Abstract
We have generated a transgenic mouse line that reaches a hematocrit concentration of 0.85 due to constitutive overexpression of human erythropoietin in an oxygen-independent manner. Unexpectedly, this excessive erythrocytosis did not lead to thrombembolic complications in all investigated organs at any age. Thus, we investigated the mechanisms preventing thrombembolism in this mouse model. Blood analysis revealed an age-dependent elevation of reticulocyte numbers and a marked thrombocytopenia that matched the reduced megakaryocyte numbers in the bone marrow. However, platelet counts were not different from wild-type controls, when calculations were based on the distribution (eg, plasma) volume, thereby explaining why thrombopoietin levels did not increase in transgenic mice. Nevertheless, bleeding time was significantly increased in transgenic animals. A longitudinal investigation using computerized thromboelastography revealed that thrombus formation was reduced with increasing age from 1 to 8 months in transgenic animals. We observed that increasing erythrocyte concentrations inhibited profoundly and reversibly thrombus formation and prolonged the time of clot development, most likely due to mechanical interference of red blood cells with clot-forming platelets. Transgenic animals showed increased nitric oxide levels in the blood that could inhibit vasoconstriction and platelet activation. Finally, we observed that plasmatic coagulation activity in transgenic animals was significantly decreased. Taken together, our findings suggest that prevention of thrombembolic disease in these erythrocytotic transgenic mice was due to functional consequences inherent to increased erythrocyte concentrations and a reduction of plasmatic coagulation activity, the cause of which remains to be elucidated.
Introduction
A high hematocrit is expected to be associated with an increased risk of thrombosis or embolism. Numerous reports from patients with polycythemia vera and pseudopolycythemia confirm a correlation of elevated hematocrit levels and the incidence of thrombosis.1, 2, 3
The Framingham study established a positive correlation between the hematocrit value and the risk of cerebral infarction,4 and in a prospective study a hematocrit level higher than 0.51 was found to be an independent risk factor for stroke.5 In cyanotic congenital heart disease, exceedingly high hematocrit values of up to 0.80 have been recorded,6 and cerebral and pulmonary infarcts7 as well as cerebral venous thrombosis correlate with hematocrit levels.8,9 Some studies even suggest a procoagulatory role for erythrocytes put forth on platelets.10,11
A functional dissection of the relationship between the hematocrit, the activation of hemostasis and coagulation, and the presence of thrombembolism had been difficult to investigate due to the lack of an adequate animal model. Thus, we generated a suitable mouse model by establishing a transgenic mouse line characterized by an isolated erythrocytosis.12 These transgenic mice, constitutively overexpressing the human erythropoietin gene in an oxygen-independent manner, reach a hematocrit plateau of 0.80 to 0.85 within the first 2 months without altering their blood pressure, heart rate, or cardiac output.13 We showed that in these transgenic mice, endothelial nitric oxide synthase levels and nitric oxide–mediated endothelium-dependent relaxation was significantly increased12 despite concomitant expression of the vasoconstrictor endothelin-1.14 Apart from this, another transgenic mouse line lacking the receptor tyrosine kinase c-kit that exhibits hematopoietic defects causing perinatal death could be rescued by breeding with our erythropoietin-overexpressing transgenic mouse.15 Of note, despite the fact that erythropoietin was found to activate components of oxidative metabolism pathways in the brain of our transgenic mouse that could be related to neuroprotective effects of erythropoietin,16 we found markedly increased cerebral infarction volumes in our transgenic mice on permanent occlusion of the middle cerebral artery.17
With aging, we observed a significantly increased mortality in erythrocytotic transgenic mice after 6 to 8 months.13 Obviously, thrombosis or impaired coagulation (or both) might contribute to their reduced life expectancy. Therefore, the aim of this study was to analyze the erythropoietin-overexpressing transgenic mouse line for any signs of thrombosis or embolism, characterize its hemostasis and coagulation system, and functionally investigate the contribution of raised erythrocyte concentrations to clot characteristics and thrombosis.
Materials and methods
The transgenic mouse line termed tg6 carries the human erythropoietin cDNA driven by the platelet-derived growth factor β (PDGF-β) promotor as described earlier.12 The transgene is inherited as an autosomal dominant trait. Hemizygous transgenic males were mated to wild-type C57Bl/6 females, and the wild-type littermates served as controls in all experiments. Transgenic mice reached plasma erythropoietin levels of 123.8 ± 29.0 U/L compared with 8.4 ± 17.9 U/L in wild-type control siblings. Animals were kept in conventional housing conditions and had unrestricted access to food and water up to the experiment. All investigations were performed with the approval of the state animal ethics committee.
In anesthetized mice (inspiratory isoflurane concentration 1%-1.5% supplemented with oxygen), subaquatic bleeding time was measured from standardized dissection of the tail tip. It was cut at a diameter of 0.16 mm and placed in 37°C sterile physiologic saline solution; the time until the stream of erythrocytes stopped was recorded. If after 15 minutes the bleeding had not stopped spontaneously, the wound was cauterized and a bleeding time of 15 minutes was used for statistical calculations.
Histologic slides (1-3 μm) were cut from paraffin-embedded material and stained with hematoxylin and eosin or Masson Goldner trichrome stain.
Blood was sampled from anesthetized mice from the retro-orbital plexus for microhematocrit determination and from the caudal vena cava for all other studies. After a median laparotomy, the caudal vena cava was exposed, cannulated with a 26-gauge catheter (Abbocath-T, Abbott Ireland, Sligo, Republic of Ireland), and the mouse exsanguinated at a rate of 1 mL blood per minute. Typical blood volumes collected were 800 to 1200 μL and 1500 to 2700 μL for wild-type and transgenic mice, respectively,13 gaining 300 to 450 μL and 200 to 350 μL plasma, respectively. Because hypobaric pressures can lead to rupture of the erythrocyte membrane, activate coagulation, and thus disturb coagulation measurements, particular care was taken to avoid negative pressures during blood withdrawal.
The distribution volume of the coagulation inhibitor sodium citrate is the plasma volume. For determination of the activated partial thromboplastin time, the prothrombin time, and the thrombin time, the amount of sodium citrate added was calculated based on the plasma fraction (1 – hematocrit value) of the blood. Adding sodium citrate to whole blood in the normal ratio of 1:10 had been reported to be the cause of false pathologic coagulation test results in patients with polycythemia vera.18 Hematologic parameters were measured with a VetABC blood counter (ABX Diagnostics, Montpellier, France); coagulation and clinical chemistry parameters were measured with a KC-10 (Fa Amelung, Lemgo, Germany) and a Han Aeroset analyzer (Abbott, Wiesbaden, Germany), respectively. Computerized thromboelastograms were performed with a TEG 5000 analyzer (Haemoscope, Niles, IL). All tests described above were carried out in 5 groups of male mice aged 1, 2, 4, 6, and 8 months.
For thromboelastogram analysis performed on native whole blood, the standardized blood withdrawal time was 1 minute, and the total elapsed time until the beginning of the measurement was 2 minutes after the start of the blood sampling. To investigate the functional role of the erythrocyte and platelet concentrations on the thrombelastogram, the respective cell concentration of whole blood was adjusted by addition of platelet-rich plasma or platelet-poor plasma. Platelet-rich plasma and platelet-poor plasma were obtained after centrifugation of citrated whole blood (4°C 10 minutes) at 850g and 2550g, respectively.
To determine the osmotic fragility, washed erythrocytes from wild-type and transgenic mice were resuspended in 0.9% NaCl and the hematocrit level adjusted to 0.45. Erythrocyte suspension (10 μL) was added to 100 μL lysis solution containing decreasing concentrations of NaCl (120-45 mM corresponding to an osmolality of 210-82 mOsm/kg), incubated for 5 minutes at room temperature, and centrifuged at 2000g for 2 minutes. The absorbance of the supernatant was determined at 550 nm.
Nitrate concentrations in plasma were measured with the Griess reaction (R & D Systems, Minneapolis, MN); plasma concentrations of thrombopoietin and erythropoietin were measured by enzyme-linked immunosorbent assay (ELISA; Mouse TPO Immunoassay, R & D Systems, and EPO-ELISA Medac, Medac, Wedel, Germany). Total RNAwas extracted from liver,19 reverse transcribed, and thrombopoietin mRNA quantified by competitive polymerase chain reaction (PCR) normalized against glyceraldehydes-3-phosphate dehydrogenase (GAPDH) expression. The primer sequences for thrombopoietin competitive PCR were ctctgtccagccccgtagc (forward) and ccccaagaggaggcgaac (reverse) yielding a product length of 314 bp.20
All data were tested with parametric tests (Student t test or ANOVA with Bonferroni post hoc test as applicable) for statistical significance of differences (P < .05), except erythropoietin and thrombopoietin plasma protein concentrations, which were analyzed with the nonparametric Mann-Whitney test. Results are presented as mean values ± SD unless stated otherwise.
Results
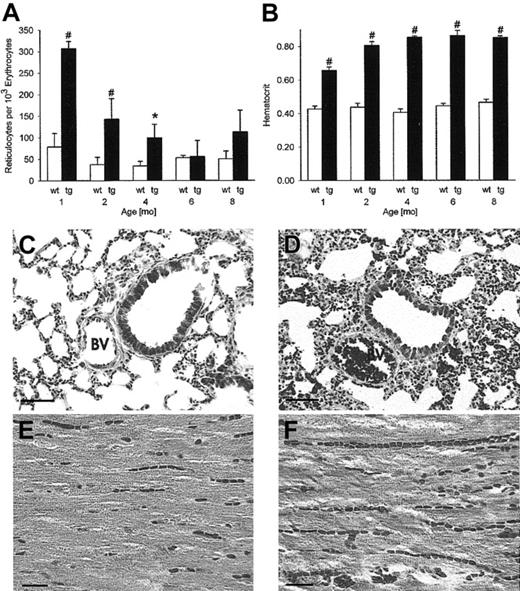
Excessive erythrocytosis implies increased numbers of young erythrocytes and reticulocytes. Indeed, at 1, 2, and 4 months the reticulocyte count in peripheral blood was increased significantly in transgenic mice (Figure 1A), ultimately resulting in the marked rise of the hematocrit level up to the plateau of 0.85 (Figure 1B).
Lack of histologic evidence of thrombosis or emboli in any organ despite a hematocrit value of 0.85. Reticulocyte numbers (A) were increased in erythropoietin transgenic mice, most prominently in the first 4 months (n = 5 per group). Time course of the hematocrit value (B) shows a plateau of 0.85 from 2 months onward in transgenic mice (n = 30 per group). BV indicates bronchial vein; wt, wild-type; tg, transgenic. *P < .01, #P < .001, compared with age-matched wild-type controls. Tissue sections from the lung and the heart of 8-month-old mice (C,E, wild-type mice; D,F, transgenic mice) demonstrate the plethora of erythrocytes in the vasculature of transgenic mice without signs of thrombosis or emboli. Original magnification, × 200 for panels C and D, trichrome, scale bar 50 μm. Original magnification, × 400 for panels E and F, hematoxylin and eosin, scale bar 20 μm.
Lack of histologic evidence of thrombosis or emboli in any organ despite a hematocrit value of 0.85. Reticulocyte numbers (A) were increased in erythropoietin transgenic mice, most prominently in the first 4 months (n = 5 per group). Time course of the hematocrit value (B) shows a plateau of 0.85 from 2 months onward in transgenic mice (n = 30 per group). BV indicates bronchial vein; wt, wild-type; tg, transgenic. *P < .01, #P < .001, compared with age-matched wild-type controls. Tissue sections from the lung and the heart of 8-month-old mice (C,E, wild-type mice; D,F, transgenic mice) demonstrate the plethora of erythrocytes in the vasculature of transgenic mice without signs of thrombosis or emboli. Original magnification, × 200 for panels C and D, trichrome, scale bar 50 μm. Original magnification, × 400 for panels E and F, hematoxylin and eosin, scale bar 20 μm.
Histology
Histologic sections from 8-month-old male transgenic mice suffering from excessive erythrocytosis were carefully evaluated for signs of tissue infarction (Figure 1), because an elevated hematocrit level is known to predispose to thrombosis and embolization. The plethora of blood vessels, most evident for veins and capillaries, was prominent in all tested tissues. We did not find any evidence of thrombosis, embolization, or tissue infarction in lung, heart (Figure 1), brain, liver, kidney, spleen, testes, or skeletal muscle (data not shown). Of note, no hemorrhage was observed in any tissue.
Hemostasis
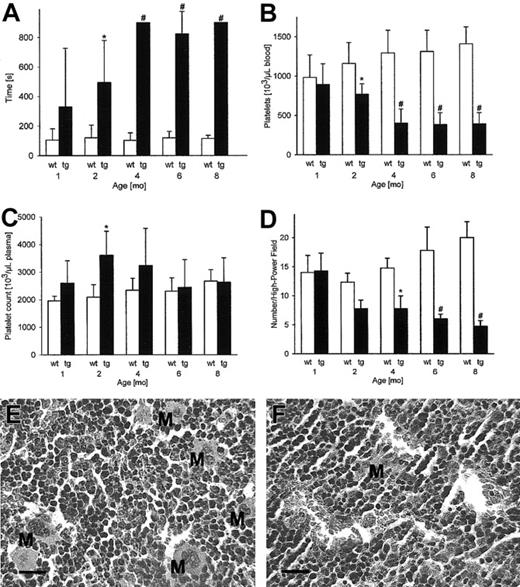
The lack of thrombosis despite the extremely high hematocrit value of 0.85 in adult transgenic mice led us to further investigate hemostasis and coagulation. Standardized subaquatic tail bleeding time measurements provided evidence that from 2 months onward hemostasis was significantly impaired in transgenic mice (Figure 2A). Platelets are known to contribute to hemostasis and thus concentrations thereof were subsequently measured. Apparently, at 1 month, platelet concentrations in transgenic mice were within the normal range, but in older mice a more than 50% decrease of platelet numbers was found (Figure 2B). However, considering that erythrocytes contribute 85% to the blood volume, we calculated the platelet concentration in the plasma volume fraction. As shown in Figure 2C, platelet concentrations in transgenic mice were no longer decreased when the platelet distribution volume in plasma was taken into account. Platelets are released from megakaryocytes mainly in response to the glycoprotein thrombopoietin. However, neither thrombopoietin mRNA (wild-type, 15.0 ± 4.2 versus transgenic, 10.6 ± 3.5 aM/fM; not significant) nor plasma levels (wild-type, 377 ± 128 versus transgenic, 474 ± 411 pg/mL; not significant) were significantly decreased in transgenic mice.
Subaquatic bleeding time, platelet concentrations, and bone marrow histology showing reduced megakaryocyte numbers. Subaquatic bleeding time was measured to assess hemostasis (A). Apparently reduced platelet numbers in transgenic mice (B) were calculated to be in the same range or even increased in comparison with wild-type mice when the highly reduced distribution volume of platelets; that is, the plasma fraction, was taken into account (C). Sternal bone marrow was quantified for the presence of megakaryocytes per high-power (× 400) visual field (D) showing a progressive reduction of megakaryocyte numbers with increasing age (n = 5 per group). *P < .01, #P < .001, compared with age-matched wild-type controls. Panels E (wild-type) and F (transgenic) illustrate the reduced number of megakaryocytes in 8-month-old transgenic mice compared with age-matched wild-type controls. M indicates megakaryocyte. All sections were stained with hematoxylin and eosin (original magnification, × 400), scale 20 μm.
Subaquatic bleeding time, platelet concentrations, and bone marrow histology showing reduced megakaryocyte numbers. Subaquatic bleeding time was measured to assess hemostasis (A). Apparently reduced platelet numbers in transgenic mice (B) were calculated to be in the same range or even increased in comparison with wild-type mice when the highly reduced distribution volume of platelets; that is, the plasma fraction, was taken into account (C). Sternal bone marrow was quantified for the presence of megakaryocytes per high-power (× 400) visual field (D) showing a progressive reduction of megakaryocyte numbers with increasing age (n = 5 per group). *P < .01, #P < .001, compared with age-matched wild-type controls. Panels E (wild-type) and F (transgenic) illustrate the reduced number of megakaryocytes in 8-month-old transgenic mice compared with age-matched wild-type controls. M indicates megakaryocyte. All sections were stained with hematoxylin and eosin (original magnification, × 400), scale 20 μm.
Because nitric oxide inhibits hemostasis, we next measured the plasma concentration of nitrate, the stable conversion product, or nitric oxide. Notably, we found a significant increase of nitrate in transgenic mice compared with controls (wild-type versus transgenic, 34.8 ± 10.6 versus 54.3 ± 14.3 μM; P < .01).
Bone marrow
Subsequently, bone marrow sections were evaluated for the presence of megakaryocytes. Whereas megakaryocytes were only moderately reduced in transgenic mice until the second month (Figure 2D), megakaryocyte numbers were markedly reduced in 8-month-old transgenic mice compared with wild-type control mice (Figure 2E-F). Taken together, it is conceivable that the constant acceleration of erythrocyte production resulted in the displacement of thrombopoiesis in the bone marrow by red lineage cells in erythropoietin transgenic mice.
Thrombus formation
To learn more about the lack of thrombosis and embolization in transgenic mice, blood coagulation was further investigated by thromboelastography. Thromboelastography allows the measurement of the complete clotting process, specifically the kinetics of clot development (α-angle), the strength of the clot (maximal amplitude), and fibrinolysis (clot lysis). Most importantly, ex vivo native whole blood from 2-month-old transgenic mice showed a significantly reduced clot strength that declined further with increasing age (Figure 3; Table 1, maximal amplitude). In addition, the kinetics of clot formation were retarded in transgenic mice (Table 1, α-angle).
Computerized thromboelastography of wild-type and transgenic mice blood: the effect of hematocrit and platelet concentration. Computerized thromboelastography was used to investigate clot formation. Native whole blood from wild-type (dashed lines) and transgenic (solid lines) mice was analyzed and typical traces of the different age groups are shown (A; n = 4-6). Clot strength was reduced as early as 1 month in transgenic mice and further declined with age compared with wild-type controls. The erythrocyte concentration was increased in blood from transgenic mice and, at the same time, the concentration of platelets was decreased. To characterize the effect on the thromboelastogram of the erythrocyte concentration separate from the effect of the platelet concentration, experiments were performed where the concentration of one cell type was kept constant while the concentration of the other was varied. Increasing the hematocrit level when the platelet concentration was kept at 1000 (103/μL) (B;n = 3) resulted in a marked and progressive reduction of clot strength. When the hematocrit value was kept constant at 0.40 and the platelet concentration was varied (C;n = 3), a small reduction of clot strength with decreasing platelet concentrations was found; wt indicates wild-type; tg, transgenic; m, month; Hct, hematocrit; Plt, platelet number (103/μL).
Computerized thromboelastography of wild-type and transgenic mice blood: the effect of hematocrit and platelet concentration. Computerized thromboelastography was used to investigate clot formation. Native whole blood from wild-type (dashed lines) and transgenic (solid lines) mice was analyzed and typical traces of the different age groups are shown (A; n = 4-6). Clot strength was reduced as early as 1 month in transgenic mice and further declined with age compared with wild-type controls. The erythrocyte concentration was increased in blood from transgenic mice and, at the same time, the concentration of platelets was decreased. To characterize the effect on the thromboelastogram of the erythrocyte concentration separate from the effect of the platelet concentration, experiments were performed where the concentration of one cell type was kept constant while the concentration of the other was varied. Increasing the hematocrit level when the platelet concentration was kept at 1000 (103/μL) (B;n = 3) resulted in a marked and progressive reduction of clot strength. When the hematocrit value was kept constant at 0.40 and the platelet concentration was varied (C;n = 3), a small reduction of clot strength with decreasing platelet concentrations was found; wt indicates wild-type; tg, transgenic; m, month; Hct, hematocrit; Plt, platelet number (103/μL).
Clot formation kinetics, clot strength, and clot lysis in wild-type and transgenic mice
. | Age, mo . | α-Angle . | Maximal amplitude, mm . | Clot lysis, % . |
|---|---|---|---|---|
| Wild-type | ||||
| 1 | 72.8 ± 6.7 | 82.5 ± 0.7 | 0 ± 0 | |
| 2 | 66.1 ± 13.1 | 79.4 ± 4.9 | 0.1 ± 0.3 | |
| 4 | 76.3 ± 0.8 | 79.8 ± 0.9 | 0 ± 0 | |
| 6 | 62.3 ± 6.5 | 76.8 ± 2.5 | 0 ± 0 | |
| 8 | 68.1 ± 7.1 | 77.6 ± 3.4 | 0 ± 0 | |
| Transgenic | ||||
| 1 | 70.0 ± 4.8 | 73.2 ± 4.9 | 2.7 ± 3.4 | |
| 2 | 54.5 ± 15.2 | 55.5 ± 4.8* | 12.2 ± 11.3 | |
| 4 | 40.3 ± 13.5† | 48.9 ± 13.7† | 14.7 ± 10.3 | |
| 6 | 30.1 ± 14.5* | 49.1 ± 7.4† | 4.5 ± 10.1 | |
| 8 | 32.5 ± 6.9† | 47.0 ± 15.7† | 9.7 ± 14.6 |
. | Age, mo . | α-Angle . | Maximal amplitude, mm . | Clot lysis, % . |
|---|---|---|---|---|
| Wild-type | ||||
| 1 | 72.8 ± 6.7 | 82.5 ± 0.7 | 0 ± 0 | |
| 2 | 66.1 ± 13.1 | 79.4 ± 4.9 | 0.1 ± 0.3 | |
| 4 | 76.3 ± 0.8 | 79.8 ± 0.9 | 0 ± 0 | |
| 6 | 62.3 ± 6.5 | 76.8 ± 2.5 | 0 ± 0 | |
| 8 | 68.1 ± 7.1 | 77.6 ± 3.4 | 0 ± 0 | |
| Transgenic | ||||
| 1 | 70.0 ± 4.8 | 73.2 ± 4.9 | 2.7 ± 3.4 | |
| 2 | 54.5 ± 15.2 | 55.5 ± 4.8* | 12.2 ± 11.3 | |
| 4 | 40.3 ± 13.5† | 48.9 ± 13.7† | 14.7 ± 10.3 | |
| 6 | 30.1 ± 14.5* | 49.1 ± 7.4† | 4.5 ± 10.1 | |
| 8 | 32.5 ± 6.9† | 47.0 ± 15.7† | 9.7 ± 14.6 |
At 1, 2, 4, 6, and 8 months, coagulation was assessed by computerized thromboelastography of native whole blood from transgenic and wild-type animals. The maximal amplitude, a parameter of clot strength, as well as the α-angle, indicating the kinetics of clot formation, were significantly decreased in transgenic mice aged 4 months and older. Clot lysis was virtually absent in wild-type blood, and only a small percentage of clot lysis was found in transgenic mice. Clot lysis (%): 100 — (amplitude [mm] at 30 minutes after maximal amplitude normalized to maximal amplitude [mm] × 100). Each group had 4 to 6 mice.
P < .01, †P < .001 compared with age-matched wild-type control group.
We considered the effect of the hematocrit on the thrombus formation as a possible cause of the coagulation defect of transgenic mouse blood and in addition we were interested in elaborating the contribution of the platelet concentration to clot formation. In reconstitution experiments with wild-type blood, an increase of the hematocrit with the platelet count kept within the normal range resulted in a hematocrit-dependent decrease in clot strength and retarded clotting kinetics, indicating the interference of erythrocytes with clot formation (Figure 3). Of note, the clot strength was reduced to approximately the same extent as in old transgenic mice. When the platelet concentration was decreased in wild-type blood at a constant hematocrit level of 0.40 (Figure 3), a decrease of clot strength was noted, but that effect was much less pronounced compared with the influence of the hematocrit elevation.
These findings indicate that the elevated erythrocyte concentration is a major factor contributing to the retarded clotting in transgenic mice. Conversely, a reduction of hematocrit level would be expected to result in a normalization of the thrombelastogram and an increase in clot strength. Indeed, when transgenic blood was diluted with transgenic plasma to a hematocrit concentration of 0.40, the clot strength and the clot development kinetics were found to be close to normal values (Table 2). Similar results were obtained when wild-type plasma was added to transgenic blood for dilution (Table 2).
A reduction of the hematocrit resulted in a normalization of the clot formation kinetics and clot strength in transgenic mice
. | α-Angle . | Maximal amplitude, mm . | Clot lysis, % . | Platelets, 103/μL . | HCT value . |
|---|---|---|---|---|---|
| Tg blood diluted | 58.0 ± 7.6 | 57.5 ± 6.5 | 0.0 ± 0.0 | 732 ± 93 | 0.40 ± 0.01 |
| Tg RBC + wt plasma | 51.9 ± 12.1 | 56.3 ± 5.2 | 1.9 ± 4.3 | 812 ± 316 | 0.42 ± 0.03 |
| Wt blood + hemolysis | 62.3 ± 3.9 | 69.3 ± 1.0 | 0.0 ± 0.0 | 845 ± 16 | 0.42 ± 0.01 |
| Wt 4 mo | 76.3 ± 0.8 | 66.0 ± 6.8 | 0.0 ± 0.0 | 1010 ± 136 | 0.43 ± 0.02 |
| Tg 4 mo | 20.1 ± 5.3* | 35.1 ± 9.1* | 2.1 ± 2.3 | 338 ± 194* | 0.80 ± 0.02* |
. | α-Angle . | Maximal amplitude, mm . | Clot lysis, % . | Platelets, 103/μL . | HCT value . |
|---|---|---|---|---|---|
| Tg blood diluted | 58.0 ± 7.6 | 57.5 ± 6.5 | 0.0 ± 0.0 | 732 ± 93 | 0.40 ± 0.01 |
| Tg RBC + wt plasma | 51.9 ± 12.1 | 56.3 ± 5.2 | 1.9 ± 4.3 | 812 ± 316 | 0.42 ± 0.03 |
| Wt blood + hemolysis | 62.3 ± 3.9 | 69.3 ± 1.0 | 0.0 ± 0.0 | 845 ± 16 | 0.42 ± 0.01 |
| Wt 4 mo | 76.3 ± 0.8 | 66.0 ± 6.8 | 0.0 ± 0.0 | 1010 ± 136 | 0.43 ± 0.02 |
| Tg 4 mo | 20.1 ± 5.3* | 35.1 ± 9.1* | 2.1 ± 2.3 | 338 ± 194* | 0.80 ± 0.02* |
Clot strength (maximal amplitude) and clot kinetics (α-angle) were significantly increased after reduction of hematocrit by addition of plasma to red blood cells. For comparison, values for blood (sampled with sodium-citrate anticoagulation) from 4-month-old wild-type and transgenic mice are shown. Hemolysis (free hemoglobin concentration 3000 mg/L) did not impair the clot formation kinetics and clot strength. Clot lysis (%): 100 — (amplitude [mm] at 30 minutes after maximal amplitude normalized to maximal amplitude [mm] × 100). n = 3-5, except for hemolysis n = 2.
HCT indicates hematocrit; tg, transgenic; RBC, red blood cell; wt, wild-type.
P < .001 compared with all groups.
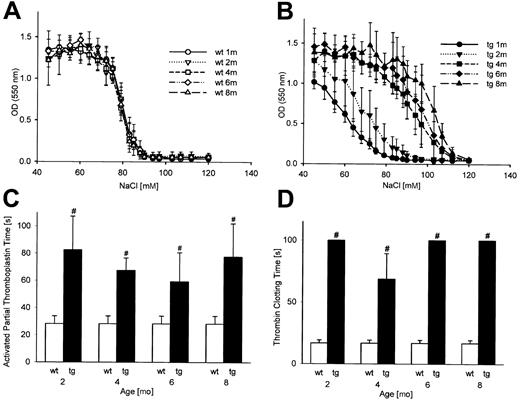
Because the plasma from transgenic mice was tinted light yellow, the presence of hemolysis products was investigated. Although the bilirubin level was normal and normal serum potassium values (Table 3) indicated adequate blood sampling techniques, the concentration of free hemoglobin (Table 3) and lactate dehydrogenase (data not shown) was significantly elevated in transgenic mice. In addition, the osmotic fragility of erythrocytes from transgenic mice was markedly increased in animals aged 4 months and older (Figure 4B), thus providing further evidence that hemolysis was present in these mice in vivo. To explore the impact of hemolysis products on clotting, wild-type blood was hemolyzed to yield the same concentration of free hemoglobin as in transgenic mice, and thromboelastograms were recorded. As shown in Table 2, no effects of elevated free hemoglobin levels on the clot strength were observed, suggesting that hemolysis is not a likely cause of the clotting deficiency in transgenic mice.
Selected coagulation and clinical chemistry data
. | Age, months . | Fibrinogen level, g/L . | Prothrombin time, s . | Total bilirubin level, μM . | Potassium level, mM . | Free hemoglobin level, mg/L . |
|---|---|---|---|---|---|---|
| Wild-type | ||||||
| 1 | ND | ND | ND | ND | 879.5 ± 303.3 | |
| 2 | 1.7 ± 0.9 | 11.8 ± 0.9 | <2 | 4.2 ± 1.1 | 747.7 ± 778.6 | |
| 4 | 1.3 ± 0.4 | 11.6 ± 0.9 | <2 | 4.3 ± 1.5 | 633.8 ± 339.8 | |
| 6 | 1.5 ± 0.7 | 12.0 ± 1.1 | <2 | 4.5 ± 1.2 | 199.3 ± 96.4 | |
| 8 | 1.4 ± 0.5 | 11.6 ± 0.9 | <2 | 4.7 ± 1.8 | 395.8 ± 182.5 | |
| Transgenic | ||||||
| 1 | ND | ND | <2 | ND | 1531.3 ± 1025.4 | |
| 2 | 1.5 ± 0.7 | 16.6 ± 0.7* | <2 | 5.3 ± 0.8 | 1794.6 ± 1506.6 | |
| 4 | 1.7 ± 1.1 | 17.5 ± 1.7* | <2 | 5.1 ± 1.3 | 9000.0 ± 2642.0* | |
| 6 | 1.5 ± 0.7 | 17.1 ± 0.9* | <2 | 4.8 ± 1.2 | 3317.5 ± 62.9* | |
| 8 | 1.3 ± 0.8 | 17.6 ± 2.3* | 4.5 ± 2.2 | 4.3 ± 1.2 | 5613.3 ± 3940.8† |
. | Age, months . | Fibrinogen level, g/L . | Prothrombin time, s . | Total bilirubin level, μM . | Potassium level, mM . | Free hemoglobin level, mg/L . |
|---|---|---|---|---|---|---|
| Wild-type | ||||||
| 1 | ND | ND | ND | ND | 879.5 ± 303.3 | |
| 2 | 1.7 ± 0.9 | 11.8 ± 0.9 | <2 | 4.2 ± 1.1 | 747.7 ± 778.6 | |
| 4 | 1.3 ± 0.4 | 11.6 ± 0.9 | <2 | 4.3 ± 1.5 | 633.8 ± 339.8 | |
| 6 | 1.5 ± 0.7 | 12.0 ± 1.1 | <2 | 4.5 ± 1.2 | 199.3 ± 96.4 | |
| 8 | 1.4 ± 0.5 | 11.6 ± 0.9 | <2 | 4.7 ± 1.8 | 395.8 ± 182.5 | |
| Transgenic | ||||||
| 1 | ND | ND | <2 | ND | 1531.3 ± 1025.4 | |
| 2 | 1.5 ± 0.7 | 16.6 ± 0.7* | <2 | 5.3 ± 0.8 | 1794.6 ± 1506.6 | |
| 4 | 1.7 ± 1.1 | 17.5 ± 1.7* | <2 | 5.1 ± 1.3 | 9000.0 ± 2642.0* | |
| 6 | 1.5 ± 0.7 | 17.1 ± 0.9* | <2 | 4.8 ± 1.2 | 3317.5 ± 62.9* | |
| 8 | 1.3 ± 0.8 | 17.6 ± 2.3* | 4.5 ± 2.2 | 4.3 ± 1.2 | 5613.3 ± 3940.8† |
Time course of some coagulation and clinical chemistry data of transgenic animals and age-matched controls; n = 3 to 6 per group.
ND indicates not determined.
P < 0.01.
P < 0.001 for age-matched intergroup comparison.
Increased erythrocyte osmotic fragility and decreased activity of the plasmatic coagulation in transgenic animals. Osmotic fragility of erythrocytes from transgenic mice (B) was markedly increased from 4 months onward compared with wild-type controls (A). Prolongation of the activated partial thromboplastin time (C) and the thrombin clotting time (D) revealed a decreased activity of the plasmatic coagulation; wt indicates wild-type; tg, transgenic; OD, optical density. *P < .01, #P < .001 compared with age-matched wild type controls.
Increased erythrocyte osmotic fragility and decreased activity of the plasmatic coagulation in transgenic animals. Osmotic fragility of erythrocytes from transgenic mice (B) was markedly increased from 4 months onward compared with wild-type controls (A). Prolongation of the activated partial thromboplastin time (C) and the thrombin clotting time (D) revealed a decreased activity of the plasmatic coagulation; wt indicates wild-type; tg, transgenic; OD, optical density. *P < .01, #P < .001 compared with age-matched wild type controls.
Plasma coagulation
Finally, we analyzed the plasma coagulation. Unexpectedly, clot formation via the intrinsic (Figure 4C) and the extrinsic (Table 3, prothrombin time) coagulation pathways was significantly impaired in transgenic mice. In addition, the thrombin time was also prolonged (Figure 4D). A reduced fibrinogen level in transgenic mice as cause of the impaired plasmatic coagulation was excluded (Table 3). Thus, the plasmatic coagulation was found to be less active in transgenic mice too.
Discussion
Hematocrit values of 0.72 to 0.91 have been reported in high-altitude inhabitants,21,22 erythrocytosis from congenital heart disease,23 neonatal erythrocytosis,24 polycythemia vera,18 and Chuvash polycythemia.25 From the published literature a hematocrit value of 0.85 was expected to be associated with thrombosis and embolization.2,3,26 Compared with sea-level population, long-term high-altitude inhabitants have an increased risk of stroke, and erythrocytosis is the common risk factor.27,28 In infants and young children with cyanotic congenital heart disease, the risk of cerebrovascular accidents is increased.8,29 However, the exact role of an increased hematocrit level as a risk factor per se for thrombembolic accidents remains a matter of debate. Indeed it has recently been reported that in adults with cyanotic congenital heart disease (mean hematocrit, 0.70 ± 0.11), the risk of stroke was not increased; that is, no stroke occurred within 748 patient-years of observation.7 In line with this observation is our finding that the erythropoietin-overexpressing transgenic mice with excessive erythrocytosis had no signs of thrombembolic complications in any organ at any age. In fact, preliminary data (not shown) indicate that the transgenic mice die from congestive cardiac failure. These findings need to be validated by longitudinal controlled studies; if they are confirmed, this observation extends the usefulness of the animal model to nonerythroid diseases.
A limitation of the mouse model presented is the difference in the molecular basis of the erythrocytosis in comparison with the human disorders. Whereas polycythemia vera is a clonal hematopoietic stem cell disorder often accompanied by an increase in platelets and neutrophils, Chuvash polycythemia30,31 has recently been found to be caused by a mutation of the von Hippel-Lindau protein leading to a constitutive elevation of the transcription factor hypoxia-inducible factor 1 (HIF-1). HIF-1 not only induces the expression of erythropoietin resulting in polycythemia, but also vascular endothelial growth factor and other genes controlled by HIF-1. Therefore, factors in addition to the erythrocytosis are likely to contribute to the thromboembolism in the human disorders discussed.
What mechanism might have prevented the clotting of the highly viscous blood at a hematocrit level of 0.85? In this study we compiled convincing evidence that high concentrations of erythrocytes interfere with clot formation. Specifically, with a hematocrit value of 0.80, clot strength was reduced and clot formation kinetics were slowed down. Possibly, the high erythrocyte concentration mechanically deters the interaction of platelets and fibrin with the extravascular tissue or the endothelium. Indeed, our reconstitution experiments showed that reversal of excessive erythrocytosis resulted in a normalization of clotting performance and vice versa. In keeping with this, in patients with hematocrit values over 0.60, preoperative phlebotomy improved the coagulation abnormalities, and blood loss at surgery was reported to be lower than in untreated patients.32
Several mouse lines transgenic for different fragments of the gene encoding erythropoietin33,34 or transgenic for a mutant human erythropoietin receptor identified in a patient with erythrocytosis35 have been generated in the past. None of these publications commented on the presence or absence of thrombosis or investigated the coagulation system. However, unlike the erythropoietin-overexpressing transgenic mouse line investigated here, the hematocrit levels of those mouse lines (0.45-0.69) did not reach the high levels reported here.
Villeval et al36 induced erythrocytosis in mice by transplanting bone marrow cells transfected with a retroviral vector carrying a monkey erythropoietin cDNA into lethally irradiated mice, resulting in extremely high hematocrit values (0.90 ± 0.05). In agreement with our observations, they found no histologic evidence of thrombosis or emboli. Tissue damage and capillary vascular permeability increase with increasing doses of irradiation.37 In contrast to all mouse models of erythrocytosis discussed so far, thoracic, muscular, and intestinal hemorrhage were noted in some of the irradiated mice. Together with the markedly shortened mean survival of 71 days, the findings suggest that irradiation effects in addition to the high hematocrit values contributed to the observed phenotype.
Another prominent hematologic abnormality in the erythropoietin transgenic mice was the reduced platelet concentration in peripheral blood. However, the distribution space of platelets in blood, in particular in flowing blood in vivo, is the plasma.38 The extremely high hematocrit level in transgenic mice reduced the distribution volume of platelets to 15%, whereas 85% of volume is taken up by erythrocytes. Recalculating the platelet concentration based on the plasma volume fraction revealed that in transgenic mice the plasma platelet concentration was not different from that in wild-type mice. Therefore, reduced clot strength or prolonged hemostasis in transgenic mice in vivo cannot be attributed to reduced platelet numbers. Based on the revised calculations, the unaltered plasma thrombopoietin concentration is in agreement with the plasma platelet concentration observed in wild-type and transgenic mice.
Clot formation was further explored by analysis of plasma coagulation. Surprisingly, we found a reduction of the activity of both the extrinsic and the intrinsic pathways. In patients with erythrocytosis caused by cyanotic congenital heart disease, decreased levels of coagulation factors as well as thrombocytopenia have been reported.39 These deficiencies have been found to be due to hepatic dysfunction from congestive cardiac failure and chronic disseminated coagulation.40 Although the erythropoietin transgenic mice develop cardiac dysfunction during their later life,13 no signs of hepatic dysfunction are known in these mice. Alternatively, because the common final pathway of the coagulation cascade, the generation of fibrin from fibrinogen through thrombin, does affect the results of the activated partial thromboplastin time as well as the prothrombin time, the presence of a thrombin inhibitor in the plasma of transgenic mice as the cause of the pathologic results cannot be excluded. Although the fibrinogen concentration was within the range reported for the mouse strain C57Bl/6,41 a relative deficiency of fibrinogen with respect to the increased number of erythrocytes might contribute to the impaired clot formation.
Thromboelastography has been shown to accurately detect fibrinolysis.42 Hemolysis, which was observed in transgenic mice, could have contributed to the coagulation defect by inducing a fibrinolytic state. But computerized thromboelastography revealed only minor fibrinolysis, present only in older transgenic animals, and reconstitution experiments showed that acute hemolysis did not affect clot formation. This is in agreement with a recent report on coagulation at high altitude43 and a study by Lugassy and Filin,44 who did not find hyperfibrinolysis in a cohort of patients with polycythemia vera. Still, fibrinolysis as a factor contributing to the coagulation abnormalities of the erythropoietin-overexpressing transgenic mice cannot be completely ruled out.
Vasoconstriction and the formation of a white thrombus, among other factors, contribute to hemostasis. Endothelial cells produce nitric oxide in response to shear stress, promoting vasodilation and inhibiting platelet activation, and nitric oxide deficiency enhances hemostasis in endothelial nitric oxide synthase knock-out mice.45 Conversely, increased levels of nitric oxide products in our mice might contribute to the defective hemostasis by weakening vasoconstriction12 and reducing responsiveness of platelets.
In this study we report the unexpected finding that erythropoietin transgenic mice living with a hematocrit level of 0.85, do not have any thrombosis or embolization. We provided evidence that hemostasis and plasmatic coagulation were significantly impaired in transgenic mice. Important protective mechanisms were the significantly reduced activity of the plasmatic coagulation cascade and, surprisingly, an inhibition of the thrombus formation by mechanical hindrance from the extremely high erythrocyte concentrations. As such, these mice may provide a useful animal model to study the adaptive changes to erythrocytosis as well as the interactions of rheology and coagulation.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-09-2814.
Supported by a grant of the Department of Anesthesiology and Resuscitation, Shinshu University (K.F.W.) and a faculty grant of the University of Lübeck (K.F.W.).
J.S. and J.H. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Ann-Katrin Hellberg, Dunja Schumacher, Jenifer Ehlert, and Alex Jurat for expert technical assistance and Claire Levine for valuable editorial help in the preparation of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal