Abstract
Toll-like receptors (TLRs) are pattern recognition receptors that trigger innate immunity. In this study we investigated the expression of 10 TLRs in human naive and memory B-cell subsets. We report that in human naive B cells most TLRs are expressed at low to undetectable levels, but the expression of TLR9 and TLR10 is rapidly induced following B-cell-receptor (BCR) triggering. In contrast, memory B cells express several TLRs at constitutively high levels. The differential expression of TLR9 correlates with responsiveness to its agonist, CpG DNA. Thus, human memory B cells proliferate and differentiate to immunoglobulin (Ig)–secreting cells in response to CpG, while naive B do so only if simultaneously triggered through the BCR. The BCR-induced expression of TLRs in human naive B cells prevents polyclonal activation in a primary response, because it restricts stimulation to antigen-specific B cells. In contrast, the constitutive expression of TLRs in memory B cells allows polyclonal activation of the entire memory pool. Thus, in human B cells TLRs are downstream of BCR and play a role both in the primary response and in the memory phase.
Introduction
Toll-like receptors (TLRs) are pattern recognition receptors that trigger innate immunity, providing both immediate protective responses against pathogens and instructing the adaptive immune response through the induction of dendritic cell recruitment and maturation.1, 2, 3, 4 TLR triggering results in nuclear factor kappa B (NF-κB)–mediated activation of inflammatory genes.5 Ten TLRs have been described in humans, and agonists have been defined for 9 of them.6 TLRs can function as homodimers or heterodimers, increasing the repertoire of specificities.7 TLR1, TLR2, and TLR6 are triggered by peptidoglycan and other microbial products,8,9 TLR3 by double-stranded RNA,10 TLR4 by lipopolysaccharide (LPS),11 TLR5 by flagellin,12 TLR7 and TLR8 by imidazoquinolines,6,13 and TLR9 by unmethylated CpG DNA.14, 15, 16, 17, 18, 19, 20, 21
TLRs are differentially expressed in dendritic cell (DC) subsets.22, 23, 24 Monocytes and myeloid DCs express TLR2 and TLR4 and respond to peptidoglycan and LPS, while plasmacytoid DCs express a reciprocal pattern—namely, TLR7 and TLR9 and respond to CpG. The induction of DC maturation by TLRs represents the functional link between innate and adaptive response.1,25 TLR expression can be regulated; for instance, interferon-γ (IFN-γ) up-regulates TLR4 expression in human phagocytes, enhancing their capacity to respond to LPS.26
Mouse B cells proliferate and differentiate in response to LPS or CpG,14,27 indicating that they express functional TLR4 and TLR9. Human B cells also respond to CpG,19 but recent data indicate that memory B cells are more responsive than naive B cells, raising the possibility that TLR expression may be differentially regulated in human naive and memory B cells.28 We report here that in human naive B cells TLRs are expressed at low to undetectable levels but their expression is rapidly up-regulated by B-cell-receptor (BCR) triggering. In contrast, memory B cells express several TLRs at constitutively high levels. The differential expression correlates with responsiveness to CpG DNA, an agonist of TLR9, and points to a relevant difference between human and mouse B cells. Thus, in humans TLRs are downstream of BCR and play a role in the late phase of acquired immunity.
Materials and methods
Cell isolation and culture
B lymphocytes were isolated from peripheral blood mononuclear cells (PBMCs) using CD19 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and further purified by cell sorting using a FACSVantage (Becton Dickinson, San Jose, CA). Cells were stained with directly labeled antibodies to CD27 (Pharmingen, San Diego, CA) (to identify memory B cells) and appropriate combinations of monoclonal or polyclonal antibodies to CD14, CD3, CD16 (Beckman Coulter, Marseille, France), immunoglobulin (Ig) M, IgD, IgG, or IgA (Jackson ImmunoResearch, West Grove, PA) to obtain pure B subpopulations. Cells were labeled with 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured at 3 × 104 cells per well in 96 flat-bottom microplates in complete RPMI 1640 supplemented with 10% fetal calf serum (FCS, HyClone Laboratories, Logan, UT). CpG oligodeoxynucleotide 2006 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) complete phosphorothioate (Microsynth, Balgach, Switzerland) was used at the optimal concentration of 2.5 μg/mL. Titration experiments showed comparable results over a range of concentrations (1-10 μg/mL). Human recombinant interleukin-2 (IL-2) (R&D Systems, Minneapolis, MN) and IL-10 (Pharmingen) were used at 10 ng/mL. F(ab′)2 fragments of goat antihuman Ig (Jackson ImmunoResearch) were used at 2 μg/mL.
Cell proliferation and Ig production
Purified B-cell populations were labeled with CFSE29 and cultured with appropriate stimuli. Cell proliferation was measured on day 5. Dead cells were gated out by the use of propidium iodide (Sigma, St Louis, MO). Ig-secreting cells (ISCs) were detected by isotype-specific enzyme linked immunospot assay (ELISPOT) on day 6 of culture. Briefly, 96-well plates (Millipore, MAIPS4510) were coated with 1 μg/mL purified goat antihuman IgG, IgA, or IgM (Southern Biotechnology, Birmingham, AL). After washing and blocking with phosphate buffered saline (PBS) 1% bovine serum albumin (BSA), appropriate numbers of cells were added to the plates and incubated overnight at 37°C. Plates were washed with PBS and PBS/0.05% Tween and incubated with isotype-specific secondary antibodies, followed by streptavidin–horseradish peroxidase (streptavidin-HRP) (Sigma). The assay was developed with 3-amino-9-ethylcarbazole (AEC) (Sigma) as a chromogenic substrate.
PCR and Taqman analysis
Total RNA was isolated from naive and memory B-cell subsets using TRIZOL Reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. RNA samples were treated with DNase I to remove contaminating genomic DNA. First-strand cDNA was synthesized at 37°C for 1 hour in 20 μL reaction mixture using random primers and Moloney murine leukemia virus reverse transcriptase (M-MLV RT) (Invitrogen). Serially diluted cDNA samples were amplified using a Taq DNA polymerase (Invitrogen). The primers for TLR1-9 were previously described.22 Other primers used for polymerase chain reaction (PCR) were as follows: TLR10: 5′-ATGAGACTCATCAGAAACATTTAC-3′ and 5′-GCGGATCCGCTACCTTCTTCATAATG-3′; RP105: 5′-CTGCTTCTTTTGGGTGGTGCT-3′ and 5′-CTCGAGATTGGATATTCCCGT-3′; MD-1: 5′-CTCTACCAGAGTTGCGATCCATTACAA-3′ and 5′-TCAGGAGCACATGATAGTAGCATTG-3′; MD-2: 5′-GGGTCTGCAACTCATCCGAT-3′ and 5′-GGTGTAGGATGACAAACTCCAAGC-3′; MyD88: 5′-TGGTGGTGGTTGTCTCTGATG-3′ and 5′-CCAGAACCAAGATTTGGTGCA-3′; Btk: 5′-ATCTGATTTCGGCCTGTCCAG-3′ and 5′-CATCTGCTTTCTCATGCCAACA-3′; Pax-5: 5′-AATCCCACCATGTTTGCCTG-3′ and 5′-CGGAGACTCCTGAATACCTTC-3′. PCR products were resolved on a 2.0% agarose gel and visualized by ethidium bromide staining.
Quantification of TLR2, TLR4, TLR7, and TLR9 mRNAs was done by real-time quantitative PCR using an ABI PRISM 7700 sequence detector (Applied Biosystems, Foster City, CA) and Taqman reagents according to manufacturer instructions. The sequences of probes and primers used were as follows for TLR7: probe: 5′-AGATACCGCAGGGCCTCCCGC-3′; primer: 5′-TTACCTGGATGGAAACCAGCTACT-3′ and 5′-TCAAGGCTGAGAAGCTGTAAGCTA-3′. The sequences of probes and primers used for TLR2, TLR4, and TLR9 were described previously.22 Amplification of β-actin was done for each experimental sample as endogenous control to account for differences in the amount and quality of total RNA added to each reaction.
Results
Differential expression of TLRs in naive and memory B-cell subsets
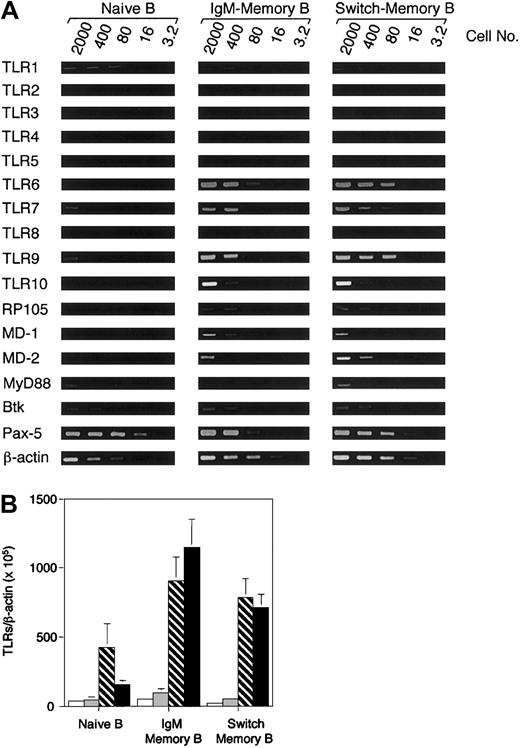
Human memory B lymphocytes can be identified according to the expression of CD27.30 Total B lymphocytes were isolated from PBMCs using CD19 microbeads and stained with antibodies to CD27 and Ig isotypes to discriminate between naive and memory B-cell subsets.30,31 Naive (CD27-), IgM memory (CD27+, IgG-, IgA-), and switch memory B cells (CD27+, IgM-, IgD-) were isolated by cell sorting. The expression of TLR1 through TLR10 was assessed by semiquantitative PCR analysis using as starting material graded numbers of B cells (Figure 1). While the expression of β-actin, Pax-5, Btk, and MyD88 was comparable in naive and memory B cells, the expression of TLRs varied remarkably. TLR1 and TLR2 were expressed at low levels in both naive and memory B cells. In contrast, TLR6, TLR7, TLR9, and TLR10 were expressed at low to undetectable levels in naive B cells and at high levels in memory B cells. The TLR-like gene RP-105 32 and the TLR4-associated receptors MD-1 and MD-2 were also expressed at higher levels in memory B cells compared with naive B cells. TLR4 and TLR8 were not detectable within the sensitivity of the assay. The expression of TLR2, TLR4, TLR7, and TLR9 was confirmed by real-time PCR (Figure 1B). TLR2 and TLR4 were expressed at very low levels and did not show consistent differences between naive and memory cells, while TLR7 and especially TLR9 levels were much higher in memory than naive B cells. Taken together, the above data indicate that in human B cells the expression of several TLRs is developmentally regulated.
TLRs are preferentially expressed on human memory B cells. (A) CD19+ cells isolated by magnetic sorting were stained with antibodies to CD27 and Ig isotypes. Naive B cells (CD27-), IgM memory B cells (CD27+, IgG-, IgA-) and switch memory B cells (IgM-, IgD-) were isolated by cell sorting and immediately analyzed for gene expression by PCR (40 cycles). The input cell number of each reverse transcriptase (RT)–PCR reaction is indicated. One representative experiment of 3 is shown. (B) Quantitation of TLR2 (□), TLR4 (▦), TLR7 (▧), and TLR9 (▪) by real-time PCR in naive and memory B-cell subsets. The data are normalized to the expression of β-actin. Data are the mean ± SD of 4 experiments.
TLRs are preferentially expressed on human memory B cells. (A) CD19+ cells isolated by magnetic sorting were stained with antibodies to CD27 and Ig isotypes. Naive B cells (CD27-), IgM memory B cells (CD27+, IgG-, IgA-) and switch memory B cells (IgM-, IgD-) were isolated by cell sorting and immediately analyzed for gene expression by PCR (40 cycles). The input cell number of each reverse transcriptase (RT)–PCR reaction is indicated. One representative experiment of 3 is shown. (B) Quantitation of TLR2 (□), TLR4 (▦), TLR7 (▧), and TLR9 (▪) by real-time PCR in naive and memory B-cell subsets. The data are normalized to the expression of β-actin. Data are the mean ± SD of 4 experiments.
BCR triggering rapidly up-regulates TLR9 expression in naive B cells
We considered the possibility that BCR triggering might upregulate TLR expression in naive B cells, thus selectively sensitizing the antigen-specific B cells to respond to polyclonal activators. We focused our analysis on TLR9 regulation, because this is a major TLR expressed by human B cells and because a potent agonist (CpG 2006) is available.18 Naive, IgM memory, and switch memory B cells were cultured in medium alone or in the presence of F(ab′)2 fragments of goat antihuman Ig antibodies (anti-Ig) or CpG 2006. Cells were harvested at different time points, and mRNA levels were measured by real-time PCR (Figure 2A). Naive B cells triggered by anti-Ig rapidly up-regulated TLR9 mRNA (about 8-fold in 2 hours), while stimulation with CpG 2006 induced a lower and variable response and did not significantly synergize with anti-Ig. IgM memory and switch memory B cells showed higher basal levels of TLR9 mRNA and a proportionally much lower increase in response to the stimuli. TLR10 was also up-regulated in naive B cells by anti-Ig but with a slower kinetics compared with TLR9 (Figure 2B). These results indicate that in human naive B cells, TLR9 expression is regulated by BCR triggering.
BCR triggering leads to rapid up-regulation of TLR9 and TLR10 in naive B cells. (A) Naive (i), IgM memory (ii), and switch memory (iii) B cells were isolated as in Figure 1 and cultured in the presence or absence of CpG 2006 and F(ab′)2 fragments of goat antihuman Ig as a BCR trigger. At different time points cells were harvested and the expression of TLR9 was measured by real-time PCR. The data are normalized to the expression of β-actin. Data are the mean ± SD of 4 experiments. ○ indicates medium; ▵, CpG; □, anti-Ig; and ⋄, CpG + anti-Ig. (B) Kinetics of TLR9 and TLR10 up-regulation in naive B cells determined by semiquantitative PCR. One representative experiment of 4.
BCR triggering leads to rapid up-regulation of TLR9 and TLR10 in naive B cells. (A) Naive (i), IgM memory (ii), and switch memory (iii) B cells were isolated as in Figure 1 and cultured in the presence or absence of CpG 2006 and F(ab′)2 fragments of goat antihuman Ig as a BCR trigger. At different time points cells were harvested and the expression of TLR9 was measured by real-time PCR. The data are normalized to the expression of β-actin. Data are the mean ± SD of 4 experiments. ○ indicates medium; ▵, CpG; □, anti-Ig; and ⋄, CpG + anti-Ig. (B) Kinetics of TLR9 and TLR10 up-regulation in naive B cells determined by semiquantitative PCR. One representative experiment of 4.
BCR triggering is required for CpG-induced activation of naive B cells but is dispensable for activation of memory B cells
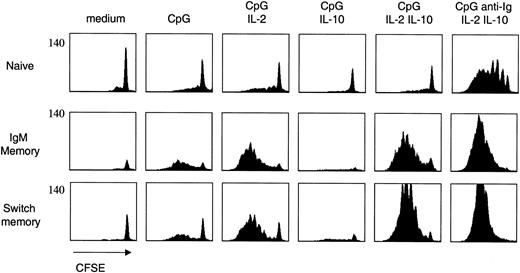
The up-regulation of TLR9 expression following BCR triggering suggests that these stimuli may synergize in the activation of naive B cells. We therefore stimulated naive and memory B-cell subsets with CpG, anti-Ig, and cytokines in various combinations and measured their proliferative response using the CFSE dilution method29 (Figure 3). Two observations were made. First, naive B cells proliferated only when stimulated by anti-Ig, CpG, IL-2, and IL-10, while the response of memory B cells was comparable in the presence or in the absence of anti-Ig. Second, IgM memory B cells, and to a lower extent switch memory B cells, were able to proliferate in response to CpG even in the absence of exogenous cytokines. This response was completely inhibited by IL-10. However, IL-10 was synergistic rather than inhibitory when added together with IL-2.
Differential requirements for naive and memory B-cell proliferation. Naive and memory B-cell subsets were isolated as in Figure 1, labeled with CFSE, and cultured with CpG 2006, anti-Ig, and cytokines in different combinations. Cell proliferation was assessed on day 5. CFSE is on a 4-log scale. One representative experiment of 10.
Differential requirements for naive and memory B-cell proliferation. Naive and memory B-cell subsets were isolated as in Figure 1, labeled with CFSE, and cultured with CpG 2006, anti-Ig, and cytokines in different combinations. Cell proliferation was assessed on day 5. CFSE is on a 4-log scale. One representative experiment of 10.
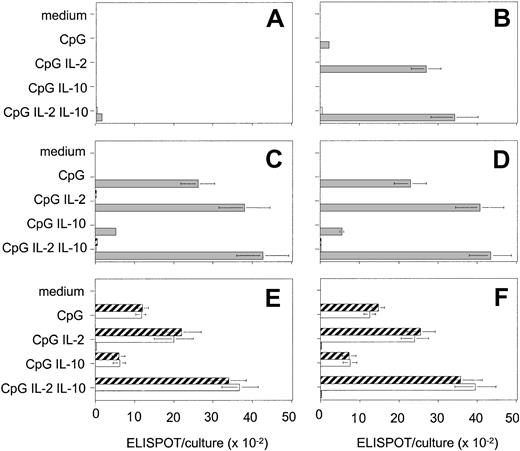
Using an isotype-specific ELISPOT assay, we measured the differentiation of naive and memory B cells to ISCs (Figure 4). Consistent with the proliferation data, we observed a striking synergy between anti-Ig, CpG, and cytokines in the activation of naive B cell. In contrast, anti-Ig did not significantly affect the response of memory B cells. IgM memory B cells generated large numbers of ISCs, which were almost exclusively IgM, indicating that these cells do not switch, at least under these culture conditions. Switch memory B cells generated IgG and IgA ISCs in relatively constant proportions irrespective of the nature of the stimulus. The inhibitory effect of IL-10 was also observed for ISC generation.
BCR triggering is required for CpG-induced differentiation of naive but not of memory B cells. Naive (A-B), IgM memory (C-D), and switch memory (E-F) B cells were cultured with CpG 2006 and cytokines in different combinations and in the absence (A,C,E) or in the presence (B,D,F) of anti-Ig. ISCs were measured by isotype-specific ELISPOT on day 6. Data are the mean ± SD of 5 experiments. ▦ indicates IgM; □, IgG; and ▨, IgA.
BCR triggering is required for CpG-induced differentiation of naive but not of memory B cells. Naive (A-B), IgM memory (C-D), and switch memory (E-F) B cells were cultured with CpG 2006 and cytokines in different combinations and in the absence (A,C,E) or in the presence (B,D,F) of anti-Ig. ISCs were measured by isotype-specific ELISPOT on day 6. Data are the mean ± SD of 5 experiments. ▦ indicates IgM; □, IgG; and ▨, IgA.
Discussion
We have shown that TLR9 is expressed at very low level in human naive B cells; it is rapidly up-regulated by BCR triggering; and it is expressed at a high constitutive levels in memory B cells. Consistent with these observations, memory B cells are directly triggered by CpG to proliferate and differentiate to ISCs, while naive B cells require synergistic stimulation by anti-Ig and CpG.
In this study we focused primarily on TLR9, because it is expressed at high level in human B cells and its ligand is a powerful B-cell mitogen. However, human B cells express several TLRs that show distinct expression patterns in naive and memory cells. TLR6, TLR7, TLR9, and TLR10 are expressed at low levels in naive B and at high levels in memory B cells. Because TLR10 is expressed at high level in B cells, the identification of TLR10 ligands will offer the opportunity to selectively target B cells without affecting myeloid or plasmacytoid DCs. Other TLRs— namely, TLR1, TLR2, and TLR4—are expressed at comparable levels by naive and memory B cells, this level being particularly low for TLR2 and TLR4. Human B cells express very low levels of TLR4 and although they express MD-2 and RP105 they fail to respond to LPS (N.L.B., unpublished data, 2002).
Previous studies in the mouse system provided evidence for a synergism between BCR and TLRs. These studies suggested that antigens containing polyclonal activator, such as LPS or unmethylated DNA, may act as a physical bridge between BCR and TLRs, which are constitutively expressed on mouse naive B cells, the cross-linking of the 2 receptors leading to B-cell proliferation and differentiation.27,33 On the basis of our results we propose a revised model based on a temporal bridge between BCR and TLR9. First, antigen binds to BCR and triggers TLR9 expression. Second, antigen and the associated TLR9 ligand is internalized in the endosomal compartment, where it interacts with newly synthesized TLR9. This model is consistent with our data as well as with the intracellular expression of TLR9.34 The induction of gene expression ensures fidelity in primary response.
CpG DNA has been traditionally considered as an adjuvant for T- and B-cell responses.15 A recent study suggests that CpG DNA potentiates the B-cell adaptive immune response by enhancing terminal differentiation.35 We have shown here that naive and memory B-cell proliferation and differentiation can be induced in the complete absence of T cells. Our study suggests that direct coupling of antigen to CpG may be sufficient to create a strong immunogen capable of eliciting highly specific T-independent antibody responses.
The constitutive expression of TLRs in memory cells provides a mechanistic explanation to our recent findings that CpG can selectively trigger memory cells.28 It should be noted that in response to CpG naive B cells do not proliferate but up-regulate CD69 and, to a lower extent, CD86 within 4 hours after stimulation.28 This indicates that in naive B cells some signal is transmitted in response to CpG. The failure of CpG to induce proliferation of naive B cells may be due to the fact that TLR9 is either expressed at too low levels or is inefficiently coupled to signal transduction pathways. In addition, our results reveal that IL-10 can potently inhibit CpG-induced B-cell proliferation, an effect that is contingent on the presence of IL-2. Indeed, when added alone IL-10 completely inhibited the CpG-induced B-cell proliferation, while in the presence of IL-2 it enhanced B-cell proliferation. These findings suggest a new role for IL-10 as a “fine-tuner” of B-cell activation induced by microbial products. In this respect, it is tempting to hypothesize that, in the absence of IL-10 polyclonal activation of IgM memory B cells by chronic infection may induce the continuous proliferation leading to the development of mucosa-associated lymphatic tissue (MALT) lymphomas.36
In conclusion, our results suggest a novel role for TLRs in the immune response. In dendritic cells, TLRs function as primary triggers for dendritic cell maturation and cytokine production and in this way bridge innate to adaptive immunity. In contrast, in human B cells, TLRs function downstream of BCR and play distinct functions in primary responses and immunologic memory. Selective TLR9 up-regulation in BCR-triggered B cells makes so that in a T-independent response only the naive B cells that have captured microbial antigens will be activated. In contrast, the constitutive expression of TLRs in memory B cells provides a mechanism to continuously generate antibody of all memory specificities, thus sustaining serologic memory.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-11-3569.
Supported by the Swiss National Science Foundation (grant 31-63885). A.L. is supported by the Helmut Horten Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Federica Sallusto for critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal