Abstract
We have utilized a free-solution isoelectric focusing technique (FS-IEF) to obtain chaperone-rich cell lysate (CRCL) fractions from clarified tumor homogenates and have previously reported on their vaccinating potential. To better understand the underlying mechanisms as well as to improve on the immunizing efficacy of tumor-derived chaperone complexes, in the present study we examined the effects of CRCL-loaded dendritic cells (DCs) against 12B1, an aggressive bcr-abl+ murine leukemia tumor. We found that DCs incubated with 12B1-derived CRCL had higher expression of CD40 and major histocompatibility complex class II (MHC-II) on their cell surface, produced more interleukin-12 (IL-12), and had superior immunostimulatory capacity in a mixed leukocyte reaction (MLR) when compared with DCs exposed to unfractionated tumor lysate or purified heat-shock protein 70 (HSP70). Vaccination of mice with 12B1 CRCL–pulsed DCs significantly prolonged their survival, with more than 80% of mice rejecting their tumors following a lethal challenge with live 12B1 compared with those immunized with tumor lysate or HSP70-loaded DCs. The protective immunity generated was tumor specific, long lasting, and both CD4+ and CD8+ T-cell dependent. Moreover, immunization with CRCL-loaded DCs resulted in a 75% cure rate in mice with pre-existing 12B1 tumors. Our findings indicate that CRCL has prominent adjuvant effects and is a very effective source of tumor antigen for pulsing DCs. FS-IEF–derived CRCL-pulsed DCs are a promising anticancer vaccine that warrants clinical research and development.
Introduction
Developing more effective anticancer vaccines has been one of the major goals of cancer immunotherapy. Purified tumor-derived chaperone proteins such as heat-shock protein 70 and 90 (HSP70 and 90), GRP94/gp96, and calreticulin (CRT) have shown promise as vaccines, capable of generating tumor-specific T-cell responses and protective antitumor immunity in numerous animal models.1, 2, 3, 4, 5, 6 These studies have indicated that it is not the chaperone proteins, per se, but rather the tumor antigen repertoire (ie, peptides) carried by the chaperones that is immunogenic. In the normal cellular environment, chaperone proteins perform their intracellular functions as multiprotein complexes consisting of chaperones, cochaperones, substrate molecules, and others. Vaccination studies have demonstrated that when purified away from their normal cellular environment, individual chaperone proteins retain effective antitumor activity. However, a remaining question has been whether multichaperone/cochaperone vaccines would be more effective than single-component HSP vaccines.
To address this question, we have utilized a free-solution isoelectric focusing technique (FS-IEF) to obtain chaperone-rich cell lysate (CRCL) fractions from clarified tumor homogenates. Using this method we are able to obtain much higher quantities of immunogenic material in a timely fashion. CRCL contains chaperone proteins carrying a broad repertoire of antigenic peptides, which may offer significant advantages over individual antigenic peptide vaccines. In our previous studies, we have shown that vaccines prepared from chaperone-rich fractions were capable of providing protective immunity in mice against tumor challenge in an A20 B-cell leukemia model.7 To explore the effectiveness of CRCL as a vaccine in other tumor models, to improve on its efficacy, to study its potency in a pre-existing tumor setting, as well as to examine its effects on DCs, we enriched for chaperone proteins by FS-IEF from 12B1, a bcr-abl+ murine leukemia. We found that vaccination of mice with 12B1-derived CRCL provides significant protection against lethal subcutaneous challenge with autologous tumor. CRCL enhances costimulatory molecule expression, interleukin-2 (IL-2) production and immmunostimulatory function of DCs in vitro. Vaccination with DCs loaded with CRCL provides a superior protective effect when compared with tumor lysate or HSP70-pulsed DCs. The T-cell immunity elicited by CRCL-loaded DCs is tumor specific and long lasting. Moreover, CRCL-loaded DCs can be used effectively to treat mice with pre-existing tumors. Our findings indicate that 12B1-derived FS-IEF–enriched CRCL is capable of both efficient antigen delivery and of DC activation resulting in generation of potent antitumor immunity.
Materials and methods
Bcr-abl+ leukemia cell line
12B1 is a murine leukemia cell line derived by retroviral transformation of BALB/c bone marrow cells with the human bcr-abl (b3a2) fusion gene, and these cells express the p210 bcr-abl protein. This is an aggressive leukemia, with the 100% lethal dose (LD100) being 102 cells after tail-vein injection and 103 cells after subcutaneous injection. The 12B1 cell line was kindly provided by Dr Wei Chen (Cleveland Clinic, Cleveland, OH). The cell line was tested monthly and found to be free of Mycoplasma contamination.
Mice
Female BALB/c (H2d) mice that were 6- to 10-weeks old (Harlan Sprague Dawley, Indianapolis, IN) were used for the experiments. The animals were housed in a dedicated pathogen-free facility and cared for according to the University of Arizona Institutional Animal Care and Use Committee guidelines.
Tumor generation
All tissue/cell culture reagents were purchased from Gibco/BRL (Gaithersburg, MD). 12B1 cells were cultured at 37°C and in 5% CO2 in RPMI medium containing 10% heat-inactivated fetal calf serum and supplemented with 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, 0.025 μg/mL amphotericin B, 0.5 × minimal essential medium nonessential amino acids, 1 mM sodium pyruvate, and 50 μM 2-mercaptoethanol. Cells were prepared for injection by washing and resuspending in Hanks balanced salt solution. The cells were counted and adjusted to a concentration of 25 × 106 cells/mL. Female BALB/c mice were injected with 0.2 mL (5 × 106 cells) subcutaneously in both flanks and were monitored for tumor development. Tumors larger than 1 cm in diameter were harvested from killed mice. In vivo passaging of tumors involved harvesting and mincing the tumor to produce a cell suspension. The cell suspension was filtered through a cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ) to remove debris and centrifuged. The cell pellet was resuspended, washed, counted, and injected into mice.
FS-IEF for chaperone enrichment and conventional purification of HSP70
Tumor tissue grown in vivo was harvested from mice and homogenized at 4°C in a motor-driven glass/Teflon homogenizer; the buffer was 10 mM Tris (tris(hydroxymethyl)aminomethane)/Cl (pH 7.4)/10 mM NaCl, 0.1% Triton X-100/0.1% Triton X-114/0.1% Igepal CA-630 (equivalent to nonidet P-40; all detergents were from Sigma Chemical, St Louis, MO), with the following protease inhibitors (all from Roche Molecular Biochemicals, Indianapolis, IN): leupeptin (2 μg/mL), pepstatin A (1 μg/mL), phenylmenthylsulfonyl fluoride (0.5 mM), and a Complete protease inhibitor cocktail tablet. This buffer was chosen for its low ionic strength and ability to solubilize membranes. The homogenate was centrifuged at 10 000g for 30 minutes at 4°C to obtain a “low-speed” supernatant. That supernatant was centrifuged at 100 000g for 60 minutes at 4°C to obtain a “high speed” supernatant. This was then dialyzed against 5 mM Tris/Cl (pH 7.4)/5 mM NaCl, 0.05% Triton X-100/0.05% Triton X-114/0.05% Igepal CA-630. Protein concentration of this dialysate was determined by the bicinchoninic acid (BCA) method (Pierce Endogen, Rockford, IL) using bovine serum albumin as a standard. This dialysate was frozen in aliquots containing 25 mg total protein. To generate vaccine, one aliquot was filtered through a 0.8-μm filter and prepared for isoelectric focusing by adding urea to 6 M; the detergents Triton X-100, Triton X-114, and Igepal each to 0.05%; and a mixture of Rotolytes (Bio Rad Laboratories, Hercules, CA) 5 mL each of solution A and B for each pH range (3.9-5.6; 4.5-6.1; and 5.1-6.8) to a total volume of around 40 to 50 mL. FS-IEF was carried out in a Rotofor device (Bio Rad Laboratories). Isoelectric focusing was conducted for 5 hours at 15 W constant power while the apparatus was cooled with recirculating water at 4°C; the anode compartment contained 0.1 M H3PO4, while the cathode compartment contained 0.1 M NaOH. Then, 20 fractions were harvested; the pH of each fraction was determined with a standard pH meter, and the protein content was analyzed by sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as previously described.3 SDS-PAGE and Western blot results indicated that following FS-IEF, several fractions ranging from pH 5.1 to pH 6.0 contained HSP70, HSP90, GRP94/gp96, and CRT. Fractions selected to be pooled for vaccines were those that contained all 4 of the above HSPs. FS-IEF utilizes small amounts of starting material to yield relatively large amounts of tumor-derived chaperone proteins. In general, 1 g of tumor can yield 1000 μg CRCL vaccine, while from the same amount of tumor, only 30 to 50 μg of individual chaperone protein such as HSP70 can be generated using conventional purification strategies. Purification of 12B1 HSP70 was done via conventional and nucleotide-affinity chromatography as previously described.3 Endotoxin level of the CRCL is lower than 0.01 endotoxic units (EU)/μg of CRCL as examined by Limulus amebocyte lysate (LAL) assay (QCL-1000; BioWhittaker, Walkersville, MD).
Preparation of chaperone-enriched vaccines
Fractions from FS-IEF that contained substantial amounts of 4 chaperone proteins (HSP70, HSP90, GRP94/gp96, and CRT), as determined by SDS-PAGE and Western blotting, were pooled and dialyzed stepwise out of urea and detergents (starting in 0.1 × phosphate-buffered saline [PBS], 4 M urea, and 0.025% detergents, and ending with 0.1 × PBS). Pooled fractions were then concentrated using Centricon devices and reconstituted in PBS. Vaccines were then passed onto an Extracti-gel D column (Pierce Endogen) to remove detergent. Protein concentrations were determined by the BCA method, and the concentrated proteins were diluted to appropriate concentration for in vivo and in vitro experiments.
Immunodepletion of chaperone proteins
Chaperone proteins were removed from CRCL by immunopreciptiation with antibodies specific for HSP/HSC70, HSP90, GRP94/gp96, and calreticulin (CRT). The primary antibodies used were anti-HSC70 (HSP73), mouse monoclonal antibody (Mab) clone 1B5 (SPA-815; Stressgen Biotechnologies, Victoria, BC, Canada); anti-HSP70 (HSP72)/HSC70, mouse Mab clone N27F3-4 (SPA-820; Stressgen); anti-HSP90, rat Mab clone 2D12 (SPA-845; Stressgen), and mouse Mab 3G3 (MA3-011; Affinity BioReagents, Golden, CO); anti-GRP94, rat Mab clone 9G10 (SPA-850; Stressgen), and a rabbit polyclonal sera specific for GRP94 (a gracious gift from Dr Chris Nicchitta, Duke University, Durham NC); rabbit polyclonal anti-CRT antibodies from Affinity BioReagents (PA3-900) and from Stressgen (SPA-600). Antibodies were incubated with CRCL overnight at dilutions of 1:200 to 1:500. Antibodies were precipitated with ImmunoPure immobilized recombinant protein A (Pierce Endogen); bridging antibodies (rabbit anti–mouse immunoglobulin G [IgG] + IgM and rabbit anti–rat IgG + IgM; Jackson ImmunoResearch, West Grove, PA) were added at 1:200 dilution as necessary. Depletion of chaperone proteins was confirmed by lack of reactivity with specific antibodies for chaperones by Western blotting. Control depletions consisted of incubations with normal mouse serum and normal rabbit serum, followed by the precipitation steps.
Generation of bone marrow–derived DCs
BALB/c mouse bone marrow DCs were generated using a slightly modified protocol from that described previously.8 Bone marrow was harvested from femurs and tibiae and filtered through a Falcon 100-μm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). Red blood cells were lysed in a hypotonic buffer and the marrow was cultured in complete RPMI medium (therapeutic grade; Gibco BRL), which contains 10% fetal calf serum, l-glutamine, human serum albumin, 50 μg/mL streptomycin sulfate, and 10 μg/mL gentamicin sulfate. Murine granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 ng/mL; Peprotech, Rocky Hill, NJ) and IL-4 (10 ng/mL) were added to the culture. After 6 days, the nonadherent and loosely adherent cells were harvested, washed, and used for in vivo and in vitro experiments. Less than 10% of these cells were contaminated by macrophages (CD14+ cells).
Flow cytometry
DCs were washed in PBS containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide (Sigma Chemical). A total of 2 × 105 cells were placed in each well of 96-well U-bottom microtiter plates. Surface expression of specific antigens was determined by incubating the cells first with an Fc receptor–blocking antibody (BD Pharmingen, San Diego, CA) for 5 minutes and then with saturating amounts of monoclonal antibodies (Pharmingen) for 30 minutes at 4°C. Antibodies used included purified fluorescein isothiocyanate (FITC)–conjugated anti–I-Ad (clone AMS-32.1, mouse IgG2b), anti-CD80 (clone 16-10A1, hamster IgG), anti-CD86 (clone GL1, rat IgG2a), anti-CD40 (clone HM40-3, hamster IgM), and purified phycoerythrin (PE)–conjugated anti-CD11c (clone HL3, hamster IgG). After a 30-minute incubation the cells were then washed 3 times in PBS containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide and fixed with PBS containing 1% paraformaldehyde (Polysciences, Warrington, PA). Using a FACScan (Becton Dickinson Immunocytometry, San Jose, CA), 10 000 cells were analyzed.
Mixed leukocyte reaction (MLR)
Day-6 BALB/c (H2d) DCs were incubated with 50 μg/mL 12B1-derived lysate, HSP70, or FS-IEF–enriched CRCL in the presence of 10 ng/mL murine GM-CSF and IL-4 for 24 hours. DCs were collected, treated with 50 μg/mL Mitomycin C (Sigma Chemical) for 20 minutes, and then washed 3 times with PBS. Splenocytes (105 per well) from naive C57BL/6 (H2b) mice were plated in U-bottom 96-well plates. DCs were serially diluted and incubated with splenocytes with the ratio of splenocytes to DCs ranging from 1:1 to 27:1. After a 4-day coculture, 20 μL of 50 μCi/mL (1.85 MBq/mL) [3H]thymidine (ICN Pharmaceuticals, Costa Mesa, CA) was added to each well. The cells were harvested 18 hours later using a 96-well Packard cell harvester and the radioactivity measured on a Packard beta counter (Packard Biosciences, Meriden, CT).
Enzyme-linked immunospot (ELISPOT) assays
ELISPOT assays were performed to measure the IL-12 secretion by DCs. Between 105 to 106 day-6 DCs were cultured with 50 μg/mL 12B1-derived lysate, HSP70, or CRCL in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours on Millipore MultiScreen-HA 96-well plates (MAHA S45; Millipore, Bedford, MA). The plates had been previously coated overnight with anti–IL-12 capture antibody (10 μg/mL, clone 9A5, rat Mab antimouse IL-12 [p70]; BD PharMingen). DCs were then washed out with copious amounts of PBST (PBS + 0.05% Tween 20). Biotinylated anti–IL-12 antibody (2 μg/mL, clone C17.8, rat Mab antimouse IL-12 [p40/p70]; BD PharMingen) was added for 2 hours. Free antibody was washed out, and the plates were incubated with horseradish peroxidase (HRP)–linked avidin (ABC Elite reagent, 1 drop each of Reagent A and Reagent B per 10 mL PBS; Vector Laboratories, Burlingame, CA) for one hour, following extensive washing with PBST, and then washing with PBS. Spots were visualized by the addition of the HRP substrate 3-amino-9-ethylcarbazole (AEC; Sigma Chemical) prepared in acetate buffer (pH 5.0) with 0.015% hydrogen peroxide. Spots were examined using a dissecting microscope. Wells of interest were photographed with a microscope-mounted Cool SNAP CCD camera (RS Photometrics, Tucson, AZ), and images were captured with RS Image, Version 1.07 (Roper Scientific, Tucson, AZ). The image of each well was electronically optimized to visualize the maximum number of spots. ELISPOT assays were also performed to measure interferon-gamma (IFN-γ) secretion from splenocytes from mice primed by vaccination with DCs pulsed with CRCL or pulsed with chaperone-immunodepleted CRCL. Splenocytes were harvested and red blood cells were lysed in hypotonic buffer. There were 1 000 000 splenocytes/well plated, as described for DCs above; those cells were restimulated with CRCL or chaperone-immunodepleted CRCL. The rest of the procedure was as described above, except that the capture antibody was rat Mab antimouse IFN-γ, clone R4-6A2 (BD PharMingen), and the detection antibody was biotinylated rat Mab antimouse IFN-γ antibody (clone XMG1.2; BD PharMingen).
In vivo tumor growth experiments
In the prophylactic model, mice were immunized with indicated vaccines (20 μg/mouse per vaccination) by subcutaneous injection into the left groin on days - 14 and - 7. On day 0, mice were challenged with 103 (LD100) viable 12B1 cells obtained from a single in vivo passage. For dosage escalation studies, 5, 10, 20, and 50 μg/mouse were used for each vaccination. In experiments with DCs, DCs were incubated with 50 μg/mL of the indicated vaccines in the presence of 10 ng/mL murine GM-CSF and IL-4 for 24 hours; then the DCs were washed with PBS 3 times, and resuspended in PBS followed by subcutaneous injection into mice. To exclude potential HSP70 or CRCL toxicity on DCs, day-6 DCs were plated in 96-well flat-bottom plates (50 000 cells/well) in the presence of increasing concentrations of 12B1 tumor–derived HSP70 or CRCL (0-100 μg/mL) for 24 hours. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, stock solution 5 mg/mL; Sigma) at 10 μL per well was added for an additional 4 hours. The supernatant was aspirated and the formazan crystals were solubilized in dimethylsulfoxide, followed by determination of optical densities at 560 nm and 690 nm using a microtiter plate reader. A total of 5 × 105 DCs were injected per mouse subcutaneously on days - 14 and - 7. In T-cell depletion experiments, CD4+, CD8+, or both T-cell populations were depleted by intraperitoneal injection of 200 μg anti-CD4 (GK1.5)9 and/or anti-CD8 (2.43)10 antibodies on days - 3, - 1, + 1, and + 3. Tumor size was measured every other day with calipers once the tumors became palpable. Tumor volume was calculated using the formula length × width2 ×π/6. Differences in mean tumor volume between groups were compared using an unpaired t test. In other experiments the Kaplan-Meier product-limit method was used to plot the time to tumor appearance and the log-rank statistic to test differences between groups.11,12 Mice with tumor were killed at the end point listed. Tumor-free mice were kept for rechallenge experiments. In rechallenge experiments, 103 live 12B1 (LD100) cells were injected into the right groin 80 to 125 days after the first challenge, whereas 106 A20 leukemia cells (LD100) were injected into the left groin.
In a pre-existing tumor model, mice were injected with 103 viable 12B1 cells at right groin on day 0. On day + 2, mice were vaccinated as indicated by subcutaneous injection into the left groin. Tumor volume was measured at indicated time points.
Results
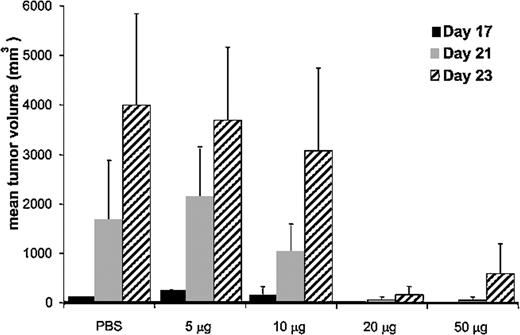
Immunization with 12B1 tumor–derived CRCL provides tumor-specific immunity against autologous tumor challenge. Dose escalation experiments were performed in order to determine the optimal amount of 12B1 CRCL needed to immunize the animals. BALB/c mice received 5, 10, 20, or 50 μg CRCL subcutaneously on days - 14 and - 7, and were challenged on the opposite side with an LD100 dose of 12B1 cells on day 0. Tumor volume measurements demonstrated that immunization of mice with 20 μg 12B1-derived CRCL provided optimal protection against tumor challenge (Figure 1). This vaccine dose was consistent with our previous studies using other tumor models.7
Effect of CRCL immunizing dose on mean tumor volume of challenged mice. BALB/c mice (n = 4 per group) were immunized with increasing quantities (5-50 μg) of 12B1-derived CRCL, or with saline on days - 14 and - 7, and were challenged in the opposite groin with 12B1 leukemia cells on day 0. Tumors were measured and volumes calculated once tumors were palpable. Mean tumor volumes for days 17 to 23 are shown. Representative data from 1 of 2 experiments are shown. Error bars represent SEM.
Effect of CRCL immunizing dose on mean tumor volume of challenged mice. BALB/c mice (n = 4 per group) were immunized with increasing quantities (5-50 μg) of 12B1-derived CRCL, or with saline on days - 14 and - 7, and were challenged in the opposite groin with 12B1 leukemia cells on day 0. Tumors were measured and volumes calculated once tumors were palpable. Mean tumor volumes for days 17 to 23 are shown. Representative data from 1 of 2 experiments are shown. Error bars represent SEM.
To demonstrate the specificity of chaperone protein–stimulated immune responses, mice were vaccinated with CRCL isolated from A20 B-cell leukemia/lymphoma or 12B1 bcr-abl+ leukemia. A20-derived CRCL did not generate protection against 12B1 challenge, confirming that the immunity elicited is tumor specific (Figure 2A). Additionally, immunization of animals with tumor lysate generated no protection (Figure 2A) as was demonstrated previously for A20 B-cell leukemia.7 These data indicate that unfractionated 12B1 tumor lysate itself is not an effective immunogen and that the CRCL enrichment is necessary to enhance its immunogenicity.
CRCL vaccine generates tumor-specific immunity. (A) Immunization with 12B1-derived CRCL provides specificity protection against 12B1 tumor challenge. BALB/c mice were immunized with 20 μg 12B1- or A20 tumor–derived CRCL, on days - 14 and - 7, followed by challenge with 103 12B1 cells (LD100) in the opposite groin on day 0 (saline versus CRCL [12B1], P < .05 from day 15 onward; n = 11-24 mice per group). (B) Immunization with purified HSP70 or CRCL from 12B1 tumor delays tumor growth. Mice were immunized with 20 μg 12B1 tumor–derived HSP70 or CRCL or unfractionated lysate on days - 14 and - 7 followed by challenge with 103 12B1 cells in the opposite groin on day 0 (saline vs HSP70 or CRCL, P < .05 from day 15 onward; n = 8 mice per group).
CRCL vaccine generates tumor-specific immunity. (A) Immunization with 12B1-derived CRCL provides specificity protection against 12B1 tumor challenge. BALB/c mice were immunized with 20 μg 12B1- or A20 tumor–derived CRCL, on days - 14 and - 7, followed by challenge with 103 12B1 cells (LD100) in the opposite groin on day 0 (saline versus CRCL [12B1], P < .05 from day 15 onward; n = 11-24 mice per group). (B) Immunization with purified HSP70 or CRCL from 12B1 tumor delays tumor growth. Mice were immunized with 20 μg 12B1 tumor–derived HSP70 or CRCL or unfractionated lysate on days - 14 and - 7 followed by challenge with 103 12B1 cells in the opposite groin on day 0 (saline vs HSP70 or CRCL, P < .05 from day 15 onward; n = 8 mice per group).
Purified tumor-derived HSP70 has been reported to be an effective vaccine.2,6 Tumor-derived CRCL contains HSP70 and at least 3 other of the reported immunogenic chaperones.7 To compare the immunogenicity of purified 12B1 HSP70 with that of CRCL, mice were immunized with 20 μg of purified HSP70 or CRCL. The protective effects of HSP70 and CRCL were comparable, with both preparations significantly delaying 12B1 tumor growth (Figure 2B).
DCs pulsed with CRCL have increased major histocompatibility complex class II (MHC-II) and costimulatory molecule expression, IL-12 production, and immunostimulatory capacity in vitro. Since DCs have been reported to express receptors for exogenous HSPs13 and since they are being used as antigen-presenting cells (APCs) in clinical vaccine trials, we examined the effect of tumor-derived chaperone proteins on bone marrow–derived DCs. Day-6 DCs were cultured with 12B1-derived CRCL or unfractionated tumor lysate or purified HSP70 for 24 hours. About 80% of these DCs are CD11c+ as assessed by flow cytometry (data not shown). The mean fluorescence intensity (MFI) of CD40, CD80, CD86, and MHC-II on CD11c+ gated cells was further determined. Tumor-derived CRCL had only a modest effect on CD80 and CD86 induction (Figure 3A). However, CRCL preparations significantly increased expression of CD40 and MHC-II on DCs.
Tumor-derived CRCL activates DCs. (A) 12B1-derived chaperone proteins stimulate DCs to express MHC-II and costimulatory molecules. Day 6 bone marrow–derived DCs, grown in GM-CSF and IL-4, were exposed to 50 μg/mL 12B1 HSP70 (□), CRCL (▪), or unfractionated lysate (▨) for 24 hours, harvested, and analyzed by flow cytometry for expression of the cell-surface markers indicated. MFI of CD40, CD80, CD86, and MHC-II (I-Ad) on CD11c+ gated nontreated DCs was considered as one. The numbers in the ordinate represent the fold increase in MFI of treated DCs when compared with nontreated DCs. Means and SEM from 3 experiments are shown. (B) DCs pulsed with CRCL have increased IL-12 production. ELISPOT assays were performed to measure IL-12 production by DCs. At day 6 of culture, 3 × 105 DCs were cultured with 50 μg/mL 12B1-derived lysate, HSP70, or CRCL in the presence of 10 ng/mL GM-CSF and IL-4 each for 24 hours (DC + CRCL or DC + HSP70 versus DC or DC + lysate, P < .05; DC + CRCL versus DC or DC + HSP70, P < .05; representative data from 3 experiments are shown). (C) 12B1-derived CRCL increases DC capacity to stimulate allogeneic splenocyte proliferation. DCs were cultured as in “Materials and methods,” harvested, treated with Mitomycin C, and washed. Splenocytes from C57BL6 mice were added (105 per well) and cultured with the indicated ratios of pretreated BALB/c DCs for 4 days. [3H]thymidine was added and the cells were cultured for an additional 18 hours before the incorporated radioactivity was counted (DC + CRCL versus DC, DC + lysate, or DC + HSP70; P < .05; representative data from 2 experiments are shown). cpm indicates counts per minute.
Tumor-derived CRCL activates DCs. (A) 12B1-derived chaperone proteins stimulate DCs to express MHC-II and costimulatory molecules. Day 6 bone marrow–derived DCs, grown in GM-CSF and IL-4, were exposed to 50 μg/mL 12B1 HSP70 (□), CRCL (▪), or unfractionated lysate (▨) for 24 hours, harvested, and analyzed by flow cytometry for expression of the cell-surface markers indicated. MFI of CD40, CD80, CD86, and MHC-II (I-Ad) on CD11c+ gated nontreated DCs was considered as one. The numbers in the ordinate represent the fold increase in MFI of treated DCs when compared with nontreated DCs. Means and SEM from 3 experiments are shown. (B) DCs pulsed with CRCL have increased IL-12 production. ELISPOT assays were performed to measure IL-12 production by DCs. At day 6 of culture, 3 × 105 DCs were cultured with 50 μg/mL 12B1-derived lysate, HSP70, or CRCL in the presence of 10 ng/mL GM-CSF and IL-4 each for 24 hours (DC + CRCL or DC + HSP70 versus DC or DC + lysate, P < .05; DC + CRCL versus DC or DC + HSP70, P < .05; representative data from 3 experiments are shown). (C) 12B1-derived CRCL increases DC capacity to stimulate allogeneic splenocyte proliferation. DCs were cultured as in “Materials and methods,” harvested, treated with Mitomycin C, and washed. Splenocytes from C57BL6 mice were added (105 per well) and cultured with the indicated ratios of pretreated BALB/c DCs for 4 days. [3H]thymidine was added and the cells were cultured for an additional 18 hours before the incorporated radioactivity was counted (DC + CRCL versus DC, DC + lysate, or DC + HSP70; P < .05; representative data from 2 experiments are shown). cpm indicates counts per minute.
We also evaluated IL-12 secretion by DCs after stimulation with tumor-derived lysate, HSP70, or CRCL. Day-6 bone marrow–derived DCs were cultured with 50 μg/mL 12B1 lysate, HSP70, or CRCL in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. Compared with lysate and HSP70, CRCL-stimulated DCs clearly had more pronounced IL-12 production as assessed by ELISPOT (Figure 3B).
We then examined the effect of 12B1-derived CRCL on DC immunostimulatory function in an MLR in vitro. BALB/c DCs were cultured with 50 μg/mL 12B1 HSP70, or CRCL, or tumor lysate for 24 hours. The DCs were treated with 50 μg/mL Mitomycin C for 20 minutes, then washed and added at different concentrations to plates containing 105 viable C57BL/6 splenocytes per well and cocultured for 4 days. [3H]thymidine was added to the plates, and the cells were cocultured for an additional 18 hours. DCs exposed to 12B1 CRCL were more potent stimulators of allogeneic responses in these MLRs (Figure 3C).
DCs pulsed with 12B1 tumor–derived CRCL induce potent T-cell–dependent antitumor immune responses. Given the in vitro stimulatory activities of CRCL on DCs, in vivo experiments exploring whether the immunizing effects of CRCL may be augmented by DCs were then conducted. Using MTT assays we first evaluated whether increasing concentrations of 12B1 CRCL or HSP70 or unfractionated lysate impaired DC viability. No changes in DC viability were observed with up to 100 μg/mL of tumor-derived proteins (data not shown). Day-6 immature bone marrow–derived DCs were then incubated with 50 μg/mL 12B1-derived CRCL or the same concentration of purified 12B1 HSP70 or unfractionated 12B1 tumor lysate for 24 hours. The DCs were washed and resuspended in PBS and then injected to mice subcutaneously (5 × 105 per mouse). Immunization with 12B1 CRCL–pulsed DCs significantly inhibited tumor growth, with more than 80% of mice remaining tumor free (Figure 4). This compares favorably with vaccination with 12B1 CRCL without DCs, resulting in rejection rates of 50% (data not shown). DCs pulsed with HSP70 were less effective, albeit also significantly better than DCs alone with about 30% protection (Figure 4). This was similar to the protection achieved with purified HSP70 without DCs (data not shown). Vaccination of mice with 12B1 lysate-pulsed DCs had only a minimal protective effect (Figure 4). These data indicate that while tumor-derived HSP70 or CRCL vaccines yield comparable protective immunity, this can be augmented by ex vivo pulsing of DCs only in the case of CRCL. These findings suggest that tumor-derived CRCL may have better capacity to activate DCs compared with unfractionated tumor lysate or purified HSPs that correlate well with our in vitro DC studies (Figure 4). We infer that chaperone proteins in CRCL are in part responsible for its vaccination potential from immunodepletion studies of CRCL. Chaperone proteins GRP94/gp96, HSP90, HSP70, and CRT were immunoprecipitated from tumor-derived CRCL; the depleted vaccine was pulsed onto DCs, which were then used to vaccinate mice as above. ELISPOT assays were performed using “full” CRCL in the restimulation phase. Vaccination with DCs pulsed with depleted CRCL resulted in an approximately 50% reduction in the IFN-γ output from those splenocytes compared with splenocytes from mice vaccinated and restimulated with nondepleted CRCL.
Immunization with DCs pulsed with 12B1-derived CRCL provides significant protection to 12B1 tumor challenge. DCs were incubated with 50 μg/mL 12B1-derived CRCL or the same concentration of purified HSP70 or unfractionated lysate protein for 24 hours. DCs (5 × 105) were injected subcutaneously into the groin on days - 14 and - 7, and the mice were challenged with 12B1 cells (103) in the opposite groin on day 0 (saline versus DC, not significant [ns]; DC versus DC + lysate, ns; DC versus DC + HSP70, P < .05; DC versus DC + CRCL, P < .0001; DC + HSP70 versus DC + CRCL, P < .005; pooled data from 2 experiments, n = 12-16 mice per group).
Immunization with DCs pulsed with 12B1-derived CRCL provides significant protection to 12B1 tumor challenge. DCs were incubated with 50 μg/mL 12B1-derived CRCL or the same concentration of purified HSP70 or unfractionated lysate protein for 24 hours. DCs (5 × 105) were injected subcutaneously into the groin on days - 14 and - 7, and the mice were challenged with 12B1 cells (103) in the opposite groin on day 0 (saline versus DC, not significant [ns]; DC versus DC + lysate, ns; DC versus DC + HSP70, P < .05; DC versus DC + CRCL, P < .0001; DC + HSP70 versus DC + CRCL, P < .005; pooled data from 2 experiments, n = 12-16 mice per group).
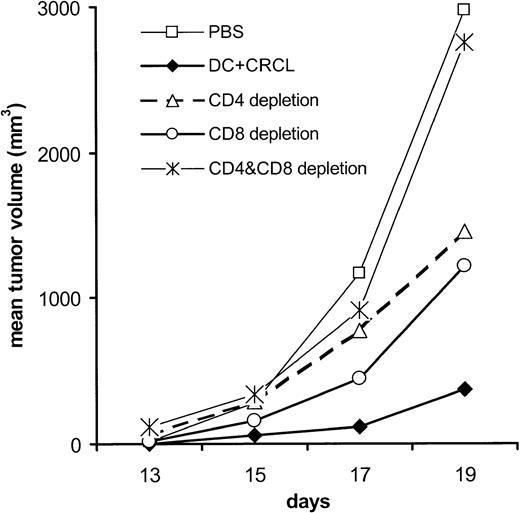
To define the roles of CD4+ and CD8+ T cells in the protective immunity induced by CRCL-loaded DCs, mice were depleted of CD4+, CD8+, or both T-cell populations. Immunity was partially abrogated when either CD4+ or CD8+ T cells were depleted. Complete loss of protection was observed in mice that were depleted of both T-cell populations, indicating that CD4+ and CD8+ T cells contribute to the antitumor immunity generated by CRCL-loaded DCs (Figure 5).
Antitumor immunity induced by DCs pulsed with 12B1-derived CRCL is both CD4+ and CD8+ T-cell dependent. On days - 14 and - 7, mice were immunized subcutaneously with DCs (5 × 105) that had been preincubated with 50 μg/mL 12B1-derived CRCL for 24 hours. Groups of mice were injected intraperitoneally with 200 μg anti-CD4 and/or anti-CD8 antibodies on days - 3, - 1, + 1, and + 3. On day 0, mice were challenged with 12B1 cells (103) in the opposite groin (saline versus CD4 and CD8 depletion, ns; CRCL versus CD4 and CD8 depletion, P < .05; n = 4 mice per group, representative data from 1 of 2 experiments).
Antitumor immunity induced by DCs pulsed with 12B1-derived CRCL is both CD4+ and CD8+ T-cell dependent. On days - 14 and - 7, mice were immunized subcutaneously with DCs (5 × 105) that had been preincubated with 50 μg/mL 12B1-derived CRCL for 24 hours. Groups of mice were injected intraperitoneally with 200 μg anti-CD4 and/or anti-CD8 antibodies on days - 3, - 1, + 1, and + 3. On day 0, mice were challenged with 12B1 cells (103) in the opposite groin (saline versus CD4 and CD8 depletion, ns; CRCL versus CD4 and CD8 depletion, P < .05; n = 4 mice per group, representative data from 1 of 2 experiments).
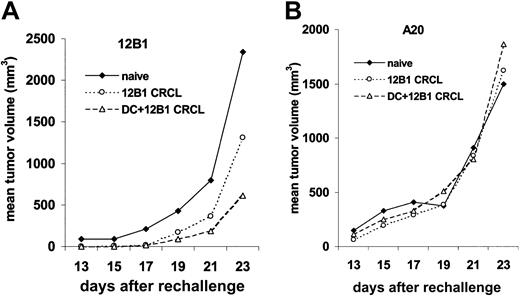
At 80 to 125 days after initial tumor challenge, surviving and naive mice, of the same ages as controls, were rechallenged with live 12B1 cells (103; LD100) in the right groin and A20 cells (106; LD100) in the left. Surviving mice from the group vaccinated with DCs pulsed with CRCL had retarded 12B1 growth, with 6 (55%) of 11 mice rejecting the rechallenge of 12B1. The group that received 12B1 CRCL vaccine (without DCs) also had delayed tumor growth, albeit less impressive than that achieved with DCs + CRCL, with 1 in 4 rejecting their 12B1 rechallenge. In contrast, all naive mice developed 12B1 tumors (Figure 6). Moreover, all of the immune and naive mice that were challenged with A20 leukemia cells in the opposite groin developed tumors with similar growth rates, confirming the specificity of the antitumor response generated by 12B1 CRCL immunizations (Figure 6).
DCs pulsed with 12B1-derived CRCL induce a long-term tumor-specific immune response. (A) At 11 or 18 weeks after initial challenge, naive mice (n = 3) or surviving mice (n = 4 for CRCL, 11 for DCs pulsed with CRCL) were rechallenged with 103 live 12B1 cells. Tumor volume was monitored. (B) The same mice were challenged with 106 A20 cells in the right groin.
DCs pulsed with 12B1-derived CRCL induce a long-term tumor-specific immune response. (A) At 11 or 18 weeks after initial challenge, naive mice (n = 3) or surviving mice (n = 4 for CRCL, 11 for DCs pulsed with CRCL) were rechallenged with 103 live 12B1 cells. Tumor volume was monitored. (B) The same mice were challenged with 106 A20 cells in the right groin.
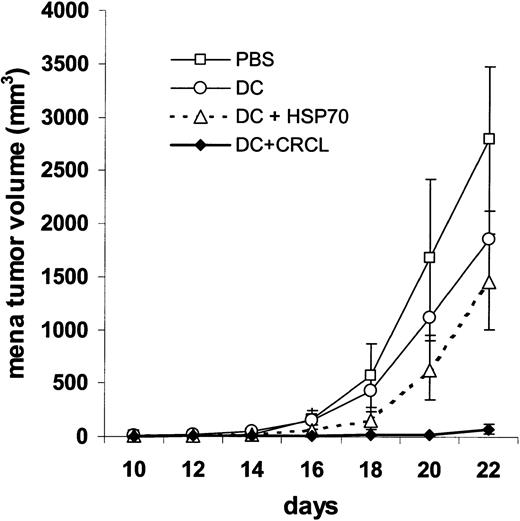
CRCL-pulsed DCs are effective therapeutic vaccines. To examine the potency of a CRCL-loaded DC vaccine in a pre-existing tumor setting, we first induced 12B1 tumor followed by a single subcutaneous immunization with chaperone-pulsed DCs. Specifically BALB/c mice received a lethal dose (LD100) of 12B1 sucutaneously on day 0 and were immunized in the opposite groin on day + 2 with DCs (5 × 105 per mouse) pulsed with HSP70 or CRCL. The group receiving HSP70-loaded DCs showed a delay in tumor growth when compared with saline controls, but this was not significantly different from mice receiving unpulsed DCs. However, CRCL-loaded DCs significantly delayed tumor development when compared with all other groups. Moreover, this therapeutic vaccine resulted in eradication of tumor growth in 12 of (75%) 16 of the mice (Figure 7).
DCs pulsed with 12B1-derived CRCL are effective therapeutic vaccines in tumor-bearing mice. DCs were incubated with 50 μg/mL 12B1-derived CRCL or the same concentration of purified HSP70 for 24 hours. Mice were injected with 12B1 cells (103) subcutaneously in the right groin on day 0 and injected with CRCL- or HSP70-loaded DCs (5 × 105) in the opposite groin on day 2 (DC versus DC + HSP70, ns; DC versus DC + CRCL, P < .05; DC + HSP70 versus DC + CRCL, P < .005; pooled data from 2 experiments, n = 8-16 mice per group).
DCs pulsed with 12B1-derived CRCL are effective therapeutic vaccines in tumor-bearing mice. DCs were incubated with 50 μg/mL 12B1-derived CRCL or the same concentration of purified HSP70 for 24 hours. Mice were injected with 12B1 cells (103) subcutaneously in the right groin on day 0 and injected with CRCL- or HSP70-loaded DCs (5 × 105) in the opposite groin on day 2 (DC versus DC + HSP70, ns; DC versus DC + CRCL, P < .05; DC + HSP70 versus DC + CRCL, P < .005; pooled data from 2 experiments, n = 8-16 mice per group).
Discussion
Chaperone proteins are the most abundant soluble intracellular molecules. Under physiologic conditions, their ability to stimulate immune responses is not expected, since the immune system normally does not attack itself. However, when presented to cells extracellularly, HSPs or chaperone proteins may act as danger signals alerting the immune system to tissue injury.14, 15, 16 Individual chaperone proteins, such as HSP70 and GRP94/gp96, have been shown to deliver partial maturation signals to DCs and to stimulate proinflammatory cytokine expression, thus inducing Th1-type immune responses.17, 18, 19, 20, 21 Stress proteins are known to perform their intracellular functions as multiprotein complexes consisting of chaperones, cochaperones, and substrate molecules. In our previous studies, we have demonstrated that chaperone proteins within the CRCL separate in complexes7 and therefore may more accurately recreate danger signals, consequently activating APCs more efficiently than individual purified chaperone proteins.
DCs are professional APCs, known to be critical activators of T-cell responses. Pulsing DCs with tumor antigens, such as peptides, tumor lysate, or apoptotic tumor cells has been demonstrated to generate tumor-specific protective immunity.22, 23, 24 In the present study, we show that immunization with DCs pulsed with 12B1-derived CRCL significantly delays or prevents tumor growth in vivo, indicating that CRCL-associated peptides are presented by DCs. Although the mechanisms are not completely clear, an increasing body of data suggests that DCs take up chaperone-peptide complexes through specific receptors and re-present the peptides on MHC-I molecules.25, 26, 27, 28, 29 Recently, receptors for HSP70 and GRP94/gp96 have been identified.13,30,31 Tumor-derived CRCL is made up of multiple chaperone proteins; therefore, additional potential chaperone receptors on the surface of APCs may be involved in the endocytosis of these complexes. Following uptake, the chaperone protein–escorted peptides are processed and represented on MHC molecules generating antigen-specific T-cell responses. In addition to HSP70, HSP90, GRP94/gp96, and CRT, other chaperone protein members are also present in the CRCL fractions, such as BiP/grp 78, grp75, small amounts of HSP72, HSP 40, and other unidentified proteins.7 We have preliminary data to indicate that immunodepletion of GRP94/gp96, HSP90, HSP70, and CRT from CRCL reduces—but does not completely abrogate— the effectiveness of CRCL-pulsed DCs to stimulate T cells (as assayed by IFN-γ release). It is therefore possible that other proteins in the CRCL may contribute to the superior immunogenicity demonstrated in these studies. Identifying the role of each of these proteins is critical and may help in the discovery of additional immunogenic chaperone proteins.
Tumor lysate has been used as a source of antigen for loading DCs in preclinical and clinical studies.32,33 In the 12B1 model and in the numerous other tumor models we have studied, such as A20 lymphoma,7 B16 melanoma, N2A neuroblastoma, Sa1 fibrosarcoma, and 4T1 mammary carcinoma34 (M.W.G., unpublished observations, June 2001), tumor lysate did not stimulate a measurable immune response when used alone, while some protection was achieved when lysates were loaded onto DCs. Moreover, when incubated with DCs, unfractionated tumor lysate did not change the phenotype of DCs or enhance their immunostimulatory function in an MLR. Subtle differences in the preparation of lysate and DC may contribute to the differential outcomes between our studies and those of others. This lack of protective immunity with unfractionated tumor lysate may be due to the presence of immunosuppressive factors in the lysate and/or to insufficient concentration of chaperone proteins and the peptides they carry (M.W.G. et al, manuscript in preparation). In contrast to lysate and consistent with our previous study,7 CRCL is very immunogenic, indicating that the FS-IEF step is essential to enhance the immunogenicity of tumor lysate preparations.
Since it has been reported that HSPs may share some common pathways with lipopolysaccharide (LPS),35 it is important to exclude the possibility that the immunogenic protection of CRCL is due to LPS contamination. Endotoxin level of CRCL is less than 0.01 EU/μg as examined by LAL assay. Moreover, A20 lymphoma–derived CRCL, which is prepared by the same method in parallel with 12B1 leukemia CRCL, showed no protection when mice were challenged with 12B1 tumor (Figure 2A). Furthermore, other studies in our laboratory36 demonstrated that CRCL from normal mouse tissues provided danger signals to apoptotic tumor cells and elicited antitumor immunity. However, the immunoprotection was not evident when mice were immunized with liver CRCL alone, indicating that liver CRCLs, per se, are not immunogenic. These data support the theory that the immunizing effects of 12B1 CRCL were not the result of LPS contamination.
It has been shown by a variety of studies that the immunogenicity of tumor-derived chaperones results from the antigenic peptides escorted by the chaperone proteins.5,6,37,38 We have recently utilized ELISPOT assays in which mice were vaccinated with DCs pulsed either with 12B1-derived CRCL or with a specific BCR-ABL peptide. Restimulation of either group's splenocytes with either 12B1 CRCL or BCR-ABL peptide resulted in substantial IFN-γ release, thus immunologically indicating that a specific BCR-ABL peptide is a component of the peptide antigen repertoire of 12B1-derived CRCL. This has also been similarly demonstrated by specific CTL assays in which splenocytes from mice primed with either 12B1 CRCL or BCR-ABL peptide were able to lyse cells “coated” either with CRCL or with the BCR-ABL peptide (Y.Z. et al, manuscript in preparation). If different chaperone proteins preferentially escort distinct peptides, CRCLs may have a broader antigenic repertoire and therefore more tumor-specific epitopes than those escorted by individual chaperone proteins, such as HSP70. We have also examined the immunogenicity of individual CRCL fractions (of those that are pooled to constitute the vaccine) by comparing fractions, distinct in overall protein profile but still containing 4 of the known immunogenic chaperones, HSP70, HSP90, GRP94/gp96, and CRT. We found that these fractions had comparable activity in prolonging survival of mice.7 Importantly, irrelevant FS-IEF fractions (ie, containing little or no detectable chaperone proteins) provided no protective benefit. Thus, the protective effect of CRCL vaccination is not an artifact of the isoelectric focusing procedure. Given the limitations of preparing tumor vaccines from autologous tumor in the clinical setting, FS-IEF is a relatively simple, rapid, and efficient procedure allowing one to obtain 30 to 50 times as much vaccinating material from the same quantity of tumor as with conventionally purified HSPs. This vaccinating material includes the 4 major immunogenic chaperones as well as other chaperone and nonchaperone proteins, many of which remain unidentified.
Using the A20 B-cell leukemia model, we reported that HSP70 preparations induced more potent tumor-specific immunity than any of the other known immunogenic chaperones isolated from the same tumor.3 In this study, we directly compared the immunogenicity of 12B1 CRCL with that of purified HSP70. We found that CRCL alone provided equivalent protection against tumor challenge when compared with 12B1 HSP70 immunizations. However, when these chaperones where incubated with DCs and utilized as a cellular vaccine, CRCL-pulsed DCs showed distinctly better antitumor immunity than HSP70-pulsed DCs in both a prophylactic and therapeutic setting. This suggests that CRCL may deliver additional signals important in activation of DCs and/or provide additional tumor-specific peptides to these APCs. Since 12B1 is a very aggressive tumor, with an LD100 of 103 cells by subcutaneous injection, achieving cures in a pre-established tumor setting by CRCL-loaded DCs attests to the effectiveness of this approach.
In conclusion, the present study demonstrates that when compared with purified HSP70 or unfractionated lysate, CRCL has superior ability to activate/mature DCs and is able to induce potent, long-lasting and tumor-specific T-cell–mediated immunity. The FS-IEF technique for enhancing for multiple chaperones from tumor lysate is relatively easy and rapid, yielding sufficient immunogenic material for clinical use. CRCL in combination with DCs may provide a practical and effective strategy for the development of a clinically useful anticancer vaccine.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-10-3108.
Supported in part by the Leukemia and Lymphoma Society of America, the Tee Up for Tots Fund, the Arizona Disease Control Research Commission, and the Michael Landon Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Luke Whitesell for his helpful comments, Marilyn Marron for help with CRCL preparations, Jane Davis for help with the chaperone depletion experiments, and Barbara Carolous for assistance with FACS analysis.

![Figure 2. CRCL vaccine generates tumor-specific immunity. (A) Immunization with 12B1-derived CRCL provides specificity protection against 12B1 tumor challenge. BALB/c mice were immunized with 20 μg 12B1- or A20 tumor–derived CRCL, on days - 14 and - 7, followed by challenge with 103 12B1 cells (LD100) in the opposite groin on day 0 (saline versus CRCL [12B1], P < .05 from day 15 onward; n = 11-24 mice per group). (B) Immunization with purified HSP70 or CRCL from 12B1 tumor delays tumor growth. Mice were immunized with 20 μg 12B1 tumor–derived HSP70 or CRCL or unfractionated lysate on days - 14 and - 7 followed by challenge with 103 12B1 cells in the opposite groin on day 0 (saline vs HSP70 or CRCL, P < .05 from day 15 onward; n = 8 mice per group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3108/5/m_h81134417002.jpeg?Expires=1764988974&Signature=RzzEk8xbDxHpGs2ExiqVrLDyzkxh3NrQhhqz~Nc8WGVaj9fvfVcC7A-Q~~BgYDk2wUYVjiVqU3Ms7QIL17yzq1cr-lgIh0jnCMjZkX8b4CCB5k53NndmbwSCuharRxIo4ghZT3fMbdIVE6P1FIvMjiABGfd3sbmXgpTG2uQysVUCet5TbZW6Z7o58BvCMp2JPAq7i5T-XTrsGUNZRevIFMB7hJdE2YBX9IJePk-QxLWRaFYSVxytTz2vgAyMBaH8pljzJuQkosevQwEzdyDWuaIaiu3trcjY8RSHFrW5ulYTBL6haJigZenBlCrbmMTsURpJT9O3I3bx-pIOHzkpbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Tumor-derived CRCL activates DCs. (A) 12B1-derived chaperone proteins stimulate DCs to express MHC-II and costimulatory molecules. Day 6 bone marrow–derived DCs, grown in GM-CSF and IL-4, were exposed to 50 μg/mL 12B1 HSP70 (□), CRCL (▪), or unfractionated lysate (▨) for 24 hours, harvested, and analyzed by flow cytometry for expression of the cell-surface markers indicated. MFI of CD40, CD80, CD86, and MHC-II (I-Ad) on CD11c+ gated nontreated DCs was considered as one. The numbers in the ordinate represent the fold increase in MFI of treated DCs when compared with nontreated DCs. Means and SEM from 3 experiments are shown. (B) DCs pulsed with CRCL have increased IL-12 production. ELISPOT assays were performed to measure IL-12 production by DCs. At day 6 of culture, 3 × 105 DCs were cultured with 50 μg/mL 12B1-derived lysate, HSP70, or CRCL in the presence of 10 ng/mL GM-CSF and IL-4 each for 24 hours (DC + CRCL or DC + HSP70 versus DC or DC + lysate, P < .05; DC + CRCL versus DC or DC + HSP70, P < .05; representative data from 3 experiments are shown). (C) 12B1-derived CRCL increases DC capacity to stimulate allogeneic splenocyte proliferation. DCs were cultured as in “Materials and methods,” harvested, treated with Mitomycin C, and washed. Splenocytes from C57BL6 mice were added (105 per well) and cultured with the indicated ratios of pretreated BALB/c DCs for 4 days. [3H]thymidine was added and the cells were cultured for an additional 18 hours before the incorporated radioactivity was counted (DC + CRCL versus DC, DC + lysate, or DC + HSP70; P < .05; representative data from 2 experiments are shown). cpm indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3108/5/m_h81134417003.jpeg?Expires=1764988974&Signature=u0wuZcvsbKFHXr-N2eirIoz8O-0QyydJSnGvxQiUnVGEOaczJ5U3TsyGAnSPzuC45ppr6~RN6LVVy0U2lIsIFAanmXW-dTZxrQjLKWJ~~sN4MxlEgQ6LAwXCpa2MbGbpZ7W8N6y2wKru0-9M~1hE5V1pDpFP~w0fgo~hcEqeELrvIW1cjregdlc7oPdHSi~dTvNGyZKHahHUg8CIdKeab1coaD301X0MIYm6sT6AVw9D4nGtsdQW9Bnd0GoZ6k36k3iWNGhNmbZVE6DzEAodY7CoUlRxB-T81zM0V7ug4QBi3h~tZf6MMXehjwk32LmQe1mWRbWe103HWiweICzUrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Immunization with DCs pulsed with 12B1-derived CRCL provides significant protection to 12B1 tumor challenge. DCs were incubated with 50 μg/mL 12B1-derived CRCL or the same concentration of purified HSP70 or unfractionated lysate protein for 24 hours. DCs (5 × 105) were injected subcutaneously into the groin on days - 14 and - 7, and the mice were challenged with 12B1 cells (103) in the opposite groin on day 0 (saline versus DC, not significant [ns]; DC versus DC + lysate, ns; DC versus DC + HSP70, P < .05; DC versus DC + CRCL, P < .0001; DC + HSP70 versus DC + CRCL, P < .005; pooled data from 2 experiments, n = 12-16 mice per group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/11/10.1182_blood-2002-10-3108/5/m_h81134417004.jpeg?Expires=1764988974&Signature=BByuwxRYDn5wXbKwQzsm8ux0a3oKXDyuazg65RtPpt7EdwU6U0P492DVyKzwVZ3iQnxstCzL6VUUdcimBPRXXaZ~1YjYAizcs042o7SclkRKpBKAxUdnwX4r0sq5k1o37boNXAY3BbcAf4xNMVHVN7~lxIJQHel33z6n7txYuL9JiT7R-Z5Tu5omOSNXASEIeW9LVlgstdspDV8JSLS7vRvsNujiVpk9Ve9yxsoeml-E0dher8Hsc1aSYIZFZQfcVTohICTDpJ1OFstOLFPZdIzvLD3y3uUk8yQ2zFpnaSNyPFq7MXnyfiy84rvL9wACBd2j9D3UdZqWC1Bpmwkw5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal