Abstract
Angiogenesis plays an important role in a variety of pathophysiologic processes, including tumor growth and rheumatoid arthritis. We have previously shown that soluble E-selectin (sE-selectin) is an important angiogenic mediator. However, the mechanism by which sE-selectin mediates angiogenesis is still unknown. In this study, we show that sE-selectin is a potent mediator of human dermal microvascular endothelial cell (HMVEC) chemotaxis, which is predominantly mediated through the Src and the phosphatidylinositiol 3–kinase (PI3K) pathways. Further, sE-selectin induced a 2.2-fold increase in HMVEC tube formation in the Matrigel in vitro assay. HMVECs pretreated with the Src inhibitor (PP2) and the PI3K inhibitor (LY294002) or transfected with Src antisense oligonucleotides or Akt dominant-negative mutants significantly inhibited sE-selectin–mediated HMVEC tube formation. In contrast, HMVECs transfected with an extracellular signal-related kinase 1/2 (ERK1/2) mutant or pretreated with the mitogen-activated protein kinase (MAPK) inhibitor PD98059 failed to show sE-selectin–mediated HMVEC tube formation. Similarly, in the Matrigel-plug in vivo assay, sE-selectin induced a 2.2-fold increase in blood vessel formation, which was significantly inhibited by PP2 and LY294002 but not by PD98059. sE-selectin induced a marked increase in Src, ERK1/2, and PI3K phosphorylation. PI3K and ERK1/2 phosphorylation was significantly inhibited by PP2, thereby suggesting that both of these pathways may be activated via Src kinase. Even though the ERK1/2 pathway was activated by sE-selectin in HMVECs, it seems not to be essential for sE-selectin–mediated angiogenesis. Taken together, our data clearly show that sE-selectin–induced angiogenesis is predominantly mediated through the Src-PI3K pathway.
Introduction
Angiogenesis, or new blood vessel formation, is a complex process that involves endothelial cell proliferation and migration, followed by capillary tube formation. Each stage in this process can be modulated by a number of factors present in normal or pathologic conditions. Vascular endothelial growth factor (VEGF) is one of the most studied factors that mediates angiogenesis through Src family kinases and phosphatidylinositiol 3–kinase (PI3K) pathways.1,2 Src kinases are activated by a variety of growth factors and function downstream of receptor tyrosine kinases.3,4 Eliceiri et al, showed that both VEGF and fibroblast growth factor (FGF) stimulate Src activation in avian endothelial cells.1 However, only VEGF-induced angiogenesis was inhibited by treatment with a retrovirus that encodes Src-251, a dominant-interfering mutant of Src. Moreover, overexpression of Src-251 in avian blood vessels induces apoptotic death, indicating that VEGF-induced activation of Src is essential for endothelial cell survival and angiogenesis. Src kinases, once activated, may in turn activate downstream PI3K. PI3K has been implicated in a number of cellular functions including cell adhesion, cell survival, and angiogenesis.2,5 The serine-threonine kinase, Akt, is a downstream target of PI3K. Cardone et al6 have shown that Akt phosphorylation promotes cell survival by phosphorylating Bcl-2/Bcl-XL agonist causing cell death (BAD), forkhead-related protein 1 (FKHR1), and caspase-9. Akt has also been shown to play an important role in angiogenesis.2
E-selectin is a calcium-dependent lectin that mediates adhesive interactions of circulating leukocytes with vascular endothelium during normal and abnormal inflammatory conditions such as rheumatoid arthritis (RA) and atherosclerosis.7,8 E-selectin is a single-chain 115-kDa glycoprotein with a lectinlike N terminal domain, an epidermal growth factor (EGF)–like motif, and a variable number of repeat units homologous to the consensus repeats of complement binding proteins.9 The lectin domain of E-selectin plays a major role in ligand recognition and binds to sialyl Lex on leukocytes as well as on endothelial cells.10-12E-selectin is rapidly synthesized and expressed on activated endothelial cells and is rapidly shed from the cellular surfaces on cellular activation.7 Elevated levels of soluble E-selectin (sE-selectin) have been found in patients with vasculoproliferative disorders such as RA13 and tumor growth.14,15 We have previously shown that sE-selectin is a potent angiogenic mediator.16 Recently, we have shown that sE-selectin mediates monocyte chemotaxis through the Src- mitogen-activated protein kinase (MAPK) pathway.17However, the signaling pathways by which sE-selectin mediates angiogenesis are still unknown.
In this study, we examined the signaling pathways by which sE-selectin mediates angiogenesis. Our results suggest that sE-selectin mediates its signal in HMVECs through the Src-PI3K pathway. Inhibition of Src, MAPK, and PI3K pathways with PP2, PD98059, and LY294002, respectively, significantly reduced sE-selectin–mediated HMVEC chemotaxis. Similarly, PP2 and LY294002 significantly inhibited Matrigel in vitro endothelial cell tube formation and Matrigel in vivo blood vessel formation. However, PD98059 failed to significantly inhibit in vitro endothelial cell tube formation as well as in vivo blood vessel formation. Taken together, these results suggest that Src- and PI3K-dependent signaling pathways are major mediators of sE-selectin–induced angiogenesis.
Materials and methods
Reagents
Recombinant human sE-selectin was purchased from R&D Systems (Minneapolis, MN). sE-selectin contained less than 0.1 ng endotoxin per 1 μg protein content (per the manufacturer). Basic fibroblast growth factor (bFGF) was purchased from Intergen (Purchase, NY). Hanks balanced salt solution (HBSS) was obtained from Life Technologies (Bethesda, MD). Orthovanadate, paranitrophenylphosphate, leupeptin, aprotinin, phenylmethylsulfonyl fluoride (PMSF), dimethyl sulfoxide (DMSO), bovine serum albumin (BSA), IGEPAL CA-630, pertusis toxin, and protein A agarose conjugate were obtained from Sigma Chemical (St Louis, MO). Protease inhibitor cocktail tablets were obtained from Boehringer Mannheim (Mannheim, Germany). PP2, LY294002, and PD98059 were purchased from Calbiochem (San Diego, CA). Rabbit polyclonal antihuman total Src antibody, phosphospecific Src antibody, rabbit polyclonal antihuman PI3K antibody, polyclonal rabbit antihuman total Akt antibody, and phosphospecific Akt antibody were purchased from Upstate Biotechnology (Lake Placid, NY). Antibodies against total extracellular signal-related kinase 1/2 (ERK1/2) and phosphospecific ERK1/2 were purchased from Biosource International (Camarillo, CA). Antitubulin antibody was purchased from Oncogene Research Products (Boston, MA). Endothelial cell basal medium (EBM) and the bullet kit containing media supplements were purchased from BioWhittaker (Walkersville, MD). Growth factor–reduced Matrigel, derived from Engelbreth-Holm-Swarm (EHS) mouse tumor, was purchased from Becton Dickinson (Bedford, MA). Fetal bovine serum (FBS) and media 199 were obtained from Gibco BRL (Grand Island, NY). Polycarbonate filters with 8-μM pores were obtained from Poretics (Livemore, CA). pcDNA3 plasmid, HB101 competentEscherichia coli cells, and Lipofectin kits were obtained from Invitrogen (Carlsbad, CA). Protein estimation reagents (bicinchoninic acid [BCA] kit) were purchased from Pierce Biotechnology (Rockford, IL). [γ-32P] adenosine triphosphate (ATP), enhanced chemiluminescence (ECL) Western blotting detection reagents, and mouse horseradish peroxidase–conjugated antibody were obtained from Amersham Life Sciences (Arlington Heights, IL).
Cell culture, cell lysis, and immunoblotting
HMVECs were purchased from BioWhittaker. HMVECs were cultured in endothelial cell growth medium (BioWhittaker) containing the bullet kit and 10% FBS. HMVECs were used between passages 3 and 12. HMVECs (2 × 105/well) were plated in 6-well plates and incubated overnight at 37°C in EBM containing the bullet kit and 10% FBS. HMVECs were further incubated for 2 hours in EBM containing no bullet and reduced serum (2% FBS) prior to stimulating with sE-selectin for various time points. At the end of each time period, supernatants were aspirated and cells lysed in extraction buffer containing 100 mM tris(hydroxymethyl)aminomethane (Tris, pH 7.4), 100 mM NaCl, 1 mM ethylenediamine-tetra acetic acid (EDTA), 1 mM ethyleneglycol-tetra acetic acid (EGTA), 1 mM NaF, 20 mM NaP2O4, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1 mM PMSF, and protease inhibitors (protease inhibitor cocktail tablets, Boehringer Mannheim, 1 tablet/10 mL). For experiments with signaling inhibitors, HMVECs were preincubated with the respective inhibitor before activation with sE-selectin. The protein content of different samples was quantitated using the bicinchoninic acid (BCA) protein assay kit. Cell lysates were mixed 1:1 with Laemmli sample buffer and boiled for 5 minutes. Of each sample, 20 μg was subjected to 10% SDS–polyacrylamide gel electrophoresis (PAGE). Separated proteins were electrophoretically transferred from the gel onto nitrocellulose membranes using a Tris-glycine buffer. To block nonspecific binding, membranes were incubated with 5% nonfat milk in Tris-buffered saline containing 0.01% Tween-20 (TBST) for 1 hour at room temperature. The blots were incubated in respective primary antibody in TBST plus 5% nonfat milk at 4°C overnight. After washing with TBST, the blots were incubated with horseradish peroxidase–conjugated sheep anti–mouse immunoglobulin G (IgG; 1:10 000) or with goat anti–rabbit IgG (1:10 000) for 45 minutes at room temperature. An ECL detection system was used to detect specific protein bands. Blots were scanned and analyzed for the measurement of the band intensities with UN-SCAN-IT version 5.1 software (Silk Scientific, Orem, UT). The immunoblots were then stripped for 30 minutes at 55°C in 67 mM Tris (pH 6.7), 2% SDS, 100 mM 2-mercaptoethanol, and reprobed with the respective pan antibody.
Plasmid constructions and transient transfections
HMVECs were transiently transfected with 4 μg pcDNA3 plasmid containing the dominant-negative mutants of ERK1/2, Akt, p38 MAPK, or c-Jun (a gift from Dr Cun-Yu Wang, University of Michigan, Ann Arbor) using Lipofectin kits (Invitrogen) per the manufacturer's instructions. An empty pcDNA3 plasmid was used as a negative control and a pcDNA3 plasmid containing CAT was used to optimize transfection efficiency. At 72 hours after transfections, HMVECs were used in chemotaxis assays (or Matrigel in vitro HMVEC tube formation assays) or harvested for Western blotting.
Preparation of oligonucleotides (ODNs) and lipofection of HMVECs
The ODNs were synthesized and purified by the Northwestern University Biotechnology Laboratory and modified with phosphorothioate. Sequences of the c-Src ODNs used in the study are as follows: antisense, GGG CTT GCT CTT GCT GCT CCC CAT; and sense, ATG GGG AGC AGC AAG AGC AAG CCC.18 HMVECs were transiently transfected with sense and antisense ODNs as described earlier.18 At 16 hours after transfection, HMVECs were used for chemotaxis assays (or Matrigel in vitro HMVEC tube formation assays) or harvested for Western blotting.
PI3K assay
HMVECs (2 × 105 cells/well) were plated in 6-well plates in EBM containing bullet and 10% FBS. Once the cells were 80% to 90% confluent, they were further incubated in EBM containing reduced serum (2% FBS) for 2 hours. HMVECs were then stimulated with sE-selectin (50 nM) for 15 minutes at 37°C. At the end of the incubation, cell lysate was prepared. For inhibitor studies, HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulation with sE-selectin. PP2 was also present during HMVEC stimulation with sE-selectin. The protein content of each sample was quantitated using a bicinchoninic acid (BCA) protein assay kit and normalized according to the protein concentration. Of each sample, 500 μg in 500 μL lysis buffer was incubated with 5 μL rabbit anti-PI3K antibody overnight at 4°C with continuous shaking. Protein A agarose conjugate (60 μL; 50% slurry in phosphate-buffered saline [PBS]) was added to each sample and further incubated for one hour at 4°C. The immunoprecipitates were collected by centrifuging at 14 000g for 10 seconds. The immunoprecipitates were then washed 3 times with buffer A (137 mM NaCl; 20 mM Tris-HCl, pH 7.4; 1 mM CaCl2; 1 mM MgCl2; and 0.1 mM sodium orthovanadate) containing 1% nonionic detergent IGEPAL CA-630, followed by 3 washes with buffer B (0.1 M Tris-HCl, pH 7.4; 5 mM LiCl; and 0.1 mM sodium orthovanadate) and 3 washes with TNE (10 mM Tris-HCl, pH 7.4; 150 mM NaCl; and 5 mM EDTA) containing 0.1 mM sodium orthovanadate. The last wash was removed as completely as possible. To each sample, the following reagents were added sequentially: 50 μL TNE, 10 μL (20 μg) phosphatidylinositol (PI, in 10 mM Tris-HCl, pH 7.4, containing 1 mM EGTA), and 10 μL 100 mM MgCl2. The PI reaction was initiated with the addition of 5 μL [γ-32P] ATP. The reaction mixture was incubated at 37°C for 15 minutes with continuous agitation. The reaction was stopped by the addition of 20 μL 6 N HCl. Radiolabeled lipid was extracted from the reaction sample by adding 160 μL CH3Cl/MeOH (1:1), vortexing, and then separating the organic and aqueous phases by centrifugation for 10 minutes at 14 000g. Radiolabeled lipid (50 μL) containing the lower organic phase was spotted onto oxalate-treated thin layer chromatography (TLC) plates (Fisher Scientific, Pittsburgh, PA) and developed in CHCl3/MeOH/H2O/NH4OH (60:47:11.3:2). The TLC plates were dried and autoradiographed.
HMVEC chemotaxis
HMVECs (3.75 × 104 cells/25 mL EBM and 0.1% FBS) were placed in the bottom wells of a 48-well Boyden chemotaxis chamber (NeuroProbe, Cabin John, MD) with gelatin-coated polycarbonate membranes (8-μM pore size; Nucleopore, Pleasanton, CA).16 The chambers were inverted and incubated in a humidified incubator with 5% CO2/95% air at 37°C for 2 hours, allowing endothelial cell attachment to the membrane. The chambers were inverted, and the test substances, PBS or positive control bFGF (60 nM) in PBS, were added, and the chamber was further incubated for 2 hours at 37°C. In the inhibitor studies, HMVECs were pretreated with the respective inhibitor (10 μM PP2, LY294002, or PD98059; or 50 ng/mL PT) for 2 hours, and inhibitors were present in the lower chamber along with HMVECs during the chemotaxis assay. The membranes were then removed, fixed in methanol for 1 minute, and stained with Diff-Quik (Dade Behring, Newark, DE). The data are expressed as the number of cells migrating through the membrane per well (the sum of 3 high-power × 40 fields per well, averaged for each quadruplicate well).
Matrigel in vitro HMVEC tube formation assay
The Matrigel in vitro assay was used to examine HMVEC tube formation in response to sE-selectin. Matrigel was plated in 8-well chamber slides after thawing on ice and allowed to polymerize at 37°C for 30 to 60 minutes. HMVECs (400 μL; 4 × 104cells/mL in media 199 containing 2% FBS and 200 mg/mL endothelial cell growth supplement [Becton Dickinson]) was added to each chamber and treated with bFGF (vehicle control) or sE-selectin with or without the respective inhibitor. The chamber slides were then incubated for 16 to 18 hours at 37°C in 5% CO2 humidified atmosphere. Culture media were aspirated off the Matrigel surface, and the cells were fixed with methanol and stained with Diff-Quick Solution II. Each chamber was photographed using a Polaroid Microcam camera (Carl Zeiss, Jena, Germany) at × 22 magnification. The number of tubes formed was quantitated as previously described.19 Briefly, a connecting branch between 2 discreet endothelial cells was counted as one “tube.”
Matrigel-plug angiogenesis in vivo assay
To examine the effect of sE-selectin on angiogenesis in vivo a Matrigel-plug assay was used.20,21 C57/BL6 mice were anesthetized by Metofane inhalation. Each mouse was shaved on its ventral aspect and given a subcutaneous injection of sterile Matrigel (500 mL/injection) with a 27-gauge needle. Matrigel containing PBS served as the negative control; Matrigel containing bFGF (1 ng/mL) served as the positive control; and Matrigel with sE-selectin (100 nM) was the test substance. In the inhibitor study, 20 μM respective inhibitor (PP2, LY294002, or PD98059) was added along with sE-selectin in the Matrigel before it was injected. After 7 to 10 days, the mice were killed by Metofane inhalation and cervical dislocation. The Matrigel plugs were then carefully dissected out, with removal of any surrounding connective tissue, and then analyzed by hemoglobin measurement or by histology.22 23 For histologic analysis, 3 plugs from each group were embedded in paraffin for tissue sectioning and staining.
Hemoglobin determination in Matrigel plugs.
Hemoglobin levels in the Matrigel plug correlate with blood vessel growth.19 The Matrigel plugs dissected from the mice were carefully stripped of any remaining peritoneum. The plugs were weighed and homogenized for 5 to 10 minutes on ice. Supernatant or standard (50 μL) was added to a 96-well plate in duplicate, followed by 50 μL tetramethylbenzidine. The plate was allowed to develop at room temperature for 15 to 20 minutes with gentle shaking, and the reaction was stopped by adding 150 μL 2N H2SO4. Absorbance was read at 450 nm, and hemoglobin levels were normalized by the weight of the plugs.
Masson trichrome staining of Matrigel plugs.
Using Masson trichrome straining, 5-mm sections were deparaffinized and stained.19 In brief, sections were hydrated in distilled water followed by incubation with Bouin solution for one hour at 56°C, washing in water, incubation with Weigert iron solution for 7 minutes, washing with water, and incubation with Biebrich scarlet-acid fuchsin solution for 2 minutes. Sections were then rinsed, incubated in phosphomolybdic-phosphotungstic acid solution for 10 minutes, dipped in aniline blue solution for 5 minutes, rinsed, dipped in glacial acetic acid solution for 3 to 5 minutes, and dehydrated in 2 changes of 95% alcohol, 100% alcohol, and 100% xylene. The slides were mounted with Cytoseal 60 (Stephens Scientific, Kalamazoo, MI).
Statistical analysis
Data were analyzed using Student t test, andP values less than .05 were considered significant.
Results
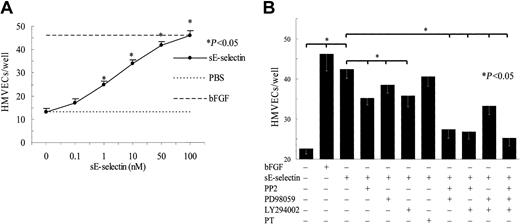
sE-selectin mediates HMVEC chemotaxis through the Src and PI3K pathways
We have previously shown that sE-selectin is a potent angiogenic mediator.16 To examine the signaling cascade by which sE-selectin mediates angiogenesis, we performed HMVEC chemotaxis assays, a facet of the angiogenic response, in 48-well modified Boyden chambers. sE-selectin induced a dose-dependent increase in HMVEC chemotaxis, reaching significance at 1 nM to 100 nM (P < .05, Figure 1A), compared with negative control PBS. bFGF was used as positive control in all the assays. To further study the role of different signaling pathways that may be involved in sE-selectin–mediated HMVEC chemotaxis, HMVECs were pretreated with the respective signaling inhibitor or combination of Src kinase inhibitor (PP2, 10 μM), PI3K inhibitor (LY294002, 10 μM), MAPK inhibitor (PD98059, 10 μM), or G-protein inhibitor (PT, 50 ng/mL) for 2 hours prior to performing the chemotaxis assays. The Src inhibitor (PP2) was most effective in inhibiting sE-selectin–mediated HMVEC chemotaxis (37%), whereas the MAPK inhibitor (PD98059), PI3K inhibitor (LY294002), and G-protein inhibitor (PT) showed 20%, 34%, and 9% inhibition, respectively (Figure 1B). HMVECs pretreated with the combination of PP2 and PD98059 showed 76% inhibition of chemotaxis, which was further enhanced by the combination of PP2 and LY294002 (79%) or the combination of PP2, PD98059, and LY294002 (87%). However, the combination of PD98059 and LY294002 showed 47% sE-selectin–mediated HMVEC chemotaxis inhibition.
sE-selectin induces HMVEC chemotaxis through Src and PI3K pathways.
(A) HMVECs (1 × 106 cells/mL) in the lower chamber were incubated with various concentrations of sE-selectin in the upper chamber for 2 hours at 37°C. PBS was used as a negative control and bFGF (60 nM) as a positive control. Results are expressed as the number of cells migrating through the membrane per well ± SEM from 3 independent experiments. sE-selectin showed a dose-dependent increase in HMVEC chemotaxis compared with negative control PBS (P < .05). *Represents a significant difference (P < .05) between the sE-selectin and negative control PBS. (B) For inhibition studies, HMVECs were pretreated with the respective inhibitor or inhibitor combination (10 μM PP2, LY294002, or PD98059; or 50 ng/mL PT) for 2 hours at 37°C and then assayed in 48-well chemotaxis chambers in response to 50 nM of sE-selectin. Results are expressed as the number of cells migrating through the membrane per well ± SEM from 6 independent experiments. PT in the figure represent pertusis toxin. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced HMVEC chemotaxis predominantly through the Src and PI3K pathways.
sE-selectin induces HMVEC chemotaxis through Src and PI3K pathways.
(A) HMVECs (1 × 106 cells/mL) in the lower chamber were incubated with various concentrations of sE-selectin in the upper chamber for 2 hours at 37°C. PBS was used as a negative control and bFGF (60 nM) as a positive control. Results are expressed as the number of cells migrating through the membrane per well ± SEM from 3 independent experiments. sE-selectin showed a dose-dependent increase in HMVEC chemotaxis compared with negative control PBS (P < .05). *Represents a significant difference (P < .05) between the sE-selectin and negative control PBS. (B) For inhibition studies, HMVECs were pretreated with the respective inhibitor or inhibitor combination (10 μM PP2, LY294002, or PD98059; or 50 ng/mL PT) for 2 hours at 37°C and then assayed in 48-well chemotaxis chambers in response to 50 nM of sE-selectin. Results are expressed as the number of cells migrating through the membrane per well ± SEM from 6 independent experiments. PT in the figure represent pertusis toxin. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced HMVEC chemotaxis predominantly through the Src and PI3K pathways.
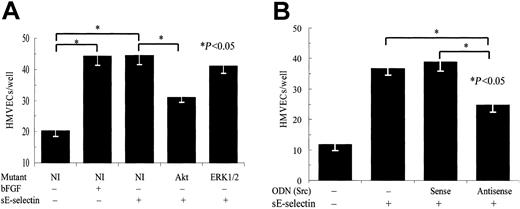
To further corroborate the results obtained in the HMVEC chemotaxis studies using signaling inhibitors, we performed a similar assay using HMVECs transiently transfected with dominant-negative mutants of ERK1/2 and Akt or HMVECs transiently transfected with c-Src antisense and sense ODNs. A pcDNA3 plasmid with no insert was used as control for the mutant studies. HMVECs transiently transfected with plasmids with no insert showed significantly more chemotaxis in response to both sE-selectin and positive control bFGF (Figure2A). HMVECs transfected with the Akt mutants showed significantly less (55%) sE-selectin–mediated HMVEC chemotaxis, whereas HMVECs transfected with the ERK1/2 mutants did not show a significant inhibition (14%). HMVECs transfected with Src antisense ODNs showed significant inhibition (53%) of sE-selectin–mediated chemotaxis compared with HMVECs transfected with sense ODNs (Figure 2B). These results from HMVEC chemotaxis assay using signaling inhibitors, dominant-negative mutants, and sense and antisense ODNs suggest that sE-selectin may be mediating HMVEC chemotaxis predominantly through the Src and PI3K pathways and partially through the MAPK pathway.
HMVECs transfected with the Akt dominant-negative mutant or Src antisense oligonucleotides showed significantly less sE-selectin–mediated chemotaxis.
(A) HMVECs were transiently transfected with pcDNA3 plasmids containing ERK1/2 or Akt dominant-negative mutants. Plasmids with no insert were used as a negative control. At 72 hours after transfections, HMVEC chemotaxis was performed. (B) HMVECs were transiently transfected with c-Src antisense ODN or control sense ODN. At 16 hours after transfections, HMVEC chemotaxis was performed. Results are expressed as the mean number of cells migrating through the membrane per well ± SEM from 4 independent experiments. *Represents a significant difference (P < .05) between the respective groups. HMVECs transfected with an Akt mutant or Src antisense ODN showed significant inhibition of sE-selectin–mediated chemotaxis, whereas HMVECs transfected with an ERK1/2 mutant failed to show significant inhibition.
HMVECs transfected with the Akt dominant-negative mutant or Src antisense oligonucleotides showed significantly less sE-selectin–mediated chemotaxis.
(A) HMVECs were transiently transfected with pcDNA3 plasmids containing ERK1/2 or Akt dominant-negative mutants. Plasmids with no insert were used as a negative control. At 72 hours after transfections, HMVEC chemotaxis was performed. (B) HMVECs were transiently transfected with c-Src antisense ODN or control sense ODN. At 16 hours after transfections, HMVEC chemotaxis was performed. Results are expressed as the mean number of cells migrating through the membrane per well ± SEM from 4 independent experiments. *Represents a significant difference (P < .05) between the respective groups. HMVECs transfected with an Akt mutant or Src antisense ODN showed significant inhibition of sE-selectin–mediated chemotaxis, whereas HMVECs transfected with an ERK1/2 mutant failed to show significant inhibition.
Signaling cascade mediated by sE-selectin in HMVECs
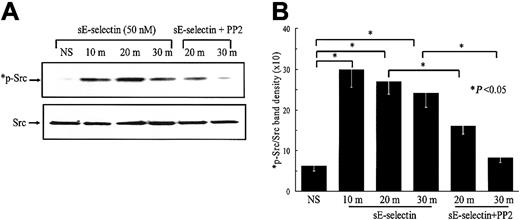
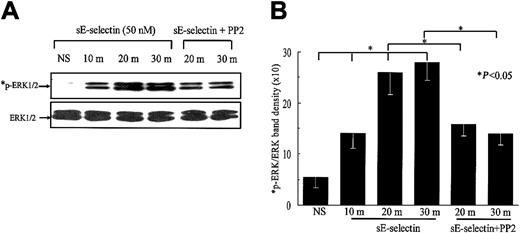
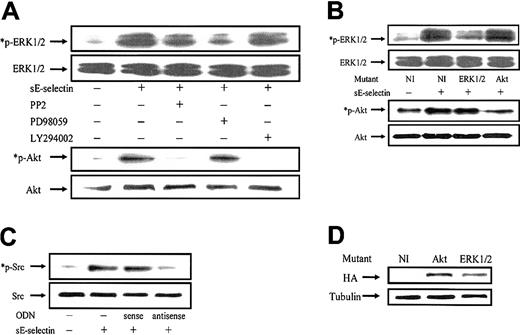
We next examined the signaling pathways involved in sE-selectin–stimulated HMVECs. sE-selectin induced protein tyrosine phosphorylation of several proteins in the molecular-weight range of 140 to 150, 130 to 140, 50 to 65, and 30 to 40 kDa (data not shown). As the predominant band was observed in the molecular-weight range of Src kinase (≈ 60 kDa), we were interested to determine whether Src family kinases were activated in HMVECs upon stimulation with sE-selectin. We performed a time-course study of Src kinase activation followed by Src inhibition using the Src inhibitor PP2. sE-selectin induced a marked increase in Src phosphorylation at 10, 20, and 30 minutes (Figure 3). Pretreatment of HMVECs with PP2 showed substantial inhibition of Src phosphorylation at 20 and 30 minutes. Equal amounts of protein samples were loaded in each lane, as confirmed by stripping the blots and reprobing with rabbit antihuman total Src antibody. We next examined whether sE-selectin could also activate the ERK1/2 and the p38 MAPK pathways in HMVECs. sE-selectin induced a time-dependent increase in phosphorylation of ERK1/2, which peaked at 30 minutes (Figure4). However, sE-selectin failed to activate p38 MAPK in HMVECs (data not shown). sE-selectin–induced ERK1/2 phosphorylation was significantly inhibited by the pretreatment of HMVECs with PP2, suggesting that Src lies upstream of ERK1/2 in sE-selectin–mediated endothelial cell signaling (Figure 4).
sE-selectin–induced Src phosphorylation is inhibited by the Src inhibitor PP2.
HMVECs were stimulated with 50 nM sE-selectin for various time points as indicated (m indicates minutes; NS, nonstimulated). In the inhibition study, HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulating with sE-selectin, and PP2 was also present during the stimulation with sE-selectin. Cell extracts were prepared, and the protein content in each sample was quantitated. Samples (20 μg each) were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-Src (*p-Src) antibody. To verify equal loading, the blots were stripped and reprobed with rabbit antihuman total Src antibody. (A) Representative blot from 3 independent experiments. (B) Ratio of p-Src to total Src band density ± SEM from 3 independent experiments. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced a marked increase in Src phosphorylation at 10, 20, and 30 minutes after stimulation. Pretreatment of HMVECs with PP2 significantly inhibited Src phosphorylation.
sE-selectin–induced Src phosphorylation is inhibited by the Src inhibitor PP2.
HMVECs were stimulated with 50 nM sE-selectin for various time points as indicated (m indicates minutes; NS, nonstimulated). In the inhibition study, HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulating with sE-selectin, and PP2 was also present during the stimulation with sE-selectin. Cell extracts were prepared, and the protein content in each sample was quantitated. Samples (20 μg each) were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-Src (*p-Src) antibody. To verify equal loading, the blots were stripped and reprobed with rabbit antihuman total Src antibody. (A) Representative blot from 3 independent experiments. (B) Ratio of p-Src to total Src band density ± SEM from 3 independent experiments. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced a marked increase in Src phosphorylation at 10, 20, and 30 minutes after stimulation. Pretreatment of HMVECs with PP2 significantly inhibited Src phosphorylation.
sE-selectin induces a time-dependent increase in ERK1/2 phosphorylation.
HMVECs were stimulated with 50 nM sE-selectin for various time points as indicated (m indicates minutes; NS, nonstimulated). In the inhibition study, HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulating with sE-selectin, and PP2 was also present during the stimulation with sE-selectin. Cell extracts were prepared and the protein content in each sample was quantified. Samples (20 μg each) were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-ERK1/2 (*p-ERK1/2) antibody. To verify equal loading, the blots were stripped and reprobed with rabbit antihuman ERK1/2 antibody. (A) Representative blot from 3 independent experiments. (B) The ratio of p-ERK1/2 to ERK1/2 band density ± SEM from 3 independent experiments. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced a marked increase in ERK1/2 phosphorylation at 10, 20, and 30 minutes after stimulation. Pretreatment of HMVECs with PP2 significantly inhibited ERK1/2 phosphorylation.
sE-selectin induces a time-dependent increase in ERK1/2 phosphorylation.
HMVECs were stimulated with 50 nM sE-selectin for various time points as indicated (m indicates minutes; NS, nonstimulated). In the inhibition study, HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulating with sE-selectin, and PP2 was also present during the stimulation with sE-selectin. Cell extracts were prepared and the protein content in each sample was quantified. Samples (20 μg each) were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-ERK1/2 (*p-ERK1/2) antibody. To verify equal loading, the blots were stripped and reprobed with rabbit antihuman ERK1/2 antibody. (A) Representative blot from 3 independent experiments. (B) The ratio of p-ERK1/2 to ERK1/2 band density ± SEM from 3 independent experiments. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced a marked increase in ERK1/2 phosphorylation at 10, 20, and 30 minutes after stimulation. Pretreatment of HMVECs with PP2 significantly inhibited ERK1/2 phosphorylation.
Because the PI3K/Akt pathway is known to play an important role in angiogenesis, we also examined whether the PI3K/Akt pathway is activated in sE-selectin–stimulated HMVECs.2 24 HMVECs stimulated with sE-selectin showed a marked increased in PI and Akt phosphorylation (Figure 5), thereby indicating that sE-selectin, in addition to activating Src and ERK1/2, also activates the PI3K/Akt pathway in HMVECs. Next, we examined whether sE-selectin–induced activation of PI3K in HMVECs was mediated via Src kinases. To study this, we first pretreated HMVECs with the Src inhibitor PP2 (10 μM) for 2 hours prior to preparing the cell lysate for both the PI3K assay and the Akt phosphorylation studies. Pretreatment of HMVECs with PP2 substantially inhibited sE-selectin–mediated PI3K activation as observed by PI3K assay and Akt phosphorylation studies (Figure 5). To further examine whether there was any cross talk between the PI3K/Akt and ERK1/2 pathways in sE-selectin–stimulated endothelial cells, HMVECs were pretreated with PP2, PD98059, or LY294002 prior to stimulation with sE-selectin. The Src inhibitor PP2, as observed earlier, inhibited both Akt and ERK1/2 activation (Figure 6A). The PI3K inhibitor (LY294002) and the ERK1/2 inhibitor (PD98059) failed to show ERK1/2 and Akt inhibition, respectively. Similarly, HMVECs transfected with the ERK1/2 mutants showed inhibition of sE-selectin–induced ERK1/2 phosphorylation, but not of Akt phosphorylation. HMVECs transfected with the Akt mutants showed inhibition of Akt phosphorylation but not ERK1/2 phosphorylation (Figure 6B). These results suggest that sE-selectin mediates its signal in HMVECs through PI3K/Akt and ERK1/2 pathways via Src kinases. HMVECs transfected with antisense Src ODNs showed significant inhibition of sE-selectin–meditated Src phosphorylation compared with sense Src ODNs (Figure 6C). HMVECs transfected with dominant-negative mutants of Akt and ERK1/2 showed similar relative transfection efficiency as assessed by Western blotting with the tagged protein hemagglutinin (HA) (Figure 6D).
sE-selectin induces PI3K and Akt activation in HMVECs.
HMVECs were stimulated with sE-selectin (50 nM) for 15 minutes. In the inhibition study HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulation with sE-selectin, and PP2 was also present during the stimulation with sE-selectin. (A) PI3K was immunoprecipitated from the cell lysate and then the kinase assay performed using PI as the substrate and [γ-32P]–labeled ATP as the donor of phosphate ions. The [γ-32P]–labeled lipids were resolved by thin layer chromatography (TLC) and autoradiographed. This is a representative blot from 3 independent experiments. sE-selectin induced a marked increase in PI phosphorylation (*PI3P) compared with nonstimulated cells. PP2-pretreated HMVECs exhibited substantial inhibition of PI3K activation, thus indicating that PI3K lies downstream of Src kinase. (B) Cell extracts were prepared and the protein content in each sample was quantitated. Samples (20 μg) were resolved by 10% SDS-PAGE and probed with mouse monoclonal antihuman phospho-Akt (*p-Akt) antibody. To verify equal loading, the blots were stripped and reprobed with rabbit anti-Akt antibody. This is a representative blot from 3 independent experiments. (C) The ratio of p-Akt to Akt band density ± SEM from 3 independent experiments. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced a marked increase in Akt phosphorylation. Pretreatment of HMVECs with PP2 significantly inhibited Akt phosphorylation.
sE-selectin induces PI3K and Akt activation in HMVECs.
HMVECs were stimulated with sE-selectin (50 nM) for 15 minutes. In the inhibition study HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulation with sE-selectin, and PP2 was also present during the stimulation with sE-selectin. (A) PI3K was immunoprecipitated from the cell lysate and then the kinase assay performed using PI as the substrate and [γ-32P]–labeled ATP as the donor of phosphate ions. The [γ-32P]–labeled lipids were resolved by thin layer chromatography (TLC) and autoradiographed. This is a representative blot from 3 independent experiments. sE-selectin induced a marked increase in PI phosphorylation (*PI3P) compared with nonstimulated cells. PP2-pretreated HMVECs exhibited substantial inhibition of PI3K activation, thus indicating that PI3K lies downstream of Src kinase. (B) Cell extracts were prepared and the protein content in each sample was quantitated. Samples (20 μg) were resolved by 10% SDS-PAGE and probed with mouse monoclonal antihuman phospho-Akt (*p-Akt) antibody. To verify equal loading, the blots were stripped and reprobed with rabbit anti-Akt antibody. This is a representative blot from 3 independent experiments. (C) The ratio of p-Akt to Akt band density ± SEM from 3 independent experiments. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced a marked increase in Akt phosphorylation. Pretreatment of HMVECs with PP2 significantly inhibited Akt phosphorylation.
sE-selectin induces phosphorylation of ERK1/2 and Akt independent of each other.
(A) HMVECs were stimulated with 50 nM sE-selectin for 30 minutes. In the inhibition study, HMVECs were pretreated with either PP2, PD98059, or LY294002 (10 μM) for 2 hours prior to stimulating with sE-selectin. Cell extracts were prepared and the protein content in each sample was quantitated. Of each sample, 20 μg was resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-ERK1/2 (*p-ERK1/2) or rabbit antihuman phospho-Akt (*p-Akt) antibodies. To verify equal loading, the blots were stripped and reprobed with rabbit antihuman ERK1/2 or Akt antibody. (B) HMVECs transfected with pcDNA3 containing no insert or ERK1/2 or Akt mutant were stimulated with sE-selectin for 30 minutes and cell lysates were prepared. Cell extracts were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-ERK1/2 (*p-ERK1/2) or rabbit antihuman phospho-Akt (*p-Akt) antibodies. Equal loading was verified by stripping the blots and reprobing with rabbit anti-ERK1/2 or Akt antibody. (C) HMVECs transfected with Src sense or antisense ODNs were stimulated with sE-selectin for 20 minutes and cell lysates were prepared. Cell extracts were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-Src (*p-Src) antibody. Equal loading was verified by stripping the blots and reprobing with rabbit anti-Src antibody. (D) Cell lysates from HMVECs transfected with pcDNA3 containing no insert, Akt, or ERK mutants were resolved by 10% SDS-PAGE and probed with mouse monoclonal anti-HA antibody. Equal loading was verified by stripping the blots and reprobing with mouse monoclonal antitubulin antibody. sE-selectin induced a time-dependent increase in Akt and ERK1/2 phosphorylation via Src kinase.
sE-selectin induces phosphorylation of ERK1/2 and Akt independent of each other.
(A) HMVECs were stimulated with 50 nM sE-selectin for 30 minutes. In the inhibition study, HMVECs were pretreated with either PP2, PD98059, or LY294002 (10 μM) for 2 hours prior to stimulating with sE-selectin. Cell extracts were prepared and the protein content in each sample was quantitated. Of each sample, 20 μg was resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-ERK1/2 (*p-ERK1/2) or rabbit antihuman phospho-Akt (*p-Akt) antibodies. To verify equal loading, the blots were stripped and reprobed with rabbit antihuman ERK1/2 or Akt antibody. (B) HMVECs transfected with pcDNA3 containing no insert or ERK1/2 or Akt mutant were stimulated with sE-selectin for 30 minutes and cell lysates were prepared. Cell extracts were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-ERK1/2 (*p-ERK1/2) or rabbit antihuman phospho-Akt (*p-Akt) antibodies. Equal loading was verified by stripping the blots and reprobing with rabbit anti-ERK1/2 or Akt antibody. (C) HMVECs transfected with Src sense or antisense ODNs were stimulated with sE-selectin for 20 minutes and cell lysates were prepared. Cell extracts were resolved by 10% SDS-PAGE and probed with rabbit antihuman phospho-Src (*p-Src) antibody. Equal loading was verified by stripping the blots and reprobing with rabbit anti-Src antibody. (D) Cell lysates from HMVECs transfected with pcDNA3 containing no insert, Akt, or ERK mutants were resolved by 10% SDS-PAGE and probed with mouse monoclonal anti-HA antibody. Equal loading was verified by stripping the blots and reprobing with mouse monoclonal antitubulin antibody. sE-selectin induced a time-dependent increase in Akt and ERK1/2 phosphorylation via Src kinase.
The Src inhibitor (PP2) and the PI3K inhibitor (LY294002) inhibit sE-selectin–mediated tube formation in the Matrigel assay in vitro
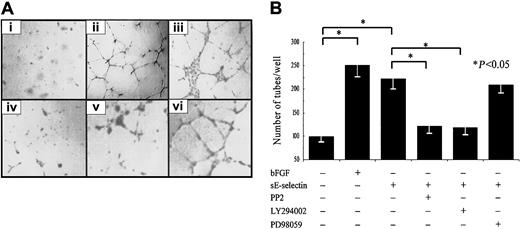
We performed 2 assays to examine the signaling pathways involved in sE-selectin–mediated angiogenesis. In the first assay, the Matrigel in vitro HMVEC tube formation assay, we used PP2, LY294002, and PD98059 inhibitors to study the role of Src kinases, PI3K, and MAPK, respectively. The Src kinase inhibitor (PP2) and the PI3K inhibitor (LY294002) significantly inhibited sE-selectin–mediated HMVEC tube formation (47% and 49%, respectively) in the Matrigel in vitro assay (P < .05; Figure7). However, the MAPK inhibitor (PD98059) failed to show significant inhibition of HMVEC tube formation (5%) compared with sE-selectin–treated cells. The same assay was performed using HMVECs transfected with Akt or ERK1/2 dominant-negative mutants or with HMVECs transfected with Src sense or antisense ODNs. HMVECs transfected with pcDNA3 plasmids containing no insert were used as the negative control (Figure 8). Similar to the inhibitor studies, HMVECs transfected with the Src antisense ODNs or Akt dominant-negative mutants showed significant inhibition of sE-selectin–mediated tube formation, whereas HMVECs transfected with ERK1/2 mutants or Src sense ODNs did not show significant inhibition. These results suggest that sE-selectin mediates HMVEC tube formation in Matrigel in vitro through the Src and PI3K pathways but not through the MAPK pathway.
sE-selectin induces endothelial cell tube formation on Matrigel in vitro.
The Matrigel in vitro HMVEC tube formation assay was performed in 8-well chamber slides. (A) Photomicrographs of representative assays for DMSO; bFGF; sE-selectin; sE-selectin + PP2; sE-selectin + LY294002; and sE-selectin + PD98059. Original magnification, × 20. (B) Quantitative data for endothelial cell tube formation are expressed as number of tubes/well ± SEM from 3 independent experiments. bFGF is used as positive control. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced significantly more endothelial tube formation than vehicle control, and this tube formation was inhibited upon treatment with PP2 and LY294002. However, HMVECs treated with PD98059 did not show significant inhibition of sE-selectin–induced endothelial cell tube formation.
sE-selectin induces endothelial cell tube formation on Matrigel in vitro.
The Matrigel in vitro HMVEC tube formation assay was performed in 8-well chamber slides. (A) Photomicrographs of representative assays for DMSO; bFGF; sE-selectin; sE-selectin + PP2; sE-selectin + LY294002; and sE-selectin + PD98059. Original magnification, × 20. (B) Quantitative data for endothelial cell tube formation are expressed as number of tubes/well ± SEM from 3 independent experiments. bFGF is used as positive control. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced significantly more endothelial tube formation than vehicle control, and this tube formation was inhibited upon treatment with PP2 and LY294002. However, HMVECs treated with PD98059 did not show significant inhibition of sE-selectin–induced endothelial cell tube formation.
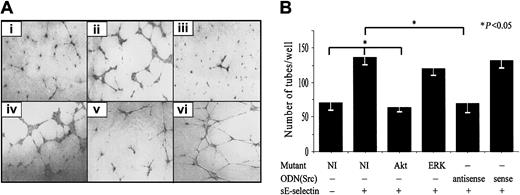
HMVECs transfected with Akt dominant-negative mutants or Src antisense ODNs show significantly less sE-selectin–induced tube formation.
HMVECs were transiently transfected with a pcDNA3 plasmid containing ERK1/2 and Akt dominant-negative mutants or with Src sense or antisense ODN. Plasmid with no insert was used as a negative control. HMVECs transfected with mutants were used for Matrigel in vitro HMVEC tube formation assays 72 hours after transfection, whereas HMVECs transfected with Src ODNs were used 16 hours after transfection. (A) Photomicrographs of a representative assay for (i) HMVECs transfected with no insert, (ii) HMVECs transfected with no insert and stimulated with sE-selectin, (iii) HMVECs transfected with an Akt mutant, (iv) HMVECs transfected with an ERK1/2 mutant, (v) HMVECs transfected with Src antisense ODNs and stimulated with sE-selectin, and (vi) HMVECs transfected with Src sense ODNs. Original magnification, × 20. (B) Quantitative data for HMVEC tube formation are expressed as number of tubes/well ± SEM from 4 independent experiments. *Represents a significant difference (P < .05) between groups. HMVECs transfected with Akt dominant-negative mutants and Src antisense ODNs showed significantly less tube formation compared with HMVECs transfected with no-insert plasmid.
HMVECs transfected with Akt dominant-negative mutants or Src antisense ODNs show significantly less sE-selectin–induced tube formation.
HMVECs were transiently transfected with a pcDNA3 plasmid containing ERK1/2 and Akt dominant-negative mutants or with Src sense or antisense ODN. Plasmid with no insert was used as a negative control. HMVECs transfected with mutants were used for Matrigel in vitro HMVEC tube formation assays 72 hours after transfection, whereas HMVECs transfected with Src ODNs were used 16 hours after transfection. (A) Photomicrographs of a representative assay for (i) HMVECs transfected with no insert, (ii) HMVECs transfected with no insert and stimulated with sE-selectin, (iii) HMVECs transfected with an Akt mutant, (iv) HMVECs transfected with an ERK1/2 mutant, (v) HMVECs transfected with Src antisense ODNs and stimulated with sE-selectin, and (vi) HMVECs transfected with Src sense ODNs. Original magnification, × 20. (B) Quantitative data for HMVEC tube formation are expressed as number of tubes/well ± SEM from 4 independent experiments. *Represents a significant difference (P < .05) between groups. HMVECs transfected with Akt dominant-negative mutants and Src antisense ODNs showed significantly less tube formation compared with HMVECs transfected with no-insert plasmid.
sE-selectin mediates blood vessel formation through Src and PI3 kinase pathways
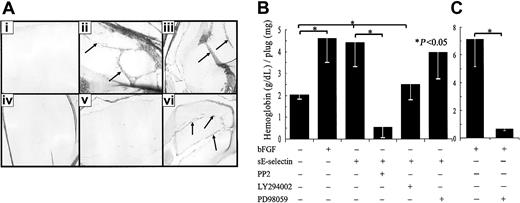
We used a bioassay, the Matrigel-plug in vivo assay, to examine whether sE-selectin mediates its biologic function through similar signaling pathways as observed in HMVEC tube formation by the Matrigel in vitro assay. Each inhibitor (PP2, LY294002, or PD98059) was incorporated in the Matrigel along with sE-selectin before Matrigel was injected into the mice. Similar to the results observed with the in vitro HMVEC tube formation assay, PP2 and LY294002 significantly (P < .05) inhibited sE-selectin–mediated blood vessel formation (65% and 44%, respectively) in the Matrigel in vivo assay (Figure 9). However, the MAPK inhibitor PD98059 did not show significant inhibition of sE-selectin–mediated blood vessel formation (8%). In the same assay system, the MAPK inhibitor PD98059 significantly inhibited bFGF-mediated blood vessel formation (Figure 9C). These results along with the in vitro tube formation assay as well as HMVEC chemotaxis assay suggest that that sE-selectin mediates its biologic function in HMVECs predominantly through Src and PI3K pathways.
PP2 and LY294002 inhibit sE-selectin–mediated blood vessel formation in Matrigel plugs in vivo.
(A) A representative assay showing Masson trichrome staining of blood vessels in paraffin sections of Matrigel plugs treated with PBS; bFGF; sE-selectin; sE-selectin + PP2; sE-selectin + LY294002; and sE-selectin + PD98059. Arrows indicate blood vessels. Original magnification, × 40. (B) sE-selectin induced significantly more blood vessel formation (*P < .05, n = 18) compared with the PBS group. The values represent the concentration of hemoglobin (g/dL)/Matrigel-plug weight (mg) ± SEM, with n indicating the number of plugs. The Src inhibitor (PP2) and PI3K inhibitor (LY294002) significantly inhibited sE-selectin–mediated blood vessel formation. However, the MAPK inhibitor (PD98059) failed to significantly inhibit sE-selectin–mediated blood vessel formation. (C) bFGF-induced blood vessel formation was significantly inhibited by the MAPK inhibitor PD98059.
PP2 and LY294002 inhibit sE-selectin–mediated blood vessel formation in Matrigel plugs in vivo.
(A) A representative assay showing Masson trichrome staining of blood vessels in paraffin sections of Matrigel plugs treated with PBS; bFGF; sE-selectin; sE-selectin + PP2; sE-selectin + LY294002; and sE-selectin + PD98059. Arrows indicate blood vessels. Original magnification, × 40. (B) sE-selectin induced significantly more blood vessel formation (*P < .05, n = 18) compared with the PBS group. The values represent the concentration of hemoglobin (g/dL)/Matrigel-plug weight (mg) ± SEM, with n indicating the number of plugs. The Src inhibitor (PP2) and PI3K inhibitor (LY294002) significantly inhibited sE-selectin–mediated blood vessel formation. However, the MAPK inhibitor (PD98059) failed to significantly inhibit sE-selectin–mediated blood vessel formation. (C) bFGF-induced blood vessel formation was significantly inhibited by the MAPK inhibitor PD98059.
Discussion
In the present study, we have examined the role of different signaling pathways by which sE-selectin mediates HMVEC chemotaxis and angiogenesis. We have previously shown that sE-selectin is a potent angiogenic mediator,16 and sE-selectin levels are up-regulated in patients with vasculoproliferative disorders such as RA25 and tumor growth.14 15 However, the signaling pathways by which sE-selectin mediates HMVEC chemotaxis and angiogenesis are still unknown. Our results from the present study show that sE-selectin mediates HMVEC chemotaxis and angiogenesis predominantly through the Src and PI3K pathways.
Previously, Simon et al26 have shown that neutrophil tethering on intact transmembrane E-selectin transduces signals in endothelial cells through the MAPK signaling pathway, and cross-linking of endothelial cell–surface E-selectin with antibodies led to up-regulation of mRNA for c-fos, an early response gene, through the Ras-MAPK pathway.27 We have recently shown that sE-selectin is a potent chemotactic factor for monocytes, and it mediates monocyte chemotaxis through the Src-MAPK pathway.17 However, to our knowledge this is the first report studying the signaling cascade involved in soluble E-selectin–mediated endothelial cell chemotaxis and angiogenesis. HMVECs stimulated with sE-selectin showed phosphorylation of a number of proteins corresponding to molecular weights of 140 to 150, 130 to 140, 55 to 65, and 30 to 40 kDa (data not shown). As the predominant band obtained using anti–phosphotyrosine antibody was observed in the molecular-weight range of Src kinase, and the Src family kinases are known to play an important role in a number of physiologic functions including monocyte adherence to endothelial cells,28endothelial cell differentiation,29 endothelial cell survival,30 and endothelial cell proliferation and angiogenesis,1 we examined the role of Src kinase in sE-selectin–stimulated HMVECs. sE-selectin induced a marked increase in HMVEC Src kinase phosphorylation, which was substantially inhibited by PP2 or by transfecting the HMVECs with Src antisense ODNs, thus indicating that Src may be an important mediator in sE-selectin–induced signaling in HMVECs. We have previously shown in monocytes that sE-selectin activates ERK1/2 and p38 MAPK pathways downstream of Src kinase.17 We next examined whether sE-selectin also activates ERK1/2 and p38 MAPK in HMVECs. sE-selectin induced a time-dependent increase in ERK1/2 phosphorylation, which was significantly inhibited by PP2. However, sE-selectin failed to activate p38 MAPK in HMVECs. These results suggest that sE-selectin may be using similar signaling pathways in HMVECs as observed previously in monocytes,17 except that in HMVECs sE-selectin does not activate p38 MAPK.
PI3K, a heterodimer of an 85-kDa adaptor subunit and 100-kDa catalytic subunit,31,32 is activated by a number of growth factors including VEGF, FGF, platelet-derived growth factor (PDGF), and EGF.24,33 It has been implicated in the regulation of cell proliferation,24 differentiation,34 cell survival,35 and angiogenesis.2 To test whether sE-selectin also mediates its signal in HMVECs through PI3K, we examined the activation of PI3K by studying the phosphorylation of 2 downstream PI3K substrates: phosphatidylinositol (PI) and the serine-threonine kinase Akt. sE-selectin induced markedly higher PI and Akt phosphorylation in HMVECs compared with nonstimulated cells. A number of studies have shown that PI3K and its substrate Akt can be activated by Src family kinases.1,30,36 In order to examine whether sE-selectin induces PI3K activation via Src family kinases, HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulation with sE-selectin. Pretreatment of HMVECs with PP2 significantly inhibited sE-selectin–mediated PI phosphorylation as well as Akt phosphorylation (50%-60%). These results indicate that sE-selectin–induced PI3K/Akt activation is mediated via Src kinase. To further understand whether there was any cross-regulation of PI3K/Akt and ERK1/2 pathways in sE-selectin–stimulated endothelial cells, HMVECs were pretreated with the ERK1/2 inhibitor (PD98059) or the PI3K inhibitor (LY294002) prior to stimulation with sE-selectin. Similar studies were done using HMVECs transfected with ERK1/2 or Akt mutants that were subsequently stimulated with sE-selectin. Our results indicate that both PI3K/Akt and ERK1/2 pathways are activated by sE-selectin in HMVECs independently of each other via Src kinase. A previous study33 has shown that EGF also uses a similar signaling cascade as observed with sE-selectin. It may be important to point out that E-selectin contains an EGF motif in its sequence.9 However, sE-selectin failed to show EGF receptor phosphorylation in endothelial cells (data not shown). It is possible that sE-selectin may be using a similar receptor molecule to mediate its signal. Currently, only the lectin-binding part of the sE-selectin receptor (sialyl Lex) is known and is present on leukocytes as well as on endothelial cells.10-12 The structure of the protein portion of the receptor molecule is unknown.
Next, to examine the functional role of sE-selectin, we performed HMVEC chemotaxis assays, Matrigel in vitro HMVEC tube formation assays, and Matrigel-plug in vivo blood vessel formation assays. The results from HMVEC chemotaxis using signaling inhibitors suggest that sE-selectin mediates chemotaxis predominantly through the Src and PI3K pathways. Inhibition of the Src pathway by PP2 provided the maximum inhibition (36%), followed by the PI3K inhibitor LY294002 (33%) and MAPK inhibitor PD98059 (20%). However, none of the inhibitors completely blocked sE-selectin–mediated HMVEC chemotaxis, thereby suggesting that sE-selectin may be mediating HMVEC chemotaxis through multiple pathways. Pretreatment of HMVECs with a combination of PP2, PD98059, and LY294002 was most effective and showed 87% inhibition of sE-selectin–mediated HMVEC chemotaxis, whereas combination of PP2 plus LY294002 or PP2 plus PD98059 showed 79% and 76% inhibition, respectively. However, combination of PD98059 plus LY294002 was much less effective and showed only 46% inhibition. These results suggests that Src may also be activating other pathways that are independent of PI3K/Akt or ERK1/2 to mediate HMVEC chemotaxis as Src inhibitor PP2 is needed to mediate the most significant inhibition. To further corroborate our results using inhibitors, we performed endothelial cell chemotaxis assays using HMVECs transiently transfected with dominant-negative mutants of ERK1/2 and Akt or HMVECs transfected with Src antisense and sense ODNs. HMVECs transfected with the Akt mutant or Src antisense ODNs showed significant decrease in sE-selectin–mediated HMVEC chemotaxis (55% and 53%, respectively) compared with HMVECs transfected with control vector alone or Src sense ODNs. In contrast, HMVECs transfected with an ERK1/2 mutant did not show significant inhibition (14%). Taken together, these results from HMVEC chemotaxis assay using signaling inhibitors as well as dominant-negative mutants or Src antisense ODNs suggest that sE-selectin may be mediating HMVEC chemotaxis predominantly through the Src and PI3K pathways, and partially through the ERK1/2 MAPK pathway. Abu-Ghazaleh et al37 and Mochizuki et al38have also shown that Src and PI3K pathways play a predominant role in the endothelial cell migration in response to VEGF and angiopoietin 2.
Angiogenesis is critical in the development of tumors2 and inflammatory disorders such as RA.39,40 We have previously reported that sE-selectin is a potent angiogenic mediator.16 In the present study we examined the signaling cascade by which sE-selectin mediates angiogenesis. We used 2 assays to study angiogenesis, the Matrigel in vitro HMVEC tube formation assay and Matrigel-plug in vivo assay. sE-selectin induced a 2.2-fold increase in endothelial cell tube formation compared with vehicle control. PP2 and LY294002 significantly (P < .05) inhibited sE-selectin–mediated endothelial cell tube formation (47% and 49%, respectively). Similarly, HMVECs transfected with Src antisense ODNs or Akt dominant-negative mutants showed significant inhibition of sE-selectin–mediated tube formation compared with HMVECs transfected with control plasmids or Src sense ODNs. However, HMVECs transfected with an ERK1/2 mutant or treated with an ERK1/2 inhibitor (PD98059) failed to show significant inhibition. In the Matrigel in vivo assay, sE-selectin also induced significant (2.2-fold) increase in blood vessel formation, which was significantly inhibited (P < .05) by treatment with PP2 (65%) or LY294002 (44%). Similar to these results obtained in the Matrigel in vitro tube formation assay, treatment with PD98059 failed to significantly inhibit sE-selectin–mediated blood vessel formation in Matrigel plugs in vivo (8%). These results suggest that even though the MAPK pathway is activated by sE-selectin in HMVECs and might play a partial role in sE-selectin–mediated HMVEC chemotaxis, sE-selectin–mediated blood vessel formation both in vitro as well as in vivo is predominantly mediated through the Src and PI3K pathways. Previously, Hartwell et al41 have shown that E-selectin–deficient and wild-type mice exhibit similar blood vessel formation in response to bFGF and tumor necrosis factor-α (TNF-α). It could be that bFGF and TNF-α do not require E-selectin molecules to mediate angiogenesis. In contrast, Aoki et al have shown that E-selectin plays an important role in VEGF-mediated angiogenesis as antimouse E-selectin antibody suppressed tube formation induced by recombinant VEGF.42 Taken together, our present data indicate that sE-selectin mediates angiogenesis predominantly through the Src-PI3K-Akt pathway. Our study also suggests a potentially novel method to inhibit sE-selectin–mediated angiogenesis via Src and PI3K pathways.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-04-1237.
Supported by the Gallagher Professorship for Arthritis Research and funds from the Veterans' Administration Research Service. Additional support included funds from National Institute of Health grants AI-40987 and HL-58695.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alisa E. Koch, Department of Medicine, Division of Rheumatology, Northwestern Medical School, 303 E Chicago Ave, Ward 3-315, Chicago, IL 60611; e-mail:ae-koch@northwestern.edu.

![Fig. 5. sE-selectin induces PI3K and Akt activation in HMVECs. / HMVECs were stimulated with sE-selectin (50 nM) for 15 minutes. In the inhibition study HMVECs were pretreated with PP2 (10 μM) for 2 hours prior to stimulation with sE-selectin, and PP2 was also present during the stimulation with sE-selectin. (A) PI3K was immunoprecipitated from the cell lysate and then the kinase assay performed using PI as the substrate and [γ-32P]–labeled ATP as the donor of phosphate ions. The [γ-32P]–labeled lipids were resolved by thin layer chromatography (TLC) and autoradiographed. This is a representative blot from 3 independent experiments. sE-selectin induced a marked increase in PI phosphorylation (*PI3P) compared with nonstimulated cells. PP2-pretreated HMVECs exhibited substantial inhibition of PI3K activation, thus indicating that PI3K lies downstream of Src kinase. (B) Cell extracts were prepared and the protein content in each sample was quantitated. Samples (20 μg) were resolved by 10% SDS-PAGE and probed with mouse monoclonal antihuman phospho-Akt (*p-Akt) antibody. To verify equal loading, the blots were stripped and reprobed with rabbit anti-Akt antibody. This is a representative blot from 3 independent experiments. (C) The ratio of p-Akt to Akt band density ± SEM from 3 independent experiments. *Represents a significant difference (P < .05) between the respective groups. sE-selectin induced a marked increase in Akt phosphorylation. Pretreatment of HMVECs with PP2 significantly inhibited Akt phosphorylation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/10/10.1182_blood-2002-04-1237/4/m_h81034300005.jpeg?Expires=1769084893&Signature=FxKqP38NyFSW8kScq3NsXWJCZbqb5OAW7ql2doiVI0k2dDBkT2-MelCJFWJZxSOqkaVilOoEs5k-teFel3qvPWAko83-Dwwkk9tC0TKwp1dBZND2EcOlHQlq7crOtUUDbwV7qTFIH-P18ymqiV3JiHplrSn~LXFHfz2qJi012YZ58QyMVYm9EKrphbQlz3l3e1oZ5HVVVLG4c5YK8-rsvBX5jv2R8XUduS4RylP4wesSUpuHQr6efNqF0IvvYiME1ZbPeZ1rxQnYwYm~B4y0UNGy811gCmgEcbTi~hMDxEoH396Ej54JNM-aP~YaKzyKSIzjBTx9ll1WxZwfE9Vt-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal