Abstract
Four human CD8+ T-cell subsets, naive (CCR7+CD45RA+), central memory (TCM, CCR7+CD45RA–), effector memory (TEM, CCR7–CD45RA–), and CD45RA+ effector memory cells (TEMRA, CCR7–CD45RA+) were compared for their capacity to proliferate and differentiate in response to antigen or homeostatic cytokines. Cytokine responsiveness and interleukin-15 receptor expression were low in naive T cells and progressively increased from TCM to TEM and TEMRA. In contrast, the capacity to accumulate in response to T-cell receptor (TCR) or cytokine stimulation showed a reciprocal pattern and was associated with resistance to cell death and Bcl-2 expression. Whereas all TCR-stimulated cells acquired a CD45RA–CCR7– phenotype, cytokine-stimulated cells maintained their phenotype with the exception of TCM cells, which expressed CCR7, CD45RA, and perforin in various combinations. Single CD8+ TCM cells, but not TEM cells, could be expanded with cytokines, and the obtained clones displayed several distinct phenotypes, suggesting that TCM cells are heterogeneous. Consistently, CCR4 expression in the CD8+ TCM pool discriminated CCR4+ type 2 polarized cells (Tc2) and CCR4–CTL precursors. Finally, ex vivo bromodeoxyuridine (BrdU) incorporation experiments revealed that memory subsets have different in vivo proliferation rates, with CCR4–TCM having the highest turnover and TEMRA the lowest. These results show that human CD8+ memory T-cell subsets have different proliferation and differentiation potentials in vitro and in vivo. Furthermore, they suggest that TEMRA cells are generated from a TCM subset upon homeostatic proliferation in the absence of antigen.
Introduction
Memory T lymphocytes are heterogeneous and comprise distinct populations that can be distinguished based on surface markers and effector functions, such as cytokine secretion and cytotoxicity. In the CD8+ compartment loss of CD28 identifies a subset of antigen-experienced T cells with high cytotoxic potential and reduced proliferative capacity, which frequently contain in vivo–expanded clones in the elderly or in human immunodeficiency virus (HIV)–infected individuals.1, 2, 3 Van Lier and colleagues defined 2 CD8+ memory T-cell subsets based on the expression of CD45 isoforms and CD27: CD45RO+CD27+ memory cells, which lacked immediate cytolytic function, and CD45RA+ CD27– effector cells with low proliferative capacity and high levels of perforin and cytotoxicity.4 Further, heterogeneity was revealed by the analysis of CCR7, a chemokine receptor for lymph node homing, which discriminated perforin-central memory T cells (TCM, CCR7+ CD45RA–) from tissue homing CCR7– effector memory T cells, which are either perforinlo CD45RA– (TEM) or perforinhi CD45RA+ (TEMRA).5 The differentiation pathways that lead to the generation of these cells, in particular the CCR7–CD45RA+ cells, are uncertain. A progressive and irreversible differentiation from naive to TCM and TEM has been demonstrated for CD4+ T cells and suggested for CD8+ T cells,5 but the precise relationship between the various CD8+ subpopulations is still a matter of debate. Further, complexity is added by the finding that some CD8+ memory T cells share characteristics with Tc2 cells, which are not cytotoxic but produce interleukin-4 (IL-4) and IL-13.6,7
There is considerable interest in understanding the distribution of CD8+ T cells generated in response to viruses or tumors within different subsets.8, 9, 10, 11, 12, 13 HIV-infected patients lack antigen-specific cells of the CCR7–CD45RA+ subset, suggesting that HIV inhibits certain steps of T-cell differentiation as a strategy to subvert immune response.9 Other studies indicated that CD45RA– and CD45RA+ memory T cells are generated in response to different antigens from the same virus and with different kinetics.10, 11, 12, 13 However, it is not clear from these studies whether these phenotypic and functional differences are due to the persistence of antigen, homeostatic mechanisms, or both.
T-cell homeostasis ensures that the pools of naive and memory T cells are independently maintained at a constant size under changing environmental conditions.14 Studies by Sprent and coworkers established that under steady-state conditions memory T cells turn over continuously at a relatively high rate, whereas naive T cells proliferate poorly.15 However, naive T cells undergo a massive homeostatic expansion when transferred to lymphopenic animals and differentiate to cells that share some characteristics with memory and effector cells.16, 17, 18 It is now well established that both maintenance and homeostatic proliferation of CD8+ T cells depend on cytokines signaling via the common γ chain (γc).19, 20, 21, 22, 23, 24 Thus, whereas CD8+ naive T cells require T-cell receptor (TCR) tickling by self-major histocompatibility complex (MHC)25,26 and IL-7,27,28 CD8+ memory T cells survive and proliferate in the absence of self-MHC29 but require IL-15 or IL-7.19, 20, 21, 22, 23, 24 While IL-7 and IL-15 are constitutively expressed by a variety of cells including dendritic cells (DCs),30,31 IL-2 is produced by antigen-activated T cells and opposes the effect of IL-15 on memory maintenance.32 Bystander T-cell activation by cytokines can lead to either proliferation33 or death,34 possibly resulting in memory attrition.
Comparatively little is known about the homeostasis of human T cells and in particular how the functional heterogeneity of the memory pool is maintained. Based on the length of telomeres and in vivo bromodeoxyuridine (BrdU) incorporation, it was estimated that human memory, but not naive, T cells divide approximately once or twice per year under steady-state conditions, and that they can perform up to 15-30 divisions under lymphopenic conditions.35, 36, 37 γc cytokines, including IL-7 and IL-15, have been reported to induce proliferation of human T cells in the absence of TCR stimulation,38, 39, 40, 41 and the enhanced T-cell turnover in lymphopenic HIV patients is associated with increased levels of IL-7.42 These findings suggest that γc cytokines have a similar role in memory T-cell maintenance in mice and man.
We previously reported that human CD4+ memory T cells proliferate in response to IL-7 and IL-15 and that cytokinestimulated TCM cells can differentiate to TEM cells.43 We therefore considered the possibility that the phenotype and function of CD8+ memory T cells may be influenced by homeostatic mechanisms. Consequently, we investigated the proliferation and differentiation potential of human CD8+ T-cell subsets in response to antigen or homeostatic cytokines. The results reported indicate that memory T-cell subsets have different proliferative capacities in vitro and in vivo and that central memory T cells have the unique ability to differentiate in an antigen-independent, but not antigen-dependent, fashion into CCR7–CD45RA+ effector cells.
Materials and methods
Antibodies and reagents
Allophycocyanin (APC)– or phycoerythrin (PE)–labeled antibodies specific for IL-7 receptor (R) α and CD45RA were purchased from Beckman Coulter (Marseille, France). All recombinant cytokines (IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, and IL-15), PE-labeled antibodies specific for perforin, BCL-2, CCR4, IL-2/15Rβ and the common γ chain, neutralizing anti–IL-2 antibody, and unlabeled and biotinylated antibody pairs for the enzyme linked immunosorbent assay (ELISA) were purchased from Pharmingen (San Diego, CA). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Molecular Probes (Eugene, OR). The anti-CCR7 antibody (3D12) was kindly provided by M. Lipp (Berlin, Germany).
Cell culture and cloning
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors. CD8+ T cells were then isolated by positive selection with anti-CD8–coated magnetic beads (MACS, Miltenyi, Bergisch Gladbach, Germany). The cells obtained were more than 98% CD8+ CD3+. Naive and memory T-cell subpopulations were purified to more than 99% by cell sorting, using anti-CD45RA and anti-CCR7 antibody.5 The distribution between the 4 subsets of 10 donors was the following: naive, 40% +/– 25%; TCM, 15% +/– 12%; TEM, 32% +/– 12%; and TEMRA, 13% +/– 8%. Labeling of T cells with CFSE was performed as described.44 Monocytes were purified by positive selection with anti-CD14 antibodies coupled to magnetic beads (Miltenyi) as described.45 CD14+ cells were cultured for 4 days in RPMI 1640 containing 10% fetal calf serum (Hyclone, Logan, UT), 2 mM glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 50 μg/mL kanamycin (Gibco, Grand Island, NY), 50 ng/mL granulocyte-macrophage colony-stimulating factor (Novartis, Basel, Switzerland), and 1000 units (U)/mL IL-4. The DCs obtained were stimulated for 24 hours with 100 ng/mL lipopolysaccharide (from Salmonella abortus equi, Sigma, St Louis, MO). T cells were cultured with DCs at a 5:1 ratio in RPMI 1640 containing 100 U/mL IL-2 (Roche, Basel, Switzerland), 5% human serum, 2 mM glutamine, 1% nonessential amino acids, 1% sodium pyruvate, and 50 μg/mL kanamycin. Recombinant cytokines were used at concentrations determined in preliminary dose-response experiments at either 25 ng/mL (IL-7, IL-15, IL-21; R&D, Minneapolis, MN) or 10 ng/mL (IL-6, IL-10, IL-4, IL-12; Pharmingen), unless otherwise indicated. Central and effector memory cells were cloned in U-bottom 96-well plates with a mix of recombinant cytokines (IL-7, IL-15, IL-6, and IL-10) or with 10 μg/mL phytohemagglutinin (PHA, Sigma), 100 U/mL IL-2, and irradiated PBMCs as feeder cells.
Flow cytometry
Cell staining of CFSE-labeled T cells was performed with PE- or APC-labeled antibodies, with the exception of the anti-CCR7 Ab, which was detected either with a mouse-adsorbed PE-labeled antirat antibody or with a biotinylated antirat antibody, followed by incubation with streptavidin-APC (Pharmingen). Expression of the IL-15Rα chain was assessed by incubating cells with a biotinylated human IL-15 fusion protein (Alexis, Carlsbad, CA), followed by staining with streptavidin-APC. Perforin and Bcl-2 expression was assessed after fixation with paraformaldehyde and permeabilization with saponin. Cells were analyzed on a FACSCalibur with CellQuest software (Becton Dickinson, Mountain View, CA).
ELISA
Cytokine-producing capacity was assessed after stimulation of purified cell populations at 5 × 105/mL for 24 hours with 50 nM phorboldibutyrate (PdBu) and 0.5 μg/mL ionomycin or immobilized anti-CD3 and anti-CD28. Cytokine concentrations of supernatants were then assessed by ELISA following a standard protocol and analyzed with the Softmax program (Molecular Devices, Sunnyvale, CA).
Ex vivo BrdU labeling
The assay was performed as described15,37 with slight modifications. Briefly, fresh PBMCs from healthy volunteers were immediately cultured with or without 10 μg/mL BrdU (Sigma) for 16 hours. In some experiments the superantigen toxic shock syndrome toxin (TSST, 100 ng/mL) and IL-2 (100 U/mL) were added as control. CD8+ T cells were then purified with magnetic beads and stained with anti–CD45RA-PE or anti–Cy-Chrome, anti–CCR4-PE, and anti-CCR7 followed by antirat APC. Cells were fixed and permeabilized with 1% paraformaldehyde in phosphate buffered saline (PBS), 1% Tween-20 for 15 minutes at 37°C, treated with 50 μg/mL DNAse (Boehringer, Mannheim, Germany) in PBS 4 mM MgCl2, pH 5 for 30 minutes, stained with anti–BrdU-fluorescein isothiocyanate or isotype control antibody (Becton Dickinson). At least 106 events were acquired by flow cytometry.
Results
Expansion potential of naive and memory CD8+ T cells activated by antigen or homeostatic cytokines
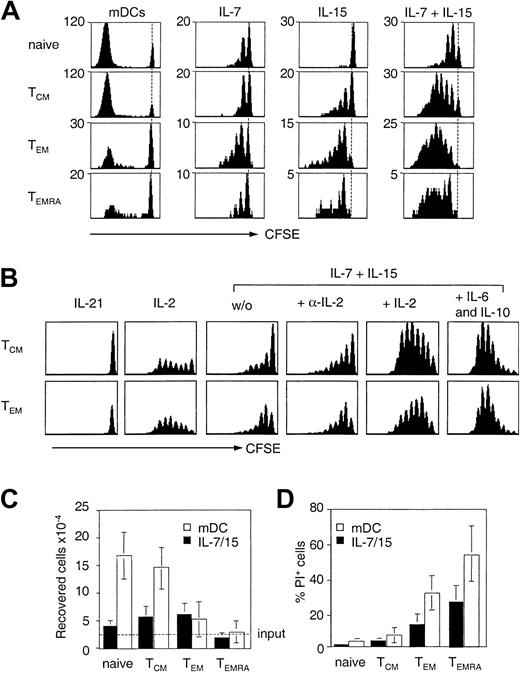
Subsets of human naive and memory CD8+ T cells were purified according to CCR7 and CD45RA expression, labeled with CFSE, and compared for their capacity to proliferate in response to antigen or cytokines implicated in homeostasis (Figure 1A-B). Antigenic stimulation provided by mature allogeneic DCs resulted in massive expansion of naive T cells and TCM cells, but TEM cells and especially TEMRA cells performed fewer divisions and were recovered in much lower numbers (Figure 1C). Similar results were obtained in the absence or presence of exogenous IL-2 and with anti-CD3 stimulation (data not shown). The low expansion potential of TCR-stimulated TEM and TEMRA cells was associated with a high rate of cell death as revealed by propidium iodide staining (Figure 1D). Death of TEMRA cells was not prevented by neutralizing anti–tumor necrosis factor (TNF) or anti–Fas ligand antibodies (data not shown), but was associated with low expression of the antiapoptotic BCL-2 protein (Table 1).
Expansion potential of human CD8+ T-cell subsets following cytokine or TCR stimulation. (A) Naive, central memory (TCM), effector memory (TEM), and CD45RA+ effector memory (TEMRA) cells were isolated from peripheral blood according to the expression of CCR7 and CD45RA, labeled with CFSE, and cultured with allogeneic mature DCs (mDCs) or stimulated with 25 ng/mL IL-7 and IL-15. Cell division was measured after 7 days. Dotted line indicates CFSE intensity of undivided cells. One representative experiment of 4 is shown. (B) Purified, CFSE-labeled TCM and TEM cells were stimulated with 25 ng/mL IL-21, 103 U/mL IL-2, 10 ng/mL IL-7 and IL-15 in the absence or presence of 103 U/mL IL-2, 2 μg/mL anti–IL-2, or 10 ng/mL IL-6 and IL-10. Proliferation was assessed by flow cytometry on day 7. The dotted line indicates the number of input cells. Number of viable cells (C) and percentage of dead (propidium iodide+) cells (D) recovered on day 7 after stimulation with allogeneic mDCs (□) or IL-7 and IL-15 (▪). Mean of 4 independent experiments.
Expansion potential of human CD8+ T-cell subsets following cytokine or TCR stimulation. (A) Naive, central memory (TCM), effector memory (TEM), and CD45RA+ effector memory (TEMRA) cells were isolated from peripheral blood according to the expression of CCR7 and CD45RA, labeled with CFSE, and cultured with allogeneic mature DCs (mDCs) or stimulated with 25 ng/mL IL-7 and IL-15. Cell division was measured after 7 days. Dotted line indicates CFSE intensity of undivided cells. One representative experiment of 4 is shown. (B) Purified, CFSE-labeled TCM and TEM cells were stimulated with 25 ng/mL IL-21, 103 U/mL IL-2, 10 ng/mL IL-7 and IL-15 in the absence or presence of 103 U/mL IL-2, 2 μg/mL anti–IL-2, or 10 ng/mL IL-6 and IL-10. Proliferation was assessed by flow cytometry on day 7. The dotted line indicates the number of input cells. Number of viable cells (C) and percentage of dead (propidium iodide+) cells (D) recovered on day 7 after stimulation with allogeneic mDCs (□) or IL-7 and IL-15 (▪). Mean of 4 independent experiments.
Cytokine receptor and BCL-2 expression of CD8+ T-cell subsets
. | TN . | TCM . | TEM . | TEMRA . |
|---|---|---|---|---|
| IL-7Rα | 30 ± 10 | 35 ± 12 | 30 ± 8 | 25 ± 9 |
| IL-15Rα | 2 ± 1 | 4 ± 1 | 7 ± 2 | 7 ± 2 |
| Common γ | 9 ± 3 | 7 ± 2 | 5 ± 3 | 5 ± 2 |
| IL-2/15Rβ | 2 ± 1 | 6 ± 2 | 12 ± 3 | 13 ± 4 |
| Bcl-2 | 87 ± 9 | 72 ± 6 | 59 ± 5 | 44 ± 5 |
. | TN . | TCM . | TEM . | TEMRA . |
|---|---|---|---|---|
| IL-7Rα | 30 ± 10 | 35 ± 12 | 30 ± 8 | 25 ± 9 |
| IL-15Rα | 2 ± 1 | 4 ± 1 | 7 ± 2 | 7 ± 2 |
| Common γ | 9 ± 3 | 7 ± 2 | 5 ± 3 | 5 ± 2 |
| IL-2/15Rβ | 2 ± 1 | 6 ± 2 | 12 ± 3 | 13 ± 4 |
| Bcl-2 | 87 ± 9 | 72 ± 6 | 59 ± 5 | 44 ± 5 |
Purified CD8+ T cells were stained for the expression of CD45RA, CCR7, and various cytokine receptors or fixed, permeabilized, and stained for BCL-2 expression. The numbers indicate the mean fluorescence intensity after subtraction of the background of isotype-matched control antibodies and represent the mean of at least 3 independent experiments.
Proliferation in response to IL-7 was comparable and low in all subsets. In contrast, responsiveness to IL-15 was low in naive T cells, intermediate in TCM cells, and high in effector memory T cells (Figure 1A). IL-2 also selectively stimulated memory T cells and boosted responses to IL-7 and IL-15, whereas addition of anti–IL-2 or stimulation with IL-21 had no effect (Figure 1B). As reported for CD4+ T cells,43 addition of DCs or DC-derived cytokines IL-6 and IL-10 boosted cytokine responsiveness of both CD8+ naive and memory cells, leading to extensive and comparable proliferation of TCM cells and TEM cells (Figure 1B).
Cytokine responsiveness correlated with the expression of the relevant cytokine receptors (Table 1). Thus, whereas the IL-7R was expressed on all subsets to a comparable level, IL-15Rα and IL-2/15Rβ chain expression were low on naive T cells, intermediate on TCM cells, and high on TEM and TEMRA cells. Consistent with their high cytokine responsiveness, TCM and TEM cells were recovered at higher numbers after stimulation with IL-15 and IL-7 as compared to naive T cells (Figure 1C). In contrast, TEMRA cells failed to accumulate despite the fact that they expressed high levels of IL-15R and nearly all cells entered cell division. The failure of cytokine-stimulated TEMRA cells to accumulate was associated with a high rate of cell death (Figure 1D), which was observed throughout the entire stimulation period and in response to all cytokine combinations tested (data not shown). These results suggest that the intrinsic high propensity to cell death compromises the accumulation of TEMRA cells in response to homeostatic cytokines in spite of their high cytokine responsiveness.
Altogether, these results show that whereas antigen-dependent expansion, cell viability, and Bcl-2 expression are progressively lost from naive and TCM to TEM and TEMRA cells, IL-15R expression and cytokine responsiveness have a reciprocal pattern and are progressively acquired with differentiation.
Generation of TEMRA cells by cytokine-stimulated TCM cells
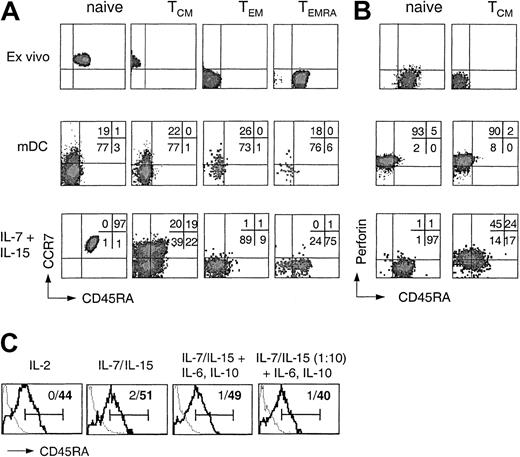
In order to assess the differentiation potential of CD8+ T-cell subsets, CFSE-labeled purified naive and memory CD8+ T-cell subsets were stimulated with either allogeneic DCs or IL-7 and IL-15. On day 7 CCR7 and CD45RA expression was analyzed on cells that had performed the same number of divisions (Figure 2A). Antigenic stimulation of any of the 4 CD8+ subsets resulted in the generation of rather homogeneous CCR7–CD45RA– effector cells. In contrast, whereas naive, TEM, and TEMRA cells proliferating in response to cytokines largely maintained their phenotype, TCM cells gave rise to cells expressing CCR7 and CD45RA in all possible combinations. Thus, some cytokine-stimulated TCM cells maintained their phenotype, whereas others generated cells with a TEM, a TEMRA, or, surprisingly, an apparent naive phenotype.
Differentiation potential of human CD8+ T-cell subsets following cytokine or TCR stimulation. (A) CD8+ T-cell subsets isolated by cell sorting were labeled with CFSE and stimulated with allogeneic mDCs or with IL-7 and IL-15. After 7 days cells that performed the same number of divisions were analyzed for the expression of CD45RA and CCR7. One representative experiment of 4 is shown. (B) Purified CD8+ naive and TCM cells were either analyzed for the expression of perforin and CD45RA ex vivo or labeled with CFSE, stimulated with allogeneic mDCs or with IL-7 and IL-15, and analyzed after 7 days for CD45RA and perforin expression. One representative experiment of 3 is shown. (C) Purified CFSE-labeled CD45RA–CD8+ TCM cells were stimulated in medium containing 103 U/mL IL-2, 25 or 2.5 ng/mL (1:10) IL-7 and IL-15 in the absence or presence of 10 ng/mL IL-6 and IL-10 in either uncoated (thick line) or anti-CD3–coated wells (dotted line). Viable dividing cells were analyzed on day 7 for CD45RA expression. Bars indicate positive staining and numbers percentage of CD45RA+ cells in the absence (bold) or presence (nonbold) of anti-CD3 antibody.
Differentiation potential of human CD8+ T-cell subsets following cytokine or TCR stimulation. (A) CD8+ T-cell subsets isolated by cell sorting were labeled with CFSE and stimulated with allogeneic mDCs or with IL-7 and IL-15. After 7 days cells that performed the same number of divisions were analyzed for the expression of CD45RA and CCR7. One representative experiment of 4 is shown. (B) Purified CD8+ naive and TCM cells were either analyzed for the expression of perforin and CD45RA ex vivo or labeled with CFSE, stimulated with allogeneic mDCs or with IL-7 and IL-15, and analyzed after 7 days for CD45RA and perforin expression. One representative experiment of 3 is shown. (C) Purified CFSE-labeled CD45RA–CD8+ TCM cells were stimulated in medium containing 103 U/mL IL-2, 25 or 2.5 ng/mL (1:10) IL-7 and IL-15 in the absence or presence of 10 ng/mL IL-6 and IL-10 in either uncoated (thick line) or anti-CD3–coated wells (dotted line). Viable dividing cells were analyzed on day 7 for CD45RA expression. Bars indicate positive staining and numbers percentage of CD45RA+ cells in the absence (bold) or presence (nonbold) of anti-CD3 antibody.
We next compared the capacity of perforin– naive T cells and TCM cells to acquire perforin following stimulation with antigen or cytokines (Figure 2B). Antigenic stimulation induced perforin in both naive T cells and TCM cells, but the cells were CD45RA–. In contrast, whereas naive T cells proliferating in response to IL-7 and IL-15 remained perforin–, a large fraction of TCM cells acquired perforin under the same condition, including cells that had up-regulated CD45RA. The addition of inhibitory anti–MHC class I antibody had no effect on cytokine-induced proliferation or perforin acquisition (data not shown). Cytokine-stimulated TEM cells, which constitutively express low levels of perforin, further up-regulated perforin expression (data not shown) but failed to acquire high levels of CD45RA (Figure 2A). Thus, cells coexpressing high levels of CD45RA and perforin were generated rather exclusively from cytokine-stimulated TCM cells. Importantly, CD45RA re-expression on dividing cytokine-stimulated TCM cells was largely unaffected by the composition and concentration of the cytokine mix but was in all cases prevented by the presence of anti-CD3 antibody (Figure 2C), showing that CD45RA re-expression requires cytokine-driven proliferation in the absence of TCR stimulation but is not under the exclusive control of a single cytokine.
Altogether, these results suggest that cytokine-stimulated TCM cells can self-renew and generate different types of effector cells, including TEMRA cells, in the absence of antigen.
Heterogeneity of the TCM pool revealed by cloning with cytokines
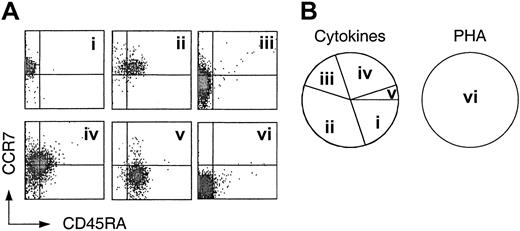
The generation of multiple fates from cytokine-driven TCM cells raises the question of whether TCM cells are heterogeneous or oligopotent. To address this issue we cloned TCM cells with either PHA plus IL-2 as an antigen surrogate or with a mix of homeostatic cytokines. The cloning mix contained IL-7 and IL-15 as well as IL-6 and IL-10, because the latter mix induced optimal expansion of both TCM and TEM cells (Figure 1B). Cloning efficiency of TCM with PHA exceeded 50%, and approximately 20% of cytokinestimulated TCM cells generated clones of limited size (approximately 102-104 cells), consistent with the slower kinetics of cytokine-driven versus antigen-driven proliferation.43 In contrast, TEM cells had a low cloning efficiency with PHA (< 30%), and no clones were obtained from cytokine-stimulated TEM, consistent with the reduced viability and expansion potential of TEM. The obtained TCM-derived clones were analyzed for CCR7 and CD45RA expression (Figure 3). While in some cytokine-derived clones all cells retained the original phenotype (Figure 3A), in all others a large fraction of the cells either down-regulated CCR7, acquired CD45RA, or both (Figure 3B-E). In contrast, all clones obtained with PHA displayed a CCR7–CD45RA– phenotype (Figure 3F). These results suggest that TCM cells are heterogeneous in that they are programmed to generate different types of effector cells under homeostatic conditions, whereas they acquire the same effector phenotype upon antigenic stimulation.
Heterogeneity of central memory CD8+ T cells revealed by cloning with cytokines. Central memory CD8+ T cells (CD45RA–, CCR7+) were cloned by single cell deposition in the presence of a cytokine cocktail or irradiated PBMCs and PHA plus exogenous IL-2. Following expansion for 3 weeks, the clones were analyzed for the expression of CD45RA and CCR7. (Ai-v) Five representative clones generated by cytokine stimulation. Cloning efficiency was approximately 20%. (vi) Phenotype of one representative clone generated by stimulation with PHA and feeder cells (cloning efficiency > 50%). (B) Frequency of cytokine and PHA-derived clones of 4 independent experiments.
Heterogeneity of central memory CD8+ T cells revealed by cloning with cytokines. Central memory CD8+ T cells (CD45RA–, CCR7+) were cloned by single cell deposition in the presence of a cytokine cocktail or irradiated PBMCs and PHA plus exogenous IL-2. Following expansion for 3 weeks, the clones were analyzed for the expression of CD45RA and CCR7. (Ai-v) Five representative clones generated by cytokine stimulation. Cloning efficiency was approximately 20%. (vi) Phenotype of one representative clone generated by stimulation with PHA and feeder cells (cloning efficiency > 50%). (B) Frequency of cytokine and PHA-derived clones of 4 independent experiments.
CCR4 expression in the TCM pool distinguishes between cytotoxic effector cell precursors and Tc2 cells
Although CD8+ TCM cells are not cytotoxic, they produced considerable amounts of IL-4 and IL-13 upon restimulation with phorbol ester and calcium ionophore (Figure 4A) or anti-CD3 and anti-CD28 (not shown). Furthermore, IL-13, but not IL-4, was also produced by cytokine-stimulated TCM cells both in the absence and presence (data not shown) of anti–MHC class I antibody, indicating that IL-13 can be elicited in the absence of TCR stimulation. In contrast, TEM and TEMRA cells produced only low or undetectable levels of type 2 cytokines. These findings suggest that Tc2 memory cells have a TCM phenotype.
CCR4 expression discriminates Tc2 cells and CTL precursors among central memory CD8+ T cells. (A) IL-4 and IL-13 production by CD8+ T-cell subsets following stimulation with PdBu and ionomycin for 24 hours (▪) or IL-7 and IL-15 for 7 days (□). Mean of 5 independent experiments. (B) CCR7 and CCR4 expression by proliferating CFSE-labeled naive T cells stimulated by allogeneic mDCs in the presence of 10 ng/mL IL-4 or IL-12 and by freshly isolated CD45RA–CD8+ T cells. (C) Cytokine production by freshly isolated CCR4+ (▪) and CCR4– (▧) central memory CD8+T cells following stimulation with PdBu and ionomycin. Mean of 3 independent experiments. (D) Cytokine-stimulated CCR4+ and CCR4–CD8 TCM cells were analyzed on day 7 for the acquisition of perforin and CCR7 expression. One representative experiment of 3 is shown.
CCR4 expression discriminates Tc2 cells and CTL precursors among central memory CD8+ T cells. (A) IL-4 and IL-13 production by CD8+ T-cell subsets following stimulation with PdBu and ionomycin for 24 hours (▪) or IL-7 and IL-15 for 7 days (□). Mean of 5 independent experiments. (B) CCR7 and CCR4 expression by proliferating CFSE-labeled naive T cells stimulated by allogeneic mDCs in the presence of 10 ng/mL IL-4 or IL-12 and by freshly isolated CD45RA–CD8+ T cells. (C) Cytokine production by freshly isolated CCR4+ (▪) and CCR4– (▧) central memory CD8+T cells following stimulation with PdBu and ionomycin. Mean of 3 independent experiments. (D) Cytokine-stimulated CCR4+ and CCR4–CD8 TCM cells were analyzed on day 7 for the acquisition of perforin and CCR7 expression. One representative experiment of 3 is shown.
To address whether Tc2 cells were a subpopulation of TCM cells, we searched for phenotypic markers of Tc2 cells. To this aim naive CD8+ T cells were primed with allogeneic DCs under type 1 or type 2 polarizing conditions and compared for chemokine receptor expression (Figure 4B). Priming in the presence of IL-12 induced rapid CCR7 down-regulation on dividing cells, and only few cells expressed CCR4. In contrast, upon priming in the presence of IL-4, a large fraction of dividing cells retained CCR7 and up-regulated CCR4 expression. Consistent with the in vitro priming data, CCR4 also was expressed on a variable fraction (35% +/– 24%) of freshly isolated TCM cells, whereas only few TEM cells expressed CCR4 at low levels (Figure 4B).
To investigate if CD8+CCR4+ TCM cells were indeed Tc2 cells, we sorted TCM cells on the basis of CCR4 expression and analyzed their cytokine profiles. Ex vivo–stimulated CCR4+ TCM cells produced high levels of IL-4 but low levels of interferon (IFN)-γ CCR4–TCM expressed virtually no IL-4 but secreted IFN-γ (Figure 4C). In contrast, the 2 cell types produced comparable amounts of IL-2. These findings indicate that CCR4 expression identifies Tc2 cells as discrete subsets of central memory CD8+ T cells.
To determine if the 2 subsets had different capacities to generate cytotoxic effector cells (CTLs) under homeostatic conditions, we stimulated CCR4+ and CCR4–TCM with cytokines for 7 days and assessed CCR7 and perforin expression (Figure 4D). Most cytokinestimulated CCR4–TCM cells became CTLs because they down-regulated CCR7 and acquired perforin at the same time. In contrast, a smaller fraction of CCR4+ TCM cells down-regulated CCR7 expression, and only few CCR7– cells acquired low levels of perforin. Altogether, these results show that CTL precursors and Tc2 cells are distinct populations of the TCM pool that can be discriminated by CCR4 expression.
Differential in vivo turnover of CD8+ memory T-cell subsets
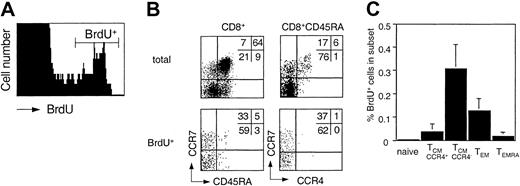
Memory T cells slowly turn over under steady-state conditions in vivo.15,37 Cells that passed the G1 cell cycle checkpoint are committed to replicate DNA and complete the cell cycle.46 The in vivo turnover of T cells can therefore be assessed by ex vivo BrdU incorporation.37 Freshly purified PBMCs from healthy donors were cultured in the presence of BrdU, and CD8+ T cells were purified and analyzed for BrdU incorporation in relation to CCR7, CCR4, and CD45RA expression (Figure 5). Although a significant fraction of small CD69–, CD25– T cells spontaneously incorporated BrdU, TSST-activated T cells (CD69+TCRVβ2+) in parallel cultures did not start to incorporate BrdU before day 2 (data not shown), indicating that the spontaneously BrdU incorporating cells had been committed to proliferation in vivo. Importantly, most cells that incorporated BrdU were CD45RA– and CCR4–. Consequently, CCR4–TCM cells had the highest and TEMRA cells the lowest turnover among memory cells, whereas the turnover of naive cells was below the detection limit. These results show that memory subsets have different rates of in vivo turnover and are consistent with the view that TEMRA cells are continuously replenished from proliferating precursors of the TCM pool.
Ex vivo BrdU incorporation of CD8+ T-cell subsets. Freshly isolated PBMCs were incubated with BrdU and processed as described in “Materials and methods.” (A) BrdU staining on total CD8+ cells. (B) Distribution of CD8+ subsets of total and BrdU+ cells. (C) Mean percentage of BrdU+ cells in CD8+. subsets in 5 healthy donors.
Ex vivo BrdU incorporation of CD8+ T-cell subsets. Freshly isolated PBMCs were incubated with BrdU and processed as described in “Materials and methods.” (A) BrdU staining on total CD8+ cells. (B) Distribution of CD8+ subsets of total and BrdU+ cells. (C) Mean percentage of BrdU+ cells in CD8+. subsets in 5 healthy donors.
Discussion
We have shown that human naive and memory CD8+ T-cell subsets have different capacities to proliferate and differentiate in response to TCR stimulation or cytokines. In particular, we show that central memory CD8+ T cells can be cloned with cytokines in the absence of TCR stimulation, and some of them give rise to cells with a characteristic CCR7–CD45RA+ phenotype, which is found in vivo following viral infection12,13 but cannot be induced in vitro by antigenic stimulation. We also provide evidence that central memory CD8+ T cells represent a heterogeneous subset comprising both CCR4+ Tc2 memory cells and CCR4–CTL precursors.
Both constitutive and lymphopenia-induced proliferation of mouse CD8+ T cells depend on the homeostatic cytokines IL-7 and IL-15.19, 20, 21, 22, 23, 24 Although the relative contribution of different γc cytokines to homeostatic proliferation in humans is difficult to address directly, the results presented here together with recent reports are consistent with the view that human and murine T cells have similar requirements.35, 36, 37, 38, 39, 40, 41, 42, 43 Thus, antigen-independent naive T-cell proliferation in the mouse requires TCR tickling, IL-7, and lymphopenia, whereas CD8+ memory T cells turn over constitutively, express high levels of the IL-2/15Rβ chain, and require IL-15 or IL-7. Consistently, we found that human CD8+ memory but not naive T cells incorporate BrdU ex vivo, proliferate in response to IL-15, and express high levels of the IL-15R. Because antigen-independent proliferation of naive T cells requires TCR tickling only when cytokines are limiting,47 it is not surprising that proliferation of CD8+ naive T cells in response to optimal amounts of IL-7 plus IL-15 is not inhibited by blocking anti–MHC I antibody (data not shown). Both murine19 and human43 CD4+ T cells express low levels of the IL-2/15Rβ chain and are consequently less responsive to cytokines as compared to CD8+ T cells. Mouse CD8+ memory cells recently were reported to be heterogeneous for the expression of the IL-2/15Rβ chain and to have differential requirements for IL-15.48 We show here that expression of a functional IL-15 receptor and cytokine responsiveness are associated with differentiation and increases from naive to central memory and effector memory T cells in humans. Thus, cytokine responses of human T cells recapitulate many aspects of homeostatic T-cell proliferation in vivo and provide a valuable tool to extend our knowledge of T-cell maintenance to the human system.
Memory T cells have an enhanced susceptibility to cell death49,50 and die upon bystander activation,34 although surviving cells may proliferate and repopulate the memory pool. We show that resistance of human CD8+ T-cell subsets to cell death is associated with the expression of the antiapoptotic Bcl-2 protein, which protects T cells from multiple forms of cell death.51 Both antigen and cytokines up-regulate Bcl-2 in all subsets, but the differences in Bcl-2 expression between the subsets are maintained (data not shown), supporting the notion that the high susceptibility to cell death is an intrinsic feature of TEMRA cells. Consequently, TEMRA cells have a low expansion potential in response to both antigen and cytokines in vitro and a low turnover in vivo.
Naive T cells expanding in lymphopenic mice differentiate, acquiring some characteristics of effector/memory cells.16, 17, 18 Similarly, naive cytokine-stimulated CD4+ cells43 and CD8+ (data not shown) cells acquire the capacity to secrete low levels of IFN-γ, but cells maintain a naive phenotype because they retain CD45RA and CCR7 expression and do not acquire perforin. In contrast, cytokine-stimulated TCM cells differentiate and generate various types of effector cells upon cytokine stimulation, which express CCR7, perforin, and CD45RA in various combinations. Notably, acquisition of perforin is associated with CCR7 down-regulation (Figure 4D), consistent with the notion that lymph node homing capacity and the acquisition of effector functions are coordinately regulated.5 Cloning experiments suggest that TCM cells are heterogeneous because they have different capacities to generate effector cells and may be committed to a particular differentiation pathway. Consistently, CCR4 expression within the TCM pool discriminates between type 2 polarized cells (Tc2) and CTL precursors. However, we cannot rule out that an early stochastic event in an oligopotent TCM cell commits the daughter cells to a particular differentiation pathway.
Tc2 cells can be generated by in vitro stimulation of naive CD8+ T cells in the presence of IL-4. Tc2 cells normally are not cytotoxic and produce IL-4 and IL-13 but not IFN-γ.6,7 We have shown that in vitro–primed Tc2 retain CCR7 and acquire CCR4, a chemokine receptor involved in DC–T-cell interactions52 and characteristic for Th2 cells53, 54, 55 and regulatory cells.56 Using CCR7 and CCR4 staining, we identified a subset of TCM cells that shares characteristics with in vitro–generated Tc2 cells. These cells produce IL-4 and IL-13 upon TCR stimulation and IL-13 alone upon cytokine stimulation, a finding that has precedents in T helper 1 (Th1) cells that produce IFN-γ in response to IL-12 and IL-18 in an antigen-independent manner.57 It is interesting that type 2 CD4+ and CD8+ T cells have different migratory potential. Th2 cells are tissue homing effector cells involved in inflammatory reactions, whereas Tc2 retain lymph node homing capacity and may have regulatory functions. Indeed, recent studies indicate that IL-4 derived from CD8+ cells can potently boost CTL responses possibly through DC stimulation58 or by promoting the generation of memory cells.59
CD45RA+ memory cells (TEMRA) are a dynamic and enigmatic population of the CD8+ memory pool. Various lines of evidence, such as the loss of CD28, CD27, and CCR7; the low proliferative capacity; the high susceptibility to apoptosis; and the presence of high levels of perforin and Fas ligand indicate that they represent the most differentiated type of memory cells.4,5,11 This notion is not inconsistent with a recent report showing that Epstein-Barr virus–specific effector cells that proliferate in response to persistent antigen have an even lower expansion potential and are CD45RA–.13 TEMRA cells have been reported to appear after the acute phase of viral infection, contain cells specific for lytic but not latent Epstein-Barr virus antigens,12,13 and are absent in persistent HIV infection.9,10 Intriguingly, we found that although TEMRA cells cannot be generated by antigenic stimulation, cells with a phenotype corresponding to TEMRA, that is, CD45RA+, CCR7–, and perforin+, are generated rather exclusively by a TCM subset upon cytokine stimulation. Importantly, TEMRA generation is largely independent of the cytokine combination and concentrations that drive proliferation but is completely prevented by TCR stimulation (Figure 2C), suggesting that TEMRA generation requires homeostatic proliferation in the absence of antigen, but not a particular cytokine environment. We also observed that some cytokine-stimulated TCM cells acquire CD45RA expression without losing CCR7, that is, an apparent naive phenotype, raising the possibility that circulating CCR7+CD45RA+ cells contain few antigen-experienced revertants. In summary these findings show that CD45RA re-expression on antigen-experienced CD8+ T cells is inhibited by antigen and promoted by homeostatic cytokines, consistent with the selective and late appearance of TEMRA cells in viral infections.
Altogether, our results indicate that CD8+ memory T-cell subsets have different capacities to proliferate, differentiate, and resist cell death in response to antigen and homeostatic cytokines. We suggest that TCM cells, due to their high expansion and differentiation potential and in vivo turnover, play an important role in maintaining the heterogeneous memory pool.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-11-3577.
Supported in part by the European Community (contract no. QLK-CT-201-0105) and by the Swiss National Science Foundation (grant no. 31-63885). A.L. is supported by the Helmut Horten Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank D. Jarossay for cell sorting, G. Bosshard for technical assistance, E. Traggiai and M. Manz for discussion, and A. Gett for critical reading and comments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal