Abstract
To analyze the relationship between immunophenotyping profile and main clinicopathological features and outcome in diffuse large B-cell lymphoma (DLBCL), we studied 128 patients (59 men, 69 women; median age 65 years) consecutively diagnosed with de novo DLBCL in a single institution. Cells from each patient were immunostained with CD20, CD79a, CD5, CD10, bcl-6, MUM1, CD138, bcl-2, p53, p27, and Ki-67 antibodies. Four immunophenotyping profiles were distinguished according to the pattern of differentiation: germinal center–CD10+ (GC-CD10+; CD10+/Bcl-6+/MUM1−/CD138−), germinal center–CD10− (GC-CD10−; CD10−/Bcl-6+/ MUM1−/CD138−), post–germinal center (pGC; CD10−/bcl-6±/ MUM1+/CD138−), and plasmablastic (CD10−/bcl-6−/MUM1+/CD138+). Rearrangement of bcl-2 was studied by polymerase chain reaction (PCR) in 57 patients. Single-antigen expression was as follows: CD5, 2%; CD10, 21%; bcl-6, 72%; MUM1, 54%; CD138, 2%; bcl-2, 59%; p53, 28%; p27, 40%. Distribution according to differentiation profiles was as follows: GC-CD10+, 24 patients, GC-CD10-, 30 patients; pGC, 60 patients; plasmablastic, 2 patients; other patterns, 12 patients. The pGC profile was associated with primary nodal presentation and immunoblastic morphology, whereas GC-CD10+ tumors showed disseminated disease, centroblastic morphology, bcl-2 rearrangement, and lower Ki-67 proliferative index. GC-CD10− patients more often presented with primary extranodal origin, early stage, normal lactic acid dehydrogenase (LDH) levels, and low or low/intermediate International Prognostic Index (IPI) scores than the others. However, no significant difference was found in terms of response or overall survival (OS) according to these profiles. Expression of bcl-2 was associated with advanced stage, high or high-intermediate IPI, and poor OS. Expression of bcl-2 maintained predictive value in multivariate analysis, with stage and LDH. In conclusion, differentiation profile was associated with particular clinicopathological features but was not essential to predicting outcome in DLBCL patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma in Western countries, representing about one third of these disorders.1 Although DLBCL is usually considered as a specific category, the diversity in the clinical presentation, morphology, genetic and molecular alterations strongly suggests that these tumors represent a heterogeneous group of neoplasms rather than a single clinicopathological entity.2 In fact, the biological and clinical heterogeneity of DLBCLs has already been recognized in the Revised European-American Lymphoid (REAL) and World Health Organization (WHO) classifications.3,4However, the different approaches to separate nosological entities within DLBCLs have failed, owing in part to their lack of reproducibility. Delineation of the possible different categories of DLBCL with clinical relevance may help to identify groups of patients with distinct clinical presentation and outcome who may benefit from specific treatments. About one half of DLBCL patients can be cured with current therapies, but most of the remaining half eventually die of the disease. Clinical prognostic systems, including the International Prognostic Index (IPI),5 although useful to assess overall prognosis, embrace patients with heterogeneous prognoses. It is likely that the prognostic assessment of patients with DLBCL might be improved by using biological features.
During the last decade, most studies dealing with the heterogeneity of DLBCL have focused on morphologic features, individual protein expression, or molecular alterations. The significance of morphological variants, including immunoblastic lymphoma, remains controversial. The low reproducibility of histopathologic criteria and the lack of objective immunophenotypic or genetic features have made it impossible to build up new categories on this basis. On the other hand, the expression of individual antigens related to different stages of B-cell differentiation, including CD5, CD10, bcl-6, MUM1/IRF4, and CD138, may help to define groups of tumors with different clinical and pathological characteristics.6-10 In fact, some studies have observed a potential prognostic value of the individual expression of these antigens.11-14 More recently, the global expression profile of DLBCL has been analyzed by means of cDNA and oligonucleotide microarrays.15-17 Two main gene patterns have been characterized according to the germinal center (GC) or post-GC (activated B-cell) origin. Besides the different biological behavior, these groups show marked differences in terms of clinical features and outcome. However, the potential clinical value of the stage of differentiation of DLBCL defined by the immunophenotypic profile is not known. In addition to morphological and phenotypic characteristics, several oncogenic and proliferation-related genes, such as bcl-2, bcl-6, p53, and p27, have been identified as potential prognostic factors in these tumors, with conflicting results among different studies.10,14 18-28
The aims of this study were to determine the clinical significance and prognostic value of different immunophenotypic profiles related to germinal and postgerminal cell differentiation in DLBCL defined by a relatively small number of single antigens, and to investigate the possible relationship of these groups of tumors with different oncogenic and proliferative markers in a homogeneous series of patients diagnosed and treated in a single institution.
Patients and methods
Patients
The patients were 128 persons consecutively diagnosed with a diffuse large B-cell lymphoma (DLBCL) between September 1987 and September 1998 and followed up in a single institution. The only criterion for inclusion was the availability of adequate histologic material for morphological and immunohistochemical studies at diagnosis. Patients with recognized disease phase of a follicular lymphoma or another type of indolent lymphoma with subsequent transformation into a large-cell lymphoma were not included. In addition, patients with immunodeficiency-associated tumors and primary mediastinal, central nervous system, intravascular, and primary effusion lymphomas were excluded from the study. Approval was obtained from the institutional review board of Hospital Clinic, Barcelona, for these studies. Informed consent was provided according to the Declaration of Helsinki.
The median age of the patients was 65 years (range, 22-93 years); 59 were men and 69 were women. Main characteristics of the patients at diagnosis are listed in Table 1. Primary extranodal origin (excluding 14 patients with primary ORL area involvement) was shown in 50 cases (39%). Overall, advanced stage (III/IV) was observed in 64 cases (50%) and extranodal involvement in 90 (70%), including bone marrow infiltration in 22 (17%). Of 115 patients with available data, 63 (55%) presented with high serum lactic acid dehydrogenase (LDH) levels, whereas the distribution according to the International Prognostic Index (IPI) was the following: low risk, 37 cases (31%); low/intermediate risk, 26 cases (22%); high/intermediate risk, 22 cases (19%); high risk, 33 cases (28%); and nonassessable, 10 cases. The main initial and evolutional variables, including the histologic parameters indicated below, were recorded and analyzed for prognosis.
Main initial characteristics of 128 patients with diffuse large-cell lymphoma
| Characteristics . | No. (%) . |
|---|---|
| Performance status (ECOG > 2; n = 125)* | 58 (46) |
| B symptoms | 50 (39) |
| Ann Arbor stage | |
| I | 27 (21) |
| II | 34 (27) |
| III | 16 (13) |
| IV | 48 (38) |
| Bulky disease | 34 (25) |
| Primary extranodal origin (excluding Waldeyer ring) | 50 (39) |
| Bone marrow (+) | 22 (17) |
| High serum LDH (n = 115)* | 63 (55) |
| High serum β2-microglobulin (n = 72)* | 30 (42) |
| International Prognostic Index (n = 118)* | |
| Low risk | 37 (31) |
| Low/intermediate | 26 (22) |
| High/intermediate | 22 (19) |
| High risk | 33 (28) |
| Characteristics . | No. (%) . |
|---|---|
| Performance status (ECOG > 2; n = 125)* | 58 (46) |
| B symptoms | 50 (39) |
| Ann Arbor stage | |
| I | 27 (21) |
| II | 34 (27) |
| III | 16 (13) |
| IV | 48 (38) |
| Bulky disease | 34 (25) |
| Primary extranodal origin (excluding Waldeyer ring) | 50 (39) |
| Bone marrow (+) | 22 (17) |
| High serum LDH (n = 115)* | 63 (55) |
| High serum β2-microglobulin (n = 72)* | 30 (42) |
| International Prognostic Index (n = 118)* | |
| Low risk | 37 (31) |
| Low/intermediate | 26 (22) |
| High/intermediate | 22 (19) |
| High risk | 33 (28) |
The median age of the patients was 65 years (range, 22-93 years); 59 were male and 69 were female.
ECOG indicates Eastern Cooperative Oncology Group scale.
The n shown reflects the number of patients for whom data were available.
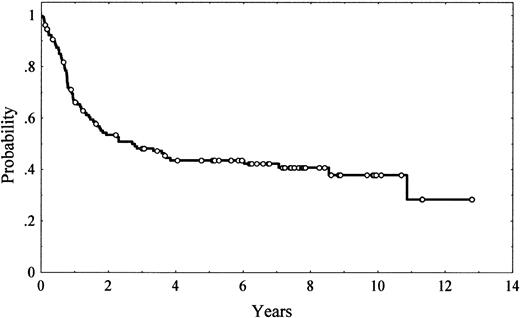
Staging maneuvers included thoracic, abdominal, and pelvic computed tomography (CT) scans, as well as bone marrow biopsies. All the patients were treated homogeneously with combination chemotherapy: adriamycin-containing regimens (in most cases CHOP [cyclophosphamide, adriamycin, vincristine, and prednisone]) in 110 patients (86%) and combination chemotherapy without adriamycin in the remaining 18 patients. Posttherapy restaging consisted of the repetition of the previously abnormal tests and/or biopsies. Response was assessed according to conventional criteria.29 Overall, 74 patients (58%) achieved a complete response (CR), 14 (11%) achieved a partial response, and 41 (32%) failed to respond to treatment. After a median follow-up of 6.5 years (range, 0.2-12.8 years) for alive patients, 72 patients had died. The 5-year overall survival (OS) was 43% (95% confidence interval [CI], 34%-52%; Figure1).
Histologic features
The diagnosis of DLCL was based on the criteria established in the WHO classification. Histologic slides were reviewed in all cases by 2 observers (L.C. and E.C.). Morphological subclassification was performed according to the following categories, based on slight modifications of the updated Kiel classification criteria30: centroblastic—90% or more of tumor cells were typical centroblasts; polymorphic centroblastic—the proportion of immunoblasts ranged from 10% to 90% of tumor cells; and immunoblastic—90% or more of tumor cells were immunoblasts. In addition, DLBCLs were considered plasmablastic when the tumor cells expressed a plasma cell differentiation phenotype (CD20−, CD79a+) with immunoblastic large cell lymphoma morphology. For comparisons among different groups in the statistical analysis, polymorphic centroblastic lymphomas were further subclassified into cases with 50% or less or more than 50% immunoblasts. The former were included in the group of centroblastic DLBCLs, whereas the latter cases, with more than 50% immunoblasts, were added to the immunoblastic subgroup. Finally, cases that did not fulfill morphological criteria were considered not further classifiable.
Immunohistochemistry
The panel of monoclonal antibodies included antibodies against the following antigens: CD20, CD79a, CD3, CD5, CD10, MUM1, CD138, bcl-2, bcl-6, Ki-67, p53, and p27. In Table2, the antibodies and the immunohistochemical conditions of use are detailed. B-cell lineage was assigned to cases with CD20 and/or CD79a positivity. A variable number of accompanying mature T cells was seen in each case. Five-micrometer paraffin sections from formalin-fixed material were dehydrated and deparaffinized according to standard procedures. A previous step of heat-induced antigen retrieval was used for CD10, bcl-2, bcl-6, MUM1, Ki-67, p53, and p27 in all cases. After incubation with the primary antibodies, the immunoreaction was developed in an automated TechMate 500 (Dako, Glostrup, Denmark), using the Dako Envision+ System peroxidase technique, with diaminobenzidine (DAB) as chromogen. The slides were counterstained with Mayer hematoxylin.
Immunohistochemical study: antibodies and conditions of use
| Antibody . | Clone . | Dilution . | Source . |
|---|---|---|---|
| CD20 | L26 | 1:80 | Dako, Carpinteria, CA |
| CD79a | JCB117 | 1:80 | Dako |
| CD3 | PS1 | 1:30 | Novocastra, Newcastle upon Tyne, England |
| CD5 | 4C7 | 1:25 | Novocastra |
| CD10 | 56C6 | 1:25 | Novocastra |
| bcl-2 | 124 | 1:50 | Dako |
| bcl-6 | PG-B6p (52) | 1:5 | Dr B. Falini, Perugia, Italy |
| CD138 | B-B4 | 1:10 | Serotec, Oxford, England |
| p53 | BP53-12 | 1:50 | Novocastra |
| p27 | 1B4 | 1:20 | Novocastra |
| Ki-67 | MIB-1 | 1:400 | Immunotech, Marseille, France |
| MUM1 | MUM1p (45) | 1:2 | Dr B. Falini |
| Antibody . | Clone . | Dilution . | Source . |
|---|---|---|---|
| CD20 | L26 | 1:80 | Dako, Carpinteria, CA |
| CD79a | JCB117 | 1:80 | Dako |
| CD3 | PS1 | 1:30 | Novocastra, Newcastle upon Tyne, England |
| CD5 | 4C7 | 1:25 | Novocastra |
| CD10 | 56C6 | 1:25 | Novocastra |
| bcl-2 | 124 | 1:50 | Dako |
| bcl-6 | PG-B6p (52) | 1:5 | Dr B. Falini, Perugia, Italy |
| CD138 | B-B4 | 1:10 | Serotec, Oxford, England |
| p53 | BP53-12 | 1:50 | Novocastra |
| p27 | 1B4 | 1:20 | Novocastra |
| Ki-67 | MIB-1 | 1:400 | Immunotech, Marseille, France |
| MUM1 | MUM1p (45) | 1:2 | Dr B. Falini |
All samples were evaluated in a semiquantitative way after the observers studied the whole immunostained slide, scoring at least 200 cells in well-preserved areas. Samples were stratified into 5 groups: 1 (0% to fewer than 10% of tumor cells were stained), 2 (10% to 25% positive cells), 3 (26% to 50% positive cells), 4 (51% to 75% positive cells), and 5 (more than 75% positive cells). CD10, bcl-6, MUM1, CD138, and p27 expression were considered positive when the score was 3 or higher (more than 25%). MUM1 positivity was considered only when tumor cells presented nuclear staining, although in some cases the cytoplasm was also stained. Finally, p53 expression was scored as positive if more than 10% of the tumor cells had nuclear staining (score 2 or higher).
Tumor proliferation was analyzed by 2 different methods: (1) analysis of mitotic index, considering a cutoff of 25 mitoses per 10 high-power fields (HPFs), and (2) proliferative index, assessed by Ki-67 immunostaining.
As detailed in Table 3, the patients were clustered into 4 groups according to immunophenotypic profile in order to assess the pattern of differentiation: germinal center origin with CD10 positivity (GC-CD10+; CD10+/bcl-6+/MUM1−/CD138−); germinal center origin–CD10 negativity (GC-CD10−; CD10−/bcl-6+/MUM1−/CD138−); post–germinal center origin (pGC; CD10−/bcl-6±/MUM1+/CD138−; and plasmablastic (PL; CD10−/bcl-6−/MUM1+/CD138+). As described in “Results,” 12 patients with different profiles or with some unavailable data were not included in these groups.
Subclassification of diffuse large B-cell lymphomas according to immunophenotypic profile to assess the degree of differentiation
| Origin . | CD10 . | Bcl-6 . | MUM1 . | CD138 . |
|---|---|---|---|---|
| Germinal center-CD10+ | + | + | − | − |
| Germinal center-CD10− | − | + | − | − |
| Post-germinal center | − | +/− | + | − |
| Plasmablastic | − | − | + | + |
| Origin . | CD10 . | Bcl-6 . | MUM1 . | CD138 . |
|---|---|---|---|---|
| Germinal center-CD10+ | + | + | − | − |
| Germinal center-CD10− | − | + | − | − |
| Post-germinal center | − | +/− | + | − |
| Plasmablastic | − | − | + | + |
Rearrangement of bcl-2/JH
DNA was extracted from paraffin-embedded tissues in 51 cases and from frozen tissues in 6 cases. In 23 samples, DNA was isolated from both frozen and paraffin-embedded tissues. DNA was isolated from frozen tissues by proteinase K/RNase treatment and phenol extraction, whereas tissue sections from formalin specimens were first deparaffinized with xylene and dehydrated with ethanol and then DNA was extracted by the standard proteinase K routine methods. Detection of the t(14;18) involving the bcl-2 major breakpoint region (MBR) and minor cluster region (mcr) was performed by polymerase chain reaction (PCR) in a Model 2400 thermal cycler (Applied Biosystems, Foster City, CA) using standard buffer conditions. Primers used were MBR: 5′ TTA GAG AGT TGC TTT ACG TGG CCT G 3′ and JH: 5′ ACC TGA GGA GAC GGT GAC C 3′ for the MBR, and MC9: 5′ TCT TGC AGG GTC TTT AAG CAG 3′ and JH for the mcr. A regimen of 35 cycles of denaturation for 1 minute at 94°C, annealing for 1 minute at 57°C, and elongation for 1 minute at 72°C was used in both reactions. Products were separated by electrophoresis on a 2% agarose gel and visualized under ultraviolet light following ethidium bromide staining.
Statistical analysis
Categorical data were compared by Fisher exact test, 2-sidedP, whereas for ordinal data, nonparametric tests were used. Bonferroni correction for multiple comparisons was applied when necessary. The actuarial survival analysis was performed according to the method described by Kaplan and Meier31 and the curves were compared by log-rank test.32 The multivariate analysis was performed with the Cox stepwise proportional hazards model.33
Results
Morphological study
The 128 DLBCLs were classified as follows: centroblastic, 49 (38%); centroblastic polymorphic with 50% or fewer immunoblasts, 18 (14%); centroblastic polymorphic with more than 50% immunoblasts, 15 (12%); immunoblastic, 12 (9%); plasmablastic, 3 (2%); other, 3 (2%; 2 of these patients had pleomorphic DLBCLs and 1 had a T cell–rich DLBCL). The remaining 28 DLBCLs were not otherwise classifiable, since they did not fulfill the criteria of any of the above-mentioned subgroups.
Immunophenotypic profile
The immunohistochemical expression of single antigens is detailed in Table 4. Patients expressing MUM1 more frequently presented with immunoblastic morphology (more than 50% immunoblasts) than did those who were MUM1-negative (37% vs 17%;P = .04), whereas, on the contrary, patients who were CD10 positive more often showed centroblastic morphology (50% or fewer immunoblasts) than did CD10-negative patients (91% vs 65%;P = .03).
Main antigen expression by immunostaining in 128 patients with diffuse large B-cell lymphoma
| Antigen . | No. assessable samples . | No. positive (%) . |
|---|---|---|
| CD20/CD79a | 128 | 128 (100) |
| CD5 | 127 | 3 (2) |
| CD10 | 128 | 27 (21) |
| bcl-2 | 126 | 74 (59) |
| bcl-6 | 127 | 91 (72) |
| MUM1 | 126 | 68 (54) |
| CD138 | 127 | 2 (2) |
| p53 | 127 | 35 (28) |
| p27 | 123 | 49 (40) |
| Antigen . | No. assessable samples . | No. positive (%) . |
|---|---|---|
| CD20/CD79a | 128 | 128 (100) |
| CD5 | 127 | 3 (2) |
| CD10 | 128 | 27 (21) |
| bcl-2 | 126 | 74 (59) |
| bcl-6 | 127 | 91 (72) |
| MUM1 | 126 | 68 (54) |
| CD138 | 127 | 2 (2) |
| p53 | 127 | 35 (28) |
| p27 | 123 | 49 (40) |
The distribution of the patients according to the immunophenotypic patterns previously described (Table 3) was as follows: GC-CD10+ profile, 24 patients (19%); GC-CD10−profile, 30 patients (23%); pGC profile, 60 patients (50%); plasmablastic profile, 2 patients (1.5%); other, 10 patients (8%; 7 patients had the profile CD10−/bcl-6−/MUM1−/CD138−and 3 had the profile CD10+/bcl-6−/MUM1+/CD138−). In 2 patients, one of them with plasmablastic morphology, one or more single-antigen determinations were not assessable, and this prevented further classification.
A significant relationship was found between the morphology and the immunophenotypic profile of the patients (Table5). Patients with pGC profiles more frequently showed an immunoblastic morphology (more than 50% immunoblasts; 18[39%] of 46 patients) than did GC-CD10−patients (5 [24%] of 21 patients) and GC-CD10+ patients (1 [5%] of 20 patients; P = .01). Of note, no GC-CD10+ patient presented with truly immunoblastic morphology (more than 90% immunoblasts). No significant relationship was found between groups with different differentiation patterns and the expression of bcl-2, p53, or p27 (Table 5).
Histological features of 114 patients with diffuse large B-cell lymphoma according to differentiation profile
| . | GC-CD10+ (n = 24), % . | GC-CD10− (n = 30), % . | pGC (n = 60), % . |
|---|---|---|---|
| Immunoblastic and polymorphic morphology (more than 50% immunoblasts)5-150 | 55-152 | 24 | 39 |
| Ki-67, 25% or less | 295-152 | 3 | 2 |
| bcl-2/JH rearrangement5-151(+) | 415-152 | 0 | 0 |
| Bcl-2 expression (+) | 67 | 50 | 62 |
| p53 (+) | 37 | 20 | 27 |
| p27 (+) | 50 | 23 | 45 |
| . | GC-CD10+ (n = 24), % . | GC-CD10− (n = 30), % . | pGC (n = 60), % . |
|---|---|---|---|
| Immunoblastic and polymorphic morphology (more than 50% immunoblasts)5-150 | 55-152 | 24 | 39 |
| Ki-67, 25% or less | 295-152 | 3 | 2 |
| bcl-2/JH rearrangement5-151(+) | 415-152 | 0 | 0 |
| Bcl-2 expression (+) | 67 | 50 | 62 |
| p53 (+) | 37 | 20 | 27 |
| p27 (+) | 50 | 23 | 45 |
n = 87 patients with centroblastic, polymorphic, or immunoblastic morphology (patients with morphology not further classifiable were excluded).
n = 57 patients in whom PCR for bcl-2/JH was performed.
P < .05 vs the other groups.
Rearrangement of bcl-2/JH
Rearrangement of bcl-2/JH was assessed in 57 patients. Seven patients showed bcl-2/JH rearrangement at MBR (4 patients) or mcr (3 patients). All 7 patients with bcl-2/JH were GC-CD10+patients (7 of 17); the incidence of bcl-2/JH rearrangement was 41%, 0%, and 0% for GC-CD10+, GC-CD10−, and pGC patients, respectively (P < .001) (Table 5). In addition, bcl-2/JH rearrangement was associated with CD10 expression (7 of 20 CD10-positive patients; P < .001), independently of MBR or mcr involvement (P = .008 and P = .028, respectively), but not with bcl-6, MUM1, CD138, or bcl-2 expression.
Proliferative activity analysis
Overall, 92 of the 118 assessable patients (78%) showed a mitotic index higher than 25 mitoses per 10 HPFs. No differences were found among morphologic subtypes or differentiation profile groups according to the mitotic index. The proliferative index, as assessed by Ki-67, was 25% or lower in 10 patients, 26% to 50% in 9 patients, 51% to 75% in 39 patients, and higher than 75% in 66 patients. The Ki-67 index of GC-CD10+ patients was significantly lower (Ki-67 < 25% in 29% of the patients) than that of GC-CD10− patients (Ki-67 < 25% in 3% of the patients;P = .02) and pGC patients (Ki-67 < 25% in 2% of the patients; P = .001).
Clinical features
The main clinical features of the patients are described in “Patients and methods” and listed in Table 1. When the clinical features were analyzed according to the patterns of differentiation (GC-CD10+, GC-CD10−, and pGC), as summarized in Table 6, GC-CD10−patients more often exhibited a primary extranodal origin, excluding Waldeyer ring areas (59%) than did GC-CD10+ patients (50%; P = .09) and pGC patients (29%;P = .02). Moreover, patients with GC-CD10−lymphomas more frequently had a localized Ann Arbor stage (I or II), normal serum LDH levels, and low- or low/intermediate–risk IPI than did pGC patients. On the other hand, 73% of GC-CD10+patients presented in an advanced stage (III or IV), in contrast to 30% of GC-CD10− patients (P = .004).
Clinical and evolutional features of 114 patients with diffuse large B-cell lymphoma according to differentiation profile
| . | GC-CD10+(n = 24) . | GC-CD10− (n = 30) . | pGC (n = 60) . |
|---|---|---|---|
| Age, y, median (range) | 63 (22-93) | 68 (33-88) | 64 (24-85) |
| Sex, no. | |||
| Male | 10 | 15 | 27 |
| Female | 14 | 15 | 33 |
| Primary extranodal origin, % | 50 | 596-151 | 29 |
| Advanced Ann Arbor stage (III/IV), % | 73 | 306-152 | 53 |
| High serum LDH, %6-150 | 54 | 326-151 | 65 |
| High/intermediate- or high-risk IPI, %6-150 | 60 | 216-152 | 53 |
| CR rate, % | 50 | 69 | 55 |
| 5-y OS, % | 43 | 57 | 40 |
| . | GC-CD10+(n = 24) . | GC-CD10− (n = 30) . | pGC (n = 60) . |
|---|---|---|---|
| Age, y, median (range) | 63 (22-93) | 68 (33-88) | 64 (24-85) |
| Sex, no. | |||
| Male | 10 | 15 | 27 |
| Female | 14 | 15 | 33 |
| Primary extranodal origin, % | 50 | 596-151 | 29 |
| Advanced Ann Arbor stage (III/IV), % | 73 | 306-152 | 53 |
| High serum LDH, %6-150 | 54 | 326-151 | 65 |
| High/intermediate- or high-risk IPI, %6-150 | 60 | 216-152 | 53 |
| CR rate, % | 50 | 69 | 55 |
| 5-y OS, % | 43 | 57 | 40 |
n = 104 patients with available data.
P < .02 vs pGC group.
P < .04 vs the other 2 groups.
Patients in whom bcl-2 expression was positive (> 25%), compared with those in whom bcl-2 expression was negative, more frequently presented with advanced Ann Arbor stage (59% vs 40%; P = .04) and high-intermediate or high risk IPI (54% vs 34%;P = .06). No other clinical relationship was observed.
Response to treatment and outcome: prognostic analysis
As described above, the complete response (CR) rate was 57% in the present series. The following variables predicted CR achievement (Table 7): ambulatory performance status (ECOG < 2); absence of bulky disease; early Ann Arbor stage; no bone marrow involvement; normal serum albumin, LDH, and β2-microglobulin levels; and IPI. No single-antigen expression predicted response to therapy. The CR rate according to differentiation pattern was 50%, 69%, and 55% for GC-CD10+, GC-CD10−, and pGC groups, respectively; these differences did not reach statistical significance. In addition, no significant difference was found according to any morphologic subtype.
Rates of complete response (CR) and overall survival (OS) among 128 patients with diffuse large B-cell lymphoma, according to the main prognostic factors
| . | n7-150 . | CR rate, % . | 5-year OS, % . | P . |
|---|---|---|---|---|
| Age, y | .004 | |||
| Younger than 60 | 45 | 69 | 60 | |
| 60 years or older | 84 | 52 | 34 | |
| B symptoms | .05 | |||
| No | 77 | 64 | 50 | |
| Yes | 50 | 47 | 34 | |
| Performance status | .00001 | |||
| ECOG 0,1 | 67 | 72 | 62 | |
| ECOG 2-4 | 58 | 40 | 22 | |
| Ann Arbor stage | .00002 | |||
| I/II | 62 | 73 | 62 | |
| III/IV | 65 | 41 | 25 | |
| Bulky disease | .001 | |||
| No | 91 | 65 | 53 | |
| Yes | 34 | 39 | 23 | |
| Extranodal involvement | ||||
| 2 or fewer sites | 98 | 61 | 49 | .004 |
| More than 2 sites | 30 | 43 | 23 | |
| Bone marrow | .016 | |||
| Negative | 102 | 62 | 49 | |
| Positive | 22 | 35 | 19 | |
| Serum LDH | .007 | |||
| Normal | 52 | 72 | 54 | |
| High | 63 | 47 | 36 | |
| IPI | < .00001 | |||
| Low risk | 37 | 83 | 82 | |
| Low/intermediate risk | 26 | 65 | 36 | |
| High/intermediate risk | 22 | 55 | 32 | |
| High risk | 33 | 26 | 16 | |
| Bcl-2 expression | .03 | |||
| Negative (25% or less) | 52 | 62 | 57 | |
| Positive (more than 25%) | 74 | 54 | 34 | |
| Differentiation pattern | NS | |||
| GC-CD10+ | 24 | 50 | 40 | |
| GC-CD10− | 30 | 69 | 54 | |
| pGC | 60 | 55 | 42 |
| . | n7-150 . | CR rate, % . | 5-year OS, % . | P . |
|---|---|---|---|---|
| Age, y | .004 | |||
| Younger than 60 | 45 | 69 | 60 | |
| 60 years or older | 84 | 52 | 34 | |
| B symptoms | .05 | |||
| No | 77 | 64 | 50 | |
| Yes | 50 | 47 | 34 | |
| Performance status | .00001 | |||
| ECOG 0,1 | 67 | 72 | 62 | |
| ECOG 2-4 | 58 | 40 | 22 | |
| Ann Arbor stage | .00002 | |||
| I/II | 62 | 73 | 62 | |
| III/IV | 65 | 41 | 25 | |
| Bulky disease | .001 | |||
| No | 91 | 65 | 53 | |
| Yes | 34 | 39 | 23 | |
| Extranodal involvement | ||||
| 2 or fewer sites | 98 | 61 | 49 | .004 |
| More than 2 sites | 30 | 43 | 23 | |
| Bone marrow | .016 | |||
| Negative | 102 | 62 | 49 | |
| Positive | 22 | 35 | 19 | |
| Serum LDH | .007 | |||
| Normal | 52 | 72 | 54 | |
| High | 63 | 47 | 36 | |
| IPI | < .00001 | |||
| Low risk | 37 | 83 | 82 | |
| Low/intermediate risk | 26 | 65 | 36 | |
| High/intermediate risk | 22 | 55 | 32 | |
| High risk | 33 | 26 | 16 | |
| Bcl-2 expression | .03 | |||
| Negative (25% or less) | 52 | 62 | 57 | |
| Positive (more than 25%) | 74 | 54 | 34 | |
| Differentiation pattern | NS | |||
| GC-CD10+ | 24 | 50 | 40 | |
| GC-CD10− | 30 | 69 | 54 | |
| pGC | 60 | 55 | 42 |
NS indicates not significant.
The n shown is the number of patients for whom data were available.
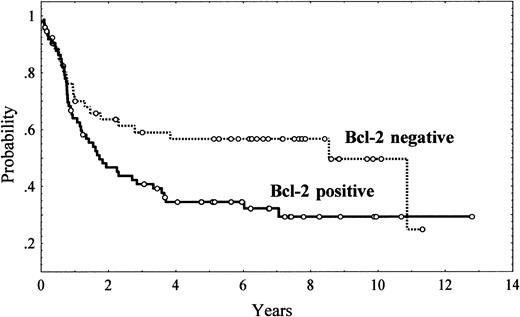
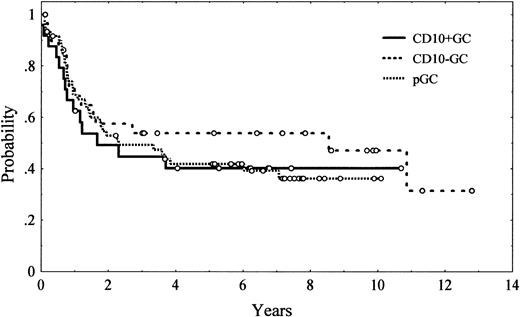
Five-year OS was 43% (95% CI, 34%-52%; Figure 1). Unfavorable clinical variables for OS were age older than 60 years (P = .004), presence of B symptoms (P = .05), poor performance status (ECOG > 2; P = .00001), presence of bulky disease (P = .001), advanced Ann Arbor stage (III-IV; P = .00002), extranodal involvement at more than 1 site (P = .004), bone marrow involvement (P = .01), low serum albumin levels (P = .02), high serum LDH levels (P = .007), and high β2-microglobulin levels (P = .02). The IPI also had a high value for predicting OS (P < .00001). Regarding immunohistologic parameters, bcl-2 expression had an unfavorable impact on OS, with a 5-year OS of 34% (95% CI, 22%-46%) and 57% (95% CI, 43%-71%) for bcl-2–positive and bcl-2–negative patients, respectively (P = .03; Figure2). No other single antigen, including CD10, bcl-6, MUM1, p53, p27, and Ki-67, showed significant influence on OS. Morphologic subtypes (centroblastic vs immunoblastic) did not reach significant prognostic value. The differences in OS found among the different patterns of differentiation did not reach statistical significance either (Table 7, Figure 3).
Overall survival of 126 patients with diffuse large B-cell lymphoma according to tumor bcl-2 expression.
P = .03.
Overall survival of 126 patients with diffuse large B-cell lymphoma according to tumor bcl-2 expression.
P = .03.
Overall survival of 114 patients with diffuse large B-cell lymphoma according to tumor immunophenotyping profile.
Tumor immunophenotyping profiles were as follows: germinal center–CD10-positive (CD10+GC), germinal center–CD10-negative (GC-CD10−GC), post–germinal center (pGC).
Overall survival of 114 patients with diffuse large B-cell lymphoma according to tumor immunophenotyping profile.
Tumor immunophenotyping profiles were as follows: germinal center–CD10-positive (CD10+GC), germinal center–CD10-negative (GC-CD10−GC), post–germinal center (pGC).
Finally, to assess the clinical interest of bcl-2 expression, a multivariate analysis was performed including all the significant variables predicting OS in the univariate analysis, namely, age (< 60 years vs > 60 years]), B symptoms, performance status (ECOG < 2 vs ECOG ≥ 2), bulky disease, Ann Arbor stage (I/II vs III/IV), extranodal involvement (< 2 sites vs ≥ 2 sites), bone marrow infiltration, bcl-2 expression (negative vs positive), and serum LDH (normal vs high). Serum albumin and β2-microglobulin were excluded because of the relatively high number of missing data. In this model, with 109 patients for whom all data were available, bcl-2 expression kept its prognostic value for OS (P = .01; relative risk [RR] = 2.1 [SE = 0.29]), along with performance status (P = .0005; RR = 1.9 [SE = 0.28]) and bulky disease (P = .05; RR = 1.74 [SE = 0.29]). Furthermore, when bcl-2 expression was included in the Cox model along with the IPI (low-risk vs low/intermediate–risk vs high/intermediate–risk vs high-risk), bcl-2 maintained a trend for independent prognostic value (P = .07; RR = 1.6 [95% CI, 1.1-2.1]), although the IPI remained the most significant variable (P < .00001; RR = 1.8 [95% CI, 1.6-2.0]).
Discussion
DLBCLs are a heterogeneous group of neoplasms in which previous morphological, phenotypic, genetic, and molecular studies have not been able to identify well-defined disease entities with clinical and therapeutic relevance. Gene expression profile examined by microarray technology has identified 2 main groups of DLBCLs: those in which the expression profile was similar to that of normal GC B cells (the so-called GC-like DLBCLs) and those with an expression pattern similar to that of in vitro–activated peripheral blood B cells (activated B-like DLBCLs).15 Besides the biological significance of these profiles as representative of different cell origins, striking differences in the outcomes of patients according to expression profile were initially reported by Alizadeh et al15 and more recently confirmed in a larger series of patients by Rosenwald et al,17 with patients having activated B-like lymphomas having a worse prognosis. An additional study using a different oligonucleotide microarray also identified these 2 groups of DLBCLs but was not able to confirm the prognostic significance of these categories.16 In addition, these microarray studies have identified different series of genes with predictive survival value independent of the IPI and the cell-of-origin category of the tumors.16 17
These interesting results lead to the question of how the microarray-based studies could be extended, using methods more widely available in clinical practice. In the present study, we evaluated the clinical and prognostic significance of the phenotypic profile related to a germinal or postgerminal follicular center differentiation stage in a large series of patients with DLBCL, and we used conventional immunophenotyping techniques to evaluate the possible impact of different oncogenic and proliferative markers on the biological behavior of the tumor. For these purposes, we selected some of the most representative antigens related to the differentiation profiles: CD10 and bcl-6 as GC markers and MUM1 and CD138 as markers associated with a B-cell post-GC profile. In addition, we studied other oncogenic and proliferative proteins, such as bcl-2, p53, p27, and Ki-67. Although the expression profile related to the stage of differentiation was not of prognostic significance in this series of tumors, these subgroups of DLBCLs were associated with different clinicopathological features of the patients, suggesting that these subgroups may represent different biological entities. In addition, bcl-2 overexpression was an independent prognostic parameter in these tumors, indicating that factors other than the differentiation profile may influence the biological behavior of DLBCLs.
Two of the antigens included in this study, CD10 and bcl-6, are normally expressed in follicular GCs and were also identified by cDNA microarray analysis as preferentially expressed in GC-derived DLBCLs. In addition, CD10 expression in DLBCL has also been associated with the presence of the t(14;18) translocation, indicating a possible origin of these tumors in germinal center–derived cells,34-36 as occurred in our tested patients, where bcl-2 rearrangement was associated with CD10 expression and GC-CD10+ patients. All our patients expressing CD10 were also positive for bcl-6, whereas a group of patients with bcl-6–positive tumors did not express CD10. Interestingly, patients with a CG CD10+ profile presented with more disseminated disease and frequently showed a centroblastic morphology, suggesting that these tumors may represent a diffuse large-cell variant of follicular lymphomas, which also present frequently with advanced stages.
The bcl-6 gene is normally expressed in B and CD4+ T cells of the follicular GCs, and it is necessary for GC formation.37,38 Rearrangements and point mutations of this gene have been detected in a number of DLBCLs, with conflicting results regarding their relationship with extranodal origin and their potential prognostic significance.39,40 Bcl-6 protein is expressed in the vast majority of follicular lymphomas and in 70% to 95% of DLBCLs, and this expression is apparently not related to gene alterations. Bcl-6 mRNA expression also segregates with other germinal center cell markers identified by microarray analysis, suggesting that DLBCLs expressing bcl-6 may originate in this type of cell. Bcl-6 protein and mRNA overexpression in DLBCL has been recently associated with a better prognosis.14 In our study, bcl-6 expression was identified in 91 (72%) of the tumors; 24 of these patients coexpressed CD10, 39 were positive for bcl-6 and MUM1, and 30 had a restricted bcl-6 reactivity. The group of patients with restricted expression of bcl-6 (GC-CD10−) presented with earlier stage, low-risk IPI, and normal LDH values. However, although this group of patients had the highest OS rate, the difference did not reach statistical significance. Similarly, no significant differences in survival were observed when all patients expressing bcl-6, patients with nodal or extranodal tumors, and patients coexpressing bcl-6 and CD10 were independently analyzed. The reasons for these discordant results between bcl-6 expression and survival in different series are not clear, but they may be related in part to the heterogeneity of the selected patients. Lossos et al observed a strong predictive value for survival of bcl-6 protein and mRNA overexpression in DLBCLs.14 However, all tumors in this study had the histologic appearance of centroblastic large-cell lymphomas. On the other hand, protein expression was examined in only 30 patients. Barrans et al recently demonstrated that a germinal center phenotype defined by CD10 and bcl-6 coexpression was associated with a significantly longer survival. However, this study was restricted to primary nodal DLBCLs and had a smaller number of patients with advanced stage and high-risk IPI than we examined in the present series.10
MUM1/IRF4 is a novel member of the interferon regulatory factor (IRF) family of genes41-43 that plays an important role in the regulation of gene expression in response to signaling by interferons and other cytokines.44-46 In normal B cells, MUM1 and bcl-6 have a pattern of mutually exclusive positivity.47Moreover, the absence of expression of MUM1 in IgD+/IgM+ follicle mantle cells,47the absence of plasma cells and the marked reduction of serum immunoglobulins in IRF4–/– mice,48and the segregation of MUM1 mRNA expression into activated B-like DLBCLs by microarray analysis15 have suggested a role for this gene in terminal phases of B-cell differentiation, and also its importance in recognizing post-GC DLBCLs. On the basis of these findings, MUM1-positive tumors in our study were assigned to a post-GC stage of differentiation. In our study, MUM1 expression was detected in 68 patients (54%), and 39 (57%) of the 68 coexpressed bcl-6. These findings are similar to those of previous studies in which MUM1 expression was observed in 51% to 75% of DLBCLs and, contrary to normal cells, coexpression with bcl6 was detected in about 50% of patients.47 The pGC phenotype was significantly associated with an immunoblastic morphology of the tumors and a primary nodal presentation.
One of the most important issues in DLBCL is to refine the standard prognostic scores, including IPI, which are mainly based on clinical parameters. Activated B-cell–like tumors, as defined by microarrays,15,17 had an unfavorable outcome. In the present series, although patients with pGC tumors showed a lower CR rate and OS than those with GC-CD10− tumors (a difference that did not reach statistical significance), the OS curves of GC-CD10+ and pGC patients could not be differentiated. These results suggest that the use of a limited number of antigens to define different subtypes of DLBCL may not capture the full spectrum of tumors with activated B-cell–like and GC B-cell–like derived profiles as defined by microarray technology. In our study, bcl-2 protein overexpression, but no other single antigen, showed predictive value for OS. In concordance with previous studies, bcl-2 overexpression was detected in 59% of our patients, and it was significantly associated with higher Ann Arbor and IPI stages and shorter overall survival.10,18,21 22 Bcl-2 overexpression kept its prognostic significance in a multivariate analysis, although IPI remained the most significant prognostic parameter.
In summary, the differentiation patterns, as assessed by immunophenotyping, were associated with particular clinicopathological features of patients with DLBCL. However, in the present series, these profiles were not the essential variable in determining the outcome of DLBCL patients.
We thank Montse Sánchez for excellent technical assistance. Montse Sánchez was supported in part by Dako.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-04-1286.
Supported in part by grant SAF 02/3261 from the Comisión Interministerial de Ciencia y Tecnologı́a (CICYT); grant FIS 99/189 from the Fondo de Investigación Sanitaria, Spain; grant AR02/37k from the Deutsche Josep Carreras Leukämie-Stiftung eV, and a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. López-Guillermo, Department of Hematology, Hospital Clı́nic, Villarroel 170, 08036 Barcelona, Spain; e-mail: alopezg@clinic.ub.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal