Abstract
Overexpression of Bcl-2 in myeloma cells results in resistance to drugs such as dexamethasone (DEX), adenovirus-mediated delivery of p53 (Ad-p53), and paclitaxel (TAX), which work through the intrinsic apoptotic pathway. Bcl-2 antisense oligodeoxynucleotides (Bcl-2-ASO) have been shown to induce apoptosis in cancer cells, as a single agent or, better, in combination with chemotherapy. We hypothesized that down-regulation of Bcl-2 by Bcl-2-ASO will sensitize drug-resistant myeloma cells to undergo apoptosis. In this paper we report a detailed time/dose study of the effect of Bcl-2-ASO on myeloma cells with varying levels of Bcl-2. Treatment of myeloma cells expressing relatively low levels of Bcl-2 with Bcl-2-ASO resulted in a substantial apoptosis concomitant with a substantial depletion of Bcl-2 protein. Maximal apoptosis was observed at 5 to 10 μg/mL Bcl-2-ASO, following 4 days of treatment. Down-regulation of Bcl-2 and apoptosis were time and dose dependent and were sequence specific. In these cell lines, apoptosis was accompanied by activation of caspase-9 and caspase-3 and by release of cytochrome c to the cytosol. In contrast, high Bcl-2–expressing myeloma cells were practically resistant to Bcl-2-ASO. Most important, however, pretreatment of myeloma cells expressing high levels of Bcl-2 with Bcl-2-ASO increased the extent of DEX-, TAX-, and Ad-p53–induced apoptosis from 10%-20% to 70%-90%. Increased apoptosis was accompanied by additional decrease in Bcl-2 protein. Similar results for down-regulation of Bcl-2 and apoptosis were obtained with freshly isolated myeloma cells. These data support development of clinical trials with combinations of Bcl-2-ASO and DEX, TAX, or Ad-p53 in the treatment of refractory myeloma patients.
Introduction
Multiple myeloma is a clonal B-cell malignancy affecting both the immune system and the skeletal system, leading to bone destruction. It is the second most frequent hematologic malignancy, afflicting 40 000 people in the United States, with 5-year survival of around 20%.1-3 Therefore, new therapeutic modalities are needed for this disease.
Bcl-2 is a highly conserved, ubiquitous, membrane protein associated mostly with the outer membranes of mitochondria and nuclei.4,5 Bcl-2 is a survival factor for many cell types,6,7 and overexpression of Bcl-2 results in chemoresistance.7,8 We have shown that Bcl-2 plays a major role in drug resistance in myeloma cell lines and in freshly isolated myeloma cells. Furthermore, apoptosis induced by dexamethasone (DEX),9-12 paclitaxel (TAX) (Taxol),12 and adenovirus delivery of p53 (Ad-p53) was blocked in cells expressing high levels of Bcl-2 and in Bcl-2–transfected ARP-1 and RPMI 8226 cells.13,14 However, Bcl-2 did not protect against melphalan,15 gemcitabine,12 or tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)–induced apoptosis, which do not work through the intrinsic apoptotic pathway.16,17
It has been shown that in vitro treatment of cells with Bcl-2 antisense oligodeoxynucleotides (Bcl-2-ASO) results in induction of apoptosis in many cancer cell types including lung,18malignant glioma,19 bladder carcinoma,20chronic lymphocytic leukemia,21 renal cell carcinoma,22 and prostate cancer.23 Bcl-2-ASO was also found effective in chemosensitization to different anticancer drugs in vivo in several mouse xenograft models of human prostate cancer,24,25 in transitional cell carcinoma,26 and in melanoma.27 A phase 1-2 clinical trial with Bcl-2-ASO as a single agent reported minor responses in patients with non-Hodgkin lymphoma (NHL).28-30 More recently however, the combination of Bcl-2-ASO plus chemotherapy has been used for the treatment of melanoma and prostate cancer with better clinical outcome.31,32
In the current study we tested the effect of Bcl-2-ASO on apoptosis and on the intracellular levels of Bcl-2 in myeloma cells expressing varying levels of Bcl-2. Most specifically, we studied the effect of pretreatment with Bcl-2-ASO on TAX-, DEX-, and Ad-p53–induced apoptosis and on the intracellular levels of Bcl-2 protein. We report here that Bcl-2-ASO induces antisense-specific apoptosis in low Bcl-2–expressing cells in a time- and dose-dependent fashion through the activation of caspase-9 and caspase-3, concomitant with down-regulation of the Bcl-2 protein and release of cytochrome c to the cytosol. In contrast, cells expressing high levels of Bcl-2 were resistant to apoptosis by Bcl-2-ASO alone. However, pretreatment with Bcl-2-ASO, at clinically relevant doses, potentiated DEX-, TAX-, and Ad-p53–induced apoptosis in drug-resistant myeloma cells and in freshly isolated myeloma cells.
Materials and methods
Cell lines and cell culture conditions
U266, RPMI 8226, IM9, HS-Sultan, and ARH-77 cell lines were from ATCC (Rockville, MD). ARP-1 cells were developed at the University of Arkansas.9 IM9, HS-Sultan, and ARH-77 cell lines were derived from multiple myeloma (MM) patients, but because they are Epstein-Barr virus–positive (EBV+), they are considered by some as lymphoblastoid cells and not “true” myeloma cells. Bcl-2 and mock-transfected cells (neo), ARP-1 and RPMI 8226 cells, were generated and maintained in G418 as described before.9 Briefly, the Bcl-2 expression plasmid (obtained from Dr S. Korsmeyer, Dana Farber Cancer Institute) contained the LTR-SV-NEO construct, driven by an SV-40 promoter and human Bcl-2 cDNA and a neomycin selection gene in a partial Blue Script plasmid (Stratagene, La Jolla, CA).9The definition of low Bcl-2–expressing cells was determined relative to the expression of Bcl-2 in the parental ARP-1 cells (low Bcl-2–expressing cells; Figure 1). Cells were cultured in RPMI 1640 medium supplemented with 15% fetal calf serum (FCS medium) (GIBCO, Grand Island, NY) in a 37°C, CO2 incubator.
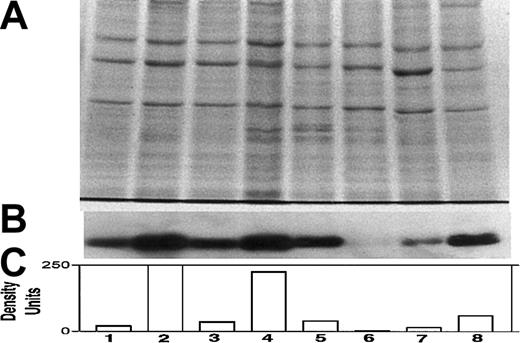
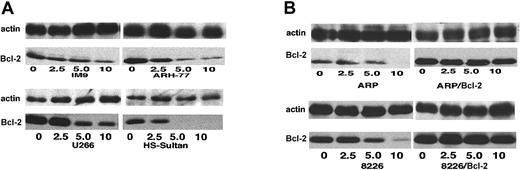
Differential expression of Bcl-2 in various myeloma cell lines.
Expression of Bcl-2 was determined in all cell lines used in this study, relative to ARP-1 cells. (A) Coomassie blue staining for loading control; (B) immunoblot for Bcl-2; and (C) quantitation of Bcl-2 bands by densitometry. Extraction of cellular protein, SDS-PAGE, immunoblotting, and quantitation of Bcl-2 bands were performed as described previously.9 Bcl-2 protein expression in ARP-1 cells and in the Bcl-2–transfected (ARP-1/Bcl-2) cells is shown in lanes 1 and 2, respectively. The expression of Bcl-2 protein in RPMI 8226 and in the Bcl-2–transfected (8226/Bcl-2) cells is depicted in lanes 3 and 4, respectively. The expression of Bcl-2 in ARH-77, HS-Sultan, IM9, and U266 cells is depicted in lanes 5, 6, 7, and 8, respectively. The level of Bcl-2 expression in ARP-1 cells was used throughout this study as a benchmark for low expression of Bcl-2. HS-Sultan and IM9 express relatively low levels of Bcl-2, whereas ARH-77, U266, and the Bcl-2–transfected ARP-1 and 8226 express relatively high levels of Bcl-2. For more details, see “Materials and methods.”
Differential expression of Bcl-2 in various myeloma cell lines.
Expression of Bcl-2 was determined in all cell lines used in this study, relative to ARP-1 cells. (A) Coomassie blue staining for loading control; (B) immunoblot for Bcl-2; and (C) quantitation of Bcl-2 bands by densitometry. Extraction of cellular protein, SDS-PAGE, immunoblotting, and quantitation of Bcl-2 bands were performed as described previously.9 Bcl-2 protein expression in ARP-1 cells and in the Bcl-2–transfected (ARP-1/Bcl-2) cells is shown in lanes 1 and 2, respectively. The expression of Bcl-2 protein in RPMI 8226 and in the Bcl-2–transfected (8226/Bcl-2) cells is depicted in lanes 3 and 4, respectively. The expression of Bcl-2 in ARH-77, HS-Sultan, IM9, and U266 cells is depicted in lanes 5, 6, 7, and 8, respectively. The level of Bcl-2 expression in ARP-1 cells was used throughout this study as a benchmark for low expression of Bcl-2. HS-Sultan and IM9 express relatively low levels of Bcl-2, whereas ARH-77, U266, and the Bcl-2–transfected ARP-1 and 8226 express relatively high levels of Bcl-2. For more details, see “Materials and methods.”
Cell culture and induction of apoptosis by Bcl-2-ASO
The 18-mer, full-phosphorothioate oligodeoxynucleotide (Bcl-2-ASO) with sequence antisense to the first 6 codons of the open reading frame of Bcl-2 (G3139, Genasense; Genta) was used throughout this study. Cells (5 × 105/mL) were cultured in FCS medium with or without Bcl-2-ASO at a concentration range of 1 to 10 μg/mL (0.2 to 2 μM) for the periods of time indicated. Aliquots were taken for determination of apoptosis by the annexin V method and for determination of Bcl-2 protein by Western immunoblotting. Reverse sense and scrambled oligodeoxynucleotides were used throughout this study as controls for specificity. All oligodeoxynucleotides used in cultures were replenished every other day. Bcl-2-ASO conjugated to fluorescein isothiocyanate (FITC–Bcl-2-ASO; Genta) was used to determine cellular uptake of Bcl-2-ASO.
Cell culture and induction of apoptosis by DEX and TAX
Cultures were initiated in T-25 tissue culture flasks in FCS medium, and TAX or DEX was added at the indicated concentrations. Apoptosis was determined by the annexin V method. In experiments where the combinations of Bcl-2-ASO and TAX or DEX were used, cells were pretreated with 5 μg/mL (1 μM) Bcl-2-ASO for 3 days. On day 3, DEX or TAX was added and cultures were continued for 2 more days. Aliquots were taken for determination of apoptosis and Bcl-2 protein following 4 or 5 days of treatment with Bcl-2-ASO alone or after 3 days of treatment with Bcl-2-ASO, followed by 24 or 48 hours of treatment with DEX or TAX. Bcl-2-ASO, reverse sense, and scrambled oligodeoxynucleotides were replenished every other day.
Detection of apoptosis by staining with annexin V
Detection of apoptosis was performed by annexin V–FITC (BioVision, Palo Alto, CA) as recommended by the manufacturer. Stained cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA). Apoptosis was quantitated by the CellQuest program (BDIS); 10 000 cells were analyzed in each sample. In several experiments, apoptosis was also determined morphologically by staining with Hoechst 33258 (Sigma, St Louis, MO) to detect fragmented nuclei as described before.12 A minimum of 200 cells were scored. Similar results were obtained with the 2 methods.
Adenovirus constructs
The virus constructs used in this study were described before.14 Ad-LacZ, a β-galactosidase–expressing, replication-deficient adenovirus (a control for virus toxicity), and Ad-p53, a wild-type p53-expressing replication-deficient adenovirus, were obtained from Dr W. El-Deiry (Pennsylvania Medical School) and were propagated as previously described.34 Titers of the adenoviruses were determined as previously described.14 We used virus particles per cell (vp/cell) as the titer unit for determination of virus infectivity. One plaque-forming unit (pfu) was equal to a virus titer of about 10 vp/cell.
Cell culture and induction of apoptosis by Ad-p53
Before virus binding, logarithmically growing cells were brought to 5 × 105 cells per 100 μL and placed in a 24-well plate in FCS medium. Ad-p53 or Ad-LacZ (control adenovirus) was added at the indicated virus titer in a volume of 100 μL, and virus was allowed to adhere for 3 hours at 37°C, after which 300 μL FCS medium was added to each well and incubation continued under same conditions for additional 24 or 48 hours. The wells were then harvested and tested for apoptosis by the annexin V method. Spontaneous apoptosis in the control wells, without virus, was below 8% for all cell lines tested. Pooled wells were used for determination of p53 expression by Western immunoblotting. Combination experiments were performed by pretreatment with Bcl-2-ASO (5 μg/mL) for 3 days before the Ad-p53 adherence step, and cultures were continued with or without Ad-p53 as described in the previous paragraph.
Blocking of Bcl-2-ASO–induced apoptosis by caspase-specific blocking peptides
To identify the downstream caspases involved in Bcl-2-ASO–induced apoptosis, we used caspase-specific blocking peptides (FMK derivatives; BioVision). We used 2 μM of each of the following peptides: YVAD (caspase-1 inhibitor), VDVAD (caspase-2 inhibitor), DEVD (caspase-3 inhibitor), LEVD (caspase-4 inhibitor), WEHD (caspase-5 inhibitor), VEID (caspase-6 inhibitor), IETD (caspase-8 inhibitor), LEHD (caspase-9 inhibitor), AEVD (caspase-10 inhibitor), and VAD (pancaspase inhibitor). FA-FMK at similar concentration was used as a control. Cells (4 × 105/mL) were cultured in 1 mL FCS medium in a 24-well plate, and Bcl-2-ASO (5 μg/mL) was added with or without the blocking peptide or with control peptide (FA-FMK). Cultures were harvested after 3 days and were tested for apoptosis by the annexin V binding method.
Determination of caspase-9 activation by fluorescent-tagged caspase-9–specific substrate peptide
To confirm the downstream caspase activation pathway used by Bcl-2-ASO, we also used FITC–caspase-9–specific substrate peptide (CaspaTag; Serologicals, Norcross, GA). Typically, cells (4 × 105/mL) were cultured in 1 mL FCS medium in a 24-well plate, and Bcl-2-ASO was added at concentrations of 0, 2.5, 5, or 10 μg/mL and cultures continued for 3 days. For measurement of caspase-9 activation, cells were further cultured for 1 hour at 37°C with FITC–caspase-9 substrate peptide; then fluorescence generated due to substrate degradation was determined by flow cytometry. Cells were then analyzed by flow cytometry as described for annexin V.
Determination of cytochrome c release from mitochondria by flow cytometry
To determine cytochrome c release from mitochondria, we used direct staining with FITC–anticytochrome c antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Typically, cells were cultured as described for caspase-9. Cultures were then harvested, and cells were fixed for 30 minutes at room temperature with 1% paraformaldehyde in phosphate-buffered saline (PBS). Cells were then washed with PBS and permeated with permeation buffer (0.05% Triton X-100 in PBS and 2% bovine serum albumin [BSA]) containing 10 μL FITC–anticytochrome c antibody. Permeation/staining was carried out at 4°C for 20 minutes. Unbound antibody was washed with PBS and cells analyzed by flow cytometry as described for annexin V. This staining method allows differential staining of cytosolic but not mitochondrial cytochrome c as indicated by confocal microscopy (Y.G., manuscript in preparation).
Determination of Bcl-2 and p53 protein expression by Western immunoblotting
Cells (1 × 106 to 2 × 106) were washed (× 2) with PBS, and total cellular protein was extracted as described before.11 Equal amounts of protein (50 μg) were loaded onto each lane, and protein bands were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). For Western immunoblotting, loading controls were performed using the housekeeping protein, β-actin (Sigma). In other experiments, loading controls were performed by first electrophoresing 5 μg protein per lane, staining with Coomassie blue, and then quantitating by densitometry. For Western immunoblotting, gel loading was corrected according to the Coomassie blue gel results. Gel electrophoresis, immunoblotting, detection of specific bands, and quantitation of protein bands by densitometry were performed as previously described.11 Antibodies to Bcl-2 were from Dako (Carpinteria, CA), and antibody to p53 was from Santa Cruz Biotechnology. A low-molecular-weight ladder of biotinylated protein markers (Biorad, Hercules, CA) was run for each gel. The p26 (Bcl-2) and p53 bands were tentatively identified according to their migration on the blot. In most experiments, up to 4 myeloma cell lines were processed in the same SDS-PAGE and immunoblotted simultaneously on the same membrane. This approach minimized experimental variation due to variations in protein transfer, immunoblotting, detection, and exposure to x-ray film and allowed for a reliable comparison of Bcl-2 expression between different cell lines or within the same cell line.
Staining for CD38+ CD45− myeloma cells and flow sorting of myeloma cells
Bone marrow samples were collected from MM patients following informed consent, and the mononuclear fraction was obtained by Ficoll-Hypaque separation. Staining for myeloma (CD38+CD45−) cells and flow sorting were performed as described before using the FACStar Plus (Turbo-Fast Sorter; BDIS).16 Myeloma cells with more than 97% purity were obtained routinely.16
Results
Bcl-2-ASO–induced apoptosis in myeloma cells expressing varying levels of Bcl-2
We first determined the relative expression of Bcl-2 protein by Western immunoblotting in each of the myeloma cell lines. Figure1 depicts the intracellular levels of Bcl-2 protein determined by Western immunoblotting; (Figure 1A) loading control stained with Coomassie blue; (Figure 1B) immunoblot for Bcl-2; and (Figure 1C) quantitation of Bcl-2 protein by densitometry. Bcl-2 expression in ARP-1 cells and in the ARP-1/Bcl-2 is shown in Figure 1C, lanes 1 and 2, respectively. Bcl-2 expression in RPMI 8226 and 8226/Bcl-2 is depicted in Figure 1C, lanes 3 and 4, respectively. Expression of Bcl-2 in ARH-77, HS-Sultan, IM9, and U266 cells is depicted in Figure 1C, lanes 5, 6, 7, and 8, respectively. Bcl-2 protein levels in the various cell lines were compared with the levels expressed in ARP-1 cells, used here as a benchmark. Thus, RPMI 8226 cells (Figure 1C, lane 3) expressed 1.6-fold Bcl-2 protein, whereas ARH-77 and U266 expressed 2.7-fold and 3-fold Bcl-2, respectively. ARP/Bcl-2 and 8226/Bcl-2 cells expressed 10- and 6-fold higher Bcl-2 protein levels, respectively, compared with ARP-1 cells, whereas HS-Sultan and IM9 expressed 0.2- and 0.7-fold Bcl-2 protein compared with ARP-1 cells (Figure 1).
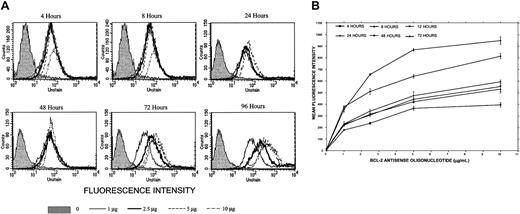
We then determined the uptake of Bcl-2-ASO at 1 to 10 μg/mL (0.2 to 2 μM) into ARH-77 cells (high Bcl-2) by employing FITC-conjugated Bcl-2-ASO. Uptake of Bcl-2-ASO at 4, 8, 24, 48, 72, and 96 hours was determined by flow cytometry. The results are depicted in Figure2A. It is clear that uptake of FITC–Bcl-2-ASO is time and dose dependent, reaching a maximum at 72 to 96 hours at 5 to 10 μg/mL FITC–Bcl-2-ASO. Similar experiments were performed with ARP-1 cells (low Bcl-2), and results are depicted in Figure 2B. The results are expressed as mean fluorescence intensity (MFI) versus time and dose of Bcl-2-ASO. MFI values directly correlate with the amount of Bcl-2-ASO per cell. Again, as was the case for ARH-77, maximal uptake was around 72 hours, at 5 to 10 μg/mL (Figure2B). At the higher doses of FITC–Bcl-2-ASO, fluorescence was detected and was localized mostly to the nuclei by confocal microscopy (results not shown).
Uptake of Bcl-2-ASO.
(A) Kinetics of Bcl-2-ASO uptake in ARH-77 myeloma cells. ARH-77 cells (high Bcl-2) were seeded at 5 × 105/mL and placed in a 24-well plate in a total volume of 1 mL FCS medium. FITC–Bcl-2-ASO was added at the indicated concentrations. FITC–Bcl-2-ASO was replenished every other day. Uptake was determined by flow cytometry using FACSCalibur, and results were analyzed by the CellQuest software; 10 000 cells were analyzed. Uptake at 4, 8, 24, 48, 72, and 96 hours is shown at 0 μg/mL, 1 μg/mL, 2.5 μg/mL, 5 μg/mL, and 10 μg/mL Bcl-2-ASO. The raw histograms are depicted for the various combinations. Note maximal uptake of FITC–Bcl-2-ASO at 5 to 10 μg/mL at 72 to 96 hours of treatment. Shaded histograms show cells with no FITC–Bcl-2-ASO. (B) Kinetics of Bcl-2-ASO uptake in ARP-1 myeloma cells. Experiment was performed as outlined in panel A except that ARP-1 cells (low Bcl-2) were used. Results are depicted as change in mean fluorescence intensity (MFI) as a function of treatment duration. Uptake was time and dose dependent, with a clear increase in the MFI between 72 and 96 hours, especially at the higher doses of FITC–Bcl-2-ASO. Note the similar kinetics of uptake for both cell lines presented in both panels. Bars are ± SD of at least 3 experiments.
Uptake of Bcl-2-ASO.
(A) Kinetics of Bcl-2-ASO uptake in ARH-77 myeloma cells. ARH-77 cells (high Bcl-2) were seeded at 5 × 105/mL and placed in a 24-well plate in a total volume of 1 mL FCS medium. FITC–Bcl-2-ASO was added at the indicated concentrations. FITC–Bcl-2-ASO was replenished every other day. Uptake was determined by flow cytometry using FACSCalibur, and results were analyzed by the CellQuest software; 10 000 cells were analyzed. Uptake at 4, 8, 24, 48, 72, and 96 hours is shown at 0 μg/mL, 1 μg/mL, 2.5 μg/mL, 5 μg/mL, and 10 μg/mL Bcl-2-ASO. The raw histograms are depicted for the various combinations. Note maximal uptake of FITC–Bcl-2-ASO at 5 to 10 μg/mL at 72 to 96 hours of treatment. Shaded histograms show cells with no FITC–Bcl-2-ASO. (B) Kinetics of Bcl-2-ASO uptake in ARP-1 myeloma cells. Experiment was performed as outlined in panel A except that ARP-1 cells (low Bcl-2) were used. Results are depicted as change in mean fluorescence intensity (MFI) as a function of treatment duration. Uptake was time and dose dependent, with a clear increase in the MFI between 72 and 96 hours, especially at the higher doses of FITC–Bcl-2-ASO. Note the similar kinetics of uptake for both cell lines presented in both panels. Bars are ± SD of at least 3 experiments.
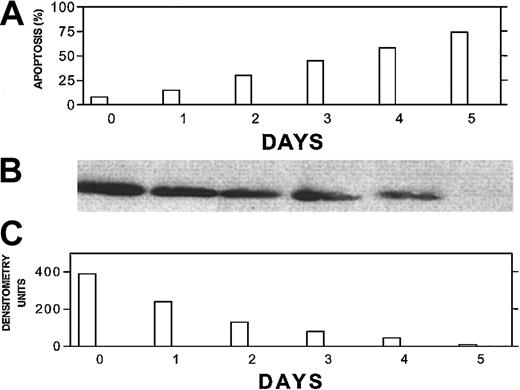
To further correlate the kinetics of Bcl-2-ASO–induced apoptosis and its effect on Bcl-2 protein levels, we treated ARP-1 cells (low Bcl-2) with 5 μg/mL Bcl-2-ASO for up to 5 days and measured apoptosis by the annexin V method and Bcl-2 levels by Western immunoblotting. The results are depicted in Figure 3. Bcl-2 protein levels decreased in a time-dependent fashion, with a 50% decrease in Bcl-2 levels as early as 24 hours after treatment with no detectable apoptosis. Hence, the decrease in Bcl-2 protein levels depicted in Figure 3B-C preceded apoptosis (Figure 3A) at each time point tested, suggesting a causative effect of Bcl-2-ASO on apoptosis.
Bcl-2-ASO–induced down-regulation of Bcl-2 correlates with apoptosis and precedes apoptosis in low Bcl-2–expressing ARP-1 myeloma cells.
ARP-1 cells (low Bcl-2) were seeded at 5 × 105/mL in T-25 flasks in FCS medium. Bcl-2-ASO was added at 5 μg/mL, and cultures were harvested following 0, 1, 2, 3, 4, and 5 days of treatment. Bcl-2-ASO was replenished every other day. Apoptosis (A) was determined by the annexin V method and Bcl-2 protein by Western immunoblotting (B). The quantitation for Bcl-2 was done by densitometry (C). Loading controls for Bcl-2 protein were performed by prerunning of gels and staining with Coomassie blue as detailed in “Materials and methods.” Other experimental details are as in Figure 1. Representative results from 2 experiments are shown. Note that down-regulation of Bcl-2 precedes apoptosis for every time point tested.
Bcl-2-ASO–induced down-regulation of Bcl-2 correlates with apoptosis and precedes apoptosis in low Bcl-2–expressing ARP-1 myeloma cells.
ARP-1 cells (low Bcl-2) were seeded at 5 × 105/mL in T-25 flasks in FCS medium. Bcl-2-ASO was added at 5 μg/mL, and cultures were harvested following 0, 1, 2, 3, 4, and 5 days of treatment. Bcl-2-ASO was replenished every other day. Apoptosis (A) was determined by the annexin V method and Bcl-2 protein by Western immunoblotting (B). The quantitation for Bcl-2 was done by densitometry (C). Loading controls for Bcl-2 protein were performed by prerunning of gels and staining with Coomassie blue as detailed in “Materials and methods.” Other experimental details are as in Figure 1. Representative results from 2 experiments are shown. Note that down-regulation of Bcl-2 precedes apoptosis for every time point tested.
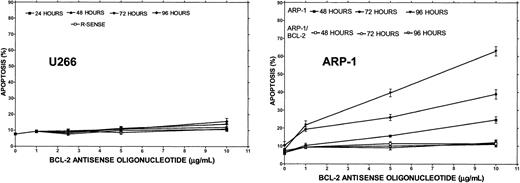
To test the effect of Bcl-2-ASO on apoptosis as a single agent and correlate it with cellular Bcl-2 levels, cells with low Bcl-2 (HS-Sultan or ARP-1) or high Bcl-2–expressing cells (ARH-77 and U266) were cultured with or without 1 to 10 μg/mL Bcl-2-ASO for up to 4 days. Reverse sense oligodeoxynucleotides and scrambled Bcl-2 oligodeoxynucleotides were used as a control for specificity. Apoptosis and Bcl-2 protein expression were assayed in the same experiment. The results obtained from HS-Sultan cells indicated a time/dose-dependent apoptosis, reaching a maximum of 70% at doses of 5 to 10 μg/mL (1 to 2 μM) following 4 days of treatment. In contrast, reverse sense oligodeoxynucleotides had no effect on apoptosis. Scrambled oligodeoxynucleotides were also ineffective (not shown). ARH-77 cells, which express higher levels of Bcl-2, were less sensitive to Bcl-2-ASO, with maximal apoptosis of 26% following 4 days of treatment with 10 μg/mL (2 μM) Bcl-2-ASO. Treatment with reverse sense Bcl-2 oligodeoxynucleotides had no effect (not shown). A similar trend was observed with U266 cells, which express even higher levels of Bcl-2 (Figure 1), with maximal apoptosis of 11% observed after 4 days of treatment with 10 μg/mL Bcl-2-ASO (Figure4). To further confirm the role of Bcl-2 protein on the resistance to Bcl-2-ASO, we studied the effect of Bcl-2-ASO on ARP-1 (low Bcl-2–expressing cells) compared with the Bcl-2–transfected ARP-1 cells, which express about 10-fold higher Bcl-2 (Figure 1). The results are depicted in Figure 4. Whereas maximal apoptosis in ARP-1 cells reached 66%, maximal apoptosis in ARP-1/Bcl-2 was only 10% (Figure 4). It is therefore clear that overexpression of Bcl-2 protects against Bcl-2-ASO–induced apoptosis in high Bcl-2–expressing cells and in Bcl-2–transfected cells. Similar results were obtained with RPMI 8226 and with Bcl-2–transfected 8226 cells (results not shown).
Bcl-2-ASO–induced apoptosis in HS-Sultan, ARH-77, U266, and ARP-1 myeloma cells.
U266 (high Bcl-2), ARP-1 cells (low Bcl-2), and ARP-1/Bcl-2 cells (high Bcl-2) were incubated with 0 to 10 μg/mL Bcl-2-ASO or with reverse sense Bcl-2 oligodeoxynucleotides for the time points indicated as described in “Materials and methods.” Apoptosis was determined by annexin V. Bcl-2-ASO and reverse sense oligodeoxynucleotides were replenished every other day. U266 cells express about 3-fold higher levels of Bcl-2, and ARP/Bcl-2 expresses about 10-fold higher Bcl-2 relative to ARP-1 cells (Figure 1). ARP/Bcl-2 cells were generated and maintained as described before.9 Bars are ± SD of at least 3 experiments. For additional experimental details, see “Materials and methods.” R-SENSE indicates reverse sense Bcl-2-ASO.
Bcl-2-ASO–induced apoptosis in HS-Sultan, ARH-77, U266, and ARP-1 myeloma cells.
U266 (high Bcl-2), ARP-1 cells (low Bcl-2), and ARP-1/Bcl-2 cells (high Bcl-2) were incubated with 0 to 10 μg/mL Bcl-2-ASO or with reverse sense Bcl-2 oligodeoxynucleotides for the time points indicated as described in “Materials and methods.” Apoptosis was determined by annexin V. Bcl-2-ASO and reverse sense oligodeoxynucleotides were replenished every other day. U266 cells express about 3-fold higher levels of Bcl-2, and ARP/Bcl-2 expresses about 10-fold higher Bcl-2 relative to ARP-1 cells (Figure 1). ARP/Bcl-2 cells were generated and maintained as described before.9 Bars are ± SD of at least 3 experiments. For additional experimental details, see “Materials and methods.” R-SENSE indicates reverse sense Bcl-2-ASO.
To correlate Bcl-2-ASO–induced apoptosis with its effect on intracellular levels of Bcl-2 protein, we studied the effect of Bcl-2-ASO on the intracellular levels of Bcl-2, following treatment with 2.5 to 10 μg/mL Bcl-2-ASO for 4 days. The results are summarized in Figure 5. Thus, 4-day treatment with 5 to 10 μg/mL Bcl-2-ASO was very effective in reducing up to 60% of Bcl-2 protein in U266 cells and 80% to 99% of the Bcl-2 protein in IM9, HS-Sultan, and ARH-77 cells (Figure 5A). In 8226 and ARP-1 cells, treatment with Bcl-2-ASO resulted in nearly undetectable levels of Bcl-2 (Figure 5B). In contrast, treatment of the Bcl-2–transfected ARP-1 and 8226 cells (× 10-fold and × 6-fold Bcl-2, respectively; Figure 1) resulted in only 10% to 25% decrease in the levels of Bcl-2 protein (Figure 5B). β-actin was used as a loading control. Reverse sense oligodeoxynucleotides did not affect Bcl-2 protein levels (results not shown). These results demonstrate a dose-dependent down-regulation of Bcl-2 by Bcl-2-ASO in low Bcl-2–expressing cells and establish an inverse correlation between Bcl-2 protein levels and apoptosis.
Bcl-2-ASO down-regulates the expression of Bcl-2 protein in low Bcl-2–expressing cells.
(A) Bcl-2 levels in Bcl-2-ASO–treated IM9 and HS-Sultan cells (low levels of Bcl-2; Figure 1) and U266 and ARH-77 cells (high Bcl-2; Figure 1). (B) Bcl-2 levels in Bcl-2-ASO–treated ARP-1 cells (low Bcl-2, mock-transfected); ARP/Bcl-2 cells (× 10-fold higher Bcl-2; Figure 1); and RPMI 8226 cells and 8226/Bcl-2 cells (× 6-fold higher Bcl-2; Figure 1). Bcl-2–transfected cells were generated and maintained as described previously.9 Cells were treated for 4 days with the indicated concentrations of Bcl-2-ASO (μg/mL). Bcl-2-ASO was replenished every other day. β-actin was used as an internal loading control. Other experimental details are as in Figure 1. Representative results from at least 3 different experiments are shown.
Bcl-2-ASO down-regulates the expression of Bcl-2 protein in low Bcl-2–expressing cells.
(A) Bcl-2 levels in Bcl-2-ASO–treated IM9 and HS-Sultan cells (low levels of Bcl-2; Figure 1) and U266 and ARH-77 cells (high Bcl-2; Figure 1). (B) Bcl-2 levels in Bcl-2-ASO–treated ARP-1 cells (low Bcl-2, mock-transfected); ARP/Bcl-2 cells (× 10-fold higher Bcl-2; Figure 1); and RPMI 8226 cells and 8226/Bcl-2 cells (× 6-fold higher Bcl-2; Figure 1). Bcl-2–transfected cells were generated and maintained as described previously.9 Cells were treated for 4 days with the indicated concentrations of Bcl-2-ASO (μg/mL). Bcl-2-ASO was replenished every other day. β-actin was used as an internal loading control. Other experimental details are as in Figure 1. Representative results from at least 3 different experiments are shown.
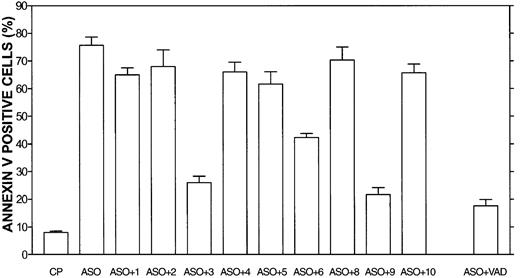
To elucidate the downstream caspases involved in Bcl-2-ASO–induced apoptosis, we used caspase-specific inhibitory peptides in 2 Bcl-2-ASO–sensitive cell lines, HS-Sultan and IM9 cells. The results obtained for HS-Sultan are depicted in Figure6. HS-Sultan cells (low Bcl-2; Figure 1) were cultured for 3 days with 5 μg/mL Bcl-2-ASO with or without 2 μM of the blocking peptides (maximal blocking concentration) or with control peptide (FA-FMK). Nonspecific toxicity of the control peptide (FA-FMK) was about 5% and of the blocking peptide was 2% to 5% above that of untreated control cells (results not shown). Of the 10 blocking peptides, the pancaspase inhibitor (VAD), caspase-3 inhibitor, and caspase-9 inhibitor blocked 70% to 80% of Bcl-2-ASO–induced apoptosis, whereas caspase-6 inhibitor blocked only 22% of Bcl-2-ASO–induced apoptosis. In contrast, inhibitors of caspase-8 and -10 and inhibitors of caspase-1, -2, -4, and -5 did not block Bcl-2-ASO–induced apoptosis. Similar results were obtained for IM9 cells (results not shown). These results suggest that the intrinsic apoptotic pathway, involving caspase-9 and -3, is the major pathway engaged in Bcl-2-ASO–induced apoptosis.
Caspase-3 and -9 blocking peptides block Bcl-2-ASO–induced apoptosis in HS-Sultan cells.
HS-Sultan cells were cultured for 72 hours with or without 5 μg/mL Bcl-2-ASO, with 2 μM of each caspase inhibitory peptide, or control peptide (CP, or FA-FMK). Nonspecific toxicity of the control peptide (CP) was around 5%. VAD indicates pancaspase inhibitor. The toxicity of the blocking peptides was 2% to 5% above that of untreated, control cells (not shown). Apoptosis was determined by annexin V. Bars are ± SD of at least 3 experiments. ASO indicates Bcl-2-ASO; ASO+1, Bcl-2-ASO plus caspase-1 blocking peptide; ASO+2, Bcl-2-ASO plus caspase-2 blocking peptide, etc. For additional experimental details, see “Materials and methods.”
Caspase-3 and -9 blocking peptides block Bcl-2-ASO–induced apoptosis in HS-Sultan cells.
HS-Sultan cells were cultured for 72 hours with or without 5 μg/mL Bcl-2-ASO, with 2 μM of each caspase inhibitory peptide, or control peptide (CP, or FA-FMK). Nonspecific toxicity of the control peptide (CP) was around 5%. VAD indicates pancaspase inhibitor. The toxicity of the blocking peptides was 2% to 5% above that of untreated, control cells (not shown). Apoptosis was determined by annexin V. Bars are ± SD of at least 3 experiments. ASO indicates Bcl-2-ASO; ASO+1, Bcl-2-ASO plus caspase-1 blocking peptide; ASO+2, Bcl-2-ASO plus caspase-2 blocking peptide, etc. For additional experimental details, see “Materials and methods.”
To further confirm the results obtained with caspase blocking peptides, we used fluorescence-tagged caspase-9 substrate-specific peptide (FITC–caspase-9 peptide). We also determined release of cytochrome c to the cytosol by using FITC–anticytochrome c antibody in fixed cells and correlated the results to the extent of apoptosis by annexin V. The combined results obtained from 72 hours of treatment of IM9 cells with Bcl-2-ASO (0 to 10 μg/mL) are depicted in Figure7. Caspase-9 activation was dependent on the dose of Bcl-2-ASO and increased from 10% to 75% positive cells with a clear shift in the mean fluorescence intensity (MFI) from 7 units at 0 μg/mL Bcl-2-ASO to 56 units at 10 μg/mL Bcl-2-ASO. Similarly, cytosolic cytochrome c was increased from 7.5% to 47% at 10 μg/mL Bcl-2-ASO, concomitant with an increase in MFI from 3 units to 9.6 at 10 μg/mL. Increase in apoptosis correlated with increase in the activation of caspase-9 and in cytochrome c release (Figure 7). The combined results in Figure 7 strongly suggest that Bcl-2-ASO–induced apoptosis is indeed carried out via the intrinsic mitochondrial pathway.
Activation of caspase-9 and cytochrome
c release in Bcl-2-ASO–treated IM9 cells. IM9 cells were cultured for 72 hours with 0 to 10 μg/mL Bcl-2-ASO. Cultures were harvested and assayed by flow cytometry for caspase-9 activation and cytochrome c release from mitochondria as described in “Materials and methods.” Apoptosis was determined by the annexin V method. At least 3 experiments were performed, and 1 representative experiment is shown. For additional experimental details, see “Materials and methods.” Note similar kinetics for apoptosis, cytochrome c release, and activation of caspase-9. Percentages indicate positive cells.
Activation of caspase-9 and cytochrome
c release in Bcl-2-ASO–treated IM9 cells. IM9 cells were cultured for 72 hours with 0 to 10 μg/mL Bcl-2-ASO. Cultures were harvested and assayed by flow cytometry for caspase-9 activation and cytochrome c release from mitochondria as described in “Materials and methods.” Apoptosis was determined by the annexin V method. At least 3 experiments were performed, and 1 representative experiment is shown. For additional experimental details, see “Materials and methods.” Note similar kinetics for apoptosis, cytochrome c release, and activation of caspase-9. Percentages indicate positive cells.
Potentiation of TAX-, DEX-, and Ad-p53–induced apoptosis by pretreatment with Bcl-2-ASO
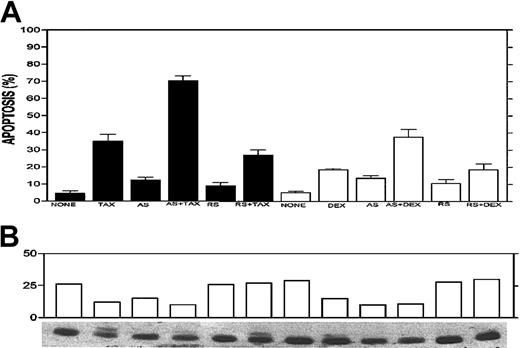
Drug combination experiments were performed with Bcl-2-ASO plus TAX, DEX, and Ad-p53 in drug-resistant myeloma cells. We first tested the effect of 3 days of pretreatment with 5 μg/mL Bcl-2-ASO on TAX- or DEX-induced apoptosis in ARH-77 cells. The results from a representative experiment are depicted in Figure8A. Treatment for 24 hours with 0.1 μM TAX or 0.5 μM DEX resulted in 34% and 16% apoptosis, respectively. Four-day treatment with Bcl-2-ASO alone at 5 μg/mL resulted in low levels of apoptosis (13% to 14%), whereas 4 days of treatment with reverse sense oligodeoxynucleotides alone resulted in only 8% to 10% apoptosis. In contrast, pretreatment with Bcl-2-ASO for 3 days followed by 24 hours of treatment with TAX or DEX resulted in 72% and 43% apoptosis, respectively (Figure 8A). In contrast, pretreatment with reverse sense Bcl-2-ASO followed by TAX or DEX resulted in only 29% and 19% apoptosis, similar to the levels of apoptosis obtained by TAX or DEX alone (Figure 8A).
Potentiation effect of Bcl-2-ASO with TAX and DEX.
Bcl-2-ASO potentiates TAX- and DEX-induced apoptosis (A) and down-regulation of Bcl-2 (B) in ARH-77 myeloma cells. ARH-77 cells were cultured for 3 days with or without 5 μg/mL Bcl-2-ASO (AS) or reverse sense oligodeoxynucleotides (RS). Cultures were continued for additional 2 days with or without 0.1 μM TAX or 0.5 μM DEX. Cells were then harvested and analyzed for apoptosis and for Bcl-2 protein expression. Cultures treated for 1 day or 2 days with TAX or DEX alone were also prepared and analyzed for apoptosis and for Bcl-2 expression. Bcl-2-ASO was replenished every other day. Bcl-2 protein was identified as a 26 kDa migrating band. Note that in TAX-treated cells a slow migrating band of about 28 kDa is visible. This was identified as the widely documented phosphorylated Bcl-2 12 and was included in the scanning for Bcl-2 content. Note the additive effect of drugs and Bcl-2-ASO on increased apoptosis (A) and decreased Bcl-2 protein (B). Other experimental details are as in Figure 1. Error bars represent ± SD for at least 3 experiments.
Potentiation effect of Bcl-2-ASO with TAX and DEX.
Bcl-2-ASO potentiates TAX- and DEX-induced apoptosis (A) and down-regulation of Bcl-2 (B) in ARH-77 myeloma cells. ARH-77 cells were cultured for 3 days with or without 5 μg/mL Bcl-2-ASO (AS) or reverse sense oligodeoxynucleotides (RS). Cultures were continued for additional 2 days with or without 0.1 μM TAX or 0.5 μM DEX. Cells were then harvested and analyzed for apoptosis and for Bcl-2 protein expression. Cultures treated for 1 day or 2 days with TAX or DEX alone were also prepared and analyzed for apoptosis and for Bcl-2 expression. Bcl-2-ASO was replenished every other day. Bcl-2 protein was identified as a 26 kDa migrating band. Note that in TAX-treated cells a slow migrating band of about 28 kDa is visible. This was identified as the widely documented phosphorylated Bcl-2 12 and was included in the scanning for Bcl-2 content. Note the additive effect of drugs and Bcl-2-ASO on increased apoptosis (A) and decreased Bcl-2 protein (B). Other experimental details are as in Figure 1. Error bars represent ± SD for at least 3 experiments.
Most important, pretreatment with Bcl-2-ASO also augmented down-regulation of Bcl-2 protein (Figure 8B). Thus, 4 days of treatment with Bcl-2-ASO alone resulted in nearly 50% decrease in Bcl-2, whereas 24 hours of treatment with TAX or DEX alone resulted in 50% and 25% decrease in Bcl-2 levels, respectively. However, pretreatment with Bcl-2-ASO for 3 days followed by 24 hours of treatment with TAX or DEX resulted in 70% and 75% decrease in Bcl-2 protein levels, respectively. In contrast, treatment with reverse sense oligodeoxynucleotides did not affect Bcl-2 protein, alone or in combination with TAX or DEX (Figure 8B). These combined results strongly suggest that Bcl-2-ASO specifically potentiates the effect of TAX and DEX by affecting both intracellular levels of Bcl-2 and apoptosis.
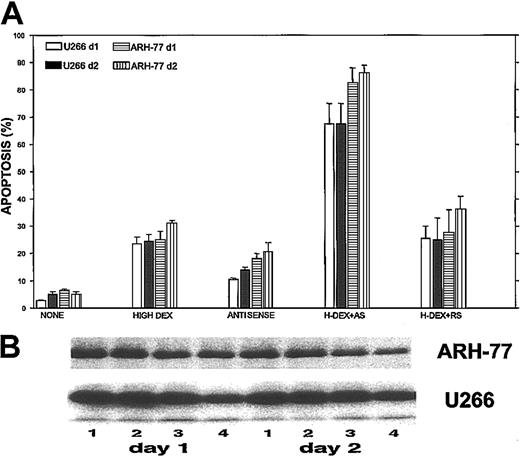
We then tested the maximal potentiation effect of Bcl-2-ASO in combination with a high dose (2 μM) of DEX on apoptosis and on down-regulation of Bcl-2 in ARH-77 and U266 cells (high Bcl-2–expressing cells; Figure 1). The results are depicted in Figure9. Treatment of ARH-77 cells with 2 μM DEX alone resulted in 25% and 32% apoptosis on days 1 and 2 of treatment, respectively. Treatment with Bcl-2-ASO alone resulted in 19% and 22% apoptosis on days 4 and 5, respectively. In contrast, pretreatment with Bcl-2-ASO for 3 days followed by 2 μM DEX resulted in 83% and 87% apoptosis on days 1 and 2, respectively (Figure 9A). Similar potentiation of DEX-induced apoptosis was observed for U266 cells (Figure 9A). In contrast to the observed apoptosis by Bcl-2-ASO, reverse sense and scrambled oligodeoxynucleotides did not potentiate DEX-induced apoptosis in these cell lines (Figure 9A).
Maximal potentiation effect of Bcl-2-ASO with DEX.
Bcl-2-ASO potentiates DEX-induced apoptosis (A) and down-regulation of Bcl-2 (B) in ARH-77 and U266 cells. U266 and ARH-77 cells were cultured with or without 5 μg/mL Bcl-2-ASO for 3 days. Cultures were further incubated with no DEX, with low DEX (1 μM), or with high DEX (2 μM) for additional 2 days. Apoptosis was determined by annexin V in cells treated with Bcl-2-ASO alone, DEX alone, or with Bcl-2-ASO (3 days) followed by 1 day or 2 days of DEX. Bcl-2-ASO was replenished every other day. Apoptosis (A) and Western immunoblotting (B) were performed as described in the legend to Figure 1. In panel B, lane 1 indicates nontreated, control cells; lane 2, cells treated for 4 (left panel) or 5 days (right panel) with Bcl-2-ASO alone; lane 3, cells treated with 2 μM DEX alone (1 day, left panel; 2 days, right panel); lane 4, cells treated with Bcl-2-ASO plus DEX for 1 day (left panel) or 2 days (right panel). Note that cells treated with Bcl-2-ASO and DEX exhibited additivity in apoptosis and in down-regulation of Bcl-2. Bars in panel A are ± SD of at least 3 experiments. One of 3 representative immunoblotting experiments is shown in panel B.
Maximal potentiation effect of Bcl-2-ASO with DEX.
Bcl-2-ASO potentiates DEX-induced apoptosis (A) and down-regulation of Bcl-2 (B) in ARH-77 and U266 cells. U266 and ARH-77 cells were cultured with or without 5 μg/mL Bcl-2-ASO for 3 days. Cultures were further incubated with no DEX, with low DEX (1 μM), or with high DEX (2 μM) for additional 2 days. Apoptosis was determined by annexin V in cells treated with Bcl-2-ASO alone, DEX alone, or with Bcl-2-ASO (3 days) followed by 1 day or 2 days of DEX. Bcl-2-ASO was replenished every other day. Apoptosis (A) and Western immunoblotting (B) were performed as described in the legend to Figure 1. In panel B, lane 1 indicates nontreated, control cells; lane 2, cells treated for 4 (left panel) or 5 days (right panel) with Bcl-2-ASO alone; lane 3, cells treated with 2 μM DEX alone (1 day, left panel; 2 days, right panel); lane 4, cells treated with Bcl-2-ASO plus DEX for 1 day (left panel) or 2 days (right panel). Note that cells treated with Bcl-2-ASO and DEX exhibited additivity in apoptosis and in down-regulation of Bcl-2. Bars in panel A are ± SD of at least 3 experiments. One of 3 representative immunoblotting experiments is shown in panel B.
Concomitant with potentiation of apoptosis, we observed an additive effect on down-regulation of Bcl-2 protein in cells pretreated with Bcl-2-ASO and DEX (Figure 9B). Thus, treatment of ARH-77 and U266 with 2 μM DEX (Figure 9B, lane 2) did not significantly change Bcl-2 protein levels compared with controls without treatment (Figure 9B, lane 1). Treatment with Bcl-2-ASO alone (Figure 9B, lane 3) resulted in 40% and 30% decrease in the level of Bcl-2 in ARH-77 and U266 cells, respectively. In contrast, treatment of ARH-77 and U266 cells with a combination of 3 days of Bcl-2-ASO followed by 2 μM DEX resulted in 75% and 60% reduction in the levels of Bcl-2, respectively (Figure9B, lane 4, left panel). A similar trend was observed in cells pretreated with Bcl-2-ASO for 3 days followed by 48 hours of DEX (Figure 9B, lane 4, right panel). Thus, pretreatment with Bcl-2-ASO clearly potentiates DEX-induced apoptosis and down-regulation of Bcl-2.
We have shown before that ARP-1 cells are sensitive to DEX and TAX, whereas Bcl-2–transfected ARP-1 cells are more than 1 log resistant to DEX and TAX.12 15 We therefore tested the hypothesis that pretreatment with Bcl-2-ASO of ARP-1/Bcl-2 cells will potentiate TAX- and DEX-induced apoptosis in these drug-resistant cells. Indeed, a low dose (0.1 μM) of TAX induced 28% and 11% apoptosis after 48 hours of treatment of ARP-1 and ARP-1/Bcl-2 cells, respectively. A high dose of TAX (0.5 μM) induced 44% and 12% apoptosis in ARP-1 and ARP/Bcl-2 cells, respectively, whereas 5 days of treatment with Bcl-2-ASO alone induced 17% and 11% apoptosis in ARP-1 and ARP/Bcl-2 cells, respectively. In contrast, the combination of 3 days of Bcl-2-ASO followed by 48 hours of low TAX resulted in 79% and 66% apoptosis in ARP-1 and ARP-1/Bcl-2 cells, respectively (not shown). A similar trend was observed in ARP-1 and ARP/Bcl-2 cells treated with Bcl-2-ASO (3 days) followed by high-dose TAX. In contrast to the synergy observed between Bcl-2-ASO and TAX in the induction of apoptosis in ARP-1/Bcl-2 cells, reverse sense oligodeoxynucleotides plus TAX did not have any additive effect over TAX alone. Similar results were obtained for Bcl-2-ASO and DEX (results not shown).
We have previously shown that U266 and ARH-77 cells are partially resistant to apoptosis induced by Ad-p53.14 These cell lines are also resistant to Bcl-2-ASO. We therefore tested for potentiation by Bcl-2-ASO of apoptosis induced by 24 and 48 hours of treatment with low-dose (1000 vp/cell) and high-dose (2000 vp/cell) Ad-p53. The results obtained after 48 hours of treatment with Ad-p53 are depicted in Figure 10A-B. Treatment with low doses of Ad-p53 for 48 hours resulted in 22% and 37% apoptosis for U266 and ARH-77 cells, respectively, whereas treatment with high doses of Ad-p53 for 48 hours resulted in 36% and 44% apoptosis for U266 and ARH-77 cells, respectively, whereas treatment for 5 days with Bcl-2-ASO alone resulted with 5% and 18% apoptosis for U266 and ARH-77 cells, respectively. In contrast, pretreatment with Bcl-2-ASO for 3 days followed by Ad-p53 for an additional 48 hours resulted in 50% and 70% apoptosis with low-dose Ad-p53 and 58% and 86% apoptosis with high-dose Ad-p53 in U266 and ARH-77 cells, respectively (Figure 10A). Similar potentiation of Ad-p53–induced apoptosis was observed for ARP-1 and the Bcl-2–transfected ARP/Bcl-2 cells (Figure 10B). In contrast to Bcl-2-ASO, treatment with reverse sense oligodeoxynucleotides and Ad-p53 did not have any additive effect over Ad-p53 alone. Likewise, Ad-LacZ did not induce apoptosis alone or in combination with Bcl-2-ASO (results not shown). Hence, as was the case for DEX and TAX, pretreatment with Bcl-2-ASO potentiated Ad-p53–induced apoptosis in drug-resistant myeloma cells.
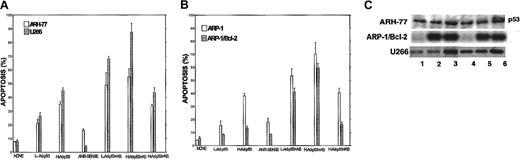
Potentiation of Ad-p53–induced apoptosis.
Bcl-2-ASO potentiates Ad-p53–induced apoptosis in ARH-77, U266 (A), and ARP-1 (B) cells. Cells were pretreated with or without 5 μg/mL Bcl-2-ASO for 3 days. Cultures were further incubated with Bcl-2-ASO plus low-dose (1000 vp/cell) or high-dose (2000 vp/cell) Ad-p53 for an additional 2 days. Cultures treated for 2 days with high- or low-dose Ad-p53 alone were also performed. Apoptosis was determined by annexin V. Bcl-2-ASO was replenished every other day. Bars are ± SD of at least 3 experiments. (C) Western immunoblotting for detection of p53 in Ad-p53–transduced cells. Cells were cultured as in panels A-B, and aliquots were taken for protein extraction and Western immunoblotting as described in the legend to Figure 1. Lane 1 indicates control, untreated cells; lanes 2 and 3, cells transduced for 1 day with low- and high-dose Ad-p53, respectively; lane 4, cells treated with Bcl-2-ASO for 5 days; lanes 5 and 6, cells pretreated for 3 days with Bcl-2-ASO followed by 2 days of transduction with low- and high-dose Ad-p53, respectively.
Potentiation of Ad-p53–induced apoptosis.
Bcl-2-ASO potentiates Ad-p53–induced apoptosis in ARH-77, U266 (A), and ARP-1 (B) cells. Cells were pretreated with or without 5 μg/mL Bcl-2-ASO for 3 days. Cultures were further incubated with Bcl-2-ASO plus low-dose (1000 vp/cell) or high-dose (2000 vp/cell) Ad-p53 for an additional 2 days. Cultures treated for 2 days with high- or low-dose Ad-p53 alone were also performed. Apoptosis was determined by annexin V. Bcl-2-ASO was replenished every other day. Bars are ± SD of at least 3 experiments. (C) Western immunoblotting for detection of p53 in Ad-p53–transduced cells. Cells were cultured as in panels A-B, and aliquots were taken for protein extraction and Western immunoblotting as described in the legend to Figure 1. Lane 1 indicates control, untreated cells; lanes 2 and 3, cells transduced for 1 day with low- and high-dose Ad-p53, respectively; lane 4, cells treated with Bcl-2-ASO for 5 days; lanes 5 and 6, cells pretreated for 3 days with Bcl-2-ASO followed by 2 days of transduction with low- and high-dose Ad-p53, respectively.
To test whether p53 protein was indeed induced following transduction with Ad-p53, we performed immunoblotting of Ad-p53–transduced ARH-77 and U266 cells (mutated p53)13and the Bcl-2–transfected ARP/Bcl-2 (p53 null) cells.13Figure 10C is a representative immunoblot depicting the increase in p53 protein in these cells transduced for 48 hours with low- (Figure 10C, lane 2) and high-dose (Figure 10C, lane 3) Ad-p53. Figure 10C, lane 4, represents cells treated for 5 days with Bcl-2-ASO. Figure 10C, lanes 5 and 6, are cells treated for 3 days with Bcl-2-ASO followed by 48 hours of treatment with low- and high-dose Ad-p53, respectively. Figure 10C, lane 1, is control, untreated cells at 48 hours. Increase in p53 protein expression was clearly observed after 48 hours of transduction of all cell lines and after 24 hours in ARP-1/Bcl-2 cells (Figure10C). There is a slight increase in the levels of p53 in the 5-day Bcl-2-ASO–treated U266 cells compared with untreated cells (Figure10C, lane 4, compare with lane 1). This observation was not consistent for this cell line and was not observed in any other cell line tested, and therefore it is considered an experimental variation.
Finally, to test whether freshly isolated myeloma cells will follow the same pattern obtained in myeloma cell lines, we isolated myeloma cells (CD38+ CD45−) from the bone marrow of 10 refractory MM patients by flow sorting of CD38+CD45− myeloma cells with more than 97% purity.16 These cells were cultured for 3 days with 5 μg/mL Bcl-2-ASO followed by additional 2 days with Bcl-2-ASO with or without 2 μM DEX. The results are depicted in Table1. As was the case for myeloma cell lines, the combination of Bcl-2-ASO plus DEX was synergistic in the induction of apoptosis in 8 of 10 patients tested (Table 1). Reverse sense Bcl-2 oligonucleotides had no effect on apoptosis alone or in combination with DEX (results not shown).
Potentiation by Bcl-2-ASO of DEX-induced apoptosis in freshly isolated myeloma cells
| MM patients (% myeloma cells in BM before sorting of myeloma cells) . | % Apoptosis . | |||||
|---|---|---|---|---|---|---|
| Day 4 ASO . | Day 1 DEX . | Day 3 ASO + day 1 DEX . | Day 5 ASO . | Day 2 DEX . | Day 3 ASO + day 2 DEX . | |
| M1 (66) | 18 | 16 | 55 | 25 | 42 | 91 |
| M2 (54) | 9 | 11 | 29 | 13 | 20 | 44 |
| M3 (40) | 8 | 19 | 55 | 18 | 27 | 78 |
| M4 (22) | 7 | 9 | 28 | 11 | 19 | 70 |
| M5 (69) | 16 | 13 | 29 | 21 | 53 | 67 |
| M6 (81) | 3 | 6 | 18 | 27 | 29 | 47 |
| M7 (55) | 7 | 27 | 64 | 28 | 44 | 98 |
| M8 (43) | 2 | 19 | 50 | 13 | 46 | 82 |
| M9 (26) | 19 | 41 | 63 | 21 | 51 | 89 |
| M10 (74) | 7 | 16 | 20 | 8 | 29 | 36 |
| MM patients (% myeloma cells in BM before sorting of myeloma cells) . | % Apoptosis . | |||||
|---|---|---|---|---|---|---|
| Day 4 ASO . | Day 1 DEX . | Day 3 ASO + day 1 DEX . | Day 5 ASO . | Day 2 DEX . | Day 3 ASO + day 2 DEX . | |
| M1 (66) | 18 | 16 | 55 | 25 | 42 | 91 |
| M2 (54) | 9 | 11 | 29 | 13 | 20 | 44 |
| M3 (40) | 8 | 19 | 55 | 18 | 27 | 78 |
| M4 (22) | 7 | 9 | 28 | 11 | 19 | 70 |
| M5 (69) | 16 | 13 | 29 | 21 | 53 | 67 |
| M6 (81) | 3 | 6 | 18 | 27 | 29 | 47 |
| M7 (55) | 7 | 27 | 64 | 28 | 44 | 98 |
| M8 (43) | 2 | 19 | 50 | 13 | 46 | 82 |
| M9 (26) | 19 | 41 | 63 | 21 | 51 | 89 |
| M10 (74) | 7 | 16 | 20 | 8 | 29 | 36 |
Fresh myeloma cells were purified from the bone marrow (BM) of refractory MM patients by flow sorting of CD38+CD45− cells. Myeloma cells with a purity of more than 97% were routinely obtained.16 Sorted cells were cultured for 3 days in FCS medium with 5 μg/mL Bcl-2-ASO, followed by an additional 2 days of culture with or without 2 μM DEX. Bcl-2-ASO was replenished every 2 days in cultures with Bcl-2-ASO. Apoptosis was determined by annexin V following 4 and 5 days of culture with Bcl-2-ASO alone; following 1 or 2 days with DEX alone; or following 3 days of treatment with Bcl-2-ASO, followed by 1 or 2 days of DEX. M1 through M10 represent myeloma cells sorted from 10 refractory MM patients. In MM patients tested, Bcl-2-ASO potentiated DEX-induced apoptosis. Background apoptosis in myeloma cells before treatment was less than 5%.
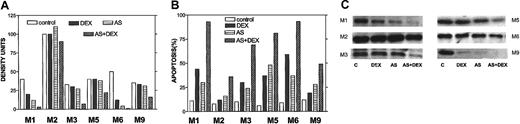
To confirm an effect of Bcl-2-ASO on Bcl-2 levels in freshly isolated myeloma cells, we repeated the sorting of CD38+CD45− myeloma cells as described in Table 1 and performed Western immunoblotting to detect the effect on Bcl-2 protein on DEX alone (2 days, 2 μM); Bcl-2-ASO alone (5 days); or 3 days with Bcl-2-ASO, followed by 3 days of Bcl-2-ASO plus 2 μM DEX. Apoptosis was determined in the same experiment. The results are depicted in Figure 11. Apoptosis (Figure 11B), Bcl-2 expression (Figure 11C), and quantitation of Bcl-2 bands by densitometry (Figure 11A) were determined in the same experiment. In 3 of 6 patients (M1, M3, and M6), DEX alone (DEX) induced significant apoptosis (30% to 60%) concomitant with a substantial decrease (10% to 50%) in Bcl-2, compared with untreated cells (Figure 11A-C). However, in all other patients, treatment with Bcl-2-ASO (AS) alone induced higher levels of apoptosis with equal or higher decrease in Bcl-2, compared with DEX alone, including patient M2 who expressed very high levels of Bcl-2. Most important, the combination of both drugs (AS+DEX) resulted in additional increase in apoptosis and an additional decrease in Bcl-2 protein. In all cases tested, the extent of apoptosis was inversely correlated with the level of Bcl-2 in the cells (compare Figure 11A with B).
Bcl-2-ASO potentiates down-regulation of Bcl-2 in DEX-treated freshly isolated myeloma cells.
Cells were treated as detailed in the footnote to Table1. The results obtained from flow-sorted myeloma cells in 6 MM patients (patients M1, M2, M3, M5, M6, and M9) outlined in Table 1 are presented. Apoptosis (B), Bcl-2 expression (C), and quantitation of Bcl-2 bands by densitometry (A) were determined in the same experiment. Note that in 3 of 6 cases, treatment with Bcl-2-ASO (AS) alone induced high levels of apoptosis with extensive down-regulation of Bcl-2 comparable or higher than DEX alone (DEX). However, the combination of both drugs (AS+DEX) resulted in all cases with additional decrease in Bcl-2 protein and additional increase in apoptosis. Also, in all cases tested, the observed decrease in Bcl-2 inversely correlated with the extent of apoptosis (A-C). “C” in panel C indicates control. Twenty-five micrograms of protein were loaded onto each gel. For further details, see the legend to Figure 1 and “Materials and methods.”
Bcl-2-ASO potentiates down-regulation of Bcl-2 in DEX-treated freshly isolated myeloma cells.
Cells were treated as detailed in the footnote to Table1. The results obtained from flow-sorted myeloma cells in 6 MM patients (patients M1, M2, M3, M5, M6, and M9) outlined in Table 1 are presented. Apoptosis (B), Bcl-2 expression (C), and quantitation of Bcl-2 bands by densitometry (A) were determined in the same experiment. Note that in 3 of 6 cases, treatment with Bcl-2-ASO (AS) alone induced high levels of apoptosis with extensive down-regulation of Bcl-2 comparable or higher than DEX alone (DEX). However, the combination of both drugs (AS+DEX) resulted in all cases with additional decrease in Bcl-2 protein and additional increase in apoptosis. Also, in all cases tested, the observed decrease in Bcl-2 inversely correlated with the extent of apoptosis (A-C). “C” in panel C indicates control. Twenty-five micrograms of protein were loaded onto each gel. For further details, see the legend to Figure 1 and “Materials and methods.”
Discussion
We have previously shown that Bcl-2 plays a major role in drug resistance in myeloma cell lines and in freshly isolated myeloma cells and that forced expression of Bcl-2 confers resistance against DEX-, TAX-, and Ad-p53–induced apoptosis.9-15 We therefore hypothesized that specific down-regulation of Bcl-2 protein by Bcl-2-ASO should result in potentiation of apoptosis in combination with drugs that work via the intrinsic apoptotic pathway.
Bcl-2-ASO enhances spontaneous apoptosis in low Bcl-2–expressing myeloma cells
A detailed study of the effect of Bcl-2-ASO on apoptosis in 6 myeloma cell lines expressing varying levels of Bcl-2 was performed. By using myeloma cells with low or high levels of Bcl-2 and Bcl-2–transfected cells and by using Bcl-2-ASO, or reverse sense oligodeoxynucleotides as controls for specificity, we established a causative effect for Bcl-2-ASO in inducing apoptosis in low Bcl-2–expressing myeloma cells. In these cells apoptosis was mediated by caspase-9 and -3 and cytochrome c release from mitochondria, suggesting that Bcl-2-ASO induces apoptosis through the intrinsic apoptosis pathway.35,36 This conclusion is also supported by the finding that down-regulation of Bcl-2 by Bcl-2-ASO precedes apoptosis at every time point tested. It has been reported that high expression of Bcl-2 can delay entry of cells into S phase.5 We examined the effect of Bcl-2 on the cell cycle in sensitive as well as in Bcl-2–resistant myeloma cells and did not observe any cell cycle effect (Y.G., unpublished observation, December 2002). Therefore, down-regulation of Bcl-2 in our study is due to specific blocking of the expression of Bcl-2 and is not affected by the relationship between Bcl-2 and the cell cycle.
In contrast to low Bcl-2–expressing cells, high Bcl-2–expressing myeloma cells (eg, ARH-77, U266) or Bcl-2–transfected cells were practically resistant to Bcl-2-ASO. Therefore, we conclude that Bcl-2-ASO might not be effective as a single agent in patients whose myeloma cells overexpress Bcl-2. Unfortunately, in the clinical setting, most drug-resistant myeloma patients have high Bcl-2–expressing myeloma cells.16,17 37 Hence, we predict that treatment of drug-resistant myeloma patients with Bcl-2-ASO as a single agent might not be clinically efficacious. Therefore, we tested the potentiation effect of Bcl-2-ASO on apoptosis induced by TAX, DEX, or Ad-p53 in myeloma cells expressing high levels of Bcl-2.
Bcl-2-ASO potentiates TAX-, DEX-, and Ad-p53–induced apoptosis drug-resistant myeloma cells
DEX is one of the most effective drugs for the treatment of multiple myeloma. However, resistance to DEX develops early in most patients.1,2 We developed an in vitro system to test for a possible synergy between Bcl-2-ASO and DEX, TAX, or Ad-p53 in myeloma cells expressing high levels of Bcl-2 and in myeloma cells with forced expression of Bcl-2. We established that pretreatment with Bcl-2-ASO potentiated DEX-, TAX-, and Ad-p53–induced apoptosis in drug-resistant myeloma cells. Similar results were obtained with myeloma cells isolated from the bone marrows of 8 of 10 myeloma patients refractory to treatment with DEX. Furthermore, immunoblotting studies of Bcl-2 levels in fresh myeloma cells sorted from 6 patients resulted in results similar to those obtained for myeloma cell lines—that is, with clear down-regulation of Bcl-2 protein by Bcl-2-ASO and additive effect on down-regulation of Bcl-2 and apoptosis when DEX was combined with Bcl-2-ASO. Furthermore, we observed an inverse correlation between the down-regulation of Bcl-2 and the extent of apoptosis in cells treated with DEX alone, Bcl-2-ASO alone, or in cells treated with both drugs, for all 6 patients tested.
The mechanism of the observed “synergy” between Bcl-2-ASO and DEX, TAX, or Ad-p53 was not studied. However, we10,15 and others have shown that DEX works by down-regulation of Bcl-2 and that TAX works by phosphorylation and down-regulation of Bcl-2.12 Therefore, one can hypothesize that additivity in apoptosis is the result of the additivity between the 2 drugs in down-regulation of Bcl-2, as our results from immunoblotting for Bcl-2 suggest. In the case of apoptosis induced by Ad-p53, it has been well documented that p53-induced apoptosis can be blocked by overexpression of Bcl-2. However, we are currently studying possible activation by p53 of the apoptosis-inducing protein, p53AIP1, which has been shown to work through the mitochondrial pathway.38 Hence, we speculate that down-regulation of Bcl-2 by Bcl-2-ASO could facilitate mitochondrial damage by p53AIP1, resulting in the additive effect observed with the combination of Ad-p53 and Bcl-2-ASO.
In earlier studies, Webb et al and Raynaud et al observed a maximum concentration (Cmax) of Bcl-2-ASO in plasma of 4 μg/mL 28,39 in lymphoma patients treated with 147.2 mg/m2/d Bcl-2-ASO. This Cmax falls within the concentration range employed in our in vitro studies. Therefore, our results are clinically relevant. Furthermore, the results obtained of our in vitro studies are consistent with the results obtained in vitro and in vivo by other groups for other types of cancers, such as xenograft mouse models of human prostate cancer,24,25 transitional cell carcinoma,26and melanoma.27
Mcl-1 is a Bcl-2 homolog that heterodimerizes with Bcl-2, bclx/l, and bax. Therefore, it has functions similar to Bcl-2, although Mcl-1 has a half-life of 3 hours compared with 17 hours for Bcl-2.5,6,40,41 Similar to Bcl-2, it has been shown that overexpression of Mcl-1 can result in drug resistance. Furthermore, drug-resistant chronic and acute lymphocytic leukemia patients were reported to have elevated levels of Mcl-1.41-43
Recently, Zhang et al44 reported that short treatment with actinomycin D (Act. D) induced apoptosis in myeloma cell lines and freshly isolated myeloma cells concomitant with substantial down-regulation of Mcl-1. Based on these observations, Zhang et al concluded that Mcl-1, not Bcl-2, is a critical survival factor for myeloma cells. Careful examination of the 3 cell lines and freshly isolated myeloma cells (from 3 MM patients) used in that study indicates that all the myeloma cells used expressed higher levels of Mcl-1 than Bcl-2 or Bclx/l. Bcl-2 and Bclx/l were also down-regulated by Act. D, albeit to a lesser extent. Hence, if one considers the short half-life of Mcl-1 (3 hours) and the fact that Act. D rapidly down-regulates protein expression by blocking transcription, it is not surprising to see effective down-regulation of proteins with shorter half-life such as Mcl-1 when compared with Bcl-2. However, in high Bcl-2–expressing cells, Bcl-2, because of its longer half-life of 17 hours,5 should constitute the rate-limiting factor for overall susceptibility to apoptosis. Nevertheless, because Act. D is not used as an anticancer drug in the treatment of MM, the clinical relevance of the findings by Zhang et al is not clear at this point.
While this work was in preparation, Derenne et al45reported the results of a study comparing the effect of antisense oligodeoxynucleotides specific to Mcl-1, Bclx/l, or Bcl-2 on apoptosis in 3 myeloma cell lines expressing high levels of Mcl-1 and in myeloma cells isolated from 2 MM patients. In these 3 cell lines, treatment with antisense to Mcl-1 was more effective in inducing apoptosis than antisense oligodeoxynucleotides to Bcl-2 or Bclx/l, although effective down-regulation of Bcl-2 and Bclx/l was demonstrated. The results obtained by Derenne et al are not surprising given the fact that the endogenous levels of Mcl-1 protein in these cell lines were higher than Bcl-2 protein levels and that Mcl-1 has a shorter half-life compared with Bcl-2.5,40 Different outcomes would be predicted in cells where Bcl-2 expression is higher than Mcl-1. Furthermore, if one considers the 3 major antiapoptotic proteins to be interchangeable in their ability to protect mitochondria from apoptosis,5,6,40,41 then the final fate of mitochondria should depend on the combined levels of all 3 major antiapoptotic proteins. Derenne et al45 also studied the combined effect of DEX plus antisense oligodeoxynucleotides to Mcl-1, Bcl-2, and Bclx/l in the 3 cell lines and reported that the combination of DEX with antisense Mcl-1 or Bcl-2-ASO were nearly equally effective in augmenting apoptosis in 2 of 3 cell line tested. These results are in concordance with the results of our study for DEX, TAX, and Ad-p53. Although we did not compare expression of Bcl-2 with Mcl-1, most patients studied by us and by others express high levels of Bcl-2.16,17,37 Interestingly, Puthier et al compared the relative expression of Bcl-2 and Mcl-1 in freshly isolated myeloma cells obtained from 6 MM patients. They observed higher expression of Bcl-2 in 3 of 6 patients, and others expressed equal amounts of Bcl-2 and Mcl-1.46
In summary, we conclude that the Bcl-2-ASO strategy is a viable option for the treatment of myeloma patients if combined with DEX, TAX, or Ad-p53.
Based on our results, a multicenter, randomized, phase 3 study in myeloma patients has been initiated by Genta to examine the clinical efficacy of DEX alone compared with Bcl-2-ASO followed by DEX.
The authors thank Mr Charles Thomas and the Institutional Flow Cytometry Core Facility for performing FACS analysis and Dr El-Deiry from Pennsylvania School of Medicine for supplying the Ad-p53 constructs. The authors also thank Genta for supplying the Bcl-2 antisense oligodeoxynucleotides (Genasense), the reverse sense and scrambled Bcl-2 oligodeoxynucleotides, and the FITC derivative of the antisense oligodeoxynucleotides.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-10-3067.
Supported partially by a grant from Genta (Berkeley Heights, CA).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yair Gazitt, Department of Medicine/Hematology, University of Texas Health Science Center, 7703 Floyd Curl Dr, San Antonio, TX 78284; e-mail:gazitt@uthscsa.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal