Abstract
Engagement of Fas (CD95) induces death of activated T cells but can also potentiate T-cell response to CD3 ligation. Yet, the effects of Fas-mediated signals on activation of naive T cells have remained controversial. We followed naive T cells responding under Fas ligation. Ligation of Fas simultaneously with activation by antigen-bearing dendritic cells promoted early death in half of the responding naive murine CD4 T cells. Surprisingly, it simultaneously accelerated cell division and interferon-γ (IFN-γ) production among surviving T cells. These cells developed quickly an activation-associated phenotype (CD44hi, CD62Llo), responded vigorously to antigen rechallenge, were partially resistant to subsequent induction of cell death via Fas, and were long-lived in vivo. Compared with cells becoming apoptotic, the surviving cells expressed lower levels of Fas and higher levels of T-cell receptor (TCR), CD4, and interleukin-2 receptor (IL-2R). Their survival was associated with expression of antiapoptotic cellular FLICE-inhibitory protein (c-FLIP), Bcl-XL, and Bcl-2. Thus, at the time of T-cell activation there is a subtle balance in the effects of Fas ligation that differs on a cell-to-cell basis. Factors that predict cell survival include expression levels of Fas, TCR, CD4, and IL-2R. Early death of some cells and a pronounced response of the surviving cells suggest that Fas ligation can both up- and down-regulate a primary T-cell response.
Introduction
An immune response can be initiated when a dendritic cell (DC) bearing antigenic material comes into contact with a naive T cell specific for the antigen. The interaction between B7.2 and B7.1 (CD86, CD80) on the DC and CD28 on the T cell provides costimulatory signals that are a prerequisite for productive activation of the naive T cell.1-3 In addition, costimulatory signals are provided to T cells by a number of other interactions,4,5 including those between tumor necrosis factor (TNF) and TNF-receptor (TNFR) family members.6However, some members of the TNFR family have an intracellular death domain and associate with intracellular death domain–containing proteins and are thus also able to activate programmed cell death.7-10 Intracellular adapter molecules recruited and downstream signaling cascades activated by ligand binding to even one given receptor of TNFR family are often multiple11 and allow “crosstalk” between both cell activation and cell death machinery.12-17
Fas (CD95) is a homotrimeric TNFR family member associated with death domains and death effector domains.18 It plays a critical role in activation-induced cell death (AICD) of repeatedly stimulated T cells.19,20 In accordance with many other TNFR family members, it also appears able to promote activation of some hematopoietic cells, especially of DCs that are resistant to its death-inducing effects.21 Also, peripheral blood T cells and thymocytes are costimulated, when promptly activated, by simultaneous Fas ligation,22,23 indicating that Fas can transduce activating signals in thymocytes and in resting T cells. However, naive T cells are not costimulated via Fas to the same extent as memory T cells,24 and Fas-deficientlpr T cells respond normally to stimulation with anti-CD3.25 These data indicate that Fas-mediated signals are not a prerequisite for normal activation of naive T cells. However, they do not rule out the possibility that Fas could modulate the evolving T-cell response. Because DCs can express Fas ligand ([FasL] CD95L) under in vitro conditions,26 and reportedly also in vivo,27 engagement of Fas by FasL on DCs could modulate the activation of naive T cells by DCs.
In the interest of determining the potential role of Fas in physiologic T-cell activation and in induction of immune tolerance, we examined the effect of ligating Fas during the interaction of naive and recently activated T cells with DCs. To bypass the impossibility of obtaining and purifying naive, conventional T cells with a single antigen specificity, we made use of T cells (OT-II T cells) of mice transgenic for a T-cell receptor with specificity to ovalbumin peptide (323-339; OT-II mice). We asked whether naive OT-II T cells receive death-promoting or/and stimulatory signals via Fas during their interaction with DCs, whether they undergo AICD later, and whether early Fas ligation affects their susceptibility to subsequent death induction via Fas. We present evidence indicating that simultaneously with a death-inducing effect on some of the responding T cells, Fas can act as a costimulatory receptor, favoring the development and promoting the responsiveness of effector T cells.
Materials and methods
Mice
Mice were bred and maintained in Central Animal Laboratory in Turku University and kept under specific pathogen–free (SPF) conditions. OT-II mice28 that express a transgenic T-cell receptor (TCR) specific for ovalbumin peptide 323-339 and have a gross overproduction of CD4 T cells with this specificity were used as CD4 T-cell donors. Rag2−/− mice on the same (C57BL/6) background were used as donors of bone marrow cells for DC propagation. Both of these mouse strains were kind gifts from Dr L. C. Harrison, the Walter and Eliza Hall Institute, Melbourne, Australia. Mice were used at 6 to 20 weeks of age. All animal experiments were approved by the Institutional Ethical Committee of Turku University.
Preparation and activation of dendritic cells and purification of naive CD4 T cells (OT-II T cells)
DCs were propagated from bone marrow cells according to the method by Inaba et al29 with slight modifications. To circumvent the need for depleting contaminating lymphocytes, Rag2−/− mice were used as bone marrow donors. Briefly, bone marrow was flushed from aseptically prepared (treated in 70% ethanol) femoral bones, red cells were lysed using hypotonic saline, and remaining white cells were plated at 2 × 106/mL in a 24-well plate in complete medium that consisted of RPMI 1640 supplemented with 10% fetal calf serum (FCS), 20 mM l-glutamine, 5 × 10−5 M 2-mercaptoethanol, penicillin/streptomycin, and 20 μg/mL recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (PharMingen, San Diego, CA). Medium was changed every 2 days, and the cells were transferred to new wells. After 5 or 6 days in culture, the cells were collected and CD11c+ dendritic cells were purified using magnetic separation (anti-CD11c magnetic cell sorting [MACS] beads and MACS columns, Miltenyi Biotech, Bergisch Gladbach, Germany). After purification, the cells were at least 95% CD11c+. To pulse these purified DCs with antigen, they were put to medium containing GM-CSF and ovalbumin (grade V; Sigma, St Louis, MO) at the following concentrations: 0, 0.002, 0.02, 0.2, and 2.0 mg/mL. To induce terminal differentiation of ovalbumin (OVA)–pulsed DCs into fully mature DCs, 10 μg/mL anti-CD40 (clone HM40-3; PharMingen) was added 2 hours later together with 1 μg/mL anti-Fas (Jo2; PharMingen) because Fas ligation can further stimulate DCs21 and we wished to dissect that effect from the effect that ligation of Fas expressed on T cells may have. After 24 hours, DCs were washed twice, recounted, and used for stimulation of OT-II T cells.
Naive ovalbumin-reactive CD4 T cells were purified from spleens and lymph nodes of OT-II mice by passing cell suspensions through nylon wool columns and by negative selection using magnetic separation. Briefly, after passage in nylon wool columns, the cells were incubated with a cocktail of biotinylated antibodies initially purified by affinity chromatography (protein G–Sepharose beads; Amersham Pharmacia Biotech, Uppsala, Sweden) from hybridoma supernatants (TIB 210, TIB 229, TIB 146, and TIB 241; ATCC, Manassas, VA) against CD8, major histocompatibility complex (MHC) class II (I-Ab,d), B cells, and CD44; washed; and then incubated with streptavidin-conjugated MACS beads (Miltenyi Biotech). After magnetic selection, purity of CD4 T cells was at least 97% and, of these, at least 99.5% were CD44lo.
Activation of naive OT-II T cells
Purified naive OT-II T cells were incubated in complete medium together with stimulated and OVA-pulsed DCs. In initial experiments, DC–T cell ratios of 1:5, 1:10, and 1:20 appeared to give optimal T-cell proliferation, whereas ratios below that gave less proliferation. Therefore, a ratio of 1:10 was chosen for all subsequent experiments. Depending on the purpose of the experiment, T cells and DCs were coincubated in flat or round-bottomed 96-well plates. Anti-Fas (Jo2), isotype-matched control immunoglobulin G (IgG) or anti-FasL (Kay10; all from PharMingen), or recombinant human FasL (R&D Systems, Minneapolis, MN) cross-linked via its FLAG tail with anti-FLAG, or anti-FLAG alone, was immediately added to these cocultures. In preliminary experiments, a range of 0.1 to 5.0 μg/mL anti-Fas gave identical effect on thymidine incorporation and cell division, and 1 μg/mL was thereafter chosen for further experiments. FasL was used at 50 ng/mL and anti-FLAG at 1 μg/mL.21 At indicated time points, supernatants were collected for analysis of cytokine production, and3H-thymidine (1 μCi [0.037 MBq] per well) was added for the last 6 hours of culture for analysis of proliferation. Cells were harvested using a semiautomated plate harvester (Tomtech MACH III; Fisher Scientific, Hampton, NH). Cytokine levels in supernatants were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) with anti–interleukin-2 (anti–IL-2) (JES6-1A12), anti–IL-4 (11B11), and anti–interferon-γ (anti–IFN-γ) (R4 6A2) as capturing antibodies and biotinylated anti–IL-2 (JES6-5H4), anti–IL-4 (BVD6-24G2), and anti–IFN-γ (XMG1.2) as detecting antibodies (all from PharMingen). Viability of cell populations was calculated from separate wells in triplicates by trypan blue staining.
Analysis of cell division versus cell death
For analysis of cell division of individual T cells, purified naive OT-II T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) prior to their coculture with activated, ovalbumin-pulsed DCs. After 3 or 6 days, cells were recovered, stained for flow cytometry with phycoerythrin-conjugated anti-CD4 (PharMingen), and gated CD4+ cells were analyzed using FACScan (Becton Dickinson, San Jose, CA) and WinMDI software version 2.8 (http://facs.scripps.edu/software.html). Viability of cells in each generation was assessed by adding 7-aminoactinomycin-D (7-AAD) (Sigma) to the cells immediately before analysis, and the proportion of 7-AAD+ cells within each cell cluster of reducing CFSE intensity was evaluated. The average number of cell divisions per population was determined in 2 steps. The percentage of cells in each generation was first multiplied by the number of cell divisions it had completed. To obtain the average number of cell divisions for the whole population, the products of all cell generations were then added together and divided by 100%.
Cell surface phenotype of activated OT-II T cells
For phenotyping, OT-II T cells were stained prior to activation and, at days 3 and 6 thereafter, for the expression of CD95 (Fas), CD95L (FasL), CD4, Vα2 (TCR), CD25, CD44, CD45RB, and CD62L. Directly fluorochrome-conjugated antibodies (all from PharMingen) were used, the exceptions being Fas and Vα2, which were detected by indirect stainings. For Fas, the first-step antibody was the same antibody (Jo2) that was used for Fas ligation in the initiation of culture of certain cells, and the second-step antibody was fluorescein isothiocyanate (FITC)–conjugated antihamster IgG (clone G 192-1; PharMingen). Vα2 was detected with biotinylated antimouse Vα2 (B20.1; PharMingen) and streptavidin-conjugated Alexa488 (Molecular Probes) as the second-step reagent. Controls included stainings with fluorochrome-conjugated rat IgG1 and rat IgG2a (PharMingen) control monoclonal antibodies (mAbs).
Restimulation of OT-II T cells
After 6 days of coculture with activated, ovalbumin-pulsed DCs, OT-II T cells were becoming small and round in size and shape, suggesting that they were returning into resting state. At this stage, cells were recovered from culture wells and subjected to gradient centrifugation (Ficoll-Paque; Amersham Pharmacia Biotech) to purify viable cells. These viable cells were then plated on new culture wells and reactivated using fresh DCs that were ovalbumin-pulsed and then activated as before, in the presence of anti-Fas (Jo2) or control IgG or in the presence of cross-linked FasL or anti-FLAG alone. Proliferation was measured at 24 hours from activation, and supernatants were collected for cytokine analysis. Viability was calculated from separate wells after trypan blue staining.
Adoptive transfer of preactivated OT-II T cells
In certain experiments, preactivated OT-II T cells were adoptively transferred into Rag2−/− mice to allow determination of their long-term survival and responsiveness. Briefly, in vitro–activated OT-II T cells were washed and viable cells obtained through gradient centrifugation (Ficoll-Paque). Then, 9 × 106 viable cells were injected intravenously into the tail vein of each recipient mouse. These recipients were killed after 25 days, and their spleen cells and lymph node lymphocytes were prepared, stained for flow cytometry, and analyzed for the presence of transferred OT-II T cells. To measure the percentage of OT-II T cells among host cells, cells were stained with phycoerythrin-conjugated anti-CD4 and FITC-conjugated anti-Vβ5.1 TCR. To measure the responsiveness of transferred OT-II T cells to antigen, spleen cell suspensions were cultured in the presence of antigen (ovalbumin).
Analysis of c-FLIP, Bcl-2, and Bcl-XL
The presence and relative abundance of cellular FLICE-inhibitory protein (c-FLIP), Bcl-2, and Bcl-XL in OT-II T cells was evaluated on the RNA level by reverse transcriptase–polymerase chain reaction (RT-PCR). Briefly, total RNA was prepared from OT-II T cells with the Ultraspec-II RNA Isolation system (Biotecx, Houston, TX). Then, 0.1 to 1 μg of total RNA samples were treated with RQ1 RNase-Free DNase (Promega, Madison, WI) to remove any remaining genomic DNA. The cDNA synthesis was done with the ThermoScript RT-PCR System (Invitrogen, San Diego, CA) using oligo (dT) primers at 50°C for 1 hour.
PCR amplification of β-actin, c-FLIP,Bcl-2, and Bcl-XL cDNAs was done with DyNAzyme II DNA polymerase (Finnzymes, Espoo, Finland) using gene-specific primers (Table 1) and the following conditions: 40 seconds at 94°C, 40 seconds at 60°C, and 60 seconds at 73°C for 40 cycles. The PCR products were analyzed in a 1.5% agarose gel with ethidium bromide.
Gene-specific primers
| Gene of interest . | Upstream primer . | Downstream primer . |
|---|---|---|
| β-actin | 5′-TGTGATGGTGGGAATGGGTCAG-3′ | 5′-TTTGATGTCACGCACGATTTCC-3′ |
| FLIP* | 5′-GTGGAAGAGTGTCTTGATGAAG-3′ | 5′-GAGCGAAGCCTGGAGAGTATT-3′ |
| Bcl-XL* | 5′-TGGAGTAAACTGGGGTCGCATC-3′ | 5′-AGCCACAGTCATGCCCGTCAGG-3′ |
| Bcl-2* | 5′-CTCGTCGCTACCGTCGTGACTTCG-3′ | 5′-GTGGCCCAGGTATGCACCCAG-3′ |
| Gene of interest . | Upstream primer . | Downstream primer . |
|---|---|---|
| β-actin | 5′-TGTGATGGTGGGAATGGGTCAG-3′ | 5′-TTTGATGTCACGCACGATTTCC-3′ |
| FLIP* | 5′-GTGGAAGAGTGTCTTGATGAAG-3′ | 5′-GAGCGAAGCCTGGAGAGTATT-3′ |
| Bcl-XL* | 5′-TGGAGTAAACTGGGGTCGCATC-3′ | 5′-AGCCACAGTCATGCCCGTCAGG-3′ |
| Bcl-2* | 5′-CTCGTCGCTACCGTCGTGACTTCG-3′ | 5′-GTGGCCCAGGTATGCACCCAG-3′ |
Intron-flanking primers.
Results
Fas ligation at the time of activation reduces proliferation of naive CD4 T cells on the whole population level due to increased cell death in the first generations of T-cell progeny
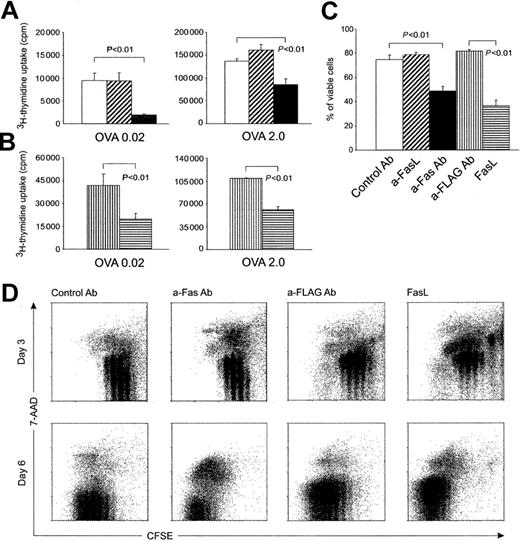
To elucidate the roles of Fas as a death-inducing and potentially costimulatory receptor on naive T cells, we determined the effect that Fas ligation has on T cells that are being activated by antigen-bearing dendritic cells. Purified naive (CD44lo) OVA-specific CD4 T cells (OT-II T cells) were incubated together with bone marrow–derived, OVA-pulsed syngeneic DCs in the presence of various concentrations of anti-Fas mAb or an isotype-matched control mAb. A function-blocking anti-FasL (Kay10) mAb was added to parallel wells to control the effects of AICD occurring in response to FasL expression. To include optimal and suboptimal levels of TCR ligation in these experiments, we used a wide range of OVA concentrations (every 10-fold concentration from 0.002 mg/mL to 2.0 mg/mL) to pulse DCs. Thymidine incorporation was invariably diminished in cultures containing agonistic anti-Fas mAb or cross-linked FasL (Figure 1A-B). In these cultures, the proportion of viable cells after 3 days of activation was significantly lower, which indicated that early Fas ligation induced cell death during the primary response (Figure 1C).
Fas ligation at the time of activation promotes early apoptosis in naive CD4 T cells (OT-II T cells).
(A) Proliferation of T cells in the presence of control (■) or anti-FasL (▨) or anti-Fas (▪) mAb at day 3 during coculture with dendritic cells pulsed with a low or high concentration of antigen: 0.02 mg/mL (left) or 2.0 mg/mL (right) ovalbumin. Without antigen, proliferation was always less than 200 cpm (not shown). (B) Proliferation of T cells in the presence of anti-FLAG alone (▥) or cross-linked FasL (ie, FasL plus anti-FLAG) (▤). (C) Viability of T cells responding in the presence of the indicated antibodies or FasL at day 3. Viability was calculated after trypan blue staining by counting living and dead cells in triplicate cultures in each experiment. DCs were pulsed with 2 mg/mL ovalbumin. (D) Viability of responding T cells activated in the presence of the indicated antibodies or FasL-plotted against cell division. Viability was determined by 7-AAD staining of CFSE-labeled T cells at day 3 and day 6. A representative staining from 1 of 3 experiments is shown. In panels A-C, results are the mean ± SEM of 3 individual experiments. P values were calculated using the Studentt test.
Fas ligation at the time of activation promotes early apoptosis in naive CD4 T cells (OT-II T cells).
(A) Proliferation of T cells in the presence of control (■) or anti-FasL (▨) or anti-Fas (▪) mAb at day 3 during coculture with dendritic cells pulsed with a low or high concentration of antigen: 0.02 mg/mL (left) or 2.0 mg/mL (right) ovalbumin. Without antigen, proliferation was always less than 200 cpm (not shown). (B) Proliferation of T cells in the presence of anti-FLAG alone (▥) or cross-linked FasL (ie, FasL plus anti-FLAG) (▤). (C) Viability of T cells responding in the presence of the indicated antibodies or FasL at day 3. Viability was calculated after trypan blue staining by counting living and dead cells in triplicate cultures in each experiment. DCs were pulsed with 2 mg/mL ovalbumin. (D) Viability of responding T cells activated in the presence of the indicated antibodies or FasL-plotted against cell division. Viability was determined by 7-AAD staining of CFSE-labeled T cells at day 3 and day 6. A representative staining from 1 of 3 experiments is shown. In panels A-C, results are the mean ± SEM of 3 individual experiments. P values were calculated using the Studentt test.
To determine the relationship between the number of division cycles and cell death induction, responding naive CD4 T cells were labeled with CFSE before activation. After 3 or 6 days, the responding cells were recovered from culture wells, stained for CD4 and for membrane integrity by using 7-AAD, and analyzed by flow cytometry. Analysis of 7-AAD staining in each generation indicated that cell death affected most severely cells that had divided only a few times (Figure1D).
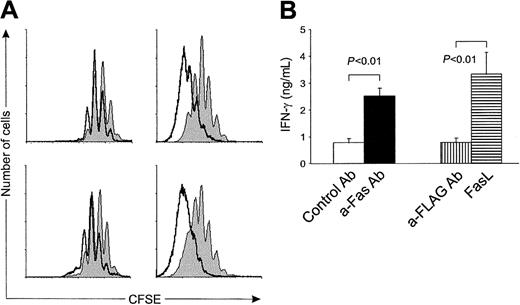
Surviving CD4 T cells proliferate faster and produce more IFN-γ than cells activated without Fas ligation
CFSE labeling of responding CD4 T cells indicated that cells that had not died due to early Fas ligation had divided on an average more times than cells not stimulated via Fas (Figure2A). This was true for various levels of TCR ligation, because it was seen when DCs were pulsed either with high (2 mg/mL) or low (0.02 mg/mL, not shown) concentrations of ovalbumin. After 3 days (Figure 2A), cells activated under control conditions had gone through a mean of 2.1 cell divisions, whereas cells activated under Fas ligation had gone through a mean of 2.9 divisions. After 6 days (Figure 2A), the difference in average number of divisions was even greater, 5.5 times versus 7.3 times. To determine the ability of the responding cells to develop into effector T cells, we measured their production of IFN-γ and IL-4. Effector cytokine production required that DCs were pulsed with the highest concentration (2 mg/mL) of ovalbumin. Under these conditions, ligation of Fas on responding cells caused a 2- to 3-fold increase in the production of IFN-γ (Figure 2B) although the percentage of viable cells was lower than under control conditions. In contrast to IFN-γ, IL-4 was not detected under any circumstances although standard dilutions of IL-4 gave positive readings. Because Fas ligation increased the response of surviving cells, this effect is hereafter referred to as Fas costimulation.
Fas ligation at the time of activation accelerates cell division and IFN-γ production in the surviving cells.
Naive OT-II T cells were activated in coculture with dendritic cells pulsed with 2 mg/mL OVA. (A) Cell division was determined at day 3 (left panels) and day 6 (right panels). Upper panels: cells costimulated with anti-Fas (open histograms) or control antibody (shaded histograms). Lower panels: cells costimulated with cross-linked FasL (open histograms) or anti-FLAG (shaded histograms). OT-II T cells were CFSE-labeled prior to coculture. A representative staining from 1 of 3 separate experiments for each panel is shown. Cells were stained with phycoerythrin-conjugated anti-CD4 and 7-AAD. Events were gated for viable (7-AAD−) CD4 T cells. (B) IFN-γ production in cocultures of naive OT-II T cells and DCs. DCs were pulsed with 2 mg/mL ovalbumin, and either control (■) or anti-Fas (a-Fas) (▪) or anti-FLAG (a-FLAG) (▥) mAb or FasL (▤) was added at the start of coculture. IFN-γ concentration was determined from supernatants at day 5 using specific ELISA. Results are the mean ± SEM of 3 individual experiments. P values were calculated using the Student t test.
Fas ligation at the time of activation accelerates cell division and IFN-γ production in the surviving cells.
Naive OT-II T cells were activated in coculture with dendritic cells pulsed with 2 mg/mL OVA. (A) Cell division was determined at day 3 (left panels) and day 6 (right panels). Upper panels: cells costimulated with anti-Fas (open histograms) or control antibody (shaded histograms). Lower panels: cells costimulated with cross-linked FasL (open histograms) or anti-FLAG (shaded histograms). OT-II T cells were CFSE-labeled prior to coculture. A representative staining from 1 of 3 separate experiments for each panel is shown. Cells were stained with phycoerythrin-conjugated anti-CD4 and 7-AAD. Events were gated for viable (7-AAD−) CD4 T cells. (B) IFN-γ production in cocultures of naive OT-II T cells and DCs. DCs were pulsed with 2 mg/mL ovalbumin, and either control (■) or anti-Fas (a-Fas) (▪) or anti-FLAG (a-FLAG) (▥) mAb or FasL (▤) was added at the start of coculture. IFN-γ concentration was determined from supernatants at day 5 using specific ELISA. Results are the mean ± SEM of 3 individual experiments. P values were calculated using the Student t test.
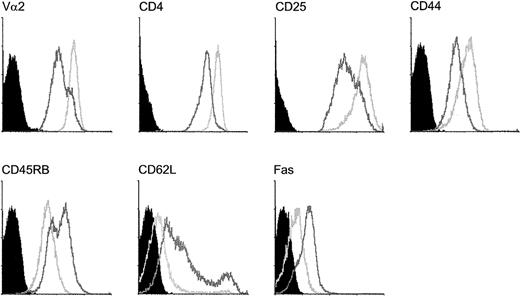
CD4 T cells that survive Fas ligation express lower levels of Fas and higher levels of TCR Vα2 and CD4 than cells that become apoptotic
To compare the cells that survived Fas ligation with cells that become apoptotic in response to Fas ligation, we analyzed separately viable and apoptotic cells for their surface expression of Fas, TCR Vα2 chain, CD4, activation markers IL-2 receptor (IL-2R), CD62L, CD44, and CD45RB (Figure 3). Apoptotic cells were analyzed as 7-AAD+ cells that still retained forward light scatter values typical for living cells. Apoptotic cells expressed lower levels of TCR Vα2 chain, coreceptor (CD4) and activation markers CD25 and CD44, and more CD45RB and CD62L than viable cells. They also expressed higher levels of Fas than surviving cells.
Differential expression of cell surface markers on responding CD4 T cells that either survive Fas ligation or become apoptotic.
Expression of TCR (Vα2), CD4, CD25, CD44, CD45RB, CD62L, and Fas was detected on responding T cells at day 3. Cells that were surviving Fas ligation (light gray open histograms) or becoming apoptotic (dark gray open histograms) were analyzed separately as explained in “Materials and methods.” Black histograms represent appropriate control stainings.
Differential expression of cell surface markers on responding CD4 T cells that either survive Fas ligation or become apoptotic.
Expression of TCR (Vα2), CD4, CD25, CD44, CD45RB, CD62L, and Fas was detected on responding T cells at day 3. Cells that were surviving Fas ligation (light gray open histograms) or becoming apoptotic (dark gray open histograms) were analyzed separately as explained in “Materials and methods.” Black histograms represent appropriate control stainings.
CD4 T cells that survive Fas ligation develop a phenotype of activated/memory cells but remain Faslo
To further characterize the possible impact of Fas ligation on T-cell activation, we followed the phenotype of cells that were activated under Fas ligation and control conditions. After 3 days of activation, Fas-ligated cells expressed slightly less CD45RB and, after a further 3 days, slightly more CD44 compared with their controls. Importantly, CD62L expression was lost almost completely from Fas-ligated cells at this time, whereas control cells still retained a population of CD62Lhi cells. These results indicate that cells surviving Fas ligation had become activated and were developing a phenotype of antigen-experienced T cells and that these changes took place even sooner than in control-activated T cells. Expression of FasL was seen after 6 days of activation and was equal in cells activated with or without Fas ligation (Figure 4A). In cells activated under control conditions, expression of Fas was up-regulated during the first 72 hours. Surprisingly, this up-regulation was absent in cells activated under Fas ligation (Figure 4B).
Fas-costimulated and control-activated CD4 T cells are phenotypically different.
(A) Expression of TCR (Vα2), CD4, CD25, and CD45RB on viable CD4 T cells was determined at day 3 and of CD44, CD62L, and FasL at day 6 on Fas-costimulated (light gray open histograms) and control-activated (dark gray open histograms) cells. Black histograms: staining control. (B) Expression of Fas on naive CD4 T cells and after 3 and 6 days of activation on Fas-costimulated and on control-activated cells.
Fas-costimulated and control-activated CD4 T cells are phenotypically different.
(A) Expression of TCR (Vα2), CD4, CD25, and CD45RB on viable CD4 T cells was determined at day 3 and of CD44, CD62L, and FasL at day 6 on Fas-costimulated (light gray open histograms) and control-activated (dark gray open histograms) cells. Black histograms: staining control. (B) Expression of Fas on naive CD4 T cells and after 3 and 6 days of activation on Fas-costimulated and on control-activated cells.
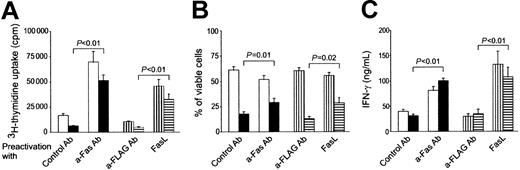
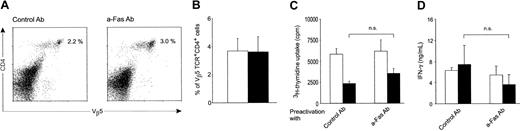
Fas costimulation of naive CD4 T cells produces effector cells that respond vigorously to antigen rechallenge and are partially insensitive to death induction via Fas engagement
To determine whether Fas-costimulated cells are destined to cell death after their initial burst or remain viable and able to respond to antigen rechallenge, viable cells were purified after 6 days of primary activation by gradient centrifugation (Ficoll-Paque) and rechallenged with antigen-pulsed DCs. All preactivated cells responded to antigen rechallenge by proliferation and cytokine secretion during the first 24 hours of restimulation. However, Fas-costimulated cells proliferated 4 times more vigorously than their controls (Figure5A). These Fas-costimulated T cells were also partially insensitive to death induction via Fas ligation, because their proliferation and the percentage of viable cells (Figure 5B) were less affected by Fas ligation. Fas-costimulated cells also produced significantly higher amounts of IFN-γ upon rechallenge than cells preactivated under control conditions (Figure 5C). IFN-γ production was not affected by Fas ligation at this stage in Fas-costimulated or control cells.
Fas-costimulated T cells respond more vigorously to antigen rechallenge and are partially resistant to death induction via Fas engagement.
After purification of viable cells, OT-II T cells were rechallenged with ovalbumin-pulsed DCs 6 days after the start of initial activation. (A) Proliferation of OT-II T cells initially activated in the presence of control mAb (“Control Ab”) or anti-Fas mAb (“a-Fas Ab”) cross-linked FasL (“FasL”) or the cross-linker (“a-FLAG Ab”) alone, during the first 24 hours of reactivation. (B) Viability of the same cells was assessed by trypan blue staining in triplicates from cultures made in parallel with panel A at day 2. (C) IFN-γ production was measured after 24 hours by ELISA as explained in Figure 2B. Results in all panels are pooled from 3 separate experiments (mean ± SEM). Cells were rechallenged in the presence of control antibody (■) or anti-Fas antibody (▪) or in the presence of cross-linker (▥) or cross-linked FasL (▤).P values were calculated by the Student t test and are given for differences between cells preactivated under different conditions and rechallenged under Fas ligation.
Fas-costimulated T cells respond more vigorously to antigen rechallenge and are partially resistant to death induction via Fas engagement.
After purification of viable cells, OT-II T cells were rechallenged with ovalbumin-pulsed DCs 6 days after the start of initial activation. (A) Proliferation of OT-II T cells initially activated in the presence of control mAb (“Control Ab”) or anti-Fas mAb (“a-Fas Ab”) cross-linked FasL (“FasL”) or the cross-linker (“a-FLAG Ab”) alone, during the first 24 hours of reactivation. (B) Viability of the same cells was assessed by trypan blue staining in triplicates from cultures made in parallel with panel A at day 2. (C) IFN-γ production was measured after 24 hours by ELISA as explained in Figure 2B. Results in all panels are pooled from 3 separate experiments (mean ± SEM). Cells were rechallenged in the presence of control antibody (■) or anti-Fas antibody (▪) or in the presence of cross-linker (▥) or cross-linked FasL (▤).P values were calculated by the Student t test and are given for differences between cells preactivated under different conditions and rechallenged under Fas ligation.
Fas-costimulated CD4 T cells survive in adoptive hosts as memory cells and retain their responsiveness to in vitro restimulation
To explore the effect of initial Fas ligation on survival rate of preactivated CD4 T cells, we transferred into Rag−/− mice equal numbers of cells preactivated for 7 days in vitro in the presence of either anti-Fas mAb or control mAb. Recipient mice were bled after 12 days to measure the percentage of circulating Vβ5.1+ CD4 T cells (the phenotype of OT-II T cells). Equal percentages of transferred cells were detected in recipients of Fas-costimulated and control-stimulated OT-II T cells (Figure 6A), indicating that Fas-costimulated cells were not dying faster than control cells. After 25 days, recipients were killed and cells were recovered from their lymph nodes and spleen for enumeration, phenotypic analysis, and in vitro antigen rechallenge. Cells had survived in adoptive hosts equally well regardless of Fas ligation during their original in vitro activation (Figure 6B). Their phenotype was consistent with that of memory cells, all cells being small in size and CD44hi (not shown). They proliferated in vitro equally (Figure 6C) and produced IFN-γ (Figure 6D) when stimulated with antigen. At this stage, both groups of memory cells were relatively insensitive to Fas ligation.
Equal survival of Fas-costimulated and control-stimulated T cells after in vivo transfer.
Following activation under control conditions or Fas ligation, viable cells were purified and equal numbers injected into Rag−/− recipient mice. (A) A blood sample was drawn 12 days after transfer, and white blood cells were stained for CD4 and Vβ5 using directly fluorochrome-conjugated mAbs and analyzed by flow cytometry. Percentages are indicative of positively stained (phenotype of OT-II T cells) cells in 2 individual mice. (B) At 25 days after transfer, mice were killed and, using the same methods as for panel A, spleen cells were stained and analyzed for the percentage of OT-II T cells (■: mice received control cells; ▪: mice received Fas-costimulated cells). (C) At the same time, spleen cells were placed in culture with ovalbumin (2 mg/mL) in the presence of control (■) or a-Fas (▪) Ab. Proliferation in response to antigen rechallenge was measured during a 72-hour incubation period. (D) Production of IFN-γ was measured from culture supernatants at day 3 using specific ELISA. Results in panels B-C are mean ± SEM of 6 mice per group, and results in panel D are mean ± SEM of 8 mice.
Equal survival of Fas-costimulated and control-stimulated T cells after in vivo transfer.
Following activation under control conditions or Fas ligation, viable cells were purified and equal numbers injected into Rag−/− recipient mice. (A) A blood sample was drawn 12 days after transfer, and white blood cells were stained for CD4 and Vβ5 using directly fluorochrome-conjugated mAbs and analyzed by flow cytometry. Percentages are indicative of positively stained (phenotype of OT-II T cells) cells in 2 individual mice. (B) At 25 days after transfer, mice were killed and, using the same methods as for panel A, spleen cells were stained and analyzed for the percentage of OT-II T cells (■: mice received control cells; ▪: mice received Fas-costimulated cells). (C) At the same time, spleen cells were placed in culture with ovalbumin (2 mg/mL) in the presence of control (■) or a-Fas (▪) Ab. Proliferation in response to antigen rechallenge was measured during a 72-hour incubation period. (D) Production of IFN-γ was measured from culture supernatants at day 3 using specific ELISA. Results in panels B-C are mean ± SEM of 6 mice per group, and results in panel D are mean ± SEM of 8 mice.
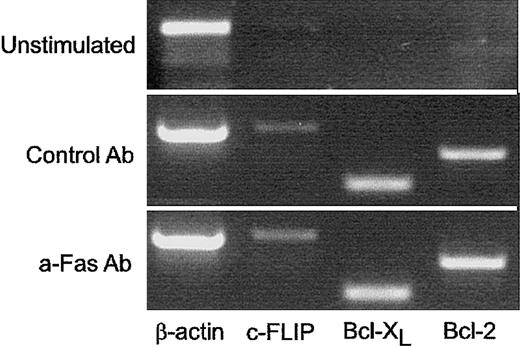
CD4 T cells that survive Fas ligation express c-FLIP, Bcl-XL, and Bcl-2
To further elucidate the mechanisms that made Fas-costimulated cells partially resistant to Fas-mediated apoptosis, we measured c-FLIP, Bcl-2, and Bcl-XL expression using RT-PCR from unstimulated and day 6–activated OT-II T cells. Unstimulated cells expresssed very low levels of c-FLIP, Bcl-2, and Bcl-XL. After 6 days of activation, mRNA for all of these was detectable both in cells that had responded under Fas ligation and in their controls and at comparable levels (Figure7).
Fas-costimulated and control-activated T cells express equal levels of c-FLIP, Bcl-XL, and Bcl-2 after initial activation.
To evaluate the effect of Fas costimulation on expression of antiapoptotic molecules, RT-PCR was run with specific primers for c-FLIP, Bcl-XL, and Bcl-2 for RNA from freshly isolated (unstimulated) T cells and after 6 days of stimulation. Similar results were obtained in 2 separate PCR reactions with 2 different sets of RNA samples.
Fas-costimulated and control-activated T cells express equal levels of c-FLIP, Bcl-XL, and Bcl-2 after initial activation.
To evaluate the effect of Fas costimulation on expression of antiapoptotic molecules, RT-PCR was run with specific primers for c-FLIP, Bcl-XL, and Bcl-2 for RNA from freshly isolated (unstimulated) T cells and after 6 days of stimulation. Similar results were obtained in 2 separate PCR reactions with 2 different sets of RNA samples.
Discussion
In this work, we have found that Fas ligation has a dualistic effect on naive T cells during their activation by antigen-bearing dendritic cells. Although it is known that, in addition to being a death receptor,30-37 Fas can stimulate T cells,23,24,38 this has been studied only during T-cell activation with TCR cross-linking, and under those conditions it is selective for memory T cells.24 Inasmuch as confirming that T cells can be stimulated by Fas also when activated by DCs, our findings add an important new level to the dualistic effects of Fas ligation on T-cell response. Although a part of the responding naive T cells die due to simultaneous Fas ligation, the surviving T cells are costimulated for proliferation and cytokine production, respond vigorously to antigen rechallenge, and become partially insensitive to Fas-induced cell death (AICD). Importantly, addition of the function-blocking anti-FasL mAb Kay10 to control cells did not increase the number of viable cells or their responsiveness. This indicates that spontaneous AICD did not affect the response of the control cells and, thus, that the effects of Fas ligation were not a consequence of blocking AICD. Therefore, our results are in favor of the notion that depending on the timing, Fas ligation can have various effects.23,24 38
Although early Fas ligation clearly increased the T-cell response to antigen rechallenge 6 days from initial activation, after passage for 3 weeks in immunoincompetent mice these T cells responded similarly to control cells. They had also survived equally well to their controls. These facts bring up 2 aspects that have not been addressed in earlier studies—namely, that Fas-costimulated cells do not appear to remain hyperactive indefinitely and that early Fas ligation does not affect long-term survival of the cells. In agreement with the results of Alderson et al22 and Chun et al,38 Fas ligation increased proliferation of our T cells. However, this was not evident when measuring thymidine incorporation, a difference that could be due to differences in the cell populations used (naive CD4 T cells versus total T cells) or the experimental system (dendritic cells presenting antigen versus immobilized antibodies without accessory cells). Thus, in the above-mentioned studies, Fas ligation apparently stimulated the cells more, resulting in even greater proliferative effect.
Consistent with the findings of Desbarats et al,24 a proportion of naive T cells activated under Fas ligation underwent cell death. Unlike in AICD induced, for example, by superantigens,39 the death-inducing effect of early Fas ligation was independent of cell cycling analogously to the rapid, division-independent apoptosis that murine CD4 T cells undergo when they receive a strong signal via TCR without costimulation.40 In our experiments, however, T cells always received appropriate costimulation (from activated DCs) at different levels of TCR engagement. Thus, experiments by Kishimoto et al40 and those of ours represent 2 different situations in which apoptosis is not under control of the cell cycle.41In fact, dead cells were most frequent among undivided cells and cells that had not divided more than 1 to 3 times, whereas cells that had divided several times contained fewer nonviable cells. This suggests that rapidly cycling T cells had converted Fas signaling away from apoptotic pathways. Previously, Suda et al42 described an analogous dichotomy in that naive T cells that were not induced to proliferate were effectively killed by recombinant murine FasL, whereas T cells that were proliferating in response to TCR/CD3 ligation became resistant to its apoptotic effect. Our results addressed the differential effects of Fas ligation on naive T cells that were all synchronously activated and revealed that such a dichotomy exists also among naive T cells that are all being activated.
One factor determining the outcome of Fas ligation during activation of an individual T cell could be the sum of other stimuli that the cell receives from the antigen-presenting cell (APC). In a cell culture system, a limited number of DCs could restrict the interaction of some T cells with an APC in terms of available time or cell surface area so that these T cells would receive suboptimal signals for survival and proliferation. However, we used a ratio of DC–T cells (1:10) that was higher than what supported maximal proliferation of our T cells (1:20) in preliminary experiments and, thus, it should have saturated the requirements for contacts between T cells and APCs. Therefore, we believe that the outcome of early Fas ligation is determined by T cell–derived factors rather than by factors associated with availability of APC contact.
To better characterize what distinguishes cells costimulated via Fas from the dying cells and from conventionally activated cells, we determined the phenotype of these cells in terms of cell surface receptor expression. Expression of CD4 and TCR and of CD25 (IL-2Rα) and CD44 was found to be higher, and expression of Fas, CD45RB, and CD62L lower in the surviving than in apoptotic cells. Differences in levels of TCR and CD4 could reflect differences in the general competence of individual T cells, higher levels being characteristic for strong responders that would survive, and lower levels for poorer responders that would have a lower threshold for apoptotic behavior. Differences in the levels of CD25, CD44, and CD45RB probably reflect activation-associated changes in the surviving cells. The difference in surface expression of Fas is potentially important, because it can affect the sensitivity to AICD. The more vigorous response of Fas-costimulated cells to antigen rechallenge and their partial insensitivity to Fas ligation at that stage are consistent with this notion. It is likely that early Fas ligation induces a negative feedback loop that inhibits the normal activation-associated up-regulation of Fas expression on cell surface, because on cells surviving early Fas ligation the level of Fas expression remained on the same level as in naive T cells. In contrast, Fas expression increased on cells activated under control conditions as well as on cells that became apoptotic in response to early Fas ligation. The small but consistent differences in expression of CD44 and CD45RB between Fas-costimulated and control-activated cells, and the more pronounced difference in CD62L expression, may reflect a difference in the strength of activation between Fas-ligated and control cells.
Expression of FasL on DCs has been proposed to distinguish tolerance-inducing from immunity-inducing DCs.27 Although this notion is an oversimplification, FasL expression on genetically engineered DCs has been reported to induce tolerance to alloantigens43 and against delayed-type hypersensitivity response.44 Our results and those of Desbarats et al24 would predict simultaneous activation of some responding T cells, a fact that could complicate tolerance induction. However, in the studies of Min et al43 and Matsue et al44 DCs were not deliberately activated and therefore presumably not terminally differentiated to provide high (co)stimulatory activity that could explain their capacity to induce “pure” tolerance. In any case, our results warrant caution in attempts to achieve immunosuppression via engineered FasL expression on dendritic cells.
In the resistance of freshly activated T cells to Fas-mediated death signaling, c-FLIP plays a central role.45 c-FLIP binds to the death-inducing signaling complex with its 2 death effector domains but either lacks the procaspase-8 domain (c-FLIPS) or its catalytic activity (cFLIPL). c-FLIPL can also recruit receptor-interacting protein (RIP) and TNF receptor-associated factor-1 (TRAF-1)/TRAF-2 46 to the signaling complex and possibly thereby regulate the activity of the transcription factor nuclear factor–κB (NF-κB) and kinases extracellular signal-regulated kinase (ERK) and Jun N-terminal kinase (JNK).47,48 Therefore, we determined whether expression of c-FLIP was associated with resistance to apoptosis and increased responsiveness in cells that had survived 6-day activation in the presence of Fas ligation. Not surprisingly, c-FLIP was expressed in day-6 cells that survived and proliferated in response to Fas ligation, supporting the role of c-FLIP as an important determinant of the downstream effects of Fas ligation. Also Bcl-XL and Bcl-2 expression was detectable in Fas-costimulated cells, suggesting in the case of Bcl-XL that Fas can also synergize with C2849to increase resistance to apoptosis.
In recent years, the number of cell surface molecules known to possess costimulatory activity or to function as their receptors has grown rapidly. This applies also to ligands and receptors that mediate T-cell apoptosis. Receptors of the TNFR family possess a dualistic role as inducers of both costimulation and cell death. In this work we elucidated the dichotomy of the effects of Fas ligation and found that the balance between the opposing outcomes of Fas ligation can be very subtle even in a highly homogenous T-cell population. Although Fas is dispensable for T-cell proliferation induced, for example, by CD3 cross-linking,25 our results suggest that Fas can nevertheless act as a costimulatory molecule that has a hitherto unrecognized function in activation of naive CD4 T cells.
We thank Mrs Anne Sovikoski-Georgieva for excellent secretarial assistance and Mrs Anitta Niittymäki for taking care of the animals.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-06-1904.
Supported by the Finnish Academy, Sigrid Juselius Foundation (Finland), and Juvenile Diabetes Foundation International.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arno Hänninen, MediCity Research Laboratory, University of Turku, Tykistökatu 6A, 20520 Turku, Finland; e-mail: arno.hanninen@utu.fi.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal