Abstract
The vasodilator-stimulated phosphoprotein (VASP) plays an important role in cGMP-induced platelet inhibition. Since VASP is an in vitro substrate for cGMP-dependent protein kinase (PKG), it has been presumed that VASP phosphorylation induced by cGMP is mediated by PKG. Here we show that, in human platelets, phosphorylation of VASP at Ser239 induced by either cGMP analogs or nitric oxide (NO) donor glyco-SNAP1 is inhibited by PKA inhibitors KT5720, PKI, Rp-Br-cAMPS, and H89, but not by PKG inhibitors KT5823 or Rp-pCPT-cGMPS. Unlike human platelets, cGMP analog–induced phosphorylation of VASP in mouse platelets is inhibited by both PKG and PKA inhibitors. Ineffectiveness of PKG inhibitors in inhibiting VASP phosphorylation in human platelets is not due to an inability to inhibit PKG, as these PKG inhibitors but not PKA inhibitors inhibit a different cGMP-induced intracellular signaling event: phosphorylation of extracellular signal–responsive kinase. Furthermore, PKA inhibitors reverse cGMP-induced inhibition of thrombin-induced platelet aggregation, whereas PKG inhibitors further enhance the inhibitory effect of cGMP analogs. Thus, PKA plays a predominant role in the cGMP-induced phosphorylation of VASP and platelet inhibition in human platelets.
Introduction
The roles of cGMP in regulating platelet activation have been controversial since the dawn of this field, because increases in platelet cGMP levels had been observed in response to either platelet agonists (thrombin, adenosine diphosphate, or collagen) or inhibitors (nitric oxide [NO] or NO donors).1, 2, 3, 4 This controversy has been explained by our recent finding that cGMP induces biphasic platelet response, an early stimulatory response that promotes platelet activation and a delayed inhibitory response in which preincubation of platelets with cGMP analogs inhibits platelet activation.5 We have further shown that the early stimulatory response of platelets to cGMP requires cGMP-dependent protein kinase (PKG) and PKG-dependent activation of extracellular signal–responsive kinase (ERK) pathway.5,6 The mechanism for the late phase inhibitory effect of cGMP is not clear. It is currently believed that the inhibitory effect of cGMP involves several cGMP-regulated signaling pathways such as cGMP-induced phosphorylation of vasodilator-stimulated phosphoprotein (VASP) and thromboxane A2 receptor.7, 8, 9 The involvement of VASP in platelet inhibition is strongly indicated by the finding that VASP knockout mice showed an enhanced platelet activity and a diminished inhibitory effect of cGMP.7,8
VASP, initially found in platelets,10 is a member of a family of proline-rich proteins designated the Ena/VASP protein family.11 VASP interacts with actin filaments zyxin, profilin, and vinculin and has been found to be associated with focal adhesions, stress fibers, and cell-cell contacts in intact cells.12 VASP is an in vitro substrate for both cAMP-dependent protein kinase (PKA) and cGMP-dependent protein kinase (PKG).13,14 Three phosphorylation sites have been identified in VASP: Ser157, Ser239, and Thr278, all of which can be phosphorylated by either PKA or PKG in vitro.14 In a purified assay system, PKA has been reported to prefer the Ser157 site and PKG the Ser239 site.15 Phosphorylation at Ser157 but not Ser239 nor Thr278 is associated with an upward shift in the apparent molecular mass in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) from 46 kDa to 50 kDa.15 Among these sites, Ser239 phosphorylation has been extensively studied, thanks to the development of a monoclonal antibody, 16C2, specifically recognizing the phosphorylated Ser239 site.15 In intact cells, either cGMP-enhancing agents such as NO donors or cAMP-enhancing agents such as prostaglandin E1 (PGE1) can stimulate phosphorylation of VASP.10,16 It is a dominant presumption that VASP phosphorylation induced by cGMP-enhancing agents is mediated by PKG. However, this presumption did not take into consideration a possible cross-talk between cGMP and cAMP signaling pathways. In fact, it has been shown that cGMP may activate the cAMP-PKA signaling pathway by inhibiting phosphodiesterase 3.2,17, 18, 19 PKA-dependent cellular responses to cGMP also have been shown in PKG-deficient mice or patients.20,21 Thus, it is necessary to clarify whether cGMP-induced VASP phosphorylation in intact platelets is mediated directly by PKG or involves cAMP-PKA pathway.
In this study, we show that cGMP analog– or NO donor–induced phosphorylation of VASP in human platelets is not, as previously thought, PKG-dependent but is predominantly mediated by PKA. Furthermore, cGMP-induced inhibition of human platelet aggregation is reversed by a PKA inhibitor but not by the PKG inhibitors, suggesting that cGMP-induced PKA activation plays an important role in cGMP-induced human platelet inhibition.
Materials and methods
Materials
The cGMP analogs 8-bromo-guanosine 3′, 5′-cyclic monophosphate (8-bromo-cGMP), and 8-(4-chlorophenylthio)-guanosine 3′, 5′-cyclic monophosphate (8-pCPT-cGMP), nitric oxide donor, L-penicillaminamide-[N-(β-D-glucopyranosyl)-N2-acetyl-S-nitroso-D (Glyco-SNAP-1), PKG inhibitors, Rp-isomer-8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphorothioate (Rp-pCPT-cGMPS), Rp-isomer-N2-etheno-8-bromo-β-phenyl-1-guanosine 3′, 5′-cyclic monophosphorothioate (Rp-Br-PET-cGMPS), and KT5823, PKA inhibitors, Rp-isomer-8-bromo-adenosine3′,5′-cyclic monophosphorothioate (Rp-Br-cAMPS), N-[2-([p-bromocinnamyl]amino)ethyl]-5-isoquinolinesulfonamide, HCl) (H89), KT5720, and myristoylated protein kinase A inhibitor 14-22 amide (PKI), and forskolin were purchased from Calbiochem (San Diego, CA). Monoclonal antibodies against the phosphorylated Ser239 site of VASP, 16C2, and against the phosphorylated Ser157 site of VASP, 5C6, have been described previously.15,22 An antibody specific for phosphorylated Thr202/Tyr204 site of extracellular signal–regulated kinase (ERK) was purchased from New England Biolabs (Beverly, MA).
Preparation of washed platelets
Fresh blood from healthy volunteers was anticoagulated with one-seventh volume of ACD (2.5% trisodium citrate, 2% D-glucose, and 1.5% citric acid). In experiments using platelet rich plasma (PRP), one-tenth volume of 3.8% trisodium citrate was used as anticoagulant. For the preparation of mouse platelets, C57/B mice (6-8 weeks old) were anesthetized by intraperitoneal injection of pentobarbital. Whole blood from C57/B mice was collected from the inferior vena cava using one-seventh volume of ACD as anticoagulant. Platelets were washed with CGS buffer (sodium chloride 0.12 M, trisodium citrate 0.0129 M, and D-glucose 0.03 M, pH 6.5) and resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer) (145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM HEPES, and 10 mM D-glucose, pH 7.4) to give a concentration of 3 × 108/mL and allowed to incubate at 37°C for 1-2 hours as previously described.23,24 Washed platelets were preincubated at 37°C for 10 minutes with various PKG or PKA inhibitors and were incubated for an additional 10 minutes after adding cGMP analogs NO donor or forskolin to stimulate VASP phosphorylation. Platelets were then solubilized in the SDS-containing sample buffer containing 0.2 mM E64, 2 mM phenylmethylsulfonyl fluoride for analysis by SDS-PAGE and Western blot.
Platelet aggregation
Platelet aggregation was measured using a turbidometric platelet aggregometer (Chrono-Log) at 37°C. Washed platelets in Tyrode solution, pH7.4, were used in platelet aggregation induced by α-thrombin (Enzyme Research Laboratories). von Willebrand factor (VWF)–dependent platelet aggregation was induced by addition of ristocetin to PRP. To differentiate GPIb-IX–mediated platelet agglutination from the second wave of integrin-dependent platelet aggregation, final concentrations of ristocetin were adjusted in each experiment to achieve a 2-wave curve of aggregation.
Western blot analysis of VASP phosphorylation
Platelet lysates were analyzed by SDS-PAGE on 4% to 15% gradient gel and electrotransfered to polyvinylidenefluoride membranes. The membranes were blocked with 5% nonfat dry milk in Tris (tris(hydroxymethyl)-aminomethane)-buffered saline (TBS) (20 mM Tris-HCl,150 mM NaCl, pH7.5), incubated with the monoclonal antibody 16C2, specific for the phosphorylated Ser239 site of VASP (0.2 μg/mL) or 5C6, specific for the phosphorylated Ser157 site of VASP (0.5 μg/mL) at 22°C for 2 hours. After 3 washes in TBS containing 0.05% Tween 20, the membranes were incubated with horseradish peroxidase–conjugated goat anti–mouse IgG (0.5 μg/mL) for 45 minutes. After further washing, reactions were visualized using an Amersham-Pharmacia enhanced chemiluminescence kit (Piscataway, NJ).
Analysis of intracellular cAMP levels
Washed platelets (3 × 108/mL) were resuspended in Tyrode solution and incubated with 0.1 mM 8-pCPT-cGMP or 8-bromo-cGMP for 5 minutes at 37°C in a platelet aggregometer. The reaction was stopped by addition of equal volumes of ice-cold 12% (wt/vol) trichloroacetic acid. Samples were mixed and centrifuged at 2000 g for 15 minutes at 4°C. Supernatant was washed with 5 volumes of water-saturated diethyl ether 4 times and then lyophilized. cAMP levels were measured using a cAMP enzyme immunoassay kit from Amersham-Pharmacia Biotech. The specificity of the anti-cAMP antibodies for cAMP versus cGMP is about 1:0.000 0067. To further exclude the possible influence of cGMP analogs on cAMP assay, the cAMP standard curves for the cGMP analog–treated samples were determined in the presence of the same concentration of the corresponding cGMP analog (8-pCPT-cGMP and 8-bromo-cGMP, respectively).
Western blot analysis of ERK phosphorylation
Washed platelets (1 × 109/mL) were preincubated in the presence of various kinase inhibitors or vehicles (water or dimethyl sulfoxide [DMSO]) at 37°C for 5 minutes and then stimulated with cGMP analogs 8-bromo-cGMP or 8-pCPT-cGMP, or a NO donor, glyco-SNAP1, in a turbidometric platelet aggregometer at 37°C for 30 seconds. Platelets were then solubilized and immunoblotted with an antibody specific for the phosphorylated form of ERK. In preliminary studies, we examined the effects of DMSO on the levels of ERK phosphorylation. While DMSO slightly increased the stimulatory effect of cGMP analogs, it had no influence on the background level of ERK phosphorylation in the absence of cGMP analogs (data not shown). Thus, in subsequent studies, the levels of ERK activity in platelets treated in the absence of cGMP analogs were used as background levels of ERK phosphorylation. However, in the cGMP analog–stimulated platelets, platelets treated with the same volume of DMSO alone were used as controls for kinase inhibitors dissolved in DMSO.
Results
PKG inhibitors failed to inhibit cGMP analog–induced phosphorylation of VASP in human platelets but inhibited VASP phosphorylation in mouse platelets
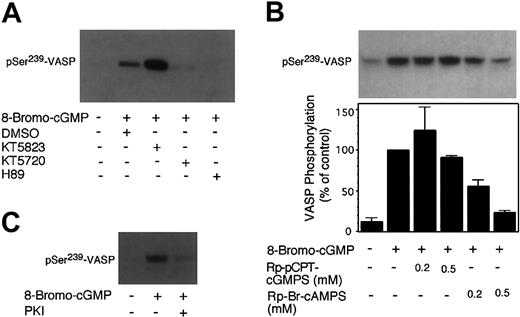
It has been presumed that cGMP-induced VASP Ser239 phosphorylation in platelets is mediated by PKG. If this presumption is true, membrane-permeable PKG inhibitors should abolish or reduce cGMP-stimulated VASP phosphorylation. To determine if PKG is required for cGMP-induced phosphorylation of VASP Ser239, PKG inhibitors Rp-pCPT-cGMPS or KT5823 were incubated with washed human platelets prior to adding cGMP analog 8-bromo-cGMP (50 μM) to induce VASP phosphorylation. The platelets were then solubilized and immunoblotted with a monoclonal antibody that specifically recognizes the phosphorylated form of Ser239 in VASP, 16C2. KT5823 had no inhibitory effects but rather enhanced cGMP-induced Ser239 phosphorylation (Figure 1). Since a previous report suggested that inability of KT5823 to inhibit VASP phosphorylation was due to the ineffectiveness of KT5823 in inhibiting PKG activity,22 we also examined the effects of the other specific PKG inhibitor, Rp-pCPT-cGMPS, which competitively inhibits cGMP binding to PKG. We found that Rp-pCPT-cGMPS was also ineffective in inhibiting cGMP-induced Ser239 phosphorylation (Figure 1).
Effects of PKG and PKA inhibitors on 8-bromo-cGMP–induced phosphorylation of VASP at Ser239 in human platelets. Washed platelets (3 × 108/mL) were preincubated with (A) PKG inhibitor KT5823 (5 μM), PKA inhibitor KT5720 (5 μM), or PKA inhibitor H89 (50 μM); and (B) PKG inhibitor Rp-pCPT-cGMPS (0.2 and 0.5 mM), or PKA inhibitor Rp-Br-cAMPS (0.2 and 0.5 mM); and (C) myristoylated PKI peptide (14-22) (5 μM) for 10 minutes. Platelets were also preincubated with buffer or DMSO as controls. Platelets were further incubated with 8-bromo-cGMP (50 μM) at 37°C in the platelet aggregometer for 10 minutes (shorter incubation with 8-bromo-cGMP failed to induce significant VASP phosphorylation) and solubilized directly in SDS-PAGE sample buffer. Phosphorylation of VASP at Ser239 was detected by immunoblotting with a monoclonal antibody specifically recognizing Ser239-phosphorylated VASP, 16C2. Pictures shown in the figure are representative of 3 independent experiments. The bar graph depicts the quantitative results from 3 experiments obtained by scanning the 16C2 reactive bands and quantifying optical density using NIH Image software.
Effects of PKG and PKA inhibitors on 8-bromo-cGMP–induced phosphorylation of VASP at Ser239 in human platelets. Washed platelets (3 × 108/mL) were preincubated with (A) PKG inhibitor KT5823 (5 μM), PKA inhibitor KT5720 (5 μM), or PKA inhibitor H89 (50 μM); and (B) PKG inhibitor Rp-pCPT-cGMPS (0.2 and 0.5 mM), or PKA inhibitor Rp-Br-cAMPS (0.2 and 0.5 mM); and (C) myristoylated PKI peptide (14-22) (5 μM) for 10 minutes. Platelets were also preincubated with buffer or DMSO as controls. Platelets were further incubated with 8-bromo-cGMP (50 μM) at 37°C in the platelet aggregometer for 10 minutes (shorter incubation with 8-bromo-cGMP failed to induce significant VASP phosphorylation) and solubilized directly in SDS-PAGE sample buffer. Phosphorylation of VASP at Ser239 was detected by immunoblotting with a monoclonal antibody specifically recognizing Ser239-phosphorylated VASP, 16C2. Pictures shown in the figure are representative of 3 independent experiments. The bar graph depicts the quantitative results from 3 experiments obtained by scanning the 16C2 reactive bands and quantifying optical density using NIH Image software.
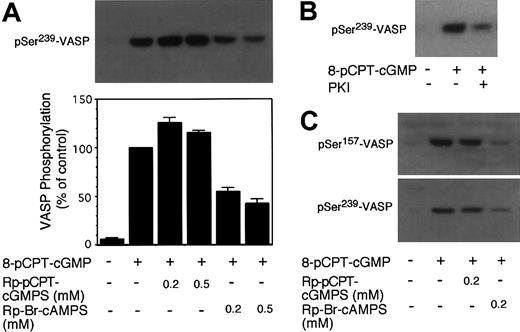
To exclude the possibility that 8-bromo-cGMP may have different properties from other cGMP analogs, 8-pCPT-cGMP also was used to induce VASP phosphorylation (Figure 2). Similar to 8-bromo-cGMP, 8-pCPT-cGMP induced VASP phosphorylation at Ser239, which was not inhibited but was rather enhanced by either Rp-pCPT-cGMPS (Figure 2) or KT5823 (not shown). We also examined cGMP-induced VASP phosphorylation at a different site, Ser157, using an antibody specific for the phosphorylated Ser157 site. We found that, similar to Ser239, cGMP-induced phosphorylation at Ser157 was not inhibited by the PKG inhibitor Rp-pCPT-cGMPS (Figure 2C). These results suggest that either the cGMP-induced VASP phosphorylation is not PKG dependent or both PKG inhibitors (KT5823 and Rp-pCPT-cGMPS) do not inhibit PKG in human platelets.
Effects of PKG and PKA inhibitors on 8-pCPT-cGMP–induced phosphorylation of VASP in human platelets. Washed platelets were preincubated with (A) PKG inhibitor Rp-pCPT-cGMPS (0.2 and 0.5 mM) or PKA inhibitor Rp-Br-cAMPS (0.2 and 0.5 mM); and (B) PKA inhibitor, myristoylated PKI peptide (14-22) (5 μM) for 10 minutes. Platelets were also preincubated with buffer or DMSO as controls. Platelets were further treated with 8-pCPT-cGMP (20 μM) at 37°C in the platelet aggregometer for 10 minutes and solubilized in SDS-PAGE sample buffer. Phosphorylation of VASP was then detected by immunoblotting as described in Figure 1. Pictures shown in the figure are representative of 3 independent experiments. The bar graph depicts the quantitative results from 3 experiments obtained by scanning the 16C2 reactive bands and quantifying using NIH Image software. (C) Washed platelets were preincubated with Rp-pCPT-cGMPS (0.2 mM) or Rp-Br-cAMPS (0.2 mM) for 10 minutes. Platelets were then treated with 100 μM 8-pCPT-cGMP at 37°C for 10 minutes. Phosphorylation of VASP at Ser239 was detected by immunoblotting with the monoclonal antibody 16C2. Phosphorylation of VASP at Ser157 was detected by immunoblotting with the monoclonal antibody 5C6.
Effects of PKG and PKA inhibitors on 8-pCPT-cGMP–induced phosphorylation of VASP in human platelets. Washed platelets were preincubated with (A) PKG inhibitor Rp-pCPT-cGMPS (0.2 and 0.5 mM) or PKA inhibitor Rp-Br-cAMPS (0.2 and 0.5 mM); and (B) PKA inhibitor, myristoylated PKI peptide (14-22) (5 μM) for 10 minutes. Platelets were also preincubated with buffer or DMSO as controls. Platelets were further treated with 8-pCPT-cGMP (20 μM) at 37°C in the platelet aggregometer for 10 minutes and solubilized in SDS-PAGE sample buffer. Phosphorylation of VASP was then detected by immunoblotting as described in Figure 1. Pictures shown in the figure are representative of 3 independent experiments. The bar graph depicts the quantitative results from 3 experiments obtained by scanning the 16C2 reactive bands and quantifying using NIH Image software. (C) Washed platelets were preincubated with Rp-pCPT-cGMPS (0.2 mM) or Rp-Br-cAMPS (0.2 mM) for 10 minutes. Platelets were then treated with 100 μM 8-pCPT-cGMP at 37°C for 10 minutes. Phosphorylation of VASP at Ser239 was detected by immunoblotting with the monoclonal antibody 16C2. Phosphorylation of VASP at Ser157 was detected by immunoblotting with the monoclonal antibody 5C6.
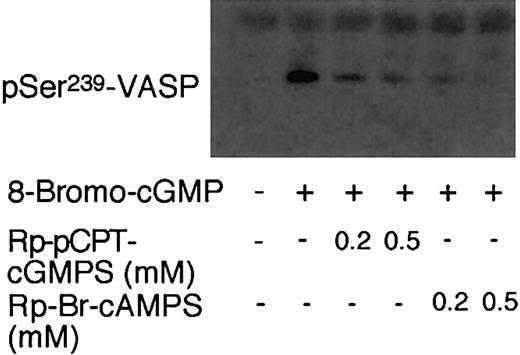
It has been previously reported that cGMP-induced VASP phosphorylation is inhibited in PKG knockout mouse platelets, as detected by a lack of cGMP-induced upwards shift in VASP migration on SDS-polyacrylamide gels.25 To determine if cGMP-induced phosphorylation at Ser235 of mouse VASP (corresponding to human Ser239) is PKG dependent, mouse platelets were pretreated with the PKG inhibitor Rp-pCPT-cGMPS and then exposed to 8-bromo-cGMP to induce VASP phosphorylation. Consistent with previous results obtained with PKG knockout mice, cGMP analog–induced VASP phosphorylation in wild-type mouse platelets was diminished by the PKG inhibitor (Figure 3), indicating that mouse platelets are different from human platelets in that cGMP-induced VASP phosphorylation at Ser235 in mouse platelets requires PKG. The finding that Rp-pCPT-cGMPS inhibited VASP phosphorylation in mouse platelets also suggests that the PKG inhibitor is effective in inhibiting PKG activity in intact platelets.
Effects of PKG and PKA inhibitors on the cGMP-induced VASP phosphorylation in mouse platelets. Washed mouse platelets (3 × 108/mL) were preincubated with Rp-pCPT-cGMPS (0.2 mM and 0.5 mM) or Rp-Br-cAMPS (0.2 mM or 0.5 mM) for 10 minutes. Platelets were then treated with 8-bromo-cGMP (100 μM) at 37°C for 10 minutes. Phosphorylation of VASP was detected by immunoblotting with monoclonal antibody 16C2 against the phosphorylated Ser239 site of VASP. Data shown are a representative of 3 independent experiments.
Effects of PKG and PKA inhibitors on the cGMP-induced VASP phosphorylation in mouse platelets. Washed mouse platelets (3 × 108/mL) were preincubated with Rp-pCPT-cGMPS (0.2 mM and 0.5 mM) or Rp-Br-cAMPS (0.2 mM or 0.5 mM) for 10 minutes. Platelets were then treated with 8-bromo-cGMP (100 μM) at 37°C for 10 minutes. Phosphorylation of VASP was detected by immunoblotting with monoclonal antibody 16C2 against the phosphorylated Ser239 site of VASP. Data shown are a representative of 3 independent experiments.
PKG inhibitors inhibit cGMP analog–induced ERK phosphorylation
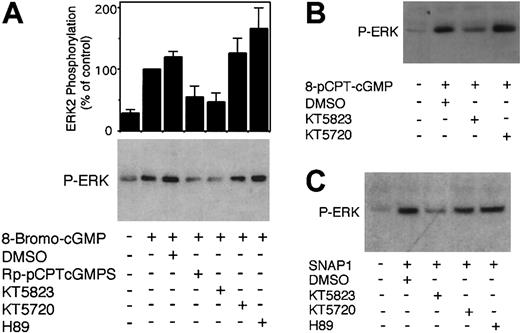
It has been shown previously that, in vascular smooth muscle cells, endothelial cells, and in platelets, cGMP induces a different intracellular signaling event; that is, activation of ERK MAPK (mitogen-activated protein kinase) pathway and phosphorylation of ERKs.6,26,27 Thus, to determine if PKG inhibitors inhibit PKG activity in human platelets, we examined the effects of these PKG inhibitors on cGMP analog–induced ERK phosphorylation in human platelets. Figure 4 shows that addition of cGMP analogs 8-bromo-cGMP or 8-pCPT-cGMP or an NO donor, glyco-SNAP1, induced phosphorylation of ERKs as detected by immunoblotting with a phosphorylation-dependent anti-ERK antibody. As a control, a cAMP analog, 8-bromo-cAMP (PKA activator), had no such effect (not shown). Both PKG inhibitors, KT5823 (5 μM) or Rp-pCPT-cGMPS (500 μM), inhibited cGMP-stimulated phosphorylation of ERKs (Figure 4). In contrast, PKA inhibitors KT5720 (5 μM) and H89 (50 μM) had no such inhibitory effects. These data demonstrate that cGMP analogs induce activation of the ERK pathway in a PKG-dependent manner. These data also indicate that both KT5823 and Rp-pCPT-cGMPS are effective in inhibiting PKG activity in intact human platelets. Thus, the failure of the PKG inhibitors to inhibit VASP Ser239 and Ser157 phosphorylation is not likely to result from their ineffectiveness in inhibiting PKG activity. Based on these data, we conclude that the cGMP analog– or NO donor–stimulated phosphorylation of VASP Ser239 in intact human platelets is not PKG dependent.
Effects of PKG and PKA inhibitors on 8-bromo-cGMP–induced phosphorylation of ERK MAP kinase. Washed platelets (1 × 109/mL) were preincubated with PKG inhibitors Rp-pCPT-cGMPS (500 μM) or KT5823 (5 μM), or PKA inhibitors KT5720 (5 μM) or H89 (50 μM) for 10 minutes. Platelets were also preincubated with buffer or the same concentration of DMSO (0.25%) as controls. DMSO had no effect on ERK phosphorylation level in resting platelets in the absence of cGMP analogs (data not shown). Platelets were then stimulated for 30 seconds in the platelet aggregometer with (A) 8-bromo-cGMP (0.1 mM), (B) 8-pCPT-cGMP, or (C) NO donor, glyco-SNAP1 (1μM), solubilized in SDS-PAGE sample buffer, separated by SDS-PAGE, and immunoblotted with a rabbit antibody specific for phosphorylated Thr202/Tyr204 site of ERKs. Pictures shown in the figure are representative of 3 independent experiments. The bar graph depicts the quantitative results from 3 experiments obtained by scanning and quantifying the immunoblotting results using NIH Image software. To quantify the relative effects of PKG or PKA inhibitors on cGMP-induced ERK phosphorylation, the level of ERK phosphorylation induced by the cGMP analog was set as 100%.
Effects of PKG and PKA inhibitors on 8-bromo-cGMP–induced phosphorylation of ERK MAP kinase. Washed platelets (1 × 109/mL) were preincubated with PKG inhibitors Rp-pCPT-cGMPS (500 μM) or KT5823 (5 μM), or PKA inhibitors KT5720 (5 μM) or H89 (50 μM) for 10 minutes. Platelets were also preincubated with buffer or the same concentration of DMSO (0.25%) as controls. DMSO had no effect on ERK phosphorylation level in resting platelets in the absence of cGMP analogs (data not shown). Platelets were then stimulated for 30 seconds in the platelet aggregometer with (A) 8-bromo-cGMP (0.1 mM), (B) 8-pCPT-cGMP, or (C) NO donor, glyco-SNAP1 (1μM), solubilized in SDS-PAGE sample buffer, separated by SDS-PAGE, and immunoblotted with a rabbit antibody specific for phosphorylated Thr202/Tyr204 site of ERKs. Pictures shown in the figure are representative of 3 independent experiments. The bar graph depicts the quantitative results from 3 experiments obtained by scanning and quantifying the immunoblotting results using NIH Image software. To quantify the relative effects of PKG or PKA inhibitors on cGMP-induced ERK phosphorylation, the level of ERK phosphorylation induced by the cGMP analog was set as 100%.
cGMP-induced phosphorylation of VASP is inhibited by PKA inhibitors
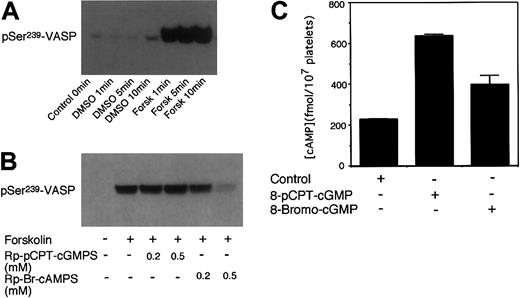
It has been established previously that VASP Ser239 is not only a PKG substrate but also a PKA substrate.14,16 In platelets, either cGMP or cAMP-elevating agents induce VASP phosphorylation.10,14 Since cGMP has been shown to enhance cAMP levels by inhibiting phosphodiesterase 3,2,17, 18, 19 we investigated the possibility that PKA may play a role in cGMP analog–induced VASP phosphorylation in platelets. To do this, washed platelets were preincubated with PKA inhibitors and then treated with 8-bromo-cGMP to induce VASP phosphorylation. Three different specific PKA inhibitors were used: KT5720 (catalytic domain inhibitor), Rp-Br-cAMPS (cAMP competitor), and a membrane-permeable PKI peptide (highly specific pseudosubstrate). These PKA inhibitors are structurally different and inhibit PKA by different mechanisms. In addition, we also used a less specific PKA inhibitor, H89. Figure 1 shows that all these PKA inhibitors inhibited 8-bromo-cGMP–stimulated VASP phosphorylation at Ser239. Similarly, these PKA inhibitors also inhibited 8-pCPT-cGMP–stimulated phosphorylation at Ser239 and Ser157 of VASP (Figure 2). The PKA inhibitor Rp-Br-cAMPS also inhibited VASP phosphorylation in mouse platelets (Figure 3). Thus, PKA is important in cGMP analog–stimulated VASP phosphorylation in platelets. To verify whether PKA activation is sufficient to induce Ser239 phosphorylation in platelets, platelets were treated with forskolin, which elevates intracellular cAMP levels. Figure 5 shows that forskolin induced a rapid phosphorylation of Ser239 in a PKA-dependent manner, indicating that PKA activation is indeed sufficient to induce phosphorylation of VASP Ser239 in platelets. It is interesting to note that forskolin-induced VASP phosphorylation reached maximum within 1 minute, much faster than cGMP analog–induced VASP phosphorylation (which does not reach maximum even after 10 minutes of incubation),5 suggesting that the effects of cGMP analogs on VASP phosphorylation may be indirect.
Phosphorylation of VASP induced by forskolin and effects of cGMP on platelet cAMP levels. (A) Washed platelets were stimulated for increasing lengths of time with 1 μM of forskolin. (B) Platelets were preincubated with Rp-pCPT-cGMPS (0.2 and 0.5 mM) or Rp-Br-cAMPS (0.2 and 0.5 mM) for 10 minutes prior to stimulation with forskolin (1 μM) for 5 minutes. Phosphorylation of VASP was then detected by immunoblotting as described in Figure 1. Data shown are representative of at least 3 independent experiments. (C) Washed platelets (3 × 108/mL) in Tyrode solution were incubated with 8-pCPT-cGMP (0.1 mM) or 8-bromo-cGMP (0.1 mM) for 5 minutes at 37°C. The reaction was stopped by addition of equal volumes of ice-cold 12% (wt/vol) trichloroacetic acid. Platelet cAMP levels then were determined using a cAMP enzyme immunoassay kit from Amersham-Pharmacia Biotech. To exclude and correct possible influence of cGMP analogs on cAMP enzyme immunoassay analysis, cAMP standards also were analyzed in the presence of identical concentrations of the cGMP analogs. Shown in the figure are data (mean ± SD) from 3 samples. Please note that cAMP levels in 8-bromo-cGMP– or 8-pCPT-cGMP–treated platelets were significantly higher than control platelets (P < .001) as determined by Students t test.
Phosphorylation of VASP induced by forskolin and effects of cGMP on platelet cAMP levels. (A) Washed platelets were stimulated for increasing lengths of time with 1 μM of forskolin. (B) Platelets were preincubated with Rp-pCPT-cGMPS (0.2 and 0.5 mM) or Rp-Br-cAMPS (0.2 and 0.5 mM) for 10 minutes prior to stimulation with forskolin (1 μM) for 5 minutes. Phosphorylation of VASP was then detected by immunoblotting as described in Figure 1. Data shown are representative of at least 3 independent experiments. (C) Washed platelets (3 × 108/mL) in Tyrode solution were incubated with 8-pCPT-cGMP (0.1 mM) or 8-bromo-cGMP (0.1 mM) for 5 minutes at 37°C. The reaction was stopped by addition of equal volumes of ice-cold 12% (wt/vol) trichloroacetic acid. Platelet cAMP levels then were determined using a cAMP enzyme immunoassay kit from Amersham-Pharmacia Biotech. To exclude and correct possible influence of cGMP analogs on cAMP enzyme immunoassay analysis, cAMP standards also were analyzed in the presence of identical concentrations of the cGMP analogs. Shown in the figure are data (mean ± SD) from 3 samples. Please note that cAMP levels in 8-bromo-cGMP– or 8-pCPT-cGMP–treated platelets were significantly higher than control platelets (P < .001) as determined by Students t test.
Platelet cAMP elevation induced by cGMP analogs
To determine whether cGMP analogs indirectly activate the PKA pathway by elevating cAMP, we examined if the cGMP analogs 8-bromo-cGMP and 8-pCPT-cGMP elevate intracellular cAMP levels. Figure 5C shows that both 8-pCPT-cGMP and 8-bromo-cGMP elevated intracellular cAMP levels in intact platelets. The effects of these cGMP analogs on cAMP levels were not caused by the possible cross-reactivity with the anti-cAMP antibody used to detect cAMP levels, as the standard curves of the cAMP assays were corrected in the presence of the same concentrations of these cGMP analogs. Interestingly, 8-pCPT-cGMP is much more potent in inducing cAMP elevation than 8-bromo-cGMP in our experiments, which is associated with much stronger inhibitory effects of 8-pCPT-cGMP on platelet aggregation (see Figure 7). These results are apparently different from a previous report from Butt et al that 8-pCPT-cGMP failed to inhibit phosphodiesterase 3 activity in platelet homogenate.28 Although the reason for this apparent discrepancy remains to be further investigated, it is noted that Butt et al only measured the cyclic nucleotide hydrolysis in platelet homogenate but did not directly examine the effect of 8-pCPT-cGMP on cAMP level in intact platelets. In contrast, we show that 8-pCPT-cGMP potently elevates intracellular cAMP level in intact platelets. Thus, our results indicate that cGMP analogs induce PKA activation by elevating intracellular cAMP levels in human platelets.
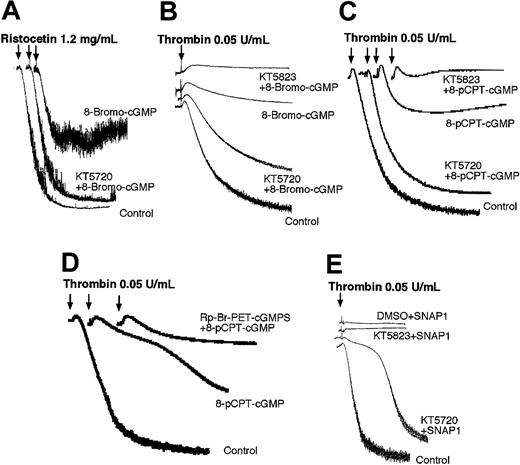
PKA inhibitor KT5720 but not PKG inhibitor KT5823 reverses cGMP-induced inhibition of platelet aggregation. Platelets were preincubated with DMSO (as a control), PKA inhibitor KT5720 (5 μM), or PKG inhibitors KT5823 (5 μM) (A-C,E) or Rp-Br-PET-cGMPS (0.2 mM) (D) for 5 minutes and then incubated with 1 mM 8-bromo-cGMP (A), 3 mM 8-bromo-cGMP (B), 0.1 mM 8-pCPT-cGMP (C-D), or 100 μM NO donor glyco-SNAP 1 (E) for additional 10 minutes. Ristocetin (1.25 mg/mL) (A) or α-thrombin (0.05 U/mL)(B-E) was then added to induce platelet aggregation. Presented in the figure are the representative results of at least 3 experiments.
PKA inhibitor KT5720 but not PKG inhibitor KT5823 reverses cGMP-induced inhibition of platelet aggregation. Platelets were preincubated with DMSO (as a control), PKA inhibitor KT5720 (5 μM), or PKG inhibitors KT5823 (5 μM) (A-C,E) or Rp-Br-PET-cGMPS (0.2 mM) (D) for 5 minutes and then incubated with 1 mM 8-bromo-cGMP (A), 3 mM 8-bromo-cGMP (B), 0.1 mM 8-pCPT-cGMP (C-D), or 100 μM NO donor glyco-SNAP 1 (E) for additional 10 minutes. Ristocetin (1.25 mg/mL) (A) or α-thrombin (0.05 U/mL)(B-E) was then added to induce platelet aggregation. Presented in the figure are the representative results of at least 3 experiments.
An important role for PKA in NO donor–induced VASP phosphorylation
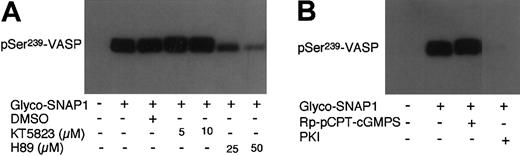
A function of VASP in platelets is to mediate downstream regulatory effects of nitrovasodilators, NO or NO donors. NO donors induce dramatic increases in endogenous cGMP levels and potently stimulate phosphorylation of VASP in platelets and other cell types. To determine whether the effects of NO donors on VASP phosphorylation may also involve PKA activity, inhibitors of PKG or PKA were preincubated with platelets. The platelets were then treated with NO donor glyco-SNAP1 (1 μM) for 5 minutes to induce VASP phosphorylation. As shown in Figure 6, glyco-SNAP1–induced Ser239 phosphorylation was not inhibited by PKG inhibitors KT5823 (5 and 10 μM) and Rp-pCPT-cGMPS (200μM) but was abolished by the specific PKA inhibitor PKI peptide. Thus, PKA also plays an important role in NO-stimulated VASP Ser239 phosphorylation in intact platelets.
Effects of PKG and PKA inhibitors on glyco-SNAP1–induced phosphorylation of VASP at Ser239. Washed platelets were preincubated with (A) KT5823 (5-10 μM) or H89 (25-50 μM), (B) Rp-pCPT-cGMPS (0.2 mM) or PKI (50 μM) for 10 minutes. Platelets also were preincubated with buffer or DMSO as controls. Platelets were then treated with glyco-SNAP1 (1 μM) at 37°C in the platelet aggregometer for 5 minutes. VASP phosphorylation was then detected by immunoblotting as described in Figure 1. Data shown are representative of at least 3 independent experiments.
Effects of PKG and PKA inhibitors on glyco-SNAP1–induced phosphorylation of VASP at Ser239. Washed platelets were preincubated with (A) KT5823 (5-10 μM) or H89 (25-50 μM), (B) Rp-pCPT-cGMPS (0.2 mM) or PKI (50 μM) for 10 minutes. Platelets also were preincubated with buffer or DMSO as controls. Platelets were then treated with glyco-SNAP1 (1 μM) at 37°C in the platelet aggregometer for 5 minutes. VASP phosphorylation was then detected by immunoblotting as described in Figure 1. Data shown are representative of at least 3 independent experiments.
The role of PKA in cGMP analog– and NO donor–induced inhibition of platelet aggregation
It is known that VASP phosphorylation is involved in cGMP-induced platelet inhibition. Thus, if cGMP-induced VASP phosphorylation involves PKA, it is likely that cGMP-induced inhibition of platelet aggregation also involves PKA. To examine this possibility, platelet-rich plasma or washed platelets were preincubated with the PKA inhibitor KT5720 and then cGMP analogs (8-bromo-cGMP or 8-pCPT-cGMP) or NO donor glyco-SNAP1. Platelet aggregation was examined after adding either low dose thrombin or ristocetin (which induces VWF binding to its platelet receptor). Platelet aggregation induced by low dose thrombin or ristocetin was partially inhibited by preincubation with cGMP analogs and NO donors (Figure 7). This inhibition was reversed by the PKA inhibitor KT5720. In contrast, the PKG inhibitor KT5823 did not reverse the inhibitory effects of cGMP analogs but further enhanced the inhibitory effects of cGMP analogs (Figure 7A-C, E). Furthermore, another PKG inhibitor, Rp-Br-PET-cGMPS, also enhanced the inhibitory effect of 8-pCPT-cGMP on thrombin-induced platelet aggregation (Figure 7D). These results are consistent with our recent report of a stimulatory role for PKG in promoting thrombin and VWF-induced platelet aggregation5 and suggest that PKA but not PKG plays a predominant role in cGMP-mediated human platelet inhibition.
Discussion
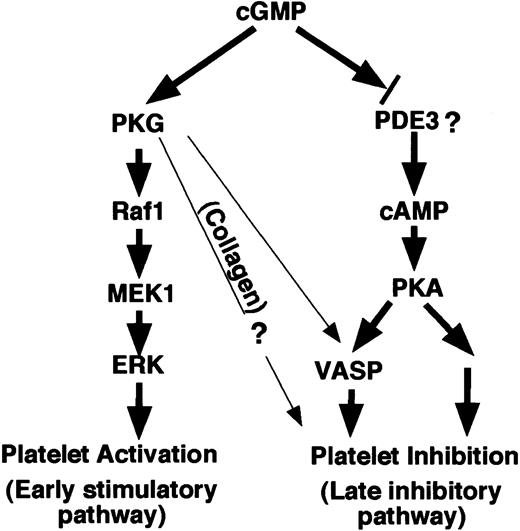
We have recently shown that the roles of cGMP in platelet activation are biphasic, consisting of an early transient stimulatory effect that promotes platelet activation and a delayed inhibitory effect that prevents the overgrowth of platelet aggregates.5 We also have shown that the stimulatory effect of cGMP during platelet activation requires PKG and PKG-dependent activation of the ERK pathway.5,6 The mechanism for the transition between cGMP-mediated platelet stimulation and cGMP-induced platelet inhibition has been unclear. The effect of cGMP analogs in inhibiting platelet activation has been thought to be PKG-dependent and involve cGMP-induced phosphorylation of VASP as evidenced by studies using PKG and VASP knockout mice.7,8,25 In this study, we show that human platelets are different from mouse platelets in that cGMP analog– or NO donor–induced VASP phosphorylation and platelet inhibition in human platelets are predominantly mediated by cGMP-induced activation of PKA. These novel findings, together with previous data obtained by us and others, depict a novel signaling mechanism that is responsible for the biphasic role of cGMP in human platelet activation (Figure 8).
The mechanisms of cGMP-induced biphasic platelet responses. Elevation of cGMP levels in platelets induces an early phase stimulatory platelet response, promoting platelet aggregation induced by certain agonists such as thrombin and VWF.5,6 This response is mediated via PKG-ERK signaling pathway.5,6 Subsequently, cGMP, possibly by inhibiting phosphodiesterase 3, causes elevation of cAMP in human platelets, activating PKA. PKA, by inducing phosphorylation of VASP and other intracellular molecules, plays a predominant role in inhibition of platelets, preventing overgrowth of hemostatic thrombus. In platelets stimulated with collagen, however, PKG is also involved in the inhibitory phase of cGMP effect in addition to a predominant role of PKA.19 PKG is important in the inhibitory phase of cGMP in collagen-induced mouse platelet aggregation and cGMP analog–induced VASP phosphorylation in mouse platelets.25
The mechanisms of cGMP-induced biphasic platelet responses. Elevation of cGMP levels in platelets induces an early phase stimulatory platelet response, promoting platelet aggregation induced by certain agonists such as thrombin and VWF.5,6 This response is mediated via PKG-ERK signaling pathway.5,6 Subsequently, cGMP, possibly by inhibiting phosphodiesterase 3, causes elevation of cAMP in human platelets, activating PKA. PKA, by inducing phosphorylation of VASP and other intracellular molecules, plays a predominant role in inhibition of platelets, preventing overgrowth of hemostatic thrombus. In platelets stimulated with collagen, however, PKG is also involved in the inhibitory phase of cGMP effect in addition to a predominant role of PKA.19 PKG is important in the inhibitory phase of cGMP in collagen-induced mouse platelet aggregation and cGMP analog–induced VASP phosphorylation in mouse platelets.25
We conclude that cGMP analog– or NO donor–induced VASP phosphorylation at Ser239 in human platelets is not PKG dependent. This is based on the finding that the membrane-permeable PKG inhibitors at concentrations that are known to inhibit PKG do not inhibit cGMP analog– or glyco-SNAP1–induced VASP phosphorylation at Ser239. Our data are consistent with a previous report showing that the PKG inhibitor KT5823 failed to inhibit cGMP-induced VASP phosphorylation in platelets.22 In the previous report, inability of KT5823 to inhibit cGMP-induced VASP phosphorylation was interpreted as its ineffectiveness in inhibiting PKG activity in human platelets, based on the presumption that VASP phosphorylation is PKG dependent. However, we show that the failure of both KT5823 and Rp-pCPT-cGMPS to inhibit VASP phosphorylation in intact human platelets is unlikely to result from their inability to inhibit PKG, because they both inhibited cGMP-induced ERK phosphorylation in intact human platelets at the same concentrations that failed to inhibit VASP phosphorylation (Figure 4). Consistent with this notion, we reported recently that these PKG inhibitors had effects similar to PKG knockout in inhibiting VWF- and thrombin-induced platelet responses.5 Also, it has been shown that, in intact endothelial cells and smooth muscle cells, cGMP induces activation of c-Raf and phosphorylation of ERKs, which are inhibited by KT5823 and Rp-pCPT-cGMPS.26,27 Burkhardt et al showed that a different inhibitor, H89, inhibited cGMP-induced VASP phosphorylation.22 However, H89 is a potent (although less specific) PKA inhibitor with a Ki for PKA 10 times less than its Ki for PKG.29 Thus, inhibition of VASP phosphorylation by H89 suggests a possible involvement of PKA in cGMP-induced VASP phosphorylation. Therefore, inability of either KT5823 or Rp-pCPT-cGMPS to inhibit VASP phosphorylation indicates that PKG is not required for cGMP-stimulated VASP phosphorylation at Ser239 and Ser157 in human platelets under these conditions. In contrast, cGMP analog–induced phosphorylation of VASP at Ser235 in mouse platelets is inhibited by PKG inhibitors (Figure 3), which is consistent with a previous report that cGMP analog–induced VASP phosphorylation was inhibited in PKG knockout mice.25 Thus, it appears that human platelets are different from mouse platelets with respect to the role of PKG in cGMP analog–induced VASP phosphorylation. Species differences in cGMP-induced cAMP elevation also have been previously reported.2 Thus, differences between species should be taken into consideration in studying the cGMP-induced VASP phosphorylation and platelet responses.
We conclude that the cGMP-induced VASP Ser239 phosphorylation in human platelets is predominantly mediated via the cAMP-PKA pathway. This conclusion is supported by the finding that cGMP analog– or NO-induced phosphorylation at Ser239 of VASP is inhibited by several different specific PKA inhibitors. These inhibitors have different molecular structures and inhibit PKA activity by totally different mechanisms. It is unlikely that these totally different inhibitors would have similar nonspecific inhibitory effects on VASP phosphorylation. Thus, it is reasonable to conclude that these inhibitors inhibit PKA-dependent VASP phosphorylation induced by cGMP. Furthermore, although the effects of cGMP in platelets can be mediated by PKG, which is the major enzyme regulated by cGMP, it is also known that NO donors induce elevation of both cGMP and cAMP.2 Also, cGMP induces elevation of cAMP by inhibiting phosphodiesterase 3 (PDE3), which hydrolyzes cAMP.2,17, 18, 19 These studies suggest that cGMP elevation may result in accumulation of cAMP in human platelets. In this respect, we have observed that cGMP analog–induced VASP phosphorylation is a delayed event requiring more than 10 minutes of cGMP analog incubation with platelets in contrast to cGMP-induced, PKG-dependent ERK phosphorylation, which reached a maximum within 1 minute.5 On the other hand, cAMP-enhancing agents induce rapid phosphorylation of VASP (within 1 minute) (Figure 5). Furthermore, we found that both 8-bromo-cGMP and 8-pCPT-cGMP elevated intracellular cAMP levels in platelets (Figure 5C), which is consistent with a recent findings that NO donor–induced cAMP elevation involves cGMP pathway.19 Thus, it appears that VASP phosphorylation in human platelets is not a direct consequence of PKG activation but rather is mediated via cGMP-induced elevation of cAMP and activation of PKA.
Although we conclude that PKA but not PKG plays a predominant role in cGMP- or NO donor–induced VASP phosphorylation at Ser239 and Ser157 in human platelets, we do not exclude the possibility that PKG also may be involved in VASP phosphorylation following an increase in intracellular cGMP. It is possible that a contribution of PKG to Ser239 and Ser157 phosphorylation was not detectable under our experimental conditions, as the inhibitory effects of PKG inhibitors could be masked by the predominant role of PKA. It is also possible that PKG may catalyze phosphorylation at sites other than Ser239 and Ser157. It would be interesting to investigate whether there are additional sites in VASP that are phosphorylated by PKG.
It is known that cGMP analog–induced platelet inhibition involves VASP.7 Also, cGMP analog–induced platelet inhibition correlates with VASP phosphorylation30 and occurs only at the cGMP analog concentrations that are sufficient to induce VASP phosphorylation.5 Thus, our finding that PKA (but not PKG) plays a predominant role in cGMP-induced VASP phosphorylation suggests that PKA may be important in cGMP-induced platelet inhibition. Indeed, PKA inhibitors reversed cGMP analog– and NO donor–induced inhibition of platelet aggregation induced by low dose thrombin and VWF. On the other hand, PKG inhibitors did not block cGMP-induced platelet inhibition but rather potentiated cGMP-induced platelet inhibition (Figure 7). Combined with our recent finding that PKG stimulates platelet activation,5 these data suggest that PKA is predominantly responsible for the cGMP-induced platelet inhibition in humans, whereas PKG plays a major role in the initial cGMP-induced stimulatory response during thrombin- or VWF-induced platelet activation. Our data are consistent with previous reports that NO donors induced elevation of intracellular cAMP levels19 and that the inhibitory effects of NO donors were attenuated by PKA inhibitors31 and an adenylyl cyclase inhibitor.19 It is interesting to note that the platelet inhibition by cGMP analogs is a significantly delayed event requiring a prolonged preincubation,5 which correlates with cGMP-induced VASP phosphorylation that also requires more than 10 minutes of cGMP analog incubation.5 In contrast, the PKA activator–induced platelet inhibition is a rapid process and correlates well with the rapid cAMP-dependent VASP phosphorylation. Since cGMP is known to elevate intracellular cAMP level by inhibiting phosphodiesterase 3, it is likely that the inhibitory effects of cGMP analogs and NO donors in human platelets are predominantly mediated via activation of PKA and subsequent phosphorylation of VASP. This conclusion, however, does not exclude the possibility that cGMP-PKG pathway may also be involved in cGMP-mediated inhibition of platelet activation induced by certain agonists such as collagen. For example, a previous study reported that an inhibitor of a PKA pathway, as well as a PKG inhibitor (to a lesser degree), partially reversed the inhibitory effect of an NO donor on collagen-induced platelet activation.19 Consistent with the possible different roles of PKG in platelet activation induced by different agonists, it was found that PKG inhibitors, which inhibited thrombin and VWF-induced platelet aggregation, did not inhibit collagen-induced platelet aggregation.5 In addition, it is known that the inhibitory effects of cGMP are not totally VASP-dependent, as cGMP has been shown to inhibit calcium mobilization in a VASP-independent manner7,8 and to induce phosphorylation of other potential PKG substrates such as the TXA2 receptor.9 Also, platelets from VASP-deficient mice still show a partial response to cGMP analog–induced platelet inhibition.7,8 Thus, it will be interesting to further investigate the roles of PKA and/or PKG in other cGMP-mediated signaling pathways.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-10-3210.
Supported in part by grants from The National Heart, Lung, and Blood Institute, National Institutes of Health (HL62350 and HL68819). Z.L. is supported by a fellowship award from the American Heart Association Midwest Affiliate. M.E. is supported by grants from the Bundesministerium für Bildung und Forschung, Interdisziplinäres Zentrum für Klinische Forschung Würzburg TP-E15 and Deutsche Forschungsgemeinschaft Sonderforschungsbereich 355 (TP-C3).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ulrich Walter for helpful discussions and Dr Randal A. Skidgel for critical reading of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal