Abstract
Acute BCR-ABL expression during in vitro hematopoietic development of embryonic stem (ES) cells causes expansion of multipotent and myeloid progenitors with a concomitant reduction in differentiation toward erythroblasts. Progenitor cell expansion is due to a rapid, cell autonomous, suppression of programmed cell death with an increase in expression of the antiapoptotic molecule BCL-XL. Other antiapoptotic effectors, including AKT, STAT5, and BCL-2 are not up-regulated by BCR-ABL in this system. In addition, the proapoptotic p38 mitogen–activated protein kinase (MAPK) pathway is suppressed by BCR-ABL expression in ES-derived hematopoietic progenitors. Inhibition of p38 MAPK by the small molecule inhibitor SB203580 expanded ES-derived hematopoietic progenitors by an antiapoptotic mechanism and is sufficient to expand ES-derived hematopoietic progenitors to levels approaching 80% of that seen following BCR-ABL expression. In the cellular context of ES-derived hematopoietic progenitors, BCR-ABL expression expands cells by suppressing programmed cell death with a set of antiapoptotic pathways distinct from those previously reported in continuous cell line studies.
Introduction
Cancer develops from an accumulation of genetic aberrations.1 Particular genetic lesions may be essential for initiation and maintenance of particular cancers. Inducible transgenic models demonstrate that sustained activation of C-MYC is essential both for the establishment and continual growth of T-cell lymphomas, myeloid leukemias, and osteogenic sarcomas.2 3In human cancer cells at the time of diagnosis, the presence of multiple genetic defects usually prevents the identification of signaling pathways and cellular changes directly altered by the primary genetic lesion.
In more than 90% of chronic myelogenous leukemia (CML) cases, the initial genetic aberration is the Philadelphia chromosome (Ph+), which results from a reciprocal translocation between chromosome 9 within the tyrosine kinase gene ABL and chromosome 22 within the BCR gene.4,5 This creates a fusion gene product BCR-ABL.6-11 The deregulated tyrosine kinase activity of BCR-ABL is required to transform cells in vitro12 and to generate a CML-like disease in vivo.13,14 Inhibition of BCR-ABL's tyrosine kinase activity with the drug imatinib mesylate (STI571; Novartis, Basel, Switzerland) is sufficient to suppress CML in patients15-17 and relapses from therapy are due to reactivation of BCR-ABL via mutation or amplification of the gene.18 19 These results demonstrate that theBCR-ABL oncogene is required both for establishment and maintenance of CML.
CML begins with a long 3- to 5-year chronic phase characterized by an expansion of progenitor and mature myeloid cells, mild anemia, and splenomegaly. This chronic phase transforms to an aggressive stage (blast crisis) associated with numerous genetic abnormalities such as trisomy 8, trisomy 19, i(17q), and loss or mutation of p53as well as epigenetic effects leading to an expansion of immature progenitors.20 21
The cellular origin of CML begins with formation of the Ph+in the hematopoietic stem cell (HSC) from where it is subsequently transmitted to all hematopoietic lineages.22,23 Studies of purified Ph+ HSCs suggest that either BCR-ABL is not expressed in this population24 or it is insufficient to affect the quiescent status of HSCs.25 Multipotent hematopoietic progenitors are receptive to BCR-ABL expression and generate an abnormal expansion of mature myeloid cells, a clinical feature of CML in chronic phase.26 27 These results demonstrate that although the Ph+ originates in HSCs, multipotent hematopoietic progenitors and not HSCs are the cell population responding to BCR-ABL in CML.
Numerous signaling pathways regulated by BCR-ABL expression have been identified over the past decade; however, their relevance toward the direct effect of BCR-ABL expression in multipotent hematopoietic progenitors remains unclear for 2 reasons. First, mostBCR-ABL studies have been conducted in fibroblast, myeloid, and pro-B cell lines, which do not represent multipotent hematopoietic progenitors.28 Second, BCR-ABL studies conducted in the cellular context of hematopoietic progenitors have largely relied on the availability of materials from patients with CML. These studies could not distinguish direct BCR-ABLconsequences versus other genetic and epigenetic effects that occurred during the evolution of these leukemic cells.
Alternative in vitro cellular systems have been developed that can be used to study BCR-ABL in the cellular context of hematopoietic progenitors. The pluripotent FDCP-Mix cell line has been used to demonstrate that growth factor independence of myeloid cells requires long-term BCR-ABL expression.29 Chronic BCR-ABL expression in human CD34+ multipotent progenitors via retroviral transduction delays apoptosis induced by serum and cytokine withdrawal.26 Embryonic stem (ES) cell–derived multipotent hematopoietic progenitors chronically expressing BCR-ABL via retroviral transduction generate a CML-like disease in mice.30 However, such studies could not evaluate the initial effect of BCR-ABL expression in multipotent hematopoietic progenitors.
We have developed an ES cell in vitro differentiation system with tetracycline-regulated BCR-ABL expression to study its effects during defined stages of hematopoietic differentiation.31 BCR-ABL expression throughout in vitro hematopoiesis leads to an expansion of mature myeloid cells with a concomitant reduction in erythropoiesis mimicking some of the bone marrow features of CML in chronic phase.31
In this study we determined which specific hematopoietic progenitor populations are responsive to initial BCR-ABL expression. Based on these results we propose a mechanism by which BCR-ABL expands such progenitors and the distinctive combination of signaling pathways that may be involved.
Materials and methods
General maintenance of feeder-independent BCR-ABL–inducible ES clones
Maintenance of OP9 stromal cell line
ES in vitro differentiation and induction of BCR-ABL expression during hematopoietic progenitor development
ES cell in vitro differentiation and induction of BCR-ABL expression was as previously described.31 32 Briefly, 104 undifferentiated ES cells, not induced for BCR-ABL expression, were cocultured on a confluent OP9 stroma per well of a 6-well plate by adding 100 ng/mL (TET). On day 5 of differentiation, both differentiated ES cells and the OP9 stroma were harvested in fresh medium in the absence of TET. Cells were replated on new dishes for 20 to 30 minutes to separate OP9 cells from differentiated ES cells. OP9 cells quickly adhered to the dish and differentiated ES cells were simply harvested by pipetting. Then, 106 differentiated ES cells were plated per 10-cm dish on a confluent OP9 cell layer. Cultures induced for BCR-ABL expression were kept without TET and control cultures had TET added. On day 7 of differentiation, hematopoietic progenitors were harvested by gentle pipetting and adherent endothelial cells were not harvested (Figure1A). BCR-ABL–expressing hematopoietic cells were identified by expression of enhanced green fluorescent protein (EGFP) and hematopoietic cells not induced for BCR-ABL expression remained EGFP−.
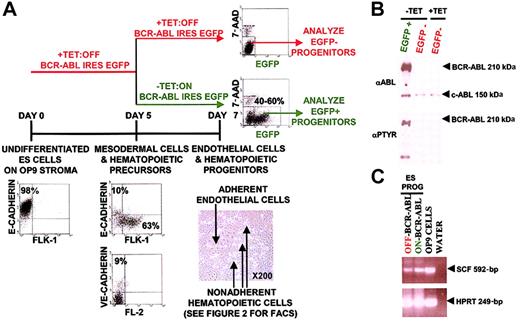
Strategy to study the initial effects of BCR-ABL expression during hematopoietic progenitor development.
(A) BCR-ABL expression was turned on at day 5 of ES cell in vitro differentiation when the majority of the cells were mesodermal cells and hematopoietic precursors as defined by FLK-1 and VE-cadherin expression. Effects were analyzed on day 7 ES in vitro–differentiated hematopoietic progenitors by harvesting nonadherent hematopoietic progenitors with gentle pipetting and not harvesting adherent endothelial cells. In this system, removal of TET on day 5 of differentiation induced BCR-ABL IRES EGFP expression in 40% to 60% of day 7 ES-derived hematopoietic progenitors as measured by flow cytometry. BCR-ABL effects were analyzed in EGFP+ cells and EGFP− cells both in BCR-ABL–induced and uninduced cultures, used as controls. (B) Exclusive BCR-ABL activation in EGFP-expressing ES-derived day 7 hematopoietic progenitor cultures without TET. EGFP+ and EGFP−ES-derived hematopoietic progenitors from BCR-ABL–induced cultures without TET were sorted to more than 95% purity using the FACSVantage along with EGFP− cells from a control BCR-ABL uninduced culture with TET. Purified cell extracts were prepared and immunoblotted with anti-ABL and antiphosphotyrosine antibodies to determine BCR-ABL expression and activity, respectively. (C) SCF is expressed in OP9 cells and ES-derived hematopoietic progenitors. RNA was extracted from OP9 cells and day 7 ES-derived hematopoietic progenitors expressing BCR-ABL and control day 7 ES-derived hematopoietic progenitors. cDNA was generated from these samples and PCR using SCF and HPRT-specific primers were conducted. SCF primers would generate a 592-bp cDNA fragment and an about 1500-bp genomic band; HPRT primers would generate a 249-bp cDNA fragment and an approximately 1100-bp genomic band.
Strategy to study the initial effects of BCR-ABL expression during hematopoietic progenitor development.
(A) BCR-ABL expression was turned on at day 5 of ES cell in vitro differentiation when the majority of the cells were mesodermal cells and hematopoietic precursors as defined by FLK-1 and VE-cadherin expression. Effects were analyzed on day 7 ES in vitro–differentiated hematopoietic progenitors by harvesting nonadherent hematopoietic progenitors with gentle pipetting and not harvesting adherent endothelial cells. In this system, removal of TET on day 5 of differentiation induced BCR-ABL IRES EGFP expression in 40% to 60% of day 7 ES-derived hematopoietic progenitors as measured by flow cytometry. BCR-ABL effects were analyzed in EGFP+ cells and EGFP− cells both in BCR-ABL–induced and uninduced cultures, used as controls. (B) Exclusive BCR-ABL activation in EGFP-expressing ES-derived day 7 hematopoietic progenitor cultures without TET. EGFP+ and EGFP−ES-derived hematopoietic progenitors from BCR-ABL–induced cultures without TET were sorted to more than 95% purity using the FACSVantage along with EGFP− cells from a control BCR-ABL uninduced culture with TET. Purified cell extracts were prepared and immunoblotted with anti-ABL and antiphosphotyrosine antibodies to determine BCR-ABL expression and activity, respectively. (C) SCF is expressed in OP9 cells and ES-derived hematopoietic progenitors. RNA was extracted from OP9 cells and day 7 ES-derived hematopoietic progenitors expressing BCR-ABL and control day 7 ES-derived hematopoietic progenitors. cDNA was generated from these samples and PCR using SCF and HPRT-specific primers were conducted. SCF primers would generate a 592-bp cDNA fragment and an about 1500-bp genomic band; HPRT primers would generate a 249-bp cDNA fragment and an approximately 1100-bp genomic band.
Phenotyping ES-derived hematopoietic progenitors
ES day 7 hematopoietic cells were stained with the following combination of antibodies from BD Pharmingen (San Diego, CA) except where stated: anti–TER-119–PE, anti-CD117–APC (c-KIT), anti-CD34–biotin with streptavidin-APC-CY7 (Caltag, Burlingame, CA), anti-CD11b–TC (Caltag); lineage panel: phycoerythrin (PE)–conjugated TER-119, B220, CD11b, CD4, CD8, and Gr-1. ES day 0 and day 5 differentiated cells were stained with anti–FLK-1–PE, E-cadherin (Zymed, South San Francisco, CA), or VE-cadherin (CD144) with antirat-TC antibody (Caltag). Data were acquired on a FACSVantage (Becton Dickinson, Franklin Lakes, NJ) and analyzed using WinMDI version 2.8 software (Scripps Research Institute, La Jolla, CA). Dead cells were excluded from analysis based on forward and side-scatter properties.
Morphologic analysis of ES-derived hematopoietic progenitors
ES-differentiated day 7 endothelial and hematopoietic cells were visualized with an inverted microscope at × 200. Cytospin preparations of ES-differentiated day 7 hematopoietic progenitors were analyzed by May-Grünwald/Giemsa-Wright staining using the HEMA 3 solution according to the manufacturer's protocol (Biochemical Sciences, Swedesboro, NJ).
BCR-ABL kinase inhibition studies in ES-derived hematopoietic progenitors
On day 5 of ES cell differentiation, the BCR-ABL tyrosine kinase inhibitor imatinib mesylate was added to cultures at a final concentration of 1 μM and 10 μM. On day 7 of ES differentiation, cells were analyzed.
Early-stage apoptosis analysis in ES-derived hematopoietic progenitors
ES-differentiated day 7 progenitors were stained with annexin V–PE (BD Pharmingen) to determine early stages of apoptosis. 7-Amino-actinomycin D (7-AAD) was used to exclude dead cells. The manufacturer's (BD Pharmingen) protocol was followed and samples were analyzed using a FACScan (Becton Dickinson).
Retroviral vectors and generation of virus stocks
Generation of BCR-ABL–expressing BaF3 cells and IL-3 stimulation
The interleukin 3 (IL-3)–dependent BaF3 cell line was maintained in Iscove medium supplemented with 10% fetal bovine serum (FBS) and 10% WEHI-3B conditioned medium (WEHI) as a source of IL-3. Cells (5 × 105) were infected in 3 mL virus supplemented with 10% WEHI and 8 μg/mL polybrene for 3 hours. After 3 hours, 7 mL BaF3 medium was added. At 48 hours after infection, EGFP+cells were sorted from BaF3 cells infected with either murine stem cell virus (MSCV) IRES EGFP or MSCV BCR-ABLP210 IRES EGFP retrovirus to more than 95% purity on a FACSVantage (Becton Dickinson).
For IL-3 stimulation, BaF3 cells were washed 3 times in BaF3 medium lacking WEHI. Washed BaF3 cells were starved for IL-3 for 6 to 8 hours in BaF3 media lacking WEHI. WEHI was added to a final concentration of 10% to IL-3–deprived cells for 10 minutes to stimulate the IL-3 signal transduction pathway. Ten minutes after IL-3 stimulation cells were harvested for analyses.
RT-PCR analyses
EGFP was used as a surrogate marker to sort for BCR-ABL–expressing ES-differentiated day 7 hematopoietic progenitors to more than 95% purity using the FACSVantage cell sorter (Becton Dickinson). EGFP−, BCR-ABL–uninduced ES-differentiated day 7 hematopoietic progenitors were similarly purified. OP9 cells were harvested by trypsinization. Total RNA was extracted from 106 cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) and 1 μg RNA was used to generate cDNA using SuperScript reverse transcriptase (RT; Gibco-BRL, Carlsbad, CA) and oligo(dT) primer according to the manufacturer's protocol. One microliter cDNA was used per polymerase chain reaction (PCR) with stem cell factor (SCF) primers sense 5′-GACTGTGTGCTCTCTTCAAC-3′ and antisense 5′-CTTGCAAAACCTCCAGAGTC-3′ generating a cDNA fragment of 592 bp and a genomic DNA band of about 1500 bp; HPRT housekeeping gene primers sense 5′-CACAGGACTAGAACACCTGC-3′ and antisense 5′-GCTGGTGAAAAGGACCTCT-3′ generating a cDNA fragment of 249 bp and a genomic DNA band of about 1100 bp.35
Protein analyses by Western blotting
BCR-ABL–expressing and control ES-differentiated day 7 hematopoietic progenitors were similarly sorted as in the RT-PCR analyses. BaF3 cells expressing BCR-ABL IRES EGFP or EGFP were similarly sorted to more than 95% purity 48 hours after infection. Cell lysates were prepared as described.36 Protein lysate (10 μg) was analyzed by immunoblotting with the following antibodies according to the manufacturer's protocol unless otherwise stated: anti-ABL was diluted 1:500,37 antiphosphotyrosine, clone 4G10 (05-321; Upstate Biotechnology, Lake Placid, NY), BCL-2 (610538; BD Transduction Laboratories, Lexington, KY), rabbit polyclonal antibodies AKT1/2 (sc-8312), ERK2 (sc-154), STAT5b (sc-835; Santa Cruz Biotechnology, Santa Cruz, CA), BCL-XL (2762), p38 MAPK (9212), phospho-p38 MAPK (Thr180/Tyr182; 9211), phospho-STAT5 (Tyr694; 9351), phospho-AKT (Ser473; 9271) (Cell Signaling Technology, Beverly, MA).
p38 MAPK inhibition studies during ES cell–derived hematopoietic progenitor development
The p38 mitogen–activated protein kinase (MAPK)–specific inhibitor SB 203580 (559398; Calbiochem, San Diego, CA) was added daily starting on day 5 of ES cell in vitro differentiation at concentrations of 0.1 μM, 1 μM, and 10 μM. An equivalent volume of dimethyl sulfoxide (DMSO) was added to control cells. ES day 7 differentiated hematopoieitic progenitors were harvested and evaluated for p38 MAPK inhibitory effects.
Results
BCR-ABL expression at the onset of hematopoiesis expands multipotent and myeloid progenitors with suppression of differentiation into erythroblasts
We developed an ES cell in vitro differentiation system with TET-regulated BCR-ABL expression to study its effects during defined stages of hematopoietic differentiation.31,32 ES cells were induced to differentiate on an OP9 stromal cell line such that by day 5 of ES cell differentiation, the majority of cells were composed of mesodermal cells and hematopoietic precursors as defined by FLK-1 expression in 60% to 80% of the cells, VE-cadherin expression in 9% to 10% of the cells, and a lack of CD45 and TER-119 expression (Figure1A).31,32,38,39 Five to 10% of day 5 differentiated ES cells remained undifferentiated and expressed E-cadherin (Figure 1A). Individual FLK-1+, VE-cadherin–negative day 4 and day 5 differentiated ES cells, and individual FLK-1+, VE-cadherin–positive day 5 differentiated ES cells have been shown to develop into both hematopoietic and endothelial cells, demonstrating that ES differentiated day 4 and day 5 cells with these markers represent mesodermal cells and hematopoietic precursors.39BCR-ABL expression was induced by TET removal on day 5 mesodermal cells and hematopoietic precursors (Figure 1A). Turning on BCR-ABL expression at earlier time points than day 5 of ES-OP9 differentiation severely hampered subsequent hematopoietic differentiation and was not a feasible approach to study BCR-ABL effects in the cellular context of hematopoietic progenitors. For example, turning on BCR-ABL expression on day 0 of ES-OP9 differentiation abrogated differentiation of ES cells into day 5 FLK-1+ and day 7 hematopoietic cells. Turning on BCR-ABL expression on day 3 of ES-OP9 differentiation resulted in 10% to 20% of day 5 cells expressing FLK-1+with most cells remaining undifferentiated and incapable of hematopoietic development (data not shown). After turning on BCR-ABL expression on day 5 of ES-OP9 differentiation, its effects during the development of hematopoietic progenitors were studied by analyzing day 7 BCR-ABL–expressing hematopoietic progenitors and control cells (Figure 1A). Nonadherent day 7 hematopoietic progenitors were collected by gentle pipetting and adherent endothelial cells were not collected and remained on the culture dish (Figure 1A). To identify BCR-ABL–expressing cells, BCR-ABL was linked to an IRES EGFP (Figure 1A-B). EGFP intensity corresponded to the level of BCR-ABL expression (Figure 1A-B). In this system, removal of TET induced BCR-ABL expression in 40% to 60% of cells as demonstrated by EGFP fluorescence and confirmed by protein expression (Figure 1A-B). EGFP− cells in BCR-ABL–induced cultures did not express BCR-ABL and were used as a control population (Figure 1A-B).
We took advantage of multiparameter cell surface marker analysis to define specific progenitor populations affected by BCR-ABL activation. C-KIT and CD34 receptors have been used in combination with lineage-depleted cells to define hematopoietic progenitors capable of in vivo multilineage reconstitution.40,41 This same set of markers was used to define multipotent hematopoietic progenitors in ES day 7 differentiated cells (Figure 2B). B- and T-cell progenitors were not generated by day 7 of ES cell in vitro differentiation and erythroblasts did not express CD34 (data not shown).31,32 38 Consequently, most experiments used the myeloid-specific CD11b integrin receptor as the sole marker for exclusion of lineage-committed progenitors from C-KIT and CD34+ multipotent progenitors (Figure 2B). Lineage-committed myeloid progenitors were defined by coexpression of C-KIT and CD34 along with CD11b (Figure 2B). Erythroblasts were identified by expression of TER-119. This was sufficient to define erythroblasts because all day 7 ES cells contain a nucleus demonstrating that no mature erythrocytes have yet formed (Figure2A).
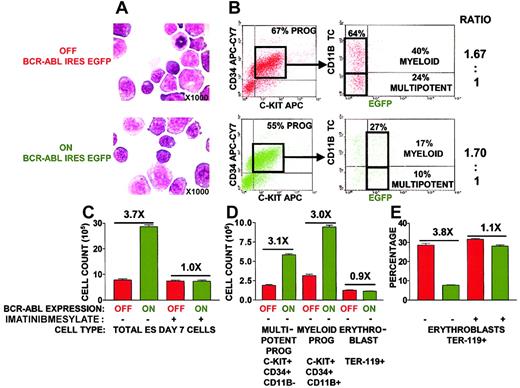
BCR-ABL expression at the onset of hematopoiesis expands multipotent and myeloid progenitors with suppression of differentiation toward erythroblasts.
(A) Morphology of day 7 ES-differentiated hematopoietic progenitors appeared unaltered on BCR-ABL induction. Cytospin preparations were stained with May-Grünwald/Giemsa-Wright. Slides were photographed at ×1000. (B) BCR-ABL expression does not skew hematopoietic differentiation toward multipotent and myeloid progenitors. The absolute percentages of multipotent and myeloid progenitors on day 7 of ES in vitro differentiation in the presence and absence of BCR-ABL induction were determined by flow cytometry using EGFP as a surrogate marker for BCR-ABL expression. Multipotent progenitors were defined by coexpression of cell surface markers C-KIT and CD34 and lack of CD11b expression or lack of lineage panel TER-119, B220, CD4, CD8, CD11b, Gr-1 expression. Myeloid progenitors were defined with cell surface markers C-KIT, CD34, and CD11b. The ratio of multipotent to myeloid progenitors was calculated from the absolute percentages in the presence and absence of BCR-ABL induction. (C) BCR-ABL tyrosine kinase activity is required to expand ES-derived hematopoietic progenitors. The effect of the BCR-ABL tyrosine kinase inhibitor imatinib mesylate on progenitor expansion was analyzed by counting the total number of ES day 7 cells after imatinib mesylate addition on ES day 5 differentiation, in the presence and absence of BCR-ABL induction. BCR-ABL–induced cultures led to BCR-ABL expression in 54% of the cells by EGFP levels, and cell counts were normalized to 100% BCR-ABL expression. Imatinib mesylate was added at both 1 μM and 10 μM concentrations with similar results. Results represent the mean ± SEM of an experiment conducted in triplicate. Similar results were obtained in 3 independent experiments. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON total cell population, multipotent progenitors, and myeloid progenitors was P < .01. Statistical analysis (paired ttest) of BCR-ABL OFF versus BCR-ABL ON and BCR-ABL ON plus imatinib mesylate versus BCR-ABL ON ES day 7 cultures wereP < .01. (D) Selective expansion of multipotent and myeloid progenitors but not erythroblasts on BCR-ABL induction. Total cell counts of day 7 ES-derived hematopoietic progenitors combined with percentages of multipotent progenitors (panel B), myeloid progenitors (panel B), and erythroblasts (defined by expression of TER-119) were used to obtain cell numbers of specific populations. BCR-ABL–induced cultures led to BCR-ABL expression in 54% of the cells by EGFP levels and cell counts were normalized to 100% BCR-ABL expression. Results represent the mean ± SEM of an experiment conducted in triplicate. Similar results were obtained in 3 independent experiments. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON multipotent and myeloid progenitors wasP < .01. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON erythroblasts wasP = .18, thereby representing an insignificant difference. (E) The tyrosine kinase activity of BCR-ABL is required to suppress hematopoietic differentiation toward erythroblasts. The effect of the BCR-ABL tyrosine kinase inhibitor imatinib mesylate on erythroblast differentiation was analyzed by examining the percentage of TER-119+ erythroblasts generated after addition of imatinib mesylate on ES day 5 differentiation, in the presence and absence of BCR-ABL induction. BCR-ABL–induced cultures led to its expression in 67% of the cells by EGFP levels and TER-119 percentages were normalized to 100% BCR-ABL expression. Imatinib mesylate was added at both 1 μM and 10 μM concentrations with similar results. Representative result of an experiment conducted in triplicate wells. Similar results were obtained in another 2 independent experiments. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON TER-119+ erythroblasts was P < .01. X indicates fold.
BCR-ABL expression at the onset of hematopoiesis expands multipotent and myeloid progenitors with suppression of differentiation toward erythroblasts.
(A) Morphology of day 7 ES-differentiated hematopoietic progenitors appeared unaltered on BCR-ABL induction. Cytospin preparations were stained with May-Grünwald/Giemsa-Wright. Slides were photographed at ×1000. (B) BCR-ABL expression does not skew hematopoietic differentiation toward multipotent and myeloid progenitors. The absolute percentages of multipotent and myeloid progenitors on day 7 of ES in vitro differentiation in the presence and absence of BCR-ABL induction were determined by flow cytometry using EGFP as a surrogate marker for BCR-ABL expression. Multipotent progenitors were defined by coexpression of cell surface markers C-KIT and CD34 and lack of CD11b expression or lack of lineage panel TER-119, B220, CD4, CD8, CD11b, Gr-1 expression. Myeloid progenitors were defined with cell surface markers C-KIT, CD34, and CD11b. The ratio of multipotent to myeloid progenitors was calculated from the absolute percentages in the presence and absence of BCR-ABL induction. (C) BCR-ABL tyrosine kinase activity is required to expand ES-derived hematopoietic progenitors. The effect of the BCR-ABL tyrosine kinase inhibitor imatinib mesylate on progenitor expansion was analyzed by counting the total number of ES day 7 cells after imatinib mesylate addition on ES day 5 differentiation, in the presence and absence of BCR-ABL induction. BCR-ABL–induced cultures led to BCR-ABL expression in 54% of the cells by EGFP levels, and cell counts were normalized to 100% BCR-ABL expression. Imatinib mesylate was added at both 1 μM and 10 μM concentrations with similar results. Results represent the mean ± SEM of an experiment conducted in triplicate. Similar results were obtained in 3 independent experiments. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON total cell population, multipotent progenitors, and myeloid progenitors was P < .01. Statistical analysis (paired ttest) of BCR-ABL OFF versus BCR-ABL ON and BCR-ABL ON plus imatinib mesylate versus BCR-ABL ON ES day 7 cultures wereP < .01. (D) Selective expansion of multipotent and myeloid progenitors but not erythroblasts on BCR-ABL induction. Total cell counts of day 7 ES-derived hematopoietic progenitors combined with percentages of multipotent progenitors (panel B), myeloid progenitors (panel B), and erythroblasts (defined by expression of TER-119) were used to obtain cell numbers of specific populations. BCR-ABL–induced cultures led to BCR-ABL expression in 54% of the cells by EGFP levels and cell counts were normalized to 100% BCR-ABL expression. Results represent the mean ± SEM of an experiment conducted in triplicate. Similar results were obtained in 3 independent experiments. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON multipotent and myeloid progenitors wasP < .01. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON erythroblasts wasP = .18, thereby representing an insignificant difference. (E) The tyrosine kinase activity of BCR-ABL is required to suppress hematopoietic differentiation toward erythroblasts. The effect of the BCR-ABL tyrosine kinase inhibitor imatinib mesylate on erythroblast differentiation was analyzed by examining the percentage of TER-119+ erythroblasts generated after addition of imatinib mesylate on ES day 5 differentiation, in the presence and absence of BCR-ABL induction. BCR-ABL–induced cultures led to its expression in 67% of the cells by EGFP levels and TER-119 percentages were normalized to 100% BCR-ABL expression. Imatinib mesylate was added at both 1 μM and 10 μM concentrations with similar results. Representative result of an experiment conducted in triplicate wells. Similar results were obtained in another 2 independent experiments. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON TER-119+ erythroblasts was P < .01. X indicates fold.
Short-term 48-hour BCR-ABL expression expanded the total population of day 7 ES cells by 3.7-fold (Figure 2C). This BCR-ABL effect was dependent on its tyrosine kinase activity because addition of the inhibitor imatinib mesylate suppressed progenitor expansion (Figure2D). Specifically, BCR-ABL expression expanded multipotent and myeloid progenitors by 3.1-fold and 3.0-fold, respectively (Figure 2D). The selective expansion of multipotent and myeloid progenitors could be due to a skewing of hematopoietic differentiation toward these cell types on BCR-ABL induction. However, BCR-ABL expression did not increase the ratio of multipotent and myeloid progenitors generated (Figure2B).
In contrast, BCR-ABL expression suppressed the development of erythroblasts with a 3.8-fold decrease in the percentage of erythroblasts generated (Figure 2E). This BCR-ABL effect was also dependent on its tyrosine kinase activity because addition of the inhibitor imatinib mesylate restored the percentage of erythroblasts generated (Figure 2E). However, suppression in erythroblast development did not reduce the absolute number of erythroblasts generated because BCR-ABL expression increased the total number of day 7 ES progenitors by 3.7-fold, which is to a similar degree as the 3.8-fold decrease in erythroblast development (Figure 2C,E). SCF has been shown to synergize with BCR-ABL leading to an expansion of granulocyte-macrophage colony-stimulating factor (GM-CSF) colonies27 and erythroid cells.42-44 The lack of BCR-ABL–induced erythroid expansion in ES-OP9 cultures may be due to a lack of SCF production. By RT-PCR, OP9 cells and ES-derived hematopoietic progenitors express SCF (Figures 1C and 2).45 Addition of SCF from day 5 to day 7 of ES-OP9 differentiation did not lead to a further increase in any day 7 hematopoietic cell populations in the presence or absence of BCR-ABL expression (data not shown).
BCR-ABL expands hematopoietic progenitors by suppression of programmed cell death
In cell line studies BCR-ABL expression has been shown to expand cells by suppressing programmed cell death in the face of apoptosis inducing agents such as tumor necrosis factor α (TNF-α), etoposide, or growth factor withdrawal.46-48 Primary Ph+progenitors are also resistant to growth factor withdrawal–induced cell death compared to normal progenitors.49 However, Ph+ progenitors remain susceptible to TNF-α–, ceramide-, or radiation-induced cell death.49-51
We next determined if BCR-ABL expression expanded ES-derived hematopoietic progenitors by suppressing programmed cell death in an environment without exogenous apoptotic stimuli. Previous analyses on the quantitative role of apoptosis following BCR-ABL activation were ambiguous because coexpressed EGFP was not used as a marker to specifically define BCR-ABL+ cells in such cultures.31
Early stages of apoptosis were measured by analyzing the level of phosphatidylserine on cell surfaces via annexin V staining. Cells that had already progressed to the stage of membrane integrity breakdown would leak out EGFP and were excluded by intercalation of 7-AAD into their DNA. BCR-ABL expression suppressed apoptosis by 3-fold, from 23% to 8% in ES-derived hematopoietic progenitors (Figure3). This was similar to the level of cell expansion mediated by BCR-ABL expression (Figure 2B). Addition of the BCR-ABL tyrosine kinase inhibitor imatinib mesylate abolished the antiapoptotic effect of BCR-ABL (Figure 3). This demonstrated that the tyrosine kinase activity of BCR-ABL was essential for its protection against programmed cell death in such progenitor cell populations.
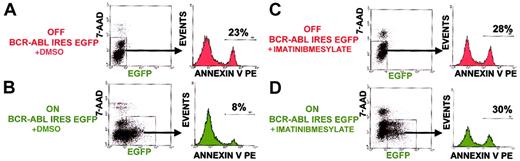
BCR-ABL tyrosine kinase activity is required to suppress apoptosis in hematopoietic progenitors.
Percent apoptotic progenitors were analyzed by annexin V binding with progenitors already progressed to the stage of membrane permeability excluded by 7-AAD intercalation into DNA. Shown are representative results of an experiment done in triplicate cultures. Similar results were obtained in another independent experiment done in triplicate cultures.
BCR-ABL tyrosine kinase activity is required to suppress apoptosis in hematopoietic progenitors.
Percent apoptotic progenitors were analyzed by annexin V binding with progenitors already progressed to the stage of membrane permeability excluded by 7-AAD intercalation into DNA. Shown are representative results of an experiment done in triplicate cultures. Similar results were obtained in another independent experiment done in triplicate cultures.
Late-stage apoptosis as measured by bromodeoxyuridine (BrdU) incorporation at the 3′-hydroxy termini of DNA strand breaks by terminal transferase (TUNEL assay from Phoenix Flow Systems, San Diego, CA) was low in these cultures (data not shown). Fluorescence-activated cell sorted (FACS) EGFP+ and EGFP− ES day 7 progenitors were permeabilized and stained with 7-AAD in combination with BrdU to determine the level of apoptosis at both the G1 and S/G2/M phases of the cell cycle. BCR-ABL expression suppressed the modest percent of late-stage apoptotic cells predominantly at the G1 phase of the cell cycle (data not shown). There was no significant increase in the percentage of cells in the S/G2/M phase of the cell cycle on BCR-ABL induction, suggesting that an increase in cell proliferation was not a major mechanism by which BCR-ABL expanded ES-derived progenitors (data not shown). These results demonstrate that antiapoptosis was the predominant mechanism by which acute BCR-ABL expression expanded ES-derived hematopoietic progenitors.
BCR-ABL suppresses programmed cell death in a cell autonomous manner
There is conflicting evidence on whether all BCR-ABL effects are cell autonomous or can occur in a nonautonomous fashion. BCR-ABL cell autonomous effects have been demonstrated in cell cocultures in which BCR-ABL–expressing cells, and not their normal counterparts, continue to expand under growth factor–limiting conditions.27,52BCR-ABL noncell autonomous effects have been demonstrated in Ph+ progenitors that secrete IL-3,53-56 in BCR-ABL–induced murine myeloproliferative disorder cells that express high levels of IL-3 and GM-CSF,57 and in medium from BCR-ABL–expressing embryoid bodies capable of supporting growth of IL-3–dependent cell lines such as BaF3 and 32D.30
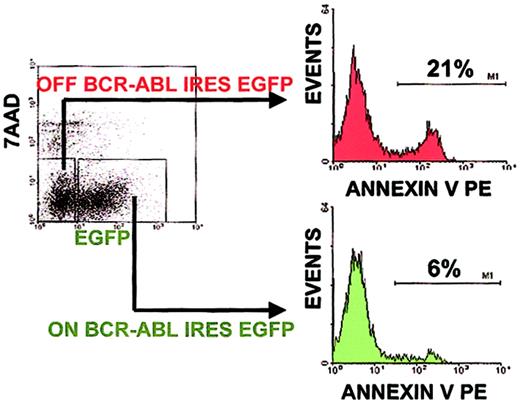
To distinguish between BCR-ABL cell autonomous versus noncell autonomous effects, EGFP+ cells were selected and compared to EGFP− cells within the same ES-derived hematopoietic progenitor culture. EGFP+ BCR-ABL–expressing progenitors had a 6% level of annexin V staining, whereas EGFP−BCR-ABL–nonexpressing progenitors had a 21% level of annexin V staining within the same culture (Figure4). When comparing the level of apoptotic suppression mediated by BCR-ABL expression in separate cultures either expressing BCR-ABL or not, the same 3-fold reduction was seen (Figure3A). A range of 2.7-fold to 3.5-fold reduction in apoptosis was observed in 3 independent experiments, each done in triplicate cell cultures under BCR-ABL ON compared to BCR-ABL OFF conditions. These results demonstrate that BCR-ABL expression suppressed apoptosis in a cell autonomous manner.
BCR-ABL suppresses programmed cell death in a cell autonomous manner.
Percent apoptotic progenitors were analyzed as in Figure 3. To determine BCR-ABL cell autonomous versus noncell autonomous effects, the percent of apoptotic progenitors was analyzed within a culture containing both BCR-ABL–expressing and control cells by gating on EGFP+ and EGFP− cells, respectively. A representative result of an experiment done in triplicate cultures is shown. Similar results were obtained in 3 independent experiments done in triplicate cultures.
BCR-ABL suppresses programmed cell death in a cell autonomous manner.
Percent apoptotic progenitors were analyzed as in Figure 3. To determine BCR-ABL cell autonomous versus noncell autonomous effects, the percent of apoptotic progenitors was analyzed within a culture containing both BCR-ABL–expressing and control cells by gating on EGFP+ and EGFP− cells, respectively. A representative result of an experiment done in triplicate cultures is shown. Similar results were obtained in 3 independent experiments done in triplicate cultures.
Of a select group of antiapoptotic molecules, only BCL-XL is up-regulated by BCR-ABL in hematopoietic progenitors
Programmed cell death generally requires the loss of mitochondrial membrane potential to release apoptogenic factors such as cytochromec into the cytoplasm. These factors activate protein caspases that degrade cellular proteins leading to apoptosis. Up-regulation of the BCL-2 family of antiapoptotic proteins such as BCL-2 and BCL-XL can prevent loss of mitochondrial membrane potential, cytochrome c release, and apoptosis. In addition, BCL-2 expression can inhibit apoptosis in a cytochromec/Apaf-1/caspase-9 “apoptosome”-independent manner.58 Conversely, induction of BCL-2 family proapoptotic proteins such as BAX and BAD can inactivate BCL-2 family antiapoptotic proteins leading to apoptosis (for a review, see Huang59). Cell death–suppressing signal transduction pathways such as the AKT and STAT5 pathways up-regulate BCL-2 antiapoptotic family members such as BCL-2 and BCL-XL to inhibit apoptosis.60-62
A broad range of antiapoptotic pathways have been connected to BCR-ABL expression in various cell systems. We examined a subset of these antiapoptotic molecules including AKT, STAT5, BCL-2, and BCL- XL. AKT and STAT5 are hyperphosphorylated in various cell lines expressing BCR-ABL60,63,64 and overexpression of dominant-negative AKT or STAT5 suppresses BCR-ABL–induced transformation of 32D cells.60,65 Both BCL-2 and BCL-XL are up-regulated by BCR-ABL expression in BaF3 cells.66,67 Antisense BCL-2 RNA induces cell death in BCR-ABL–expressing BaF3 cells.66 BCL-XLoverexpression suppresses cell death induced by imatinib mesylate in the Ph+ K562 cell line.62
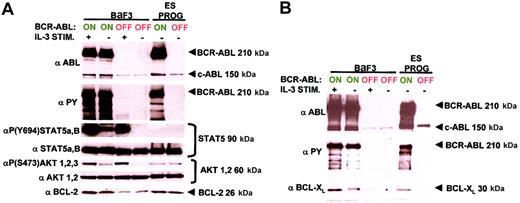
ES-derived hematopoietic progenitors expressing BCR-ABL and control cultures were sorted by FACS to more than 95% purity for EGFP+ and EGFP− cells, respectively. BaF3 cells expressing BCR-ABL have been shown to up-regulate all 4 antiapoptotic molecules AKT, STAT5, BCL-2, and BCL-XL60,61,63,64 66 and were included as positive controls. Stimulating BaF3 cells with IL-3 generated controls for AKT and STAT5 activation independent of BCR-ABL expression.
Our overall results demonstrate that AKT, STAT5, and BCL-2 were not up-regulated by BCR-ABL expression in ES-derived hematopoietic progenitors (Figure 5A). This was in contrast to control BaF3 cells and other continuous cell lines where similar levels of AKT and STAT5 protein were hyperphosphorylated at their respective active sites on BCR-ABL induction (Figure5A).60,63,64 BCL-2 was also up-regulated by BCR-ABL expression in BaF3 cells as previously reported (Figure5A).66 These results demonstrate that the response to BCR-ABL expression in ES-derived hematopoietic progenitors and BaF3 cells are dramatically different and therefore stress the importance of cell context–specific activation of signaling pathways.
BCR-ABL antiapoptotic signaling is dramatically different in ES-derived hematopoietic progenitors compared with BaF3 cells.
(A) Major antiapoptotic signaling molecules regulated by BCR-ABL expression in the pro-B BaF3 cell line are not in ES-derived hematopoietic progenitors. ES-derived hematopoietic progenitor and BaF3 cell lysates expressing BCR-ABL and control samples were prepared and equivalent protein amounts were immunoblotted with site-specific phospho-antibodies and total protein antibodies for STAT5 and AKT. IL-3–stimulated BaF3 cell lysates were included as positive controls for STAT5 and AKT activation. The levels of BCL-2 and BCR-ABL were measured with their respective antibodies, and for BCR-ABL activity a total phosphotyrosine antibody was used. Each molecular pathway was examined twice from 2 independent experiments with identical results. (B) BCL-XL is up-regulated on BCR-ABL induction in both ES-derived hematopoietic progenitors and BaF3 cells. Conditions are identical to panel A, with a BCL-XL antibody. This molecular pathway was analyzed 3 times from 3 separate experiment samples with identical results.
BCR-ABL antiapoptotic signaling is dramatically different in ES-derived hematopoietic progenitors compared with BaF3 cells.
(A) Major antiapoptotic signaling molecules regulated by BCR-ABL expression in the pro-B BaF3 cell line are not in ES-derived hematopoietic progenitors. ES-derived hematopoietic progenitor and BaF3 cell lysates expressing BCR-ABL and control samples were prepared and equivalent protein amounts were immunoblotted with site-specific phospho-antibodies and total protein antibodies for STAT5 and AKT. IL-3–stimulated BaF3 cell lysates were included as positive controls for STAT5 and AKT activation. The levels of BCL-2 and BCR-ABL were measured with their respective antibodies, and for BCR-ABL activity a total phosphotyrosine antibody was used. Each molecular pathway was examined twice from 2 independent experiments with identical results. (B) BCL-XL is up-regulated on BCR-ABL induction in both ES-derived hematopoietic progenitors and BaF3 cells. Conditions are identical to panel A, with a BCL-XL antibody. This molecular pathway was analyzed 3 times from 3 separate experiment samples with identical results.
In ES-derived hematopoietic progenitors there was a basal level of BCL-2 expression and AKT phosphorylation; however, no STAT5 phosphorylation was detectable (Figure 5A). These results demonstrate that although BCL-2 and AKT up-regulation were not required for suppression of programmed cell death by BCR-ABL, a basal level of these proteins may be required for the maintenance of ES-derived hematopoietic progenitors. STAT5 phosphorylation, however, was not required either for BCR-ABL–induced cell growth or the generation of ES-derived hematopoietic progenitors.
BCL-XL was the only antiapoptotic molecule examined that was up-regulated by BCR-ABL expression in ES-derived hematopoietic progenitors (Figure 5B). Previous reports have demonstrated that BCR-ABL induction up-regulates BCL-XL in different cell types ranging from pro-B BaF3 cells, myeloid 32D cells, to the Ph+ blast crisis K562 erythroid cell line.61 67-70 BCL-XL may be a general antiapoptotic molecule that contributes to the ability of BCR-ABL to suppress cell death in different cell lineages.
BCR-ABL expression suppresses phosphorylation of p38 MAPK at its Thr180-Gly-Tyr182 active site in hematopoietic progenitors
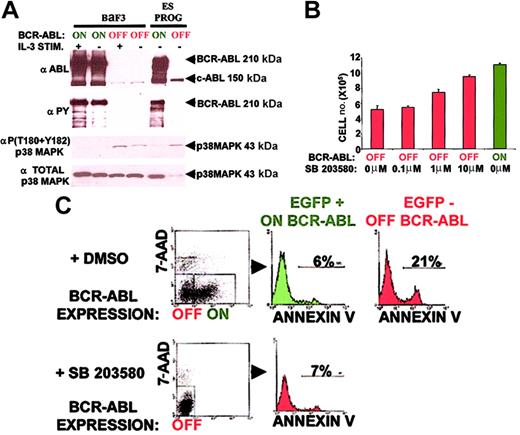
Inhibiting the expansion of Ph+ progenitors and cell lines with chemotherapeutic agents such as interferon α (IFN-α) and butyrate correlates with phosphorylation of p38 MAPK at its Thr180-Gly-Tyr182 active site.71,72 Addition of the p38 MAPK–specific inhibitor SB 203580 is sufficient to restore in vitro expansion of Ph+ cells in the face of these chemotherapeutic agents.71 72 These results demonstrate that p38 MAPK inhibition plays a critical role in the expansion of Ph+ cells. Whether p38 MAPK suppression is directly due to BCR-ABL expression or is an event that occurs during the evolution of a Ph+ leukemic clone has not been examined.
Acute BCR-ABL expression suppressed p38 MAPK phosphorylation at its Thr180-Gly-Tyr182 active site in both ES-derived hematopoietic progenitors and BaF3 cells (Figure 6A). The total amount of p38 MAPK protein, however, was significantly elevated on BCR-ABL induction in ES-derived hematopoietic progenitors (Figure 6A). These results suggest that suppression of p38 MAPK activation may prevent p38 MAPK degradation leading to an accumulation of inactive p38 MAPK in BCR-ABL–expressing ES-derived hematopoietic progenitors.
p38 MAPK inhibition can account for most of the overall antiapoptotic effect mediated by BCR-ABL expression in hematopoietic progenitors.
(A) BCR-ABL expression suppresses p38 MAPK activity in both ES-derived hematopoietic progenitors and BaF3 cells. Conditions are identical to Figure 5 with an activation site–specific p38 MAPK phospho-antibody and total p38 MAPK antibody. This molecular pathway was analyzed 3 times from 3 independent experiments with similar results. (B) The p38 MAPK small molecule inhibitor SB 203580 expands ES-derived hematopoietic progenitors in a dose-dependent manner. Doses of 0.1, 1.0, and 10 μM of SB 203580 or control DMSO were added daily from day 5 to day 7 of ES in vitro differentiation cultures. ES-differentiated day 7 total cell numbers on SB 203580 addition were compared to those in the presence and absence of BCR-ABL induction. Results represent the mean ± SEM of an experiment conducted in triplicate. Similar results were obtained in another independent experiment done in triplicate cultures. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON total cell population wasP < .01. Statistical analysis (paired t test) of BCR-ABL OFF plus 10 μM SB 203580 versus BCR-ABL OFF total cell population was P < .01. (C) The p38 MAPK small molecule inhibitor SB 203580 suppresses apoptosis in ES-derived hematopoietic progenitors similar to BCR-ABL expression. SB 203580 (10 μM) or control DMSO (10 μM) was added daily from day 5 to day 7 of ES in vitro differentiation cultures. The level of apoptosis as measured by annexin V levels (refer to Figure 3) on SB 203580 addition was compared to that in the presence and absence of BCR-ABL induction. A representative result of an experiment done in triplicate cultures is shown. Similar results were obtained in 2 independent experiments done in triplicate cultures.
p38 MAPK inhibition can account for most of the overall antiapoptotic effect mediated by BCR-ABL expression in hematopoietic progenitors.
(A) BCR-ABL expression suppresses p38 MAPK activity in both ES-derived hematopoietic progenitors and BaF3 cells. Conditions are identical to Figure 5 with an activation site–specific p38 MAPK phospho-antibody and total p38 MAPK antibody. This molecular pathway was analyzed 3 times from 3 independent experiments with similar results. (B) The p38 MAPK small molecule inhibitor SB 203580 expands ES-derived hematopoietic progenitors in a dose-dependent manner. Doses of 0.1, 1.0, and 10 μM of SB 203580 or control DMSO were added daily from day 5 to day 7 of ES in vitro differentiation cultures. ES-differentiated day 7 total cell numbers on SB 203580 addition were compared to those in the presence and absence of BCR-ABL induction. Results represent the mean ± SEM of an experiment conducted in triplicate. Similar results were obtained in another independent experiment done in triplicate cultures. Statistical analysis (paired t test) of BCR-ABL OFF versus BCR-ABL ON total cell population wasP < .01. Statistical analysis (paired t test) of BCR-ABL OFF plus 10 μM SB 203580 versus BCR-ABL OFF total cell population was P < .01. (C) The p38 MAPK small molecule inhibitor SB 203580 suppresses apoptosis in ES-derived hematopoietic progenitors similar to BCR-ABL expression. SB 203580 (10 μM) or control DMSO (10 μM) was added daily from day 5 to day 7 of ES in vitro differentiation cultures. The level of apoptosis as measured by annexin V levels (refer to Figure 3) on SB 203580 addition was compared to that in the presence and absence of BCR-ABL induction. A representative result of an experiment done in triplicate cultures is shown. Similar results were obtained in 2 independent experiments done in triplicate cultures.
Inhibition of p38 MAPK activity expands hematopoietic progenitors by suppressing apoptosis analogous to BCR-ABL expression
Inhibition of p38 MAPK suppression by SB 203580 was sufficient to expand ES-derived hematopoietic progenitors in a dose-dependent manner (Figure 6B). At the highest dose of inhibitor, 10 μM SB 203580, the level of cell expansion was 80% of that observed with BCR-ABL expression (Figure 6B). p38 MAPK inhibition has been shown to suppress cell death.73 74 In ES-derived hematopoietic progenitors, 10 μM SB 203580 induced cell expansion by suppressing apoptosis from 21% to 7% as measured by annexin V binding (Figure 6C). This 3-fold reduction was of the same magnitude as the BCR-ABL level of apoptosis inhibition (Figure 6C). These results suggest that inhibition of the p38 MAPK pathway can account for a remarkably large fraction of the overall antiapoptotic effect mediated by BCR-ABL expression in ES-derived hematopoietic progenitors.
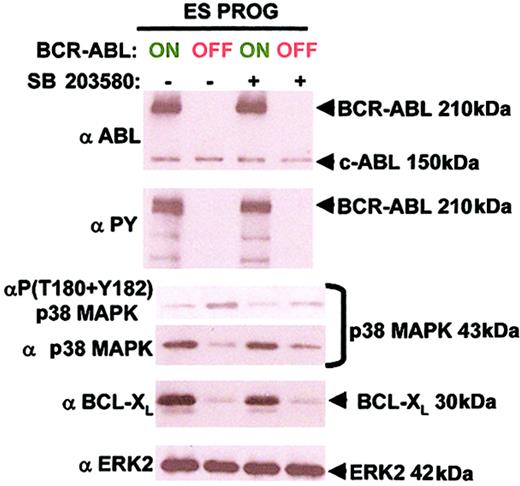
BCR-ABL suppresses p38 MAPK activity and up-regulates BCL-XL independent of each other
We examined whether inhibition of p38 MAPK led to an up-regulation of BCL-XL as a possible mechanism for suppressing apoptosis in ES-derived hematopoietic progenitors. Inhibition of p38 MAPK with 10 μM SB 203580 did not up-regulate BCL-XL in the absence of BCR-ABL expression (Figure 7). Furthermore, p38 MAPK inhibition by SB 203580 did not lead to an accumulation of total p38 MAPK unlike BCR-ABL expression (Figure 7). Similar amounts of protein lysate were examined in each condition as verified by equal levels of total ERK 2 (Figure 7). These results suggest that BCR-ABL–induced suppression of p38 MAPK activity and up-regulation of BCL-XL occur independently of each other.
BCR-ABL suppresses p38 MAPK activity and up-regulates BCL-XL independent of each other.
Conditions are identical to Figure 6, panels A and C, with an additional total ERK2 antibody. These molecular pathways were analyzed from 2 independent experiments with similar results.
BCR-ABL suppresses p38 MAPK activity and up-regulates BCL-XL independent of each other.
Conditions are identical to Figure 6, panels A and C, with an additional total ERK2 antibody. These molecular pathways were analyzed from 2 independent experiments with similar results.
Discussion
We have examined the early effects of BCR-ABL expression on the growth and development of multilineage hematopoietic progenitors. Using a BCR-ABL–inducible ES in vitro differentiation system, we demonstrate that BCR-ABL expression selectively expands multipotent and myeloid progenitors with suppression of differentiation into erythroblasts. These BCR-ABL effects require its tyrosine kinase activity and are independent of its reported scaffolding functions.75 This suppression along the erythroid lineage is extended to a reduction in mature erythrocyte development in this system.31 Together these results suggest that BCR-ABL expression may suppress hematopoietic development for the erythroid lineage leading to mild anemia in most patients with CML. The Ph+ erythroblast K562 cell line requires BCR-ABL activity to suppress hemoglobinization, providing further evidence to support this proposed mechanism.47 However, BCR-ABL expression in CD34+ progenitors can exclusively lead to an increase in erythroid colonies42-44 or GM-CSF colonies.26,27 Furthermore, embryoid body–derived BCR-ABL hematopoietic progenitors exclusively develop into erythroid cells in vitro, but differentiate into myeloid, T, and B cells in vivo.76 These apparently conflicting results suggest that microenvironmental conditions such as stromal cell contacts and growth factor levels may play pivotal roles in determining the specific lineage of cells that BCR-ABL expression affects. In our ES-OP9 differentiation system, the microenvironment may be sufficient to support expansion of BCR-ABL–expressing multipotent and myeloid progenitors and, as a consequence, reduce the development of BCR-ABL–expressing erythroblasts.
The expansion of ES-derived multipotent and myeloid progenitors was mediated by a rapid antiapoptotic effect and not by enhanced cell proliferation. Examining major antiapoptotic pathways connected to BCR-ABL expression from cell line studies revealed that AKT, STAT5, and BCL-2 were not up-regulated on BCR-ABL induction in ES-derived hematopoietic progenitors. In fact, STAT5 phosphorylation at its active site was undetectable in ES-derived hematopoietic progenitors. STAT5 has also been shown to be dispensable for generating a BCR-ABL–induced myeloproliferative disorder in vivo.77 These findings contrast with results from BCR-ABL expression in cell lines and highlight the importance of evaluating signaling in the specific cellular context of hematopoietic progenitors. In vitro ES-differentiated progenitors can contribute to long-term, multilineage hematopoiesis in vivo78 and BCR-ABL–expressing ES-derived hematopoietic progenitor clones generate an acute leukemia in vivo.30 These results suggest that ES-derived hematopoietic progenitors may be a potential cell population that can be used to study BCR-ABL effects in vivo. BCR-ABL cell context–dependent signaling has been suggested in a study where 12 genes regulated by BCR-ABL expression in a Ph+ pre-B cell line are largely unaffected in Ph+ myeloid cell lines.79
The antiapoptotic molecule BCL-XL is up-regulated in BCR-ABL–expressing ES-derived hematopoietic progenitors. Unexpectedly, this up-regulation occurred independently of STAT5 or AKT activation. Both STAT5 and AKT activation have been linked to BCL-XLup-regulation by BCR-ABL.61,62,70,80 In fact, dominant-negative forms of STAT5 have been shown to suppress BCL-XL up-regulation in cell lines expressing BCR-ABL.61,62 However, STAT5AB-deficient pro-B cells express BCL-XL at similar levels to wild-type pro-B cells, demonstrating that STAT5 is not required for BCL-XLexpression (V. Sexl, written personal communication, December 2002). Other signaling pathways that up-regulate BCL-XL include RAS,81 ERK,82 and nuclear factor κB (NF-κB).83 These signal transducers, RAS,84 ERK,85 and NF-κB,86 are activated by BCR-ABL expression in cell line studies and could contribute to BCL-XL up-regulation in ES-derived hematopoietic progenitors.
BCL-XL inhibitors have been used to examine the contribution of BCL-XL signaling in BCR-ABL–mediated suppression of apoptosis. Cell permeable peptides targeted against the BH3 domain of BCL-XL, the natural compound tetrocarcin A, the antibiotic antimycin A, and the low-molecular-weight organic compound HA14-1 have all been shown to inhibit BCL-XL (for a review, see Huang59). BCL-XL is up-regulated in a wide range of cell types expressing BCR-ABL.61 67-70BCL-XL inhibitors could be an effective treatment for patients with CML resistant to imatinib mesylate by suppressing BCR-ABL effects in all cell types.
There is conflicting evidence on whether targeting secreted factors from Ph+ cells would be a rationale treatment option for patients with CML resistant to imatinib mesylate.27,30 52-57 In our hematopoietic progenitor cultures, BCR-ABL expression suppresses apoptosis in a cell autonomous manner and does not appear to have noncell autonomous effects.
We demonstrate that suppression of the p38 MAPK pathway can largely account for the acute reduction in apoptosis mediated by BCR-ABL expression in ES-derived hematopoietic progenitors. These results are in agreement with studies showing that p38 MAPK inhibition restores BCR-ABL transformation in the face of chemotherapeutic agents such as IFN-α and butyrate.71 72
p38 MAPK inhibition may suppress apoptosis by up-regulating BCL-XL. Addition of the p38 MAPK inhibitor SB 203580 to ES-derived hematopoietic progenitors does not up-regulate BCL-XL, unlike BCR-ABL expression. These results suggest that BCR-ABL suppresses p38 MAPK and up-regulates BCL-XLindependently of each other.
BCR-ABL expression could suppress p38 MAPK at different points along its activation pathway. We did not observe any diminution in the phosphorylation of its upstream kinase MKK3/6 (data not shown). p38 MAPK phosphatases could be up-regulated on BCR-ABL induction. This is a difficult issue to examine because other MAPKs such as ERK and JNK can also be substrates for these phosphatases (for a review, see Lee et al87). BCR-ABL could also suppress TAB1 expression, a scaffolding protein that can bind to and autophosphorylate p38 MAPK.88 Alternatively, p38 MAPK suppression can occur on RAS activation in fibroblasts.89 BCR-ABL expression has been shown to increase the levels of active RAS-GTP84making this a likely pathway to be used by BCR-ABL for p38 MAPK suppression.
We have shown that BCR-ABL expression suppresses p38 MAPK activation in the face of IL-3 stimulation in BaF3 cells (Figure 6A). IL-3 is an activator of p38 MAPK in BaF3 cells (Figure 6A).90 IL-3 is also up-regulated on BCR-ABL induction30,53 with its production correlating to expansion of Ph+progenitors.55 BCR-ABL expression may enhance effects of IL-3–induced cell expansion by suppressing p38 MAPK-mediated apoptosis. This potential mechanism would give Ph+progenitors a selective advantage over normal cells to expand in the presence of IL-3.
We examined whether p38 MAPK inhibition was sufficient to expand both ES-derived hematopoietic progenitors and mature myeloid cells, similar to BCR-ABL expression. Although addition of the p38 MAPK inhibitor SB 203580 expanded ES-derived hematopoietic progenitors, mature myeloid cell production was severely suppressed (data not shown). These results demonstrate that BCR-ABL must regulate other signaling pathways in addition to p38 MAPK inhibition to expand myeloid cells. A comparison of signaling components regulated solely by p38 MAPK inhibition versus BCR-ABL activation in ES-derived hematopoietic progenitors may help to identify signaling pathways used by BCR-ABL to selectively expand myeloid elements.
We are grateful to Dr Veronica Sexl (University of Austria, Vienna) for providing unpublished results; Drs Arnie Berk, John Colicelli, Harvey Herschman, Caius Radu, Charles Sawyers, and Larry Zipursky (UCLA) for helpful discussions and critical reading of the manuscript; to Fiona Willis for technical expertise on the FACSVantage cell sorter; and to J. C. White for preparation of the manuscript.
O.N.W. is an Investigator of the Howard Hughes Medical Institute.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-11-3376.
Supported in part by grant CA76204 from the National Institutes of Health (O.N.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Owen N. Witte, HHMI/UCLA, 675 Charles E. Young Dr S, Room 5720, Los Angeles, CA 90095; e-mail:owenw@microbio.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal