Abstract
This study was designed to test the hypothesis that administration of granulocyte colony-stimulating factor (G-CSF; filgrastim) during induction chemotherapy with CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone) or CNOP (doxorubicin replaced with mitoxantrone) in elderly patients with aggressive non-Hodgkin lymphoma (NHL) improves time to treatment failure (TTF), complete remission (CR) rate, and overall survival (OS). Furthermore, the efficacy of CHOP versus CNOP chemotherapy was compared. A total of 455 previously untreated patients older than 60 years with stages II to IV aggressive NHL were included in the analysis. Patients (median age, 71 years; range, 60-86 years) were randomized to receive CHOP (doxorubicin 50 mg/m2) or CNOP (mitoxantrone 10 mg/m2) with or without G-CSF (5 μg/kg from day 2 until day 10-14 of each cycle every 3 weeks; 8 cycles). Forty-seven patients previously hospitalized for class I to II congestive heart failure were randomized to receive CNOP with or without G-CSF (not included in the CHOP versus CNOP analysis). The CR rates in the CHOP/CNOP plus G-CSF and CHOP/CNOP groups were the same, 52%, and in the CHOP with or without G-CSF and CNOP with or without G-CSF groups, 60% and 43% (P < .001), respectively. No benefit of G-CSF in terms of TTF and OS could be shown (P = .96 andP = .22, respectively), whereas CHOP was superior to CNOP (TTF/OS P < .001). The incidences of severe granulocytopenia (World Health Organization grade IV) and granulocytopenic infections were higher in patients not receiving G-CSF. The cumulative proportion of patients receiving 90% or more of allocated chemotherapy was higher (P < .05) in patients receiving G-CSF. Concomitant G-CSF treatment did not improve CR rate, TTF, or OS. Patients receiving CHOP fared better than those given CNOP chemotherapy. The addition of G-CSF reduces the incidence of severe granulocytopenia and infections in elderly patients with aggressive NHL receiving CHOP or CNOP chemotherapy.
Introduction
The age-adjusted incidence rate of non-Hodgkin lymphoma (NHL) has increased during several decades,1,2 in particular diffuse large B-cell lymphoma.3 Increasing age is associated with worse prognosis in NHL.4,5 Factors, which are partly related, contributing to a more poor prognosis include inadequate diagnostic procedures or therapy, decreased tolerance to treatment, comorbid diseases and organ dysfunction, and accumulation of certain clinical, “biologic,” and other risk factors. CHOP (cyclophosphamide, vincristine, doxorubicin, and prednisone) is considered standard chemotherapy in stages II to IV NHL and in prospective randomized studies has been shown to be as effective as later-introduced multidrug protocols.6,7 Because elderly patients with NHL probably tolerate the CHOP regimen and other more intensive chemotherapy combinations less well, many of these patients receive suboptimal (schedule/dosing) chemotherapy.8Myelosuppression is the major dose-limiting toxicity during the induction treatment in patients with aggressive NHL9,10and a large proportion of elderly patients cannot receive a planned chemotherapy dose at the scheduled intervals.11 In general, such patients have done worse than those treated at full doses.12 If granulocytopenia could be avoided or mitigated, compliance with regard to planned doses and time intervals of chemotherapy might be increased. Hypothetically, an increased relative dose intensity (RDI) might improve outcome. Administration of granulocyte colony-stimulating factor (G-CSF) has been known for more than 10 years to reduce the incidence of neutropenia, fever, and infection in patients undergoing chemotherapy.13,14 At the time of the start of the present study there was essentially no information about the potential effects of the addition of G-CSF to chemotherapy on RDI and outcome in lymphoma treatment. In addition, mitoxantrone, an anthracenedione derivative, was suggested as a substitute for doxorubicin because of better tolerance15and a potentially lower incidence of cardiomyopathy,16which could be of particular importance in the elderly patient population.
This study was designed to test the hypothesis that in elderly patients with aggressive NHL efficacy and toxicity are significantly affected by administration of G-CSF during CHOP or CNOP induction chemotherapy and the replacement of doxorubicin (CHOP) by mitoxantrone (CNOP).
Patients and methods
Patients
Between May 1992 and January 1997, 458 previously untreated patients older than 60 years from 53 centers in the Nordic countries entered the study. They had histologically diagnosed high-grade NHL according to the updated Kiel classification,17 clinical stages II to IV, and World Health Organization (WHO) performance status of 3 or less. Cases were entered on the basis of the local pathologist's diagnosis. However, a central panel with pathologists from Denmark, Finland, Norway, and Sweden reviewed all histology (see “”). Exclusion criteria included HIV infection, history of low-grade lymphoma, overt central nervous system (CNS) disease, congestive heart failure (CHF; New York Heart Association [NYHA] classifications III-IV), history of neoplasm (adequately treated basal cell carcinoma of the skin or in situ carcinoma of the uterine cervix were allowed), abnormal liver function tests (aminotransferases and alkaline phosphatase > 2.5 times the upper limit of normal, bilirubin > 50 μM), renal insufficiency (serum creatinine > 300 μM), and patients with any serious medical or psychiatric illness that would prevent informed consent or completion of protocol-prescribed treatment and follow-up.
Clinical evaluation and staging included initial hematologic and chemical survey, chest x-rays, computed tomography of the abdomen, and bone marrow biopsy. Determination of left ventricular ejection fraction was performed when clinically indicated and not as a routine procedure. Bulky disease was defined as a tumor mass more than 5 cm. Three patients were excluded due to CNS lymphoma (n = 2) and HIV infection (n = 1). Among the remaining 455 patients (median age, 71 years; range, 60-86 years), 47 patients previously hospitalized for class I to II CHF (NYHA) were randomized to receive CNOP with or without G-CSF. The remaining 408 patients were randomized in a bifactorial design to receive either CHOP plus G-CSF (n = 101), CNOP plus G-CSF (n = 103), CHOP (n = 104), or CNOP (n = 100). Ethics committee approval was obtained in all participating centers.
Treatment regimens
The CHOP regimen was administered as follows: cyclophosphamide 750 mg/m2 intravenously day 1; vincristine 1.4 mg/m2 (maximum 2.0 mg) intravenously day 1; doxorubicin 50 mg/m2 intravenously day 1, and prednisone 50 mg/m2 orally days 1 to 5. The CNOP regimen was administered in an identical manner with the exception that doxorubicin was replaced with mitoxantrone 10 mg/m2 intravenously day 1. Courses were repeated every 3 weeks unless delayed by hematologic toxicity or infection. Modifications of dosages and interval between courses were based on precise guidelines (Table 1). The first course was to be given at 100% dose. Recombinant metHu G-CSF (filgrastim, 5 μg/kg, subcutaneously) was started on day 2 and continued for a maximum of 14 days. If the absolute granulocyte count exceeded 10 × 109/L on day 11 or later, G-CSF was discontinued. Secondary G-CSF prophylaxis was not allowed in patients randomized to no G-CSF therapy.
Chemotherapy dose modification scheme
| Dosing condition . | Dosing adjustment . |
|---|---|
| Cycle 1 | No reduction allowed |
| Following cycles (if no episode of febrile granulocytopenia) and absolute granulocyte count (AGC) ≥ 1.9 × 109/L and platelet count ≥ 75 × 109/L at day of retreatment | No dose reduction |
| AGC 1.5-1.8 × 109/L or platelet count 50-74 × 109/L | Cyclophosphamide, doxorubicin/mitoxantrone reduced to 75% of full dose |
| AGC <1.5 × 109/L or platelet count < 50 × 109/L | Delayed treatment until AGC ≥ 1.5 × 109/L and platelet count ≥ 50 × 109/L; doses as above |
| If episode of fever/infection during granulocytopenia Following two such episodes, remaining cycles were to be modified as below. | Doses as below |
| AGC ≥1.9 × 109/L and platelet count ≥75 × 109/L | Cyclophosphamide, doxorubicin/mitoxantrone reduced to 75% of full dose |
| AGC < 1.9 × 109/L or platelet count < 75 × 109/L | Delayed treatment until AGC ≥ 1.9 × 109/L and platelet count ≥ 75 × 109/L; doses as above |
| Dosing condition . | Dosing adjustment . |
|---|---|
| Cycle 1 | No reduction allowed |
| Following cycles (if no episode of febrile granulocytopenia) and absolute granulocyte count (AGC) ≥ 1.9 × 109/L and platelet count ≥ 75 × 109/L at day of retreatment | No dose reduction |
| AGC 1.5-1.8 × 109/L or platelet count 50-74 × 109/L | Cyclophosphamide, doxorubicin/mitoxantrone reduced to 75% of full dose |
| AGC <1.5 × 109/L or platelet count < 50 × 109/L | Delayed treatment until AGC ≥ 1.5 × 109/L and platelet count ≥ 50 × 109/L; doses as above |
| If episode of fever/infection during granulocytopenia Following two such episodes, remaining cycles were to be modified as below. | Doses as below |
| AGC ≥1.9 × 109/L and platelet count ≥75 × 109/L | Cyclophosphamide, doxorubicin/mitoxantrone reduced to 75% of full dose |
| AGC < 1.9 × 109/L or platelet count < 75 × 109/L | Delayed treatment until AGC ≥ 1.9 × 109/L and platelet count ≥ 75 × 109/L; doses as above |
If an additional febrile episode was recorded despite dose reduction, remaining courses were to be given at 60% (cyclophosphamide, doxorubicin, mitoxantrone). If the delay exceeded 3 weeks, the patient was withdrawn from the study.
Concomitant medications and treatment
Additional antineoplastic drugs including agents that modulate the endocrine and immunologic response to cancer or white blood cell transfusions were not to be given. Prophylactic antibiotics or antifungal therapy were not allowed and antibiotics for infection or putative infection were given according to recommended guidelines.
Tumor response evaluation
Tumor response evaluation was performed after 4 chemotherapy courses. Patients in complete remission (CR) and partial response (PR) continued the treatment for 4 additional cycles and patients with stable disease (SD) or progressive disease (PD) were given optional treatment as patients with PR after 8 cycles. The WHO criteria for CR, PR, SD, and PD were used18 and all responses were evaluated by a national review committee.19 CR was disappearance of all known disease, and PR was a greater than or equal to 50% decrease in the sum of the products of the greatest perpendicular diameters of multiple lesions. It was ensured that the CR status was maintained for at least 1 month after the end of the last treatment cycle. In SD a 50% decrease in total tumor size could not be established nor could a 25% increase in the size of one or more measurable lesions be demonstrated. PD was defined as a 25% or more increase in the size of one or more measurable lesions or the appearance of new lesions. If possible, when small lesions remained biopsies of these were performed and when the result was negative for lymphoma or a CR unconfirmed/uncertain lesion20 was unchanged after 1 year, the patient was assigned a CR status.19
Granulocyte counts and infections requiring hospitalization
Granulocyte counts were determined on days 8 or 9, 11 or 12, 14 or 15, and 22. Infections requiring hospitalization were defined as fever (oral temperature > 38.5°C on 1 or > 38.0°C on 2 occasions with a minimum interval of 4 hours between recordings) and a granulocyte count less than 0.5 × 109/L.21
RDI
The relative intensity of the administered dose for each cycle of cyclophosphamide, doxorubicin, mitoxantrone was the ratio of dose intensity received to protocol dose intensity in mg/m2 body surface area per week. The cumulative RDI was computed in the same way, using cumulative data of present and previous cycles.
End points, randomization procedure, and statistical analysis
The primary end point of the study is time to treatment failure (TTF). It was measured from the date of randomization to the first observation of disease progression, death due to any cause, or early discontinuation of treatment. Secondary end points were the CR rate, overall survival (OS), disease-free survival (DFS), RDI, and the incidence of granulocytopenia and infections requiring hospitalization. OS was measured from the date of randomization to death due to any cause and DFS from first documentation of CR to the first observation of relapse or death due to any cause.
The basic design of the study is a 2 × 2 factorial design using with or without G-CSF as one factor and CHOP versus CNOP as the other factor. Patients previously hospitalized for class I to II CHF constituted a separate group that only was randomized between CNOP with or without G-CSF. At the design stage it was assumed that the effect of adding G-CSF was similar for patients treated with CHOP and CNOP (ie, no interaction was assumed). The trial was planned to accrue 450 patients to detect a hypothetical benefit of G-CSF. Assuming a low (< 1%) ineligibility rate this gave an 80% probability of detecting a relative improvement in median TTF of 37% (24-33 months) using a 2-sided log-rank test with significance level of 5%.
Patients were allocated to treatment arm by 4 national randomization offices. Those previously hospitalized for class I to II CHF were randomized in blocks of 4 and the remaining patients in blocks of 8 and the randomization was stratified according to region. All randomized patients, except the 3 patients with HIV or CNS lymphoma, were included in the efficacy comparisons according to the intention-to-treat (ITT) principle. Thus, the analyses include the patients, who, after review by a central panel of pathologists, were found to have low-grade NHL, Hodgkin lymphoma, or a diagnosis based on cytology (Table2). Granulocytopenia, granulocytopenic fever, and other toxicities in any of the cycles were also analyzed according to the randomization. Moreover, granulocytopenia and granulocytopenic fever are presented by treatment cycle for those patients who cumulatively have kept to the allocated treatment arm (per protocol analysis). RDI is also presented by treatment cycle for these patients. No significance tests have been performed for these per cycle data because the patients are selected and not comparable according to the randomization.
Distribution of patient and disease characteristics according to treatment group
| Characteristics . | CHOP + G-CSF . | CNOP + G-CSF . | CHOP . | CNOP . | CHF class I/II . | |
|---|---|---|---|---|---|---|
| CNOP + G-CSF . | CNOP . | |||||
| n | 101 | 103 | 104 | 100 | 22 | 25 |
| Age | ||||||
| Median, y | 71 | 70 | 72 | 71 | 75 | 72 |
| Older than 70 y, % | 62 | 51 | 64 | 59 | 73 | 60 |
| Sex, % | ||||||
| Male | 49 | 49 | 54 | 53 | 64 | 64 |
| Female | 51 | 51 | 46 | 47 | 36 | 36 |
| Clinical stage, % | ||||||
| I | 0 | 1 | 0 | 0 | 0 | 0 |
| II | 34 | 31 | 38 | 35 | 32 | 28 |
| III | 31 | 26 | 22 | 23 | 32 | 36 |
| IV | 35 | 42 | 40 | 42 | 36 | 36 |
| Bulky disease, % | 56 | 53 | 59 | 50 | 45 | 48 |
| Bone marrow involvement, % | 15 | 17 | 13 | 13 | 14 | 20 |
| LDH level above normal, % | 62 | 68 | 63 | 63 | 62 | 68 |
| Performance status (WHO), % | ||||||
| 0-1 | 69 | 65 | 76 | 60 | 55 | 68 |
| 2-3 | 31 | 35 | 24 | 40 | 45 | 32 |
| Extranodal sites (>1), % | 10 | 14 | 13 | 13 | 18 | 20 |
| IPI (clinical stage, LDH, and performance status), % | ||||||
| 0 | 20 | 15 | 12 | 16 | 9 | 8 |
| 1 | 24 | 26 | 40 | 24 | 29 | 28 |
| 2 | 36 | 33 | 36 | 36 | 33 | 48 |
| 3 | 20 | 26 | 12 | 24 | 29 | 16 |
| Histopathology* | ||||||
| Kiel classification, % | ||||||
| Centroblastic | 61 | 63 | 69 | 69 | 77 | 56 |
| Immunoblastic | 1 | 4 | 3 | 4 | 0 | 12 |
| Anaplastic LC | 2 | 0 | 0 | 2 | 0 | 0 |
| Lymphoblastic | 2 | 0 | 0 | 3 | 0 | 0 |
| Pleomorphic T | 10 | 6 | 8 | 2 | 9 | 0 |
| Other high-grade | 14 | 20 | 12 | 10 | 14 | 12 |
| Other (cytology, low-grade, Hodgkin lymphoma) | 10 | 7 | 8 | 10 | 0 | 20 |
| REAL classification, % | ||||||
| Diffuse large B cell | 62 | 67 | 72 | 73 | 77 | 68 |
| Anaplastic large cell | 2 | 0 | 0 | 2 | 0 | 0 |
| Lymphoblastic (B and T) | 2 | 0 | 0 | 3 | 0 | 0 |
| Peripheral T cell | 10 | 6 | 8 | 2 | 9 | 0 |
| Other high-grade | 14 | 20 | 12 | 10 | 14 | 12 |
| (B and T) | ||||||
| Other (cytology, low-grade, Hodgkin lymphoma) | 10 | 7 | 8 | 10 | 0 | 20 |
| Characteristics . | CHOP + G-CSF . | CNOP + G-CSF . | CHOP . | CNOP . | CHF class I/II . | |
|---|---|---|---|---|---|---|
| CNOP + G-CSF . | CNOP . | |||||
| n | 101 | 103 | 104 | 100 | 22 | 25 |
| Age | ||||||
| Median, y | 71 | 70 | 72 | 71 | 75 | 72 |
| Older than 70 y, % | 62 | 51 | 64 | 59 | 73 | 60 |
| Sex, % | ||||||
| Male | 49 | 49 | 54 | 53 | 64 | 64 |
| Female | 51 | 51 | 46 | 47 | 36 | 36 |
| Clinical stage, % | ||||||
| I | 0 | 1 | 0 | 0 | 0 | 0 |
| II | 34 | 31 | 38 | 35 | 32 | 28 |
| III | 31 | 26 | 22 | 23 | 32 | 36 |
| IV | 35 | 42 | 40 | 42 | 36 | 36 |
| Bulky disease, % | 56 | 53 | 59 | 50 | 45 | 48 |
| Bone marrow involvement, % | 15 | 17 | 13 | 13 | 14 | 20 |
| LDH level above normal, % | 62 | 68 | 63 | 63 | 62 | 68 |
| Performance status (WHO), % | ||||||
| 0-1 | 69 | 65 | 76 | 60 | 55 | 68 |
| 2-3 | 31 | 35 | 24 | 40 | 45 | 32 |
| Extranodal sites (>1), % | 10 | 14 | 13 | 13 | 18 | 20 |
| IPI (clinical stage, LDH, and performance status), % | ||||||
| 0 | 20 | 15 | 12 | 16 | 9 | 8 |
| 1 | 24 | 26 | 40 | 24 | 29 | 28 |
| 2 | 36 | 33 | 36 | 36 | 33 | 48 |
| 3 | 20 | 26 | 12 | 24 | 29 | 16 |
| Histopathology* | ||||||
| Kiel classification, % | ||||||
| Centroblastic | 61 | 63 | 69 | 69 | 77 | 56 |
| Immunoblastic | 1 | 4 | 3 | 4 | 0 | 12 |
| Anaplastic LC | 2 | 0 | 0 | 2 | 0 | 0 |
| Lymphoblastic | 2 | 0 | 0 | 3 | 0 | 0 |
| Pleomorphic T | 10 | 6 | 8 | 2 | 9 | 0 |
| Other high-grade | 14 | 20 | 12 | 10 | 14 | 12 |
| Other (cytology, low-grade, Hodgkin lymphoma) | 10 | 7 | 8 | 10 | 0 | 20 |
| REAL classification, % | ||||||
| Diffuse large B cell | 62 | 67 | 72 | 73 | 77 | 68 |
| Anaplastic large cell | 2 | 0 | 0 | 2 | 0 | 0 |
| Lymphoblastic (B and T) | 2 | 0 | 0 | 3 | 0 | 0 |
| Peripheral T cell | 10 | 6 | 8 | 2 | 9 | 0 |
| Other high-grade | 14 | 20 | 12 | 10 | 14 | 12 |
| (B and T) | ||||||
| Other (cytology, low-grade, Hodgkin lymphoma) | 10 | 7 | 8 | 10 | 0 | 20 |
LDH indicates lactate dehydrogenase; IPI, International Prognostic Index; LC, large cell.
According to central pathology review.
A combined variable is introduced counting the number of patients who have cumulatively per cycle received 90% or more of the correct allocated treatment. It is based on all randomized patients and significance tests are used to compare the treatment arms.
Comparisons of response to treatment and side effects and obtained RDI between treatment groups were done by Mantel-Haenszel tests stratified for CHOP/CNOP when analyzing with or without G-CSF and for use of G-CSF or not when analyzing CHOP versus CNOP. Patients previously hospitalized for class I to II CHF were not included in the comparison between CHOP and CNOP. Kaplan-Meier curves22 were calculated for TTF and OS and statistical comparisons were performed by stratified log-rank tests.23 The study is not powered to detect any interaction between the 2 factors CHOP versus CNOP and with or without G-CSF, unless it is very large, and no formal tests of interaction have been done. In the analyses it was thus assumed that the factors with or without G-CSF and CHOP versus CNOP are additive (in suitable scale), and in the case of true interaction the presented effects are averages taken over the values of the other factor. All significance tests were 2-sided and not adjusted for multiple comparisons.
Results
The distribution of patient and disease characteristics were similar in the 4 treatment groups also including patients previously hospitalized for class I to II CHF (Table 2). Five patients did not receive the first cycle according to randomization. Histopathology is also presented according to the Revised European American Lymphoma (REAL) classification.24 Low-grade lymphomas constituted 3.7% in the whole series and cases were evenly distributed among study arms.
Response
The overall CR rate in the study was 52%. Considering only patients who were randomized to all 4 treatment arms, that is, those not previously hospitalized for class I to II CHF, the CR rate was 61% in the CHOP plus G-CSF group, 41% in the CNOP plus G-CSF group, 59% in the CHOP group, and 46% in the CNOP group. In patients previously hospitalized for class I to II CHF the CR rate was 59% in the CNOP plus G-CSF group and 48% in the CNOP group. The PR rate varied between 7% and 13%. In the CHOP/CNOP plus G-CSF and the CHOP/CNOP groups the CR rate was the same, 52%. The patients treated with CHOP with or without G-CSF had a higher CR rate as compared to the CNOP with or without G-CSF group, 60% and 43%, respectively (P < .001). The CR rate was 55% in patients younger than 70 years and 50% in patients older than 70 years (P = .20).
TTF, OS, and DFS
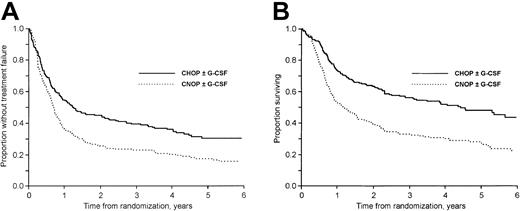
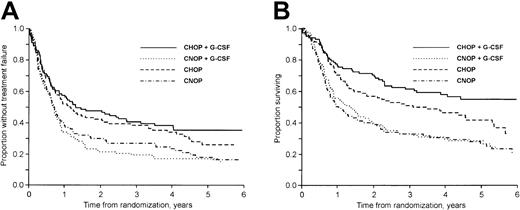
The median follow-up time was 57 months (range, 18-91 months) for surviving patients. At follow-up 35% of the patients (159 of 455) were alive. Considering first patients not previously hospitalized for class I to II CHF, the 3-year failure-free (TTF) and OS rates were 40% and 61% in the CHOP plus G-CSF group, 19% and 33% in the CNOP plus G-CSF group, 38% and 51% in the CHOP group, and 27% and 33% in the CNOP group, respectively (Figure 1A-B). In patients previously hospitalized for class I to II CHF the corresponding values were 14% and 27% in the CNOP plus G-CSF group and 16% and 27% in the CNOP group, respectively.
The addition of G-CSF to CHOP/CNOP did not affect TTF (P = .96; Figure 2A), OS (P = .22; Figure 2B), or DFS (P = .63). CNOP-treated patients had a significantly worse TTF (P < .001; Figure 3A), OS (P < .001; Figure 3B), and DFS (P = .047) than patients receiving CHOP with or without G-CSF chemotherapy. In a subgroup analysis, OS in the CHOP plus G-CSF group was significantly better than in the CHOP group (P = .045; Figure 1B). At follow-up, 62 of 104 and 45 of 101 patients in the CHOP and CHOP plus G-CSF groups, respectively, had died. The excess mortality in the CHOP group was due to more lymphoma (48 versus 39) and treatment-related (5 versus 1) deaths. However, only minor nonsignificant differences were found with regard to CR rate, DFS, and TTF (Figure 1A).
TTF and OS with/without G-CSF.
(A) TTF in patients treated with and without G-CSF. (B) OS in patients treated with and without G-CSF.
TTF and OS with/without G-CSF.
(A) TTF in patients treated with and without G-CSF. (B) OS in patients treated with and without G-CSF.
TTF and OS in patients treated with CHOP and CNOP.
(A) TTF in patients treated with CHOP and CNOP (P < .001). Patients previously hospitalized for class I to II CHF are excluded. (B) OS in patients treated with CHOP and CNOP (P < .001). Patients previously hospitalized for class I to II CHF are excluded.
TTF and OS in patients treated with CHOP and CNOP.
(A) TTF in patients treated with CHOP and CNOP (P < .001). Patients previously hospitalized for class I to II CHF are excluded. (B) OS in patients treated with CHOP and CNOP (P < .001). Patients previously hospitalized for class I to II CHF are excluded.
TTF and OS by treatment group.
(A) TTF according to treatment group. Patients previously hospitalized for class I to II CHF are excluded. (B) OS according to treatment group. Patients previously hospitalized for class I to II CHF are excluded.
TTF and OS by treatment group.
(A) TTF according to treatment group. Patients previously hospitalized for class I to II CHF are excluded. (B) OS according to treatment group. Patients previously hospitalized for class I to II CHF are excluded.
Granulocytopenia and infections requiring hospitalization
The incidence of severe granulocytopenia (WHO grade IV) in any cycle was significantly higher (P < .001; Table3) and median granulocyte nadir values lower (Table 4) in patients not receiving G-CSF. The incidence of granulocytopenic (WHO grade IV) fever requiring hospitalization in any cycle was significantly higher in the non–G-CSF groups (on average 50% versus 33%; P < .001; Table 3). The highest rate of granulocytopenic fever was recorded in the first cycle (in all treatment groups), when no dose reduction was allowed (Table 5). There were no statistical differences in the incidence of granulocytopenia or granulocytopenic fever requiring hospitalization between the CHOP with or without G-CSF and CNOP with or without G-CSF treatment groups, respectively (Tables 3-5).
Granulocytopenia, granulocytopenic (WHO grade IV) fever requiring hospitalization and other toxicities (% of patients) in any cycle according to treatment group
| Treatment group . | Granulocytopenia, <0.5 × 109/L . | Granulocytopenic fever, <0.5 × 109/L . | Mucositis3-150 . | GI toxicity3-150 . | Alopecia3-150 . | Cardiac toxicity3-150,3-151 . | Musculoskeletal pain3-150 . |
|---|---|---|---|---|---|---|---|
| CHOP + G-CSF | 553-152 | 343-152 | 5 | 153-153 | 803-155 | 5 | 103-154 |
| CNOP + G-CSF | 643-152 | 323-152 | 3 | 8 | 47 | 3 | 43-154 |
| CHOP | 89 | 50 | 4 | 103-153 | 813-155 | 3 | 2 |
| CNOP | 86 | 50 | 2 | 5 | 41 | 1 | 1 |
| Treatment group . | Granulocytopenia, <0.5 × 109/L . | Granulocytopenic fever, <0.5 × 109/L . | Mucositis3-150 . | GI toxicity3-150 . | Alopecia3-150 . | Cardiac toxicity3-150,3-151 . | Musculoskeletal pain3-150 . |
|---|---|---|---|---|---|---|---|
| CHOP + G-CSF | 553-152 | 343-152 | 5 | 153-153 | 803-155 | 5 | 103-154 |
| CNOP + G-CSF | 643-152 | 323-152 | 3 | 8 | 47 | 3 | 43-154 |
| CHOP | 89 | 50 | 4 | 103-153 | 813-155 | 3 | 2 |
| CNOP | 86 | 50 | 2 | 5 | 41 | 1 | 1 |
GI indicates gastrointestinal.
WHO grade III/IV.
Patients with class I/II CHF (NYHA) excluded.
P < .001 as compared to CHOP/CNOP.
P < .05 as compared to CNOP with/without G-CSF.
P < .001 as compared to CNOP with/without G-CSF.
P < .01 as compared to CHOP/CNOP.
Distribution of patients with granulocytopenia (WHO grade IV) and median nadir counts according to treatment group and cycle number4-150
| Treatment group . | Cycle no. . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
| CHOP + G-CSF | 94 | 87 | 85 | 79 | 79 | 73 | 66 | 63 |
| Granulocyte count less than 0.5 × 109/L | 41 (44) | 20 (23) | 13 (15) | 11 (14) | 11 (14) | 12 (16) | 4 (6) | 12 (19) |
| Median nadir (109/L) | 0.7 | 1.6 | 2.1 | 2.2 | 2.2 | 1.5 | 2.1 | 1.9 |
| CNOP + G-CSF | 117 | 119 | 109 | 98 | 88 | 78 | 71 | 68 |
| Granulocyte count less than 0.5 × 109/L | 40 (34) | 26 (22) | 24 (22) | 29 (30) | 21 (24) | 25 (32) | 20 (28) | 16 (24) |
| Median nadir (109/L) | 1.1 | 1.9 | 1.2 | 1.0 | 1.4 | 1.1 | 1.1 | 1.3 |
| CHOP | 96 | 93 | 86 | 82 | 80 | 75 | 68 | 62 |
| Granulocyte count less than 0.5 × 109/L | 66 (69) | 49 (53) | 48 (56) | 46 (56) | 43 (54) | 42 (56) | 48 (71) | 38 (61) |
| Median nadir (109/L) | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 |
| CNOP | 110 | 109 | 100 | 96 | 79 | 71 | 64 | 63 |
| Granulocyte count less than 0.5 × 109/L | 68 (62) | 48 (44) | 55 (55) | 52 (54) | 47 (59) | 38 (54) | 42 (66) | 36 (57) |
| Median nadir (109/L) | 0.3 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Total no. of patients | 417 | 408 | 380 | 355 | 326 | 297 | 269 | 256 |
| Granulocyte count less than 0.5 × 109/L | 215 (52) | 143 (35) | 140 (37) | 138 (39) | 122 (37) | 117 (39) | 114 (42) | 102 (40) |
| Median nadir (109/L) | 0.4 | 0.7 | 0.7 | 0.6 | 0.8 | 0.7 | 0.6 | 0.7 |
| Treatment group . | Cycle no. . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
| CHOP + G-CSF | 94 | 87 | 85 | 79 | 79 | 73 | 66 | 63 |
| Granulocyte count less than 0.5 × 109/L | 41 (44) | 20 (23) | 13 (15) | 11 (14) | 11 (14) | 12 (16) | 4 (6) | 12 (19) |
| Median nadir (109/L) | 0.7 | 1.6 | 2.1 | 2.2 | 2.2 | 1.5 | 2.1 | 1.9 |
| CNOP + G-CSF | 117 | 119 | 109 | 98 | 88 | 78 | 71 | 68 |
| Granulocyte count less than 0.5 × 109/L | 40 (34) | 26 (22) | 24 (22) | 29 (30) | 21 (24) | 25 (32) | 20 (28) | 16 (24) |
| Median nadir (109/L) | 1.1 | 1.9 | 1.2 | 1.0 | 1.4 | 1.1 | 1.1 | 1.3 |
| CHOP | 96 | 93 | 86 | 82 | 80 | 75 | 68 | 62 |
| Granulocyte count less than 0.5 × 109/L | 66 (69) | 49 (53) | 48 (56) | 46 (56) | 43 (54) | 42 (56) | 48 (71) | 38 (61) |
| Median nadir (109/L) | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 |
| CNOP | 110 | 109 | 100 | 96 | 79 | 71 | 64 | 63 |
| Granulocyte count less than 0.5 × 109/L | 68 (62) | 48 (44) | 55 (55) | 52 (54) | 47 (59) | 38 (54) | 42 (66) | 36 (57) |
| Median nadir (109/L) | 0.3 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Total no. of patients | 417 | 408 | 380 | 355 | 326 | 297 | 269 | 256 |
| Granulocyte count less than 0.5 × 109/L | 215 (52) | 143 (35) | 140 (37) | 138 (39) | 122 (37) | 117 (39) | 114 (42) | 102 (40) |
| Median nadir (109/L) | 0.4 | 0.7 | 0.7 | 0.6 | 0.8 | 0.7 | 0.6 | 0.7 |
Numbers of patients are given, with percentages in parentheses.
Only patients with treatment according to protocol are included. A few patients are missing due to lack of data.
Distribution of patients with granulocytopenic (WHO grade IV) fever requiring hospitalization according to treatment group and cycle number5-150
| Treatment group . | Cycle no. . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
| CHOP + G-CSF | 100 | 92 | 88 | 82 | 83 | 77 | 71 | 67 |
| Fever during granulocytopenia | 20 (20) | 7 (8) | 4 (5) | 4 (5) | 5 (6) | 3 (4) | 2 (3) | 1 (1) |
| CNOP + G-CSF | 123 | 121 | 112 | 104 | 90 | 80 | 74 | 71 |
| Fever during granulocytopenia | 22 (18) | 10 (8) | 5 (4) | 6 (6) | 2 (2) | 5 (6) | 2 (3) | 2 (3) |
| CHOP | 102 | 96 | 89 | 85 | 80 | 77 | 72 | 66 |
| Fever during granulocytopenia | 21 (21) | 6 (6) | 14 (16) | 7 (8) | 13 (16) | 10 (13) | 8 (11) | 6 (9) |
| CNOP | 123 | 116 | 109 | 103 | 87 | 77 | 71 | 68 |
| Fever during granulocytopenia | 24 (20) | 7 (6) | 11 (10) | 10 (10) | 8 (9) | 3 (4) | 13 (18) | 7 (10) |
| Total no. of patients | 448 | 425 | 398 | 374 | 340 | 311 | 288 | 272 |
| Fever during granulocytopenia | 87 (19) | 30 (7) | 34 (9) | 27 (7) | 28 (8) | 21 (7) | 25 (9) | 16 (6) |
| Treatment group . | Cycle no. . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
| CHOP + G-CSF | 100 | 92 | 88 | 82 | 83 | 77 | 71 | 67 |
| Fever during granulocytopenia | 20 (20) | 7 (8) | 4 (5) | 4 (5) | 5 (6) | 3 (4) | 2 (3) | 1 (1) |
| CNOP + G-CSF | 123 | 121 | 112 | 104 | 90 | 80 | 74 | 71 |
| Fever during granulocytopenia | 22 (18) | 10 (8) | 5 (4) | 6 (6) | 2 (2) | 5 (6) | 2 (3) | 2 (3) |
| CHOP | 102 | 96 | 89 | 85 | 80 | 77 | 72 | 66 |
| Fever during granulocytopenia | 21 (21) | 6 (6) | 14 (16) | 7 (8) | 13 (16) | 10 (13) | 8 (11) | 6 (9) |
| CNOP | 123 | 116 | 109 | 103 | 87 | 77 | 71 | 68 |
| Fever during granulocytopenia | 24 (20) | 7 (6) | 11 (10) | 10 (10) | 8 (9) | 3 (4) | 13 (18) | 7 (10) |
| Total no. of patients | 448 | 425 | 398 | 374 | 340 | 311 | 288 | 272 |
| Fever during granulocytopenia | 87 (19) | 30 (7) | 34 (9) | 27 (7) | 28 (8) | 21 (7) | 25 (9) | 16 (6) |
Numbers of patients are given, with percentages in parentheses.
Only patients with treatment according to protocol are included. A few patients are missing due to lack of data.
Other toxicities
RDI
RDI was calculated for cyclophosphamide in combination with doxorubicin/mitoxantrone. RDI according to treatment group and cycle is presented in Table 6. In the groups given G-CSF or not, the cumulative proportion of patients, who continued allocated treatment with RDI 90% or more during 8 courses, was 44% (99 of 226) and 34% (78 of 229), respectively (P < .05; Table 6) and the cumulative median RDI after 8 courses was 96.0% and 91.5%, respectively. There was a significant difference in the cumulative proportion of patients who continued allocated treatment with RDI 90% or higher between the CHOP plus G-CSF (50%; 51 of 101) and CHOP (36%; 37 of 104) groups (P < .05) but no significant differences between the CHOP with or without G-CSF and the CNOP with or without G-CSF groups or the CNOP plus G-CSF and CNOP groups, respectively (Table 6).
Cycle-specific and cumulative RDI of cyclophosphamide/doxorubicin and cyclophosphamide/mitoxantrone according to treatment group
| Treatment group . | Cycle no. . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
| CHOP + G-CSF (n = 101)6-150 | 876-151/1006-152 | 70/92 | 66/88 | 61/84 | 61/83 | 55/77 | 53/71 | 51/68 |
| Per cycle RDI, mean ± SD | 96.5 ± 8.06 | 92.4 ± 11.40 | 94.6 ± 11.94 | 91.7 ± 13.67 | 92.6 ± 13.10 | 90.0 ± 16.91 | 90.2 ± 15.13 | 91.7 ± 13.42 |
| Median | 99.5 | 96.6 | 98.8 | 97.8 | 98.1 | 98.1 | 96.7 | 98.9 |
| Cumulative RDI; mean ± SD | 96.5 ± 8.06 | 94.1 ± 8.22 | 94.0 ± 7.62 | 93.2 ± 7.87 | 93.0 ± 8.12 | 92.3 ± 8.89 | 92.5 ± 9.24 | 92.6 ± 9.40 |
| Median | 99.5 | 97.6 | 96.7 | 95.5 | 96.0 | 95.5 | 95.9 | 96.3 |
| CNOP + G-CSF (n = 125)6-150 | 1086-151/1246-152 | 96/121 | 91/112 | 81/105 | 67/90 | 59/81 | 52/75 | 48/71 |
| Per cycle RDI; mean ± SD | 97.4 ± 9.07 | 94.3 ± 12.08 | 94.9 ± 14.94 | 94.0 ± 11.79 | 94.1 ± 12.75 | 92.5 ± 14.19 | 86.6 ± 17.12 | 92.1 ± 12.89 |
| Median | 99.5 | 99.1 | 99.0 | 99.0 | 99.1 | 99.0 | 95.7 | 98.9 |
| Cumulative RDI; mean ± SD | 97.4 ± 9.07 | 95.5 ± 8.43 | 95.2 ± 8.52 | 94.8 ± 8.39 | 94.7 ± 7.22 | 93.9 ± 7.73 | 92.4 ± 8.74 | 92.3 ± 8.68 |
| Median | 99.5 | 98.1 | 97.8 | 97.9 | 97.3 | 97.2 | 94.9 | 95.4 |
| CHOP (n = 104)6-150 | 916-151/1026-152 | 71/96 | 70/89 | 59/85 | 54/80 | 46/77 | 41/72 | 37/66 |
| Per cycle RDI; mean ± SD | 97.9 ± 8.56 | 93.1 ± 11.45 | 92.0 ± 12.88 | 87.4 ± 15.62 | 87.8 ± 15.03 | 84.6 ± 19.31 | 82.4 ± 18.63 | 86.7 ± 15.13 |
| Median | 99.7 | 98.5 | 97.8 | 94.3 | 94.6 | 94.5 | 88.3 | 95.9 |
| Cumulative RDI; mean ± SD | 97.9 ± 8.56 | 95.2 ± 7.89 | 94.3 ± 8.05 | 92.5 ± 8.98 | 91.9 ± 8.75 | 90.3 ± 9.31 | 88.9 ± 10.14 | 88.8 ± 10.09 |
| Median | 99.7 | 98.3 | 97.3 | 94.6 | 94.1 | 92.9 | 91.7 | 90.9 |
| CNOP (n = 125)6-150 | 1096-151/1246-152 | 87/116 | 74/111 | 67/103 | 51/87 | 45/77 | 44/71 | 41/68 |
| Per cycle RDI; mean ± SD | 96.7 ± 9.01 | 92.1 ± 13.30 | 92.2 ± 14.68 | 90.0 ± 15.59 | 88.6 ± 17.34 | 87.0 ± 17.10 | 87.9 ± 16.19 | 86.3 ± 15.54 |
| Median | 99.1 | 99.1 | 98.9 | 98.7 | 97.3 | 97.3 | 97.0 | 96.0 |
| Cumulative RDI; mean ± SD | 96.7 ± 9.01 | 94.1 ± 9.26 | 93.0 ± 8.39 | 91.7 ± 8.97 | 90.78 ± 9.46 | 90.2 ± 9.99 | 90.3 ± 10.25 | 89.8 ± 10.17 |
| Median | 99.1 | 97.7 | 95.9 | 94.3 | 93.5 | 94.0 | 94.2 | 92.0 |
| Treatment group . | Cycle no. . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
| CHOP + G-CSF (n = 101)6-150 | 876-151/1006-152 | 70/92 | 66/88 | 61/84 | 61/83 | 55/77 | 53/71 | 51/68 |
| Per cycle RDI, mean ± SD | 96.5 ± 8.06 | 92.4 ± 11.40 | 94.6 ± 11.94 | 91.7 ± 13.67 | 92.6 ± 13.10 | 90.0 ± 16.91 | 90.2 ± 15.13 | 91.7 ± 13.42 |
| Median | 99.5 | 96.6 | 98.8 | 97.8 | 98.1 | 98.1 | 96.7 | 98.9 |
| Cumulative RDI; mean ± SD | 96.5 ± 8.06 | 94.1 ± 8.22 | 94.0 ± 7.62 | 93.2 ± 7.87 | 93.0 ± 8.12 | 92.3 ± 8.89 | 92.5 ± 9.24 | 92.6 ± 9.40 |
| Median | 99.5 | 97.6 | 96.7 | 95.5 | 96.0 | 95.5 | 95.9 | 96.3 |
| CNOP + G-CSF (n = 125)6-150 | 1086-151/1246-152 | 96/121 | 91/112 | 81/105 | 67/90 | 59/81 | 52/75 | 48/71 |
| Per cycle RDI; mean ± SD | 97.4 ± 9.07 | 94.3 ± 12.08 | 94.9 ± 14.94 | 94.0 ± 11.79 | 94.1 ± 12.75 | 92.5 ± 14.19 | 86.6 ± 17.12 | 92.1 ± 12.89 |
| Median | 99.5 | 99.1 | 99.0 | 99.0 | 99.1 | 99.0 | 95.7 | 98.9 |
| Cumulative RDI; mean ± SD | 97.4 ± 9.07 | 95.5 ± 8.43 | 95.2 ± 8.52 | 94.8 ± 8.39 | 94.7 ± 7.22 | 93.9 ± 7.73 | 92.4 ± 8.74 | 92.3 ± 8.68 |
| Median | 99.5 | 98.1 | 97.8 | 97.9 | 97.3 | 97.2 | 94.9 | 95.4 |
| CHOP (n = 104)6-150 | 916-151/1026-152 | 71/96 | 70/89 | 59/85 | 54/80 | 46/77 | 41/72 | 37/66 |
| Per cycle RDI; mean ± SD | 97.9 ± 8.56 | 93.1 ± 11.45 | 92.0 ± 12.88 | 87.4 ± 15.62 | 87.8 ± 15.03 | 84.6 ± 19.31 | 82.4 ± 18.63 | 86.7 ± 15.13 |
| Median | 99.7 | 98.5 | 97.8 | 94.3 | 94.6 | 94.5 | 88.3 | 95.9 |
| Cumulative RDI; mean ± SD | 97.9 ± 8.56 | 95.2 ± 7.89 | 94.3 ± 8.05 | 92.5 ± 8.98 | 91.9 ± 8.75 | 90.3 ± 9.31 | 88.9 ± 10.14 | 88.8 ± 10.09 |
| Median | 99.7 | 98.3 | 97.3 | 94.6 | 94.1 | 92.9 | 91.7 | 90.9 |
| CNOP (n = 125)6-150 | 1096-151/1246-152 | 87/116 | 74/111 | 67/103 | 51/87 | 45/77 | 44/71 | 41/68 |
| Per cycle RDI; mean ± SD | 96.7 ± 9.01 | 92.1 ± 13.30 | 92.2 ± 14.68 | 90.0 ± 15.59 | 88.6 ± 17.34 | 87.0 ± 17.10 | 87.9 ± 16.19 | 86.3 ± 15.54 |
| Median | 99.1 | 99.1 | 98.9 | 98.7 | 97.3 | 97.3 | 97.0 | 96.0 |
| Cumulative RDI; mean ± SD | 96.7 ± 9.01 | 94.1 ± 9.26 | 93.0 ± 8.39 | 91.7 ± 8.97 | 90.78 ± 9.46 | 90.2 ± 9.99 | 90.3 ± 10.25 | 89.8 ± 10.17 |
| Median | 99.1 | 97.7 | 95.9 | 94.3 | 93.5 | 94.0 | 94.2 | 92.0 |
Number of patients randomized.
Number of patients given 90% or more cumulatively according to protocol.
Number of patients treated cumulatively according to protocol.
Discussion
This study, which began accrual of patients 10 years ago, addressed 2 clinically important questions in elderly patients with aggressive NHL. First, does the addition of G-CSF to induction chemotherapy improve outcome in terms of TTF, OS, and CR rate and, second, does replacement of doxorubicin in CHOP with mitoxantrone (CNOP) significantly affect efficacy and toxicity?
Addition of G-CSF
Hypothetically, G-CSF therapy may decrease mortality associated with infectious complications during neutropenia and allow for an increased dose intensity in turn leading to a higher response rate and improved TTF. It has long been known that elderly patients with NHL treated with aggressive chemotherapy have an elevated risk for early death secondary to chemotherapy toxicity. Thus, Armitage and Potter25 reported that 25% of patients aged 70 years or older died during the first 2 cycles of chemotherapy from causes other than lymphoma, compared with only 2% of younger patients. In other series the risk for treatment-related death varies between 8% and 21% in similar patient populations receiving anthracycline-based aggressive chemotherapy.10 26
Patients treated with G-CSF had a lower incidence of granulocytopenia leading to a pronounced reduction of episodes of fever/infection during neutropenia requiring hospitalization. In a recently reported study, the addition of G-CSF to CHOP chemotherapy significantly reduced infections and days with antibiotics in patients older than 65 years with aggressive NHL.27 Similar findings in aggressive NHL have been observed in smaller randomized studies with the use of filgrastim in patients aged 16 to 71 years9 and 60 to 82 years,11 with lenograstim (patients aged 15-55 years28) and granulocyte-macrophage colony-stimulating factor (GM-CSF; patients aged 15-73 years29). In randomized studies lenograstim (childhood NHL30) and filgrastim (including only 74 patients31) did not reduce the number of febrile neutropenic episodes. The primary objective of all these cited studies was to evaluate hematopoietic growth factor treatment to prevent neutropenia and related episodes of fever/infection. Potential effects on RDI, CR rates, TTF, and OS remained secondary objectives. It should be noted that although patients were given quite intensive combination chemotherapy CHOP or CNOP regimens were not used in any of these studies. In addition, schedules for administration of G-CSF and GM-CSF varied considerably between studies making direct comparisons more difficult.
In the present study the cumulative proportion of patients receiving 90% or more of planned chemotherapy was moderately higher among patients treated with G-CSF. Similar results were presented by Zinzani et al11 in elderly patients with NHL randomized to receive VNCOP-B (cyclophosphamide, mitoxantrone, vincristine, etoposide, bleomycin, and prednisone) treatment with or without filgrastim. The delivered dose intensity in that study was numerically but not statistically significantly higher in the G-CSF group. In the randomized study by Doorduijn et al27 the addition of G-CSF improved the RDI of cyclophosphamide and doxorubicin; however, outcome was unaffected. Because G-CSF treatment only led to a marginally increased RDI it is not surprising that TTF, OS, and CR rates were unaffected by G-CSF therapy in the present study. This is also in good accordance with previous reports.9,11,28,29In a subgroup analysis of the superior CHOP regimen, OS was significantly better for patients treated with CHOP plus G-CSF in comparison to CHOP alone with a concomitant higher proportion of patients receiving 90% or more of scheduled chemotherapy in the G-CSF group. In a recent report, a pronounced increase in RDI has also been shown to improve outcome in elderly patients given CHOP plus G-CSF every 14 days in comparison to CHOP alone every 21 days.32However, the present subgroup analysis was not prespecified but initiated by the results of the study and, in addition, no difference in CR rate or TTF between the 2 groups could be demonstrated. Thus, the improved OS may very well be due to chance, and the results must be confirmed in another study before acceptance.
CHOP versus CNOP
When this study was planned the general opinion was that elderly patients supposedly tolerated the CHOP regimen less well leading to treatment with a suboptimal dose or schedule or both.12 In certain aspects, the favorable toxicity profile of mitoxantrone with potentially decreased cardiotoxicity and less nausea/vomiting and alopecia in comparison to doxorubicin was known.33 The probability of developing doxorubicin-induced CHF in patients older than 60 years of age at a cumulative dose of 400 mg/m2 has been reported to be 4.6%.34,35 This finding is further corroborated by the results of the present study. The choice not to monitor left ventricular ejection fraction (LVEF) was based in part on the restricted availability of uniform methods for LVEF determination. In addition, patients were planned to receive less than or equal to 400 mg/m2 doxorubicin. In fact, only 43% of CHOP-treated patients received 8 cycles at 90% or more cumulative RDI (Table 6). The results from randomized studies comparing CHOP versus CNOP36-38 and m-BACOD (cyclophosphamide, doxorubicin, vincristine, bleomycin, methotrexate, and dexamethasone) versus m-BNCOD (doxorubicin replaced with mitoxantrone [N])39-41 did not reveal inferior treatment results associated with mitoxantrone, although few patients older than 60 years were included. In addition, within the Nordic Lymphoma Group there was some experience with CNOP and other mitoxantrone-containing regimens.42 Thus, this was the rationale for the inclusion of the CHOP versus CNOP comparison in the present trial primarily focused on the potential benefit of G-CSF.
The results of the present study are very clear. CHOP was superior to CNOP (patients allocated to CNOP treatment due to previous class I-II CHF excluded) with regard to CR rate, TTF, and OS. RDI did not differ between the CHOP with or without G-CSF and CNOP with or without G-CSF groups. In addition, the incidence of granulocytopenia and infectious complications as well as cardiotoxicity did not differ between patients treated with mitoxantrone or doxorubicin. Thus, doxorubicin 50 mg/m2 was not less toxic than mitoxantrone 10 mg/m2 with regard to bone marrow function. However, as reported previously, alopecia was less pronounced in the mitoxantrone group.15 In the latter randomized study, including 145 assessable patients older than 60 years with advanced NHL of intermediate- and high-grade malignancy, patients who received CHOP every 4 weeks had a higher CR rate and better cause-specific and OS than patients given CNOP (every 4 weeks15). As in the present study there was no difference with regard to myelosuppression or cardiac side effects between treatments. Thus, in the 2 largest studies so far published comparing CHOP versus CNOP in the up-front treatment of elderly patients with aggressive NHL, CHOP therapy appears convincingly superior with regard to efficacy variables. It may be noted that both CR rates and survivals in the CHOP- and CNOP-treated patients in the present study are superior to those presented in the Sonneveld study,15 which may at least in part be explained by the shorter interval between courses and the total number of chemotherapy courses given to responding patients (8 versus 6). Much effort was focused on the evaluation of tumor responses in the present study and it was concluded that an independent review committee is a major prerequisite for a uniform response evaluation in clinical phase 3 trials.19 In contrast to these findings a large study comparing mitoxantrone (7 mg/m2) with doxorubicin (35 mg/m2) in a multiagent weekly regimen in 473 eligible patients older than 60 years with high-grade lymphoma was recently published.43 In that study, CR rates and overall but not relapse-free survival were better in patients receiving the mitoxantrone-containing regimen. In addition, leukopenia and thrombocytopenia were more common among patients given doxorubicin although severe infections did not differ between the treatment groups. A meta-analysis including all randomized trials comparing doxorubicin and mitoxantrone in aggressive NHL will be performed, and hopefully, will clarify the role of mitoxantrone in this patient category.
Recently, Coiffier et al reported that the addition of rituximab to the CHOP regimen increases the CR rate and prolongs event-free and overall survival in elderly (60-80 years old) patients with diffuse large B-cell lymphoma.44 The CR rate and 2-year OS in patients receiving CHOP alone in that study was 63% and 57%, respectively. In a German study32 comparing CHOP once every 2 and 3 weeks and CHOEP (CHOP plus etoposide) once every 2 and 3 weeks in elderly patients with aggressive lymphoma the CR rate in the patients treated with CHOP once every 3 weeks was 63% and the OS at 40 months 49%. In the present study the CR rate was 59% and OS at 24 and 40 months was 57% and 50%, respectively, in patients given CHOP alone. Thus, the results with CHOP alone were very similar in these 3 large controlled studies, which favors the notion that the superiority of CHOP versus CNOP was not attributable to an unusually good outcome among patients in the CHOP group. In addition, we believe that the results strengthen the findings reported by the French and German groups.32 44
Conclusion
In conclusion, the addition of filgrastim (G-CSF) to CHOP and CNOP reduces the incidence of severe granulocytopenia and infections requiring hospitalization. In the whole patient group G-CSF use did not significantly affect TTF, OS, or CR rate, although the cumulative proportion of patients receiving 90% or more of planned chemotherapy was moderately higher in the G-CSF group. CHOP was superior to CNOP with regard to TTF, DFS, OS, and CR rate.
The NLG would like to thank all collaborators and patients who participated in this study. The support of Roche, Amgen, Wyeth-Lederle, and the Swedish Cancer Society is gratefully acknowledged. We thank Professor George Canellos for critical review of the manuscript and Ms Britt Telander for excellent secretarial assistance.
Participants in this NLG study include the following:
Regional coordinators
Denmark: J. Myhre, Copenhagen. Finland: E. Elonen, T. Ruutu, L. Teerenhovi, Helsinki; M. Vapaatalo, Hyvinkää; K. Oksanen, Hämeenlinna; M. Pajunen, Jyväskylä; O. Kuittinen, S. Virtanen, Oulu; B. Rask, Porvoo; H. Pertovaara, Tampere; R. Huovinen, Turku. Norway: O. Mella, Bergen; A. F. Abrahamsen, H. Holte, S. Kvaløy, G. Lauvvang, Oslo; E. Wist, Tromsö; R. Telhaug, Trondheim. Sweden: A. M. Ekberg, J. H. Svensson, Borås; K. Wallman, Falun; S. Bergström, Gävle; I. Braide, T. Ekman, H. Nilsson-Ehle, Göteborg; L. Hellquist, Halmstad; B. Norberg, Jönköping; L. Nurbo, Karlskrona; S. Fredén, Karlstad; M. Mogensen, Kristianstad; M. Hjort, Lidköping; E Haapaniemi, Linköping; P Kjärgaard, Ljungby; C Myhr-Eriksson, Luleå; H. Anderson, E. Cavallin-Stahl, A Johnson, M Åkerman, Lund; O. Zettervall, Malmö; D. Fors, Piteå; J. Hallgren, Simrishamn; M. Björkholm, V. Hjalmar, B. Johansson, E. Kimby, R. Lerner, J. Liliemark, K. Merk, M. Merup, E. Ösby, Stockholm; M. Hedenus, Sundsvall; P. Johansson, Uddevalla; B. Osterman, Umeå; G. Enblad, H. Hagberg, C. Sundström, A. Taube, Uppsala; S. Hasselblom, Varberg; T. Kunze, Visby; U. Petterson, Västerås; T. Samuelsson, Växjö; M. Nordström, Örebro; K. Tholin, Östersund.
Pathology review panel
K. Bendix, Århus, Denmark; K. Franssila, Helsinki, Finland; A. Myking, Bergen, R. Langholm, Oslo, Norway; Å. Öst (coordinator), Stockholm, Sweden.
Data management
Cecilia Arnesson, Southern Swedish Regional Tumour Registry, Lund University Hospital, Lund, Sweden.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-10-3238.
Supported by Roche, Amgen, Wyeth-Lederle, and the Swedish Cancer Society.
A complete list of the members of the NLG appears in the “.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Magnus Björkholm, Division of Hematology, Department of Medicine, Karolinska Hospital, SE 17176 Stockholm, Sweden; e-mail: magnus.bjorkholm@ks.se.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal