL-2, the surviving cells of the hTERT-transduced T-cell clones in rhIL-2 were still responsive to stimulation with feeder cells and rhIL-2, as demonstrated by their ability to mount robust proliferative responses. Ectopic hTERT expression may decrease the cytokine dependence for survival of the T cells. Withdrawal of rhIL-2 during the cultures at day 14 led to massive apoptosis in untransduced cells within 24 hours and less apoptosis of hTERT-transduced cells (Figure 6B). Prolonged culture in the absence of rhIL-2 induced apoptosis of all hTERT-transduced cells, suggesting that hTERT expression may have caused a delay in cell death in the absence of rhIL-2.
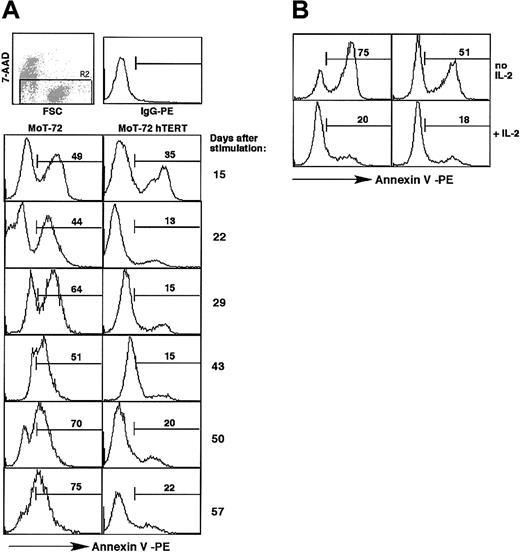
Cell death in the absence of stimulation. (A) T-cell cultures described in Figure 5 were stained weekly with annexin V and 7-AAD during the cultures to monitor cell death and survival in the absence of antigenic stimulation. Graphs show the percentage of annexin V–positive and 7-AAD–negative cells, as indicated by the numbers in the graphs, at day 15, 22, 29, 43, 50, and 57 in the cultures of MoT-72 and hTERT-transduced MoT-72. Top row: 7-AAD staining and forward scatter plot indicating the cell population (R2) of cells impermeable to 7-AAD that was analyzed, and IgG-PE control staining. Similar results were obtained with CD8+ T-cell clone AKR103 (not shown). (B) Apoptosis induced by rhIL-2 withdrawal during 24 hours. hTERT-transduced MoT-72 cells and untransduced cells cultured in rhIL-2 for 14 days were washed and cultured overnight in the presence or absence of rhIL-2. Graphs show the presence of apoptotic cells after 24 hours. Numbers in the graphs indicate the percentage of annexin V–positive cells.
Cell death in the absence of stimulation. (A) T-cell cultures described in Figure 5 were stained weekly with annexin V and 7-AAD during the cultures to monitor cell death and survival in the absence of antigenic stimulation. Graphs show the percentage of annexin V–positive and 7-AAD–negative cells, as indicated by the numbers in the graphs, at day 15, 22, 29, 43, 50, and 57 in the cultures of MoT-72 and hTERT-transduced MoT-72. Top row: 7-AAD staining and forward scatter plot indicating the cell population (R2) of cells impermeable to 7-AAD that was analyzed, and IgG-PE control staining. Similar results were obtained with CD8+ T-cell clone AKR103 (not shown). (B) Apoptosis induced by rhIL-2 withdrawal during 24 hours. hTERT-transduced MoT-72 cells and untransduced cells cultured in rhIL-2 for 14 days were washed and cultured overnight in the presence or absence of rhIL-2. Graphs show the presence of apoptotic cells after 24 hours. Numbers in the graphs indicate the percentage of annexin V–positive cells.
The difference in survival between untransduced cells and hTERT-transduced CD4+ or CD8+ T cells in the absence of stimulation was also evident from the presence of apoptotic cells that bound annexin V and that were impermeable to 7-AAD. The untransduced MoT-72 T-cell cultures contained an increasing percentage of apoptotic cells during long-term cultures in rhIL-2 (Figure 6A). In the hTERT-transduced T-cell culture, a smaller population of apoptotic cells was detected that remained present at a level between 13% and 22% over time. This difference in survival was also observed in CD8+ AKR103 T cells (data not shown). These results show that hTERT-transduced cells were more resistant than untransduced cells to apoptosis that occurs continuously during culture and is independent of proliferation. This might indicate a positive effect of hTERT expression on antiapoptotic signals such as the expression of Bcl-2.
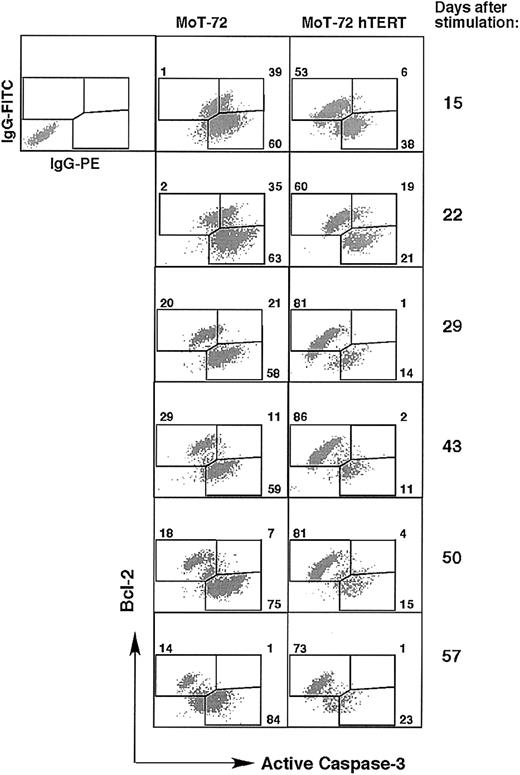
We therefore analyzed the fraction of Bcl-2–expressing cells and the level of Bcl-2 expression during the culture of both hTERT-transduced and untransduced cells of CD4+ MoT-72 T cells and CD8+ AKR103 in rhIL-2 without any form of stimulation (Figure 7). To discriminate between cells expressing Bcl-2 and cells undergoing apoptosis, we performed a double intracellular staining with an antibody against Bcl-2 together with an antibody that detects the active form of caspase-3, which represents one of the central effector caspases in the apoptosis signaling cascade.28 Double stainings for both Bcl-2 and active caspase-3 revealed 2 populations predominantly expressing either Bcl-2 or active caspase-3. During the culture, the untransduced MoT-72 cell cultures showed an increase in the number of Bcl-2–negative and active caspase-3–positive cells, whereas most of the hTERT-transduced MoT-72 cells displayed the reciprocal Bcl-2–positive, active caspase-3–negative phenotype (Figure 7). The enhanced number of Bcl-2–positive cells and active caspase-3–negative cells in hTERT-transduced cultures was confirmed in cultures of CD8+ AKR103 T cells (data not shown). These results indicate that after an initial drop in cell number in the absence of stimulation (Figure 5), a population of surviving hTERT-transduced cells remained present that expressed Bcl-2, whereas in untransduced cultures the frequency of apoptotic cells increased, resulting in a further reduction in cell number (Figures 6, 7). Ectopic hTERT expression increased the frequency of cells expressing Bcl-2 but did not affect the level of Bcl-2 expression itself, since the few surviving T cells that were still present in the untransduced cells at day 43 expressed Bcl-2 at equal levels to Bcl-2 expression in the surviving hTERT-transduced cells. Taken together, these results indicate that the prolonged survival of hTERT-transduced cells is not mediated by the induction of increased Bcl-2 protein expression and furthermore that ectopic hTERT expression favors survival of both CD4+ and CD8+ T cells in the absence of stimulation.
hTERT-transduced cell cultures contain more cells that express bcl-2 than cells expressing active caspase-3. T-cell cultures described in Figure 5 were stained intracellularly with anti–Bcl-2–FITC and antiactive caspase-3–PE mAb at day 15, 22, 29, 43, 50, and 57 during the cultures. Graphs show the double staining plots and the percentage of single- or double-positive cells in the culture of untransduced or hTERT-transduced MoT-72 cells. Top left plot shows the incubation with isotype control antibodies. In hTERT-transduced cultures an accumulation of Bcl-2–positive cells was seen, whereas the balance between Bcl-2–positive cells and cells expressing active caspase-3 was reversed in untransduced cultures. Analysis of the culture of untransduced and hTERT-transduced AKR103 cells gave similar results.
hTERT-transduced cell cultures contain more cells that express bcl-2 than cells expressing active caspase-3. T-cell cultures described in Figure 5 were stained intracellularly with anti–Bcl-2–FITC and antiactive caspase-3–PE mAb at day 15, 22, 29, 43, 50, and 57 during the cultures. Graphs show the double staining plots and the percentage of single- or double-positive cells in the culture of untransduced or hTERT-transduced MoT-72 cells. Top left plot shows the incubation with isotype control antibodies. In hTERT-transduced cultures an accumulation of Bcl-2–positive cells was seen, whereas the balance between Bcl-2–positive cells and cells expressing active caspase-3 was reversed in untransduced cultures. Analysis of the culture of untransduced and hTERT-transduced AKR103 cells gave similar results.
Ectopic hTERT expression confers resistance to oxidative stress–induced apoptosis
Throughout in vitro culture, cells may experience telomere erosion that is caused by DNA damage induced by hydroxyl radicals. In particular, telomeric sequences are extremely susceptible to cleavage by S1 nucleases that are activated by Fe2+/H2O2-mediated oxidation of DNA.8,9 Ectopic hTERT expression may protect cells from this DNA damage by exerting DNA repair at the telomeric site, which may explain the observed survival advantage in the absence of proliferation. We investigated whether hTERT-transduced cells of Th1/Th0-like clone MoT-81, Th2 clone BOY JF157, or CD8+ clone AKR103 were more resistant to apoptosis induced by oxidative stress than untransduced, wild-type cells. Figure 8 shows that treatment of untransduced T cells with increasing concentrations of H2O2 in the presence of Fe2+ ions led to an increase in the percentage of apoptotic cells, whereas treatment of hTERT-transduced T cells induced a lower degree of apoptosis at all H2O2 concentrations. hTERT transduction increased the resistance of T cells to oxidative stress both in clone MoT-81 (Th1/Th0-like) and in clone BOY JF157 (Th2), as well as in CD8+ clone AKR103. Induction of apoptosis in CD4+ T cells occurred after overnight incubation with H2O2 and increased after 48 hours of incubation (Figure 8B). Oxidative stress already triggered apoptosis of CD8+ AKR103 T cells after 5 hours of incubation (data not shown), which became more pronounced after overnight incubation (Figure 8A), indicating that the CD8+ T-cell clones were more sensitive to oxidative stress–induced apoptosis than the CD4+ T-cell clones. In our assays, Th1 and Th2 cells did not differ in sensitivity to oxidative stress, and hTERT-transduced T cells of both clones showed a decrease in sensitivity.
hTERT-transduced T cells are more resistant to oxidative stress than untransduced cells. Untransduced and hTERT-transduced cells of Th1/Th0-like clone MoT-81, Th2 clone BOY JF157, or CD8+ clone AKR103 taken 2 weeks after stimulation were depleted of dead cells and treated with H2O2 at the indicated concentrations in the presence of FeSO4 and rhIL-2. The presence of apoptotic cells was analyzed after 5 hours, 24 hours, or 48 hours of incubation by annexin V binding. In parallel cultures, apoptosis was induced by treatment with dexamethasone. Graphs show the percentage of annexin V–positive and 7-AAD–negative (apoptotic) cells after overnight incubation. (A) Percentage of apoptotic cells of clone MoT-81 after 48 hours of incubation (left panel) and clone AKR103 after 24 hours of incubation (right panel). Graphs show representative results of 2 (MoT-81) or 3 (AKR103) independent experiments. The graphs in the bottom row indicate the percentage of apoptosis induced by dexamethasone treatment. (B) Average percentage of apoptotic cells and standard deviation of CD4+ BOY JF157 T cells after 24 hours (left graph) or 48 hours (right graph) of incubation with H2O2, as measured in 3 independent experiments. ○ indicates BOY JF157; •, BOY JF157 hTERT.
hTERT-transduced T cells are more resistant to oxidative stress than untransduced cells. Untransduced and hTERT-transduced cells of Th1/Th0-like clone MoT-81, Th2 clone BOY JF157, or CD8+ clone AKR103 taken 2 weeks after stimulation were depleted of dead cells and treated with H2O2 at the indicated concentrations in the presence of FeSO4 and rhIL-2. The presence of apoptotic cells was analyzed after 5 hours, 24 hours, or 48 hours of incubation by annexin V binding. In parallel cultures, apoptosis was induced by treatment with dexamethasone. Graphs show the percentage of annexin V–positive and 7-AAD–negative (apoptotic) cells after overnight incubation. (A) Percentage of apoptotic cells of clone MoT-81 after 48 hours of incubation (left panel) and clone AKR103 after 24 hours of incubation (right panel). Graphs show representative results of 2 (MoT-81) or 3 (AKR103) independent experiments. The graphs in the bottom row indicate the percentage of apoptosis induced by dexamethasone treatment. (B) Average percentage of apoptotic cells and standard deviation of CD4+ BOY JF157 T cells after 24 hours (left graph) or 48 hours (right graph) of incubation with H2O2, as measured in 3 independent experiments. ○ indicates BOY JF157; •, BOY JF157 hTERT.
Resistance of hTERT-transduced cells to DNA damage induced by hydroxyl radicals may underlie the difference in survival between hTERT-transduced T cells and untransduced T cells. Direct activation of the apoptosis cascade by dexamethasone treatment induced apoptosis in both hTERT-transduced and in untransduced cells, indicating that the apoptosis pathway is intact in hTERT-transduced cells (Figure 8A). However, the lower degree of apoptosis induced in hTERT-transduced cells implies that hTERT transduction decreased the onset of apoptosis.
Discussion
We show here that ectopic expression of hTERT in human CD4+ T-cell clones representing different cytokine-producing subsets significantly extends the life span of these cells to more than twice the life span of the wild-type cells. hTERT-transduced T cells can be cultured for an unlimited period of time, during which large cell numbers are obtained that are identical to the untransduced cells with respect to their specificity of antigen recognition and cytokine production profile. Moreover, the cytokine production profile is not affected by long-term culture, as we did not observe any consistent changes with an increasing number of PDs or upon subcloning of the T cells. Importantly, proliferation of hTERT-transduced T cells was found to remain dependent on activating signals provided by allogeneic PBMCs or anti-CD3/anti-CD28 antibodies, as well as the presence of rhIL-2. It has been reported previously that ectopic hTERT expression allows the continuation of proliferation but does not promote entry into cell cycle by itself nor does it cause growth deregulation.29 The notion that hTERT expression mediates life span extension but not transformation also was emphasized by studies of ectopic hTERT expression in other human somatic cells that showed that these cells do not display characteristics of transformed cells.30, 31, 32
The contradicting results reported in the literature with respect to the life span–extending ability of ectopic hTERT expression in human CD8+ T cells17,19,33 led us to compare the 2 hTERT-encoding retroviruses used in the respective studies. As described here, both induced life span extension of a CD4+ as well as a CD8+ T-cell clone. Migliaccio et al reported lack of prolonged life span of human T cells by hTERT, which was based on the observation that hTERT-transduced T cells do not proliferate in the absence of stimulation.19 In fact, the results of these researchers demonstrate that repeated stimulation is required to propagate hTERT-transduced T-cell cultures. In the present report, we show that hTERT-transduced T cells can be kept in culture in a nonproliferating state in medium containing rhIL-2 and that these cells re-enter cell cycle upon stimulation. The results described by Migliaccio et al therefore do not contradict our findings but underscore the notion that hTERT-transduction does not transform human T cells.
We observed an increased survival of hTERT-transduced CD4+ or CD8+ T cells in resting cultures. Even though telomere loss may not occur in these cells, hTERT expression still prolonged the survival of these cells. The increased telomerase activity in hTERT-transduced cells may lead to more efficient repair of DNA damage that is a consequence of prolonged culture, resulting in an enhanced survival of hTERT-transduced cells. Oxidative stress activates S1 nuclease acting at cleavage sites that are frequently found in telomeric repeats.9 This particular vulnerability of telomeric sequences may represent a function of telomeres to scavenge nuclease activity in order to protect the chromosomes from DNA damage in coding regions.8 Ectopic hTERT expression has been shown to increase resistance to oxidative stress in fibroblasts by enhancing repair of cleaved telomeres following oxidative DNA damage.10 In the present study, we demonstrate that ectopic hTERT expression confers resistance to oxidative stress in human CD4+ as well as in CD8+ T cells. We analyzed the resistance of hTERT-transduced T cells to oxidative DNA damage in nonactivated T cells to exclude the presence of apoptotic cells dying from AICD. It should be considered that DNA damage is an ongoing event during the life span of T cells, which may continuously induce apoptosis of cells in culture, independent of their activation status. Therefore, increased survival of hTERT-transduced cells under oxidative stress conditions may have occurred during the entire culture period. The accumulation of hTERT-transduced T cells in mixed cultures immediately after hTERT transduction is likely to result from an increased expansion of hTERT-transduced cells, compared with their untransduced counterparts, owing to resistance to oxidative DNA damage, whereas proliferation and cell death in response to stimulation were equal. As a consequence during the mixed cultures, positive selection of hTERT-transduced cells occurred through the disappearance from the culture of untransduced cells that entered senescence.
Ectopic hTERT expression in T cells provides a tool to perform large-scale expansion of T-cell clones that specifically react with tumor antigens. Infusions of hTERT-transduced melanoma-reactive T cells in immunodeficient mice xenotransplanted with a human melanoma have shown the antitumor effect of infused human cytotoxic T cells (N.C.V. Verra, K. Weijer, A. Voordouw, P. Weder, E. Kooijberg, J. Ruizendaal, H.S., R.M.L., manuscript in preparation, March 2003). Further studies must be performed to rule out the risk of hTERT-transduced T cells to acquire a transformed phenotype after infusion in vivo. Now that sufficient numbers of T cells can be obtained, these cells may be applicable for adoptive transfer therapy in cancer patients to increase the number of effector cells that may mediate tumor regression.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-07-2018.
R.M.L. is supported by a grant (NKI 99-2058) from the Dutch Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr P. Romero (Ludwig Institute for Cancer Research, Lausanne, Switzerland) for kindly providing the hTERT-puro retroviral vector.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal