Abstract
Rhnull red cells are characteristically stomato-spherocytic. This and other evidence suggest that the Rh complex represents a major attachment site between the membrane lipid bilayer and the erythroid skeleton. As an attempt to identify the linking protein(s) between the red cell skeleton and the Rh complex, we analyzed the expression of Rh, RhAG, CD47, LW, and glycophorin B proteins in red cells from patients with hereditary spherocytosis associated with complete protein 4.2 deficiency but normal band 3 (4.2(-)HS). Flow cytometric and immunoblotting analysis revealed a severe reduction of CD47 (up to 80%) and a slower mobility of RhAG on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, possibly reflecting an overglycosylation state. Unexpectedly, 4.2−/− mice, which are anemic, displayed a normal red cell expression of CD47 and RhAG. These results suggest that human protein 4.2, through interaction with CD47, is involved in the skeleton linkage and/or membrane translocation of the Rh complex. However, these potential role(s) of protein 4.2 might be not conserved across species. Finally, the absence or low expression of red cell CD47 in CD47−/− mice and in some humans carrying RHCEgene variants (D--, D.., and RN), respectively, had no detectable effect on protein 4.2 and RhAG expression. Since these cells are morphologically normal with no sign of hemolysis, it is assumed that CD47 deficiency per se is not responsible for the cell shape abnormalities and for the compensated hemolytic anemia typical of 4.2(-) and Rhnull red cells.
Introduction
The Rh antigens are defined by the association of membrane polypeptides which are missing from or severely reduced in red blood cells (RBCs) of rare Rhnull individuals who suffer a clinical syndrome characterized by abnormalities of the red cell shape, cation transport, and membrane phospholipid organization (reviewed in Cartron1 and Huang et al2). The core of this complex is thought to be a tetramer composed of 2 Rh and 2 RhAG subunits3 to which accessory chains (CD47, LW, and glycophorin B [GPB]) are associated by noncovalent linkages.4-7 Rh and RhAG proteins are encoded by homologous genes and are erythroid specific.8,9 However, homologs of RhAG (RhBG and RhCG or RhGK) are expressed in nonerythroid tissues, thus defining a new RH gene superfamily.10
Recent studies have shed new light on potential biologic properties and functions of the Rh and Rh-related proteins.11 Rh proteins exhibit significant homology with the NH transporter of the Mep/Amt superfamily present in microorganisms that use NH as a nitrogen source.12 When expressed as recombinant proteins in yeast and Xenopusoocytes, RhAG and its kidney homolog RhGK (also called RhCG) mediate a bidirectional ammonium transport activity.13,14 Several lines of evidence also suggest that the Rh complex might participate in the association between the membrane lipid bilayer and the red cell skeleton. Indeed, initial studies have shown that the RhD antigen is in part resistant to membrane solubilization by nonionic detergent,15-17 whereas GPB and LW can be completely extracted by 1% Triton X-100.18,19 These results have been confirmed and further extended by flow cytometric analysis of blood group antigen expression on untreated and Triton X-100–treated recombinant cell lines, and erythroid progenitors during their proliferation and differentiation in vitro.20 Such studies revealed that the major members of the Rh complex, Rh(D and CcEe), RhAG, and CD47, remained predominantly associated with the detergent insoluble material of these cells. Recently, more direct evidence of the interaction between the Rh complex and the membrane skeleton has been provided by fluorescence-imaged microdeformation, a method which quantifies in situ the redistribution of fluorescently labeled red blood cell (RBC) membrane proteins during mechanically induced membrane deformation following cell aspiration into a micropipette.21 During these experiments, the behavior of Rh (D and CcEe) and CD47 proteins was intermediate between that of actin and band 3, whereas the behavior of RhAG was similar to that of actin22,23 (N. Mohandas and J.P.C., unpublished results, October 2002). Together, these results imply that the Rh complex is firmly linked to the actin-spectrin–based RBC membrane skeleton, presumably via Rh and/or RhAG and/or CD47. Taking into account the great abundance of Rh/RhAG tetramers on erythrocytes (about 100-200 000/RBC), it is assumed that the Rh complex might represent, together with the well-described band 3-ankyrin-protein 4.2 and GPC-protein 4.1-p55 complex,24-26 another major attachment site between the membrane lipid bilayer and the red cell skeleton and therefore might contribute to the membrane structure and stability of RBCs. Supporting this finding, the deleterious mutations identified in the RHor RHAG genes from Rhnull patients of the amorph or regulator types, respectively, are associated with stomato-spherocytosis and hemolytic anemias of varying severity.1 However, the precise linkages between membrane skeleton proteins and proteins of the Rh complex have not been defined.
In this study, we show that RBCs from 2 unrelated patients with hereditary spherocytosis (HS) associated with a complete lack of protein 4.2 due to distinct mutations in the EPB42 gene (4.2(-)HS)27 28 exhibit a severe reduction of CD47 level together with a modified electrophoretic pattern of RhAG. These results suggest that protein 4.2, through interaction with CD47, is involved in the linkage of the Rh complex to the red cell skeleton and/or in its translocation to the cell membrane.
Materials and methods
Blood samples
All blood samples were collected in EDTA (ethylenediaminetetraacetic acid)–coated blood tubes. They included 2 variants of protein 4.2, protein 4.2 Lisboa (C265del),27and protein 4.2 Nancy (G949del),28 causing hereditary spherocytosis associated with missing protein 4.2 (4.2(-)HS). Complete clinical data are provided in Hayette et al27 and Beauchamp-Nicoud et al.28 They also included specimens from persons presenting with the following Rh phenotypes: D--,29 D..,1 and RN,30 which carry at the homozygous state a rearranged RHCE gene causing no (D--) or low (D.., RN) expression of the RhCcEe antigens; Rhnullof the amorph (DAA) and regulator (Bra.) types carry mutations in the RH or RHAG genes, respectively.1 Blood samples from common RhD-positive (DCCee) and RhD-negative (ddccee or ddCcee) phenotypes were obtained from the Centre National de Référence sur les groupes Sanguins (CNRGS; Paris, France). Homozygous gene-targeted mice deficient for CD47 or protein 4.2 were previously described.31 32 Control mouse RBCs were from wild-type 129/Sv or Balb/c animals.
Antibodies
Murine monoclonal antibodies (mMAbs) were as follows: anti-Rh (clone BRIC69) and anti-Band3 (clone BRIC 6) were from Dr D. Anstee (Bristol, United Kingdom); anti-RhAG (clone 2D10 and LA 18.18) was from Dr A. von dem Borne (Amsterdam, The Netherlands); anti-CD47, clone 6H9, was from Dr M. J. Telen (Durham, NC), clone B6H12 was from F. Lindberg (St Louis, MO), clones 3E12, 5A3, and F8C10 were from Bioatlantic (Nantes, France); anti-LW (clone BS56) was from Dr H. H. Sonneborn (Offenbach, Germany); anti-GPA (clone R18) was from Dr P. A. W. Edwards (Cambridge, United Kingdom); anti-Lub (clone LM342) was from Dr R. H. Fraser (Glasgow, United Kingdom); anti-CD44 (clone 9D6 and A3D8) were from Bioatlantic; anti-K2 (clone F7) was from Dr P. Rubinstein (New York, NY); anti-GPC (clone MR4-130) was from Dr D. Goossens (Institut National de Transfusion Sanguine [INTS], Paris, France); anti-Fy6 (clone 2C3) has been described previously.33
Human monoclonal antibodies (hMAbs) were as follows: anti-D (clone LOR15C9) was from A. Blancher (Toulouse, France); anti-Rhc (clone RaE11), anti-RhE (clone MCG8), and anti-K1 (clone T27S) were from the INTS; and anti-C was from Diagast (Bordeaux, France).
Polyclonal antibodies (PAbs) were as follows: anti-Rhe was from the Etablissement de Transfusion Sanguine (Lyon, France); anti-s was from Oxytèle (Versailles, France); anti-S was from Bioatlantic; anti-Co3 (Sar. serum) was from the CNRGS; MPC8 raised against the C-terminal region of the Rh polypeptides was described earlier34; anti-p55 raised against synthetic peptide residues 25-47 of the human p55 cytoplasmic protein35 were from P. Bailly (INTS); anti-4.2 and anti-protein 4.1 were described elsewhere.27 36
Membrane preparations and Triton X-100 extractions
Membranes from washed RBCs were prepared by hypotonic lysis.37 All fractions were prepared from red cells that had been deep-frozen under preservative medium, a procedure known to destroy reticulocytes. Accordingly, the initial reticulocyte counts would not interfere. Solubilization by nonionic detergent was performed by adding 150 μL of 1% Triton X-100 to 50 μL of packed ghosts in phosphate-buffered saline (PBS) and gentle shaking at 4°C for 15 minutes. Supernatants and pellets were separated by cenrifugation (20 000g for 30 minutes at 4°C). Pellets were resuspended in 200 μL of 1× Laemmli buffer. Equal volume of supernatant and pellet fractions were loaded on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide) under nonreducing conditions.
Western blot analysis
For Western blot analysis, membrane proteins from whole ghost lysates or Triton X-100 soluble and insoluble fractions separated on SDS-PAGE (10% acrylamide) were transferred to filters and incubated with relevant primary antibodies. Following washing, the filters were incubated with the appropriate peroxidase-conjugated secondary antibody (anti–rabbit, anti–mouse, or anti–rat IgG) (Biosys, Compiègne, France). Immunoblots were visualized using the ECL chemiluminescent system (Amersham, Bucks, United Kingdom).
Flow cytometric analysis
Frozen blood cell samples, containing only mature RBCs from variant and control donors were resuspended in 50 μL PBS/0.2% (wt/vol) bovine serum albumin (BSA) and incubated for 30 minutes at 22°C with mMAbs or hMAbs, respectively. After several washes with PBS, the cells were incubated for 30 minutes at 22°C with fluorescein isothiocyanate (FITC)–conjugated F(ab')2 fragments of goat anti–mouse and of goat anti–human immunoglobulins (Immunotech, Marseille, France). When using anti–mouse antibodies, the cell surface antigen expression was quantified using calibration mouse IgG-coated beads (Qifikit; DAKO, Glostrup, Denmark) as standards, according to the manufacturer's instructions. The results were expressed as specific antibody binding capacity (SABC) units that proved to be directly proportional to the number of molecules bound per cell. When detected by human Mabs, antigen expression was estimated from the mean fluorescence intensity (MFI), given in arbitrary units.
Results
Flow cytometric analysis of 4.2(-)HS RBCs
Expression of the Rh complex on RBCs from the patients with the 4.2(-)Lisboa and 4.2(-)Nancy mutations was quantitatively estimated by flow cytometric analysis of Rh, RhAG, CD47, LW, and GPB expression level with specific MAbs and PAbs. Since the 4.2(-)Lisboa and 4.2(-)Nancy RBCs exhibited the DCcee and dccee phenotypes, respectively, normal RhD-positive and RhD-negative RBCs were used as controls. As shown in Table 1. this analysis revealed that CD47 expression was severely reduced (by 80%) in RBCs from both 4.2(-) patients, whereas Rh and RhAG were normally expressed. Anti–S and anti–s antibodies revealed that 4.2(-)Lisboa and 4.2(-)Nancy RBCs exhibited a normal expression level of GPB with the Ss and SS phenotypes, respectively. As expected, more LW was found on control RhD-positive than on RhD-negative RBCs, and the LW status of 4.2(-)Lisboa and 4.2(-)Nancy RBCs was in accordance with their RhD-positive and RhD-negative status, respectively.
Flow cytometric analysis of 4.2(-)HS RBCs for expression of Rh complex proteins and various blood group-carrying proteins
| Protein . | . | RhD-negative (SD) . | RhD-positive (SD) . | 4.2(-)Lisboa (SD) . | 4.2(-)Nancy (SD) . |
|---|---|---|---|---|---|
| MAb* | SABC | SABC | SABC | SABC | |
| Rh | BRIC69 | 135 200 (± 283) | 131 500 (± 7 778) | 111 500 (± 707) | 115 000 (± 657) |
| RhAG | LA18.18 | 90 450 (± 2 192) | 87 500 (± 10 182) | 109 500 (± 3 536) | 111 000 (± 4 243) |
| CD47 | 6H9 | 26 150 (± 3 182) | 23 600 (± 1 980) | 5 800 (± 141) | 5 400 (± 10) |
| CD47 | 3E12 | 19 400 (± 1 556) | 18 350 (± 1 626) | 4 750 (± 71) | 4 200 (± 141) |
| LW | BS56 | 615 (± 120) | 1 525 (± 106) | 2 050 (± 71) | 100 (± 71) |
| Band 3 | BRIC6 | 408 000 (± 15 156) | 384 500 (± 23 335) | 351 000 (± 14 142) | 349 500 (± 4 950) |
| GPA | R18 | 357 500 (± 707) | 337 000 (± 19 799) | 302 000 (± 5 657) | 288 000 (± 7 071) |
| GPC | MR4-130 | 51 650 (± 1 909) | 49 100 (± 8 344) | 61 700 (± 990) | 55 000 (± 1 414) |
| DARC | anti-Fy6 2C3 | 2 500 (± 283) | 3 250 (± 778) | 5 050 (± 212) | 3 950 (± 212) |
| Kell | anti-k F7 | 2 250 (± 71) | 4 050 (± 71) | 4 900 (± 141) | 4 450 (± 212) |
| CD44 | 9D6 | 4 150 (± 71) | 3 950 (± 636) | 13 600 (± 283) | 11 200 (± 283) |
| Lu | F241 | 1 300 (± 141) | 1 700 (± 283) | 1 800 (± 141) | 1 250 (± 71) |
| PAb† | MFI | MFI | MFI | MFI | |
| AQP1 | anti-Co3 | 256 (± 15) | 243 (± 48) | 368 (± 18) | 240 (± 33) |
| GPB | anti-S | 2 (± 0) | 39 (± 23) | 41 (± 23) | 5 (± 1) |
| GPB | anti-s | 243 (± 10) | 66 (± 2) | 41 (± 2) | 231 (± 16) |
| JK | anti-Jk3 | 68 (± 12) | 94 (± 0) | 94 (± 25) | 92 (± 26) |
| Protein . | . | RhD-negative (SD) . | RhD-positive (SD) . | 4.2(-)Lisboa (SD) . | 4.2(-)Nancy (SD) . |
|---|---|---|---|---|---|
| MAb* | SABC | SABC | SABC | SABC | |
| Rh | BRIC69 | 135 200 (± 283) | 131 500 (± 7 778) | 111 500 (± 707) | 115 000 (± 657) |
| RhAG | LA18.18 | 90 450 (± 2 192) | 87 500 (± 10 182) | 109 500 (± 3 536) | 111 000 (± 4 243) |
| CD47 | 6H9 | 26 150 (± 3 182) | 23 600 (± 1 980) | 5 800 (± 141) | 5 400 (± 10) |
| CD47 | 3E12 | 19 400 (± 1 556) | 18 350 (± 1 626) | 4 750 (± 71) | 4 200 (± 141) |
| LW | BS56 | 615 (± 120) | 1 525 (± 106) | 2 050 (± 71) | 100 (± 71) |
| Band 3 | BRIC6 | 408 000 (± 15 156) | 384 500 (± 23 335) | 351 000 (± 14 142) | 349 500 (± 4 950) |
| GPA | R18 | 357 500 (± 707) | 337 000 (± 19 799) | 302 000 (± 5 657) | 288 000 (± 7 071) |
| GPC | MR4-130 | 51 650 (± 1 909) | 49 100 (± 8 344) | 61 700 (± 990) | 55 000 (± 1 414) |
| DARC | anti-Fy6 2C3 | 2 500 (± 283) | 3 250 (± 778) | 5 050 (± 212) | 3 950 (± 212) |
| Kell | anti-k F7 | 2 250 (± 71) | 4 050 (± 71) | 4 900 (± 141) | 4 450 (± 212) |
| CD44 | 9D6 | 4 150 (± 71) | 3 950 (± 636) | 13 600 (± 283) | 11 200 (± 283) |
| Lu | F241 | 1 300 (± 141) | 1 700 (± 283) | 1 800 (± 141) | 1 250 (± 71) |
| PAb† | MFI | MFI | MFI | MFI | |
| AQP1 | anti-Co3 | 256 (± 15) | 243 (± 48) | 368 (± 18) | 240 (± 33) |
| GPB | anti-S | 2 (± 0) | 39 (± 23) | 41 (± 23) | 5 (± 1) |
| GPB | anti-s | 243 (± 10) | 66 (± 2) | 41 (± 2) | 231 (± 16) |
| JK | anti-Jk3 | 68 (± 12) | 94 (± 0) | 94 (± 25) | 92 (± 26) |
Specific antibody binding capacity (SABC), as determined by indirect immunofluorescence using QIFIKIT calibrated beads (see “Materials and methods”).
Mean fluorecence intensity (MFI; arbitrary units). The mean values of 3 independant experiments are given.
The mean fluorescence intensity (MFI) values measured with human MAbs or PAbs raised against several blood group antigens or blood group–carrying proteins, including band 3, GPA, and GPC, that belong to the ankyrin-protein 4.2-band 3 and protein 4.1-p55-GPC complexes, respectively, did not reveal any deviation from normality in the 4.2(-) RBCs (Table 1). The only exception was CD44 whose expression was found to be 3-fold higher in the 4.2(-) RBCs, as compared with controls.
Western blot analysis of 4.2(-)HS RBCs
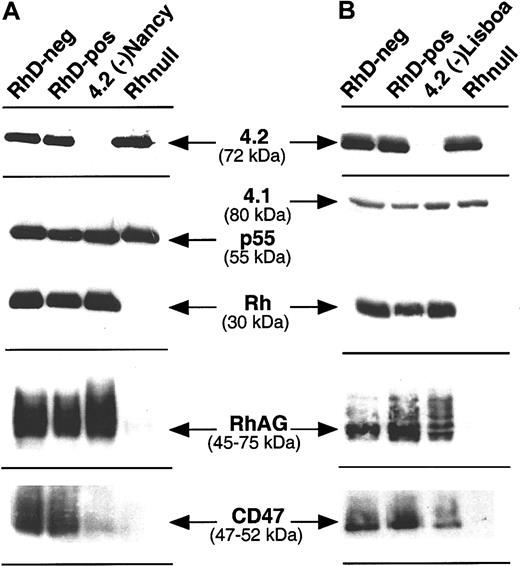
Accordingly to previous findings,27,28 we did not detect protein 4.2 in patients carrying the Lisboa and the Nancy mutations (Figure 1). The major 72-kDa protein 4.2 isoform was detected in equal amounts in RBC membrane proteins prepared from donors with the RhD-positive, RhD-negative, and Rhnull phenotypes (Figure 1A-B). As quantitative and qualitative controls, the p55 and 4.1 proteins, which are part of the GPC-4.1-p55 complex whose expression is independent of Rh, were detected with the same intensity in all samples. Then, the same blots were successively incubated with antibodies against Rh, RhAG, and CD47, the major members of the Rh complex. As positive and negative controls, anti-Rh, -RhAG, and -CD47 antibodies gave strong signals with membrane proteins from RhD-positive and RhD-negative individuals, but no staining or a very weak staining with RBC membranes from Rhnull individuals, as expected.1 In agreement with the flow cytometric analysis, a clear reduction of the amount of CD47 polypeptide and a normal level of Rh polypeptides were observed in both 4.2(-) RBC membrane preparations, using the B6H12 and MPC8 antibodies, respectively. While flow cytomeric analysis indicated normal expression of RhAG at the cell surface of 4.2(-) RBCs (see Table1), careful examination of the immunostaining experiment with MAb 2D10 which recognizes the RhAG protein (45-70 kDa) revealed a slightly lower electrophoretic mobility of the RhAG component from the 4.2(-) samples as compared with controls.
Immunostaining of RBC membrane proteins from 4.2(-)Nancy and 4.2(-)Lisboa HS erythrocytes.
RhD-positive (DCCee), RhD-negative (dccEe), and Rhnull(amorph type) membrane proteins were used as controls. Anti–protein 4.2 polyclonal antibody was used to confim the lack of the 72-kDa band in the 2 samples of 4.2(-). Antibodies against the major members of the Rh complex were as follows: anti-Rh (D+ CcEe): PAb MPC8; anti-RhD: MoAb LOR15-C9; anti-RhAG: MoAb LA18.18; anti-CD47: MoAb 6H9. PAbs against the p55 and 4.1 proteins, which belong to the GPC-4.1-p55 membrane complex, were used as controls to show that similar amounts of membrane proteins were present on each lane.
Immunostaining of RBC membrane proteins from 4.2(-)Nancy and 4.2(-)Lisboa HS erythrocytes.
RhD-positive (DCCee), RhD-negative (dccEe), and Rhnull(amorph type) membrane proteins were used as controls. Anti–protein 4.2 polyclonal antibody was used to confim the lack of the 72-kDa band in the 2 samples of 4.2(-). Antibodies against the major members of the Rh complex were as follows: anti-Rh (D+ CcEe): PAb MPC8; anti-RhD: MoAb LOR15-C9; anti-RhAG: MoAb LA18.18; anti-CD47: MoAb 6H9. PAbs against the p55 and 4.1 proteins, which belong to the GPC-4.1-p55 membrane complex, were used as controls to show that similar amounts of membrane proteins were present on each lane.
Expression of CD47 and protein 4.2 in RBCs from variant Rh phenotypes
Altered expression of CD47 was also detected in RBCs with some rare Rh variant phenotypes using several anti–CD47 MAbs (Table2). As compared with RhD-positive and RhD-negative controls, RBCs from D-- and D.. phenotypes exhibited about 70% to 75% reduction in CD47 expression, a value similar to that observed with the 4.2(-) samples. A significant albeit less important decrease of CD47 was also observed in RBCs from Rh variants of the RN phenotype. However, CD47 expression was not altered in monocytes (750 000 ± 10 000 molecules/cell) and platelets (6000 ± 1000 molecules/cell) from these variants, as compared with controls (not shown). Western blot analysis confirmed that the CD47 expression was reduced in D-- and D.. RBCs to a level similar to that observed in the 4.2(-) samples (Figure2A). As previously shown, CD47 was nearly undetectable in Rhnull membrane preparations.1The protein 4.2 level and the electrophoretic migration of RhAG were not affected by the reduction of CD47 in D-- and Rhnull RBCs (Figure 2B).
Flow cytometric analysis showing CD47 reduction in 4.2(-) and Rh variant red cells
| . | . | RhD-neg, SABC . | RhD-pos, SABC . | Rhnull (reg), SABC . | Rhnull (amorph), SABC . | 4.2(-), SABC . | D--, SABC . | D.., SABC . | RN, SABC . |
|---|---|---|---|---|---|---|---|---|---|
| Protein | MAb | ||||||||
| Rh | BRIC69 | 130 | 145 | 0 | 0 | 140.0 | 100.0 | 81.5 | 142.0 |
| RhAG | LA18.18 | 120 | 114 | 0 | 21.6 | 119.0 | 93.0 | 61.0 | 118.0 |
| CD47 | 6H9 | 28 | 28 | 2.2 | 2.2 | 6.6 | 6.2 | 6.2 | 17.4 |
| CD47 | 3E12* | 20 | 19 | 2.1 | 2.1 | 5.5 | 5.4 | 5.2 | 12.3 |
| . | . | RhD-neg, SABC . | RhD-pos, SABC . | Rhnull (reg), SABC . | Rhnull (amorph), SABC . | 4.2(-), SABC . | D--, SABC . | D.., SABC . | RN, SABC . |
|---|---|---|---|---|---|---|---|---|---|
| Protein | MAb | ||||||||
| Rh | BRIC69 | 130 | 145 | 0 | 0 | 140.0 | 100.0 | 81.5 | 142.0 |
| RhAG | LA18.18 | 120 | 114 | 0 | 21.6 | 119.0 | 93.0 | 61.0 | 118.0 |
| CD47 | 6H9 | 28 | 28 | 2.2 | 2.2 | 6.6 | 6.2 | 6.2 | 17.4 |
| CD47 | 3E12* | 20 | 19 | 2.1 | 2.1 | 5.5 | 5.4 | 5.2 | 12.3 |
Specific antibody binding capacity (SABC) values (× 10−3).
Similar values were obtained with the B6H12, 5A3, and F8C10 anti-CD47 mAbs (not shown).
Immunostaining of RBC membrane proteins from Rh variant red cells.
Antibodies used for immunoblotting were as in Figure 1. (A) Sharp reduction of CD47 in D-- and D.. red cells, characterized by the lack of expression of Cc and Ee antigens. (B) Normal migration pattern of RhAG in D-- samples, as compared with the abnormal migration in the 4.2(-) sample, used as control. 4.2-p55 stoichiometry revealed normal expression of protein 4.2 in the D-- sample. Similar results were obtained with the D.. and with other D-- samples (not shown).
Immunostaining of RBC membrane proteins from Rh variant red cells.
Antibodies used for immunoblotting were as in Figure 1. (A) Sharp reduction of CD47 in D-- and D.. red cells, characterized by the lack of expression of Cc and Ee antigens. (B) Normal migration pattern of RhAG in D-- samples, as compared with the abnormal migration in the 4.2(-) sample, used as control. 4.2-p55 stoichiometry revealed normal expression of protein 4.2 in the D-- sample. Similar results were obtained with the D.. and with other D-- samples (not shown).
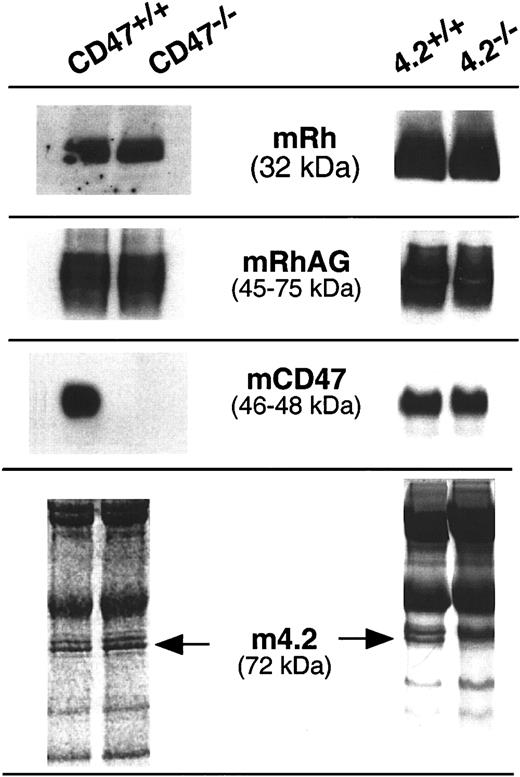
Expression of Rh-like complex in RBCs from 4.2−/− and CD47−/− mice
RBC membrane proteins isolated from control mice and 4.2−/− and CD47−/− gene-targeted mice were analyzed by Western blotting using antibodies raised against the murine homologs of RhAG and CD47 and with the MPC8 anti–human Rh antibody previously reported to cross-react with the mouse Rh polypeptide.38 As shown in Figure3, the 32-kDa and 45- to 75-kDa bands corresponding to murine Rh and RhAG polypeptides, respectively, were detected with the same intensity in all samples. Furthermore, the electrophoretic mobility of the RhAG glycoprotein was not modified in the gene-targeted samples as compared with controls. Immunostaining with the anti-CD47 antibody illustrated the lack of CD47 in CD47−/− mice and revealed a normal level of CD47 in the 4.2−/− sample. A normal CD47 expression in the absence of protein 4.2 was confirmed by flow cytometric analysis of 4.2−/− and control RBCs (MFI: 520 vs 540, respectively; not shown). Finally, Ponceau or Coomassie blue stainings revealed a normal protein 4.2 level in CD47−/− RBCs and, as expected, the lack of the 4.2-specific 72-kDa component in the 4.2−/− sample.
Western blot analysis of the Rh complex proteins in CD47−/− and 4.2−/− gene-targeted mice.
Murine Rh and RhAG proteins (mRh and mRhAG) were detected by rabbit polyclonal antibodies raised against the C-terminal regions of the human Rh polypeptides) and of the murine RhAG glycoprotein, respectively. Murine CD47 (mCD47) was detected by the miap301 MAb raised against murine CD47. Normal expression of protein 4.2 in CD47−/− mice was deduced after Coomassie or red Ponceau staining of the membrane proteins.
Western blot analysis of the Rh complex proteins in CD47−/− and 4.2−/− gene-targeted mice.
Murine Rh and RhAG proteins (mRh and mRhAG) were detected by rabbit polyclonal antibodies raised against the C-terminal regions of the human Rh polypeptides) and of the murine RhAG glycoprotein, respectively. Murine CD47 (mCD47) was detected by the miap301 MAb raised against murine CD47. Normal expression of protein 4.2 in CD47−/− mice was deduced after Coomassie or red Ponceau staining of the membrane proteins.
Comparison of detergent-solubilized properties of Rh, RhAG, and CD47 from normal and 4.2(-)HS human RBCs and from wild-type and 4.2−/− murine RBCs
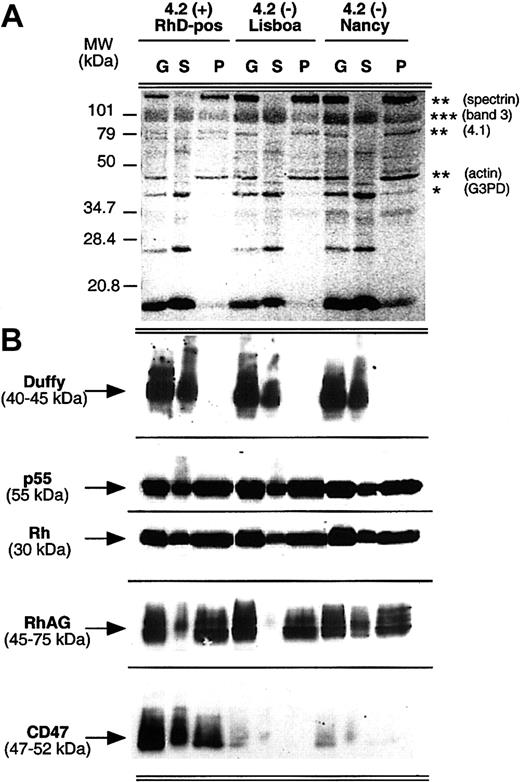
Triton X-100 extractibility of the Rh complex proteins from ghost membranes was compared for normal and 4.2(-)Lisboa and 4.2(-)Nancy RBCs. As shown in Figure 4, solubilization efficiency was identical in normal and variant samples, with skeleton proteins (spectrin, actin, protein 4.1) and G3PD or Duffy present only in the insoluble (pellet) and soluble pools, respectively. Immunostaining analysis of the Rh complex did not reveal any obvious difference of Rh and RhAG protein distribution between soluble and insoluble fractions whether protein 4.2 is present or missing. Analysis of normal RBCs revealed the presence of CD47 in both soluble and insoluble fractions. The distribution of CD47 between the soluble and insoluble fractions could not be assessed in the 4.2(-) samples, since as already shown, CD47 was barely detected in these RBCs (Figures 1and 2).
Comparison of Triton X-100 extractibility of the Rh complex from normal and 4.2(-)HS red blood cells.
Human erythrocyte ghosts (G) were extracted with 1% Triton X-100, and the soluble (S) and insoluble (pellet, P) fractions were analyzed by immunoblotting. (A) As a control of extraction, red Ponceau staining of the nitrocellulose membrane revealed the expected fractionation of proteins between the soluble (*) and pellet (**) pools, with band 3 (***) in both fractions. (B) Immunostaining of Rh complex proteins was performed as described in the legend to Figure 1A. Anti–Duffy MAb was used as a control of membrane solubilization efficiency. Anti-p55 was used as a control of the protein amount loaded for each normal and variant sample.
Comparison of Triton X-100 extractibility of the Rh complex from normal and 4.2(-)HS red blood cells.
Human erythrocyte ghosts (G) were extracted with 1% Triton X-100, and the soluble (S) and insoluble (pellet, P) fractions were analyzed by immunoblotting. (A) As a control of extraction, red Ponceau staining of the nitrocellulose membrane revealed the expected fractionation of proteins between the soluble (*) and pellet (**) pools, with band 3 (***) in both fractions. (B) Immunostaining of Rh complex proteins was performed as described in the legend to Figure 1A. Anti–Duffy MAb was used as a control of membrane solubilization efficiency. Anti-p55 was used as a control of the protein amount loaded for each normal and variant sample.
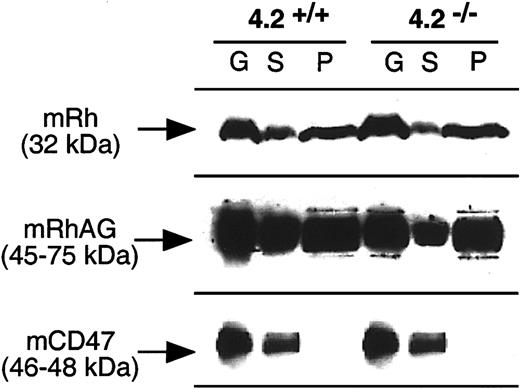
As in humans, the presence or absence of protein 4.2 in wild-type and 4.2−/− mice, respectively, did not modify the Triton solubility of mRh and mRhAG proteins (Figure5). In contrast to humans, mCD47 was detected only in the soluble fractions.
Comparison of Triton X-100 extractibility of the Rh complex for normal and 4.2−/− gene-targeted mice.
Mouse erythrocyte ghosts (G) were extracted with 1% Triton X-100 and the soluble (S) and pellet (P) fractions were analyzed by immunoblotting using anti–murine Rh complex antibodies, as described in the legend to Figure 3.
Comparison of Triton X-100 extractibility of the Rh complex for normal and 4.2−/− gene-targeted mice.
Mouse erythrocyte ghosts (G) were extracted with 1% Triton X-100 and the soluble (S) and pellet (P) fractions were analyzed by immunoblotting using anti–murine Rh complex antibodies, as described in the legend to Figure 3.
Discussion
In this study, we provide the first evidence for an association between the Rh complex and the red cell membrane skeleton component protein 4.2. Our conclusion was based on flow cytometric and Western blot analysis of the expression of the Rh complex members Rh, RhAG, CD47, GPB, and LW in RBCs from 2 unrelated patients with complete 4.2 deficiency due to distinct mutations in the EPB42 gene. The rationale of this analysis was 2-fold: (1) integral membrane proteins Rh, RhAG, and CD47 interact with the membrane skeleton of erythroid cells20 (and references herein),22,23 and (2) Rh complex deficiency and protein 4.2 deficiency are both associated with HS.1,24,25 Indeed, the observations that both 4.1(-) and GPC(-) genetic conditions are associated with hereditary elliptocytosis (HE) and that GPC is sharply reduced in 4.1(-) RBCs provided the first evidence of the linkage between the integral protein GPC and the skeletal component protein 4.1, an association which has been experimentally confirmed.26 Thus, we hypothesized that the analysis of Rh complex expression in RBCs from HS patients with a well-defined primary defect of skeletal protein(s) might likewise provide critical information for the identification of the protein partner(s) that link Rh-associated proteins to the membrane skeleton.
The major finding of this study was the observation of a severe reduction of CD47 protein in both 4.2(-) HS patients. Conversely, expression level of the other members of the Rh complex (Rh, RhAG, LW, and GPB) was normal. CD47 in 4.2(-)Lisboa and 4.2(-)Nancy RBCs, estimated from flow cytometric analysis and confirmed by immunoblotting, was decreased by 80%. As a control, CD47 expression was not altered in 4.1(-) HE red cells (not shown). Bruce et al39 discovered a new variant of protein 4.2 deficiency and independently of our preliminary report40 also found that CD47 is markedly reduced in the absence of protein 4.2. Of note, we found that CD44 expression was 3-fold higher in the 4.2(-) HS RBCs, as compared with controls. CD44 overexpression might reflect a mechanism to compensate for the loss of CD47-mediated membrane-skeleton anchoring, as postulated for the concomitant up-regulation of AE2 and NHE1 and down-regulation of AE1 in 4.2−/−RBCs.32 41
These observations suggest that protein 4.2 plays a major role in CD47 trafficking to the membrane or provides a major attachment site for the Rh complex via CD47. However, we found that the lack of protein 4.2 did not alter Triton X-100 extractability of Rh and RhAG proteins. While these experiments suggest that other component(s) are likely to be involved in the interaction of the Rh complex with the red cell skeleton, further studies will be necessary to determine to what extent protein 4.2 might modulate the strength of this attachment. A reduced electrophoretic mobility of RhAG on SDS-PAGE was also observed in both 4.2(-) samples, suggesting an overglycosylation of this glycoprotein. An overglycosylation state of RhAG was previously described in GPB-deficient RBCs42 and was correlated to a slowness of RhAG trafficking in the Golgi, suggesting that GPB plays a critical role in the translocation of RhAG to the plasma membrane. Therefore, we hypothesized that protein 4.2 and/or CD47 might be similarly associated with RhAG trafficking. However, as discussed below, analysis of Rh variants with CD47 deficiency ruled out such a role for CD47.
A reduced expression of CD47 was previously found on RBCs from Rhnull and D-- variant phenotypes.1 43We show here that these RBCs contain normal levels of protein 4.2. We further extend these observations to show that RBCs from donors with the D.. and RN variant Rh phenotypes also exhibit a reduced expression of CD47. That the (secondary) decrease of CD47, in the studied variants of the RH system, has no bearing on the amount of protein 4.2, whereas the (primary) absence of protein 4.2 caused a sharp reduction of CD47 may be accounted for by the fact that protein 4.2 is much more represented than CD47 (200 000 molecules/RBC vs 20 000-50 000 molecules/RBC) and is firmly bound to the cytoplasmic domain of band 3. Since D--, D.., and RN RBCs are characterized by the lack or decreased expression of the RhCcEe antigens, these results imply that CD47 expression is dependent on the RhCcEe polypeptide. Conversely, CD47 expression is not dependent on RhD polypeptide, as we show the same CD47 levels in RhD-positive and RhD-negative RBCs in the presence of protein 4.2. Importantly, theRHCE variants described above, despite the reduction of CD47, exhibit normal levels of RhAG and Rh polypeptides in contrast to Rhnull variants and a normal migration of RhAG on SDS-PAGE in contrast to 4.2(-) variants. Moreover, the RBC morphology is normal. These observations have 2 implications: (1) protein 4.2 but not CD47 might interfere with RhAG trafficking, and (2) the cell shape abnormalities typical of 4.2(-)HS and Rhnull RBCs do not result from the CD47 deficiency, per se, but from the lack or low expression of protein 4.2, and of Rh or RhAG, respectively. Hence, these observations strengthen the model in which Rh and RhAG define the core of the Rh complex, whereas CD47, together with GPB and LW, represent dispensable accessory chains.
The observation that gene-targeted CD47−/− mice exhibit normal expression levels of Rh, RhAG, and protein 4.2 (our present results), do not exhibit RBC abnormalities,31 and do not become anemic (E.J.B. and F. P. Lindberg, personal communication, June 2000) fully support this conclusion. In addition, this murine model shows that CD47 is not strictly required for Rh expression, at least in mice. Therefore, it is unlikely that mutant alleles of humanCD47 (not yet recognized) should be responsible for Rh deficiency.
CD47 has recently been recognized as a marker of self on RBCs. CD47-deficient mouse erythrocytes are rapidly cleared from the bloodstream by splenic red pulp macrophages (that express CD47) when transfused in wild-type (CD47+/+) recipients but not in CD47−/− recipients.44 Since it has also been demonstrated that CD47 on normal RBCs prevents this elimination by binding to the signal regulatory protein alpha (SIRPα), CD47-SIRPα interaction may represent a potential pathway for the control of hemolytic anemia. As Rhnull individuals exhibit a sharp reduction of CD47 on their RBCs, but not in other hematopoietic cells (see “Results”), it has been speculated that the elimination of Rhnull RBCs might be secondary to a reduced CD47-SIRPα signaling to splenic macrophages.44 According to this hypothesis, the hemolytic anemia in 4.2-deficient patients could be attributed, at least in part, to the decreased expression of CD47 in their RBCs. However, the observation that D-- and D.. variants exhibit no sign of hemolytic anemia, despite a reduction of CD47 in their RBCs similar to that of 4.2(-)HS or RhnullRBCs, suggests that this is not the case.
In order to investigate in an animal model the effect of a complete lack of protein 4.2 on Rh complex expression, we analyzed RBCs from gene-targeted 4.2−/− mice. Our results indicate that the absence of protein 4.2 in knock-out mice neither modifies Rh complex expression level, including mCD47, nor alters the Triton X-100 solubilization properties of murine Rh, RhAG, and CD47 proteins. Furthermore, murine CD47, in contrast with human CD47, was totally recovered in the soluble fraction after detergent extraction. These results as well as the previously reported absence of 4.2 protein in several mammalian species45 suggest that interaction between the Rh complex and protein 4.2 might be restricted to a few species. Hence, future data concerning interactions between the Rh complex and the red cell skeleton should be regarded with caution when provided by a murine model.
In conclusion, we provide evidence that cell surface expression of the Rh complex member CD47 depends on the skeletal protein 4.2. Since involment of the Rh complex in the linkage between the membrane lipid bilayer and the red cell skeleton is increasingly evident, we hypothesize that CD47 and protein 4.2 interact in RBCs. Additional studies will be necessary to determine (1) whether protein 4.2 is also involved in the membrane translocation of the Rh-related proteins, as suggested by the abnormal glycosylation of RhAG in the absence of protein 4.2, and (2) whether the skeleton association and the emerging transporter activity of Rh and RhAG might be modified by the absence of protein 4.2.
We thank all our colleagues who provided useful antibodies, Dr M. E. dos Santos (Hopital de Santo Antonio dos Capuchos, Lisboa, Portugal) and Dr O. Agulles (CTS, Nancy, France) for providing 4.2(-) blood samples.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-04-1285.
Supported in part by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique (CNRS), the Institut National de Transfusion Sanguine (INTS), and National Institutes of Health grant HL64885 (L.L.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yves Colin, INSERM U76, Institut National de Transfusion Sanguine, 6 rue Alexandre Cabanel, 75015 Paris, France; e-mail: colin@idf.inserm.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal