Abstract
The parasite ligand Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) and host endothelial receptors represent potential targets for antiadhesive therapy for cytoadherence. In the present study, the major host receptor CD36 was targeted in vitro and in vivo with a recombinant peptide, PpMC-179, corresponding to the minimal CD36-binding domain from the cysteine-rich interdomain region 1 (CIDR1) within the MCvar1 PfEMP1. The in vitro inhibitory effect of PpMC-179 on human dermal microvascular endothelial cells (HDMECs) expressing multiple relevant adhesion molecules was investigated using a parallel-plate flow chamber. Pretreatment of endothelial monolayers with PpMC-179 (2 μM) inhibited the adhesion of infected erythrocytes (IRBCs) from all clinical isolates tested by 84.4% on resting and 62.8% on tumor necrosis factor α (TNF-α)–stimulated monolayers. Adhesion to stimulated cells was further inhibited (90.4%) when PpMC-179 was administered with an inhibitory anti–intercellular adhesion molecule 1 (ICAM-1) monoclonal antibody 84H10 (5 μg/mL). To determine the in vivo effectiveness of PpMC-179, we used a human/severe combined immunodeficiency (SCID) mouse chimeric model that allowed direct visualization of cytoadherence on intact human microvasculature. In unstimulated skin grafts, PpMC-179 inhibited adhesion by 86.3% and by 84.6% in TNF-α–stimulated skin grafts. More importantly, PpMC-179 administration resulted in the detachment of already adherent IRBCs by 80.7% and 83.3% on resting and stimulated skin grafts, respectively. The antiadhesive effect of PpMC-179 was rapid and sustained in vivo for at least 30 minutes. Our data indicate that targeting cytoadhesion in vivo is feasible and may offer a rapid antimalarial therapy.
Introduction
Plasmodium falciparum malaria is an acute febrile illness characterized by fever, chills, headache, anemia, and splenomegaly. In some patients, particularly in nonimmune individuals, the infection can become severe and complicated by multiorgan dysfunction.1 Death most commonly results from cerebral malaria, severe anemia, metabolic acidosis, or pulmonary edema. Although the underlying mechanisms leading to severe falciparum malaria and death are still not completely understood, there is little doubt that much of the pathogenicity of P falciparum depends on its unique ability to adhere to host vascular endothelium and syncytiotrophoblasts in the placenta, a characteristic not shared with the other 3 species of human malaria parasites.2 Although antimalarial drug therapy can be very effective, its use has been hampered by the emergence of multiple drug resistance and the length of time required until drug therapy becomes effective. This is particularly true in comatose patients in whom an immediate effect may be lifesaving. One possible therapy that might stop the chain of pathophysiologic events is the reversal of parasite sequestration.
Cytoadherence on vascular endothelium is mediated by a number of adhesion molecules in a synergistic fashion under flow conditions in vitro and in vivo, mimicking the adhesive events in the leukocyte recruitment cascade.3-6 Infected red blood cells (IRBCs) can tether and roll on several host receptors including intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and P-selectin. These low-affinity interactions do not by themselves lead to the arrest of the interacting cells but enhance the subsequent adhesion of nearly all clinical isolates tested to CD36. ICAM-1 and VCAM-1 mediate their effect by increasing the percentage of rolling cells that become adherent, whereas P-selectin increases the total number of rolling and adherent IRBCs.6 Peptide mapping studies on CD36 reveal that residues 145 to 171 are important for IRBC adhesion.7 Furthermore, adhesion by diverseP falciparum clones and isolates to CD36 can be inhibited by monoclonal antibody (mAb) OKM5 that is thought to bind to residues 155 to 183.8
P falciparum erythrocyte membrane protein 1 (PfEMP1) is the parasite protein directly involved in adhesive interactions with microvascular endothelium.9 PfEMP1 is a highly variable protein encoded by the large var gene family.10,11 A single IRBC only expresses one vargene product during the mature stages of the parasite, whereas the remaining genes in this family are inactivated by an as yet unknown silencing mechanism.12 However, a single parasite clone can bind to more than one receptor molecule through its distinct binding modules: the Duffy binding–like domains (DBLs) and the cysteine-rich interdomain regions (CIDRs).12-14 A critical region of PfEMP1 involved in binding to CD36 is localized to a 179–amino acid sequence within the CIDR1 region.15 The recombinant 179–amino acid peptide (r179) from the Malayan Camp (MC)var1 gene has been shown to bind to CD36 and inhibit and reverse the adhesion of several CD36-binding laboratory-adapted parasite strains in static and flow chamber–based assays in vitro.16
Unlike the broad-range effect of the r179 on parasite adhesion, antibodies against r179 appear to be primarily strain specific and inhibit the binding of parasites expressing the homologous region.10,15,17 Immunization of Aotus monkeys with this region also generated a strain-specific immune response that protected from challenge with the homologous strain but not from the heterologous virulent FVO strain.18 Collectively, the results suggest that targeting CD36 with r179 may be more effective for inhibiting the adhesion of diverse parasite isolates than antibodies against this region of PfEMP1.
In this study, we tested the antiadhesive effects of the recombinant peptide r179 expressed in yeasts (PpMC-179) on clinical parasite isolates. Experiments were conducted in a parallel-plate flow chamber in vitro using microvascular endothelium,5 and in a human/severe combined immunodeficiency (SCID) mouse chimeric model in vivo.6 This human/SCID mouse model allowed us to directly visualize IRBC-endothelial cell interactions in an intact human microvasculature in a skin graft and to verify the ability of PpMC-179 to inhibit and reverse IRBC adhesion in vivo. The results indicate that the adhesion of clinical parasite isolates to microvascular endothelium was inhibited and reversed by PpMC-179 both in vitro and in vivo. The finding strongly suggests that PpMC-179 may be a suitable therapeutic agent for antiadhesive therapy.
Materials and methods
Tissue culture reagents
Unless otherwise stated, all tissue culture reagents were obtained from Gibco BRL Life Technologies (Gaithersburg, MD).
Parasites
Cryopreserved parasite isolates from adult Thai patients with well-documented severe P falciparum malaria according to World Health Organization criteria19 were thawed and studied during the first cycle in culture as described.3 The collection of specimens was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Informed consent was provided according to the Declaration of Helsinki. The parasite isolates used in the in vitro experiments were P99-14, P99-34, P00-27, and P00-28. The parasite isolates used in the in vivo studies were P94-59, P98-10, P99-14, P99-26, and P00-24. Their receptor specificity with respect to CD36 and ICAM-1 was determined using stable CD36 and ICAM-1 L-cell transfectants in a static binding assay as described.20 The number of adherent cells per 1000 target cells was counted under oil immersion.
Production in Pichia pastoris and purification of PpMC-179 from P falciparum
The recombinant PpMC-179 protein was expressed in P pastoris. In brief, a synthetic gene of the MC-179 (optimized for codon usage in P pastoris and containing a His6-tag on the C-terminus) was cloned into pPIC9K vector containing the α-factor secretion signal that directs the recombinant protein into the secretory pathway. The protein was expressed in shaker flasks and harvested at 48 hours after induction. The protein was purified using nickel-nitriltriacetic acid-agarose (Ni-NTA) followed by size exclusion chromatography on a Superdex 75 column (Amersham Pharmacia Biotech, Piscataway, NJ) and reversed phase high-performance liquid chromatography (HPLC) using a C4 column (Grace Vydac, Hesperia, CA).
Protein characterization
Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 4% to 20% gradient gels (Invitrogen, San Diego, CA) as per the manufacturer's instructions. Gels were either stained with Coomassie brilliant blue or prepared for electrophoretic transfer to polyvinylidene difluoride (PVDF) membranes. Amino acid sequencing of samples bound to PVDF by automated Edman degradation and electron spray mass spectroscopy analysis of liquid samples were performed at the Biological Resources Branch, National Institute of Allergy and Infectious Diseases. Protein concentrations were determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL).
CD36-binding assay
PpMC-179 was assayed for binding to CD36 using a protocol similar to that previously described.15 Briefly, various amounts of PpMC-179 (5-0.05 μg) were bound to 50 μL Ni-NTA beads (Qiagen, Mississauga, ON, Canada) in phosphate-buffered saline (PBS). Soluble recombinant CD3610 was added to the beads and incubated with shaking at 25°C for 2 hours. Beads were washed extensively with PBS and proteins bound were eluted by boiling in SDS-PAGE sample buffer. Proteins were separated on gels and transferred to PVDF as above; Western blots were performed by standard methods. CD36 bound on the membrane was detected by incubating blots with a monoclonal antibody termed MoAb-17915 followed by incubation with horseradish peroxidase (HRP)–conjugated goat antimouse IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Blots were developed using the enhanced chemiluminescence (ECL) detection system (Qiagen) according to the manufacturer's instructions.
Adhesion inhibition assay
The blockade of IRBC adhesion to CD36 protein by PpMC-179 was tested using the CD36-binding parasite line FMG as described.15 Briefly, recombinant CD36 was captured on a Falcon 1001 Petri dish by the MoAb-179. Blocking solutions containing 1% bovine serum albumin (BSA) alone in RPMI 1640, pH 6.8, or BSA plus PpMC-179 (50 μg/mL), or BSA plus yPyCSP (50 μg/mL) were added to the immobilized protein for 1 hour at room temperature. After washing, IRBCs from FMG strain were added at 3% parasitemia and 2% hematocrit in blocking solution. Binding was allowed to proceed for 1 hour at room temperature. Nonadherent IRBCs were washed off, and the adherent IRBCs were fixed in 2% glutaraldehyde and stained with Giemsa. Three high-power fields (× 40) in duplicate spots were counted.
Antibody and cytokine
The anti–ICAM-1 mAb 84H10, known to inhibit IRBC interactions with ICAM-1, was purchased from R & D Systems (Minneapolis, MN). Recombinant tumor necrosis factor α (TNF-α) was purchased from BD Biosciences (Bedford, MA). The concentration of cytokine used for the stimulation of human dermal microvascular endothelial cells (HDMECs) in 35-mm tissue culture dishes (Corning, NY) was TNF-α 10 ng/mL for 24 hours. For in vivo experiments, 100 ng TNF-α in 50 μL sterile PBS was injected intradermally at the edge of the human skin graft 4 hours prior to the start of the experiments.
Endothelial cell culture
HDMECs were harvested from discarded neonatal human foreskins using 0.5 mg/mL type IA collagenase (Boerhringer Mannheim Biochemicals, Indianapolis, IN) as described previously.5 The protocol was approved by the Conjoint Health Research Ethics Board of the University of Calgary. The cells were maintained in endothelial basal medium (EBM) (Biowhittaker, Walkerville, MD) with supplements provided by the manufacturer. Experiments were performed with cells from passages 1 to 5 on which adhesion molecule expression was shown to be stable.5 We and others have shown previously that HDMECs express CD36 and ICAM-1 constitutively.5,21,22Stimulation with TNF-α for 4 hours up-regulates ICAM-1 expression, and at 24 hours, ICAM-1 expression is further augmented, whereas VCAM-1 expression is induced.5 22 In inhibition studies, HDMEC monolayers were preincubated with 2 μM PpMC-179 with or without 5 μg/mL anti–ICAM-1 mAb for 30 minutes at 37°C before being used in flow chamber experiments.
Adhesive interactions under flow conditions in vitro
IRBC-endothelial cell interactions at fluid shear stresses approximating those in the microvasculature were studied using a parallel plate flow chamber as described.5 In previous studies, we have established that infusion of a 1% IRBC suspension over endothelial cell monolayers at 1 dyne/cm2 allowed us to optimally visualize the adhesive interactions in real time. A rolling IRBC was defined as one that displayed a typical end-on-end rolling motion at a velocity of less than 150 μm/s, compared to a centerline red blood cell flow rate of more than 1000 μm/s and a velocity of more than 150 μm/s for noninteracting cells in close proximity to the endothelial monolayer. The flux of rolling IRBCs was determined as the number that rolled past a fixed line on the monitor screen for the duration of the experiment and expressed as the number of rolling IRBC/min/mm2. An IRBC was considered adherent if it remained stationary for more than 10 seconds, and the results were expressed as the number of adherent IRBCs per square millimeter of surface area.
Preparation of human skin graft in SCID mice
CB-17 SCID/beige mice (Harlan Teklad, Madison, WI) were grafted with split-thickness human skin as described previously23 under a protocol approved by the Animal Care Committee and the Conjoin Health Research Ethics Board of the University of Calgary. Briefly, split-thickness grafts were prepared using a 1-mm dermatome from discarded human skin from donors undergoing plastic surgery. Recipient mice were anesthetized using halothane. A 0.5 × 0.5-cm defect was excised from the posterior thorax and covered with human skin anchored using skin staples (US Surgical, Norwalk, CT). The graft was allowed to heal for 3 weeks before the animal was used for intravital microscopy experiments. The microvessels in the human skin graft express human platelet-endothelial cell adhesion molecule 1 (PECAM-1), CD36, ICAM-1, and E-selectin by immunohistochemical staining.6 21
Intravital microscopy
Animals were prepared for intravital microscopy as previously described.6 The procedure was approved by the Animal Care Committee, University of Calgary. Briefly, the jugular vein of anesthetized animals was cannulated for administration of additional anesthetic, boluses of IRBCs, and recombinant peptide. A midline dorsal incision was made from the neck to the lower back, without disrupting the lateral dermal blood supply. The skin was reflected onto a pedestal and examined using an upright microscope (Nikon Optiphot) with a × 20 water-immersion objective (Nikon). To identify human vessels, 100 μg fluorescein isothiocyanate (FITC)–Ulex europaeus (Sigma Aldrich, St Louis, MO) was injected intravenously immediately before microscopic visualization. FITC-derived fluorescence was visualized by epi-illumination at 450 to 490 nm, using a 520-nm emission filter. IRBCs, but not noninfected red cells, were labeled with the nuclear dye rhodamine 6G (25 μg/mL) and visualized by excitation at 510 to 560 nm, using a 590-nm emission filter. Each 200-μL bolus of IRBCs contained IRBCs at approximately 50% hematocrit and 5% to 7% parasitemia. Images of the labeled IRBCs and human microvessels were visualized using a silicon-intensified CCD camera (C-2400-08; Hamamatsu Photonics, Hamamatsu City, Japan) and recorded with a VCR for playback analysis. The numbers of rolling and adherent IRBCs were determined off-line. IRBC rolling was expressed as rolling flux fraction, determined by counting the IRBCs interacting in an individual vessel over the period of observation and expressing this relative to the total number of IRBCs passing through the vessel over the same period (determined by frame-by-frame analysis). IRBCs that remained stationary on the vascular wall for at least 30 seconds were defined as adherent. Adhesive interactions in 3 to 7 postcapillary venules per skin graft were counted.
Statistical analysis
All data are presented as mean ± SEM. In the in vivo experiments, n values are based on the total number of vessels observed in the skin grafts of 1 or 2 mice studied under each condition for each clinical isolate.24 Data between 2 groups were compared by Student t test for paired samples.P ≤ .05 was considered statistically significant.
Results
PpMC-179 purification and characterization
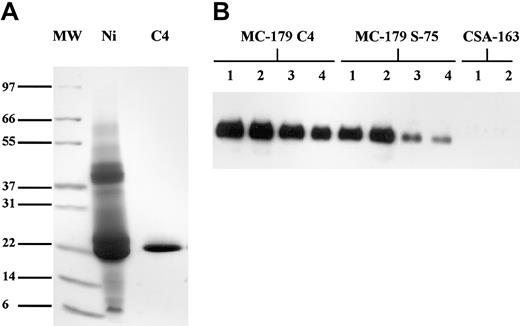
The final purified product is shown in Figure1A. N-terminal sequence analysis of the first 12 amino acid residues of the purified PpMC-179 yielded the sequence YVEDKIMSYNAF (single-letter amino acid codes). The first 2 amino acids correspond to the SnaB1 cloning site in the pPIC9K vector, proving that the α-factor secretion signal was efficiently removed during secretion from the cell. The next 10 amino acids represent the N-terminus of MC-179. There are no glycosylation sites on the y179 sequence, which is consistent with the molecular size of about 22 kDa as determined by electron spray mass spectroscopy.
Purification and binding of PpMC-179.
(A) Purification of PpMC-179 from culture supernatant. Proteins were separated on a nonreducing SDS-PAGE gel and stained with Coomassie blue. MW indicates molecular weight standards; Ni, protein eluate from Ni-NTA chromatography; and C4, pooled protein fractions from reverse phase C4-HPLC column. Sizes of molecular weight standards are illustrated. (B) Binding of PpMC-179 to CD36. CD36 bound to PVDF membranes was detected using MoAb-179 followed by HRP-conjugated goat antimouse IgG and visualized using chemiluminescence. Proteins bound to Ni-NTA beads are shown and include PpMC-179 purified on a C4 column (final product), PpMC-179 from the Superdex-75 (S-75) column, and CSA-163 as the negative control. Lanes 1 to 4 had 5 μg, 1 μg, 0.5 μg, and 0.05 μg recombinant protein on beads, respectively.
Purification and binding of PpMC-179.
(A) Purification of PpMC-179 from culture supernatant. Proteins were separated on a nonreducing SDS-PAGE gel and stained with Coomassie blue. MW indicates molecular weight standards; Ni, protein eluate from Ni-NTA chromatography; and C4, pooled protein fractions from reverse phase C4-HPLC column. Sizes of molecular weight standards are illustrated. (B) Binding of PpMC-179 to CD36. CD36 bound to PVDF membranes was detected using MoAb-179 followed by HRP-conjugated goat antimouse IgG and visualized using chemiluminescence. Proteins bound to Ni-NTA beads are shown and include PpMC-179 purified on a C4 column (final product), PpMC-179 from the Superdex-75 (S-75) column, and CSA-163 as the negative control. Lanes 1 to 4 had 5 μg, 1 μg, 0.5 μg, and 0.05 μg recombinant protein on beads, respectively.
CD36 binding
PpMC-179 was tested for its ability to bind to CD36 to determine whether it had the correct conformation. Because the protein was purified under potentially denaturing conditions by reversed phase chromatography, the final product was compared for binding to the protein purified by size exclusion. Results of the assay demonstrate that PpMC-179 bound to CD36 in a dose-dependent manner (Figure 1B). Furthermore, the product purified by reverse phase bound to CD36 at least as well, if not better, than the protein purified by size exclusion. This may reflect the higher purity and subsequent higher specific activity of the reverse phase–purified protein. CSA-163 produced in Saccharomyces cerevisiae (C.B., unpublished data, 2000) was used as the negative control in the assay. This protein is the homologous region to MC-179 in the CIDR1 domain from the FCR3-CSA strain of P falciparum. This CIDR1 domain is known not to bind to CD3625 and served to demonstrate the specificity of the assay to detect proteins that specifically bind to CD36. Furthermore, PpMC-179 inhibited the binding of IRBCs from the FMG strain to recombinant CD36 in a static binding assay, whereas an unrelated yeast recombinant protein yPyCSP had no effect (Table1).
Inhibition of IRBC (FMG strain) adhesion to recombinant CD36 protein by PpMC-179
| . | No. adherent IRBC/high-power field* . | |
|---|---|---|
| Spot 1 . | Spot 2 . | |
| Control | 96 ± 10 | 122 ± 14 |
| PpMC-179 | 6 ± 2 | 10 ± 2 |
| yPyCSP | 116 ± 15 | 122 ± 24 |
| . | No. adherent IRBC/high-power field* . | |
|---|---|---|
| Spot 1 . | Spot 2 . | |
| Control | 96 ± 10 | 122 ± 14 |
| PpMC-179 | 6 ± 2 | 10 ± 2 |
| yPyCSP | 116 ± 15 | 122 ± 24 |
Values are mean ± SD of 3 fields in each of 2 duplicate CD36 spots.
Inhibition of IRBC cytoadherence on HDMECs by recombinant PpMC-179 in vitro
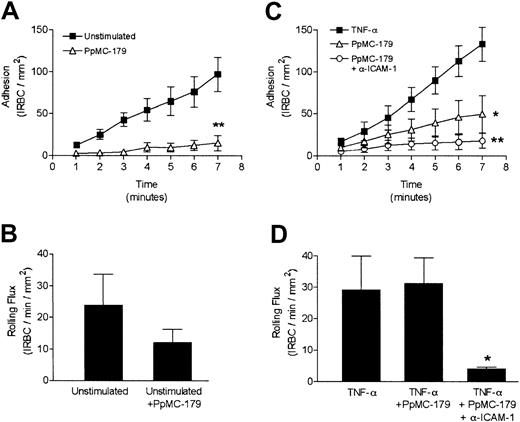
We first tested if PpMC-179 was capable of inhibiting the rolling and adhesion of IRBCs from clinical parasite isolates on HDMECs in vitro. Resting endothelial monolayers were preincubated with 2 μM PpMC-179 for 30 minutes prior to flow chamber experiments. In the 7 minutes of IRBC infusion, PpMC-179 inhibited adhesion by 84.4% (Figure2A) and the total number of adherent IRBCs decreased from 96.8 ± 20.5 to 15.1 ± 9.0/mm2(P < .01). The results presented in Figure 2, panels A and B, are the means of 4 independent experiments with 3 different clinical isolates. Rolling flux, however, was not significantly affected by PpMC-179, suggesting that the same number of IRBCs interacted with the endothelium and that in the absence of CD36, IRBC rolling could be mediated by other adhesion molecules such as ICAM-1 (Figure 2B).
Inhibition of cytoadherence on resting and stimulated HDMECs by PpMC-179 in vitro.
Resting and TNF-α–stimulated (10 ng/mL, 24 hours) endothelial monolayers were preincubated with 2 μM PpMC-179 (30 minutes at 37°C). IRBCs were then perfused over the monolayers at 1 dyne/cm2. In some experiments, monolayers were preincubated with PpMC-179 or both PpMC-179 and the inhibitory anti–ICAM-1 mAb 84H10 at 5 μg/mL. (A,C). Adhesion of IRBCs on unstimulated (n = 4) and TNF-α–stimulated HDMECs (n = 5) was significantly reduced by PpMC-179, and the combination of PpMC-179 and anti–ICAM-1 (n = 2). *P < .05 and **P < 0.01 compared to control. (B,D). Rolling flux on resting or stimulated HDMECs was unaffected by PpMC-179, but was inhibited by the combination of PpMC-179 and an anti–ICAM-1 mAb. *P < .05 compared to control.
Inhibition of cytoadherence on resting and stimulated HDMECs by PpMC-179 in vitro.
Resting and TNF-α–stimulated (10 ng/mL, 24 hours) endothelial monolayers were preincubated with 2 μM PpMC-179 (30 minutes at 37°C). IRBCs were then perfused over the monolayers at 1 dyne/cm2. In some experiments, monolayers were preincubated with PpMC-179 or both PpMC-179 and the inhibitory anti–ICAM-1 mAb 84H10 at 5 μg/mL. (A,C). Adhesion of IRBCs on unstimulated (n = 4) and TNF-α–stimulated HDMECs (n = 5) was significantly reduced by PpMC-179, and the combination of PpMC-179 and anti–ICAM-1 (n = 2). *P < .05 and **P < 0.01 compared to control. (B,D). Rolling flux on resting or stimulated HDMECs was unaffected by PpMC-179, but was inhibited by the combination of PpMC-179 and an anti–ICAM-1 mAb. *P < .05 compared to control.
On TNF-α–stimulated endothelium, treatment of monolayers with only PpMC-179 inhibited IRBC adhesion by 62.8%, that is, 133.2 ± 20.4 to 49.5 ± 21.8 IRBC/mm2 (P < .05; Figure 2C), without affecting the number of rolling cells (Figure 2D). The combination of an inhibitory anti–ICAM-1 mAb and PpMC-179 inhibited adhesion by 90.4% ± 3.69% (P < .01; Figure 2C) and rolling flux by 78.3% ± 11.5% (P < .05; Figure 2D). The inhibitory effect was measured in 5 separate experiments using 4 different clinical isolates. An IgG isotype-matched mAb CL3 directed against E-selectin had no inhibitory effect on IRBC rolling or adhesion on HDMECs (data not shown). The results demonstrate that the inhibitory effect of PpMC-179 was not limited to a particular isolate or strain and that blocking CD36 alone significantly inhibits adhesion even in the presence of ICAM-1 and VCAM-1 expression.
Inhibition of IRBC cytoadherence in human microvasculature by recombinant PpMC-179 in vivo
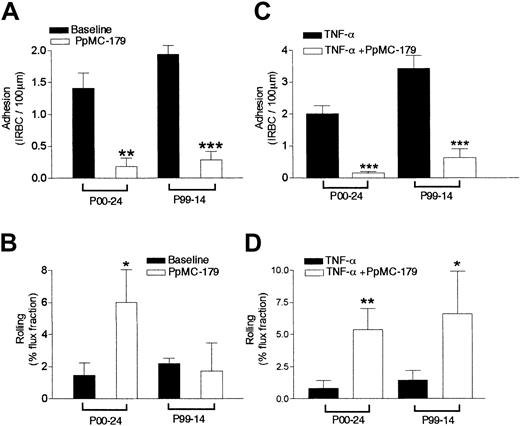
To determine if PpMC-179 could inhibit the adhesive interactions between IRBCs and human microvessels in vivo, we measured the adhesive interactions of IRBCs in the microvasculature of human skin grafted onto an SCID mouse by intravital microscopy. The receptor profiles of the parasite isolates used in these experiments are given in Table2. In inhibition experiments, the grafted mice received a bolus of 2 μM PpMC-179 via the jugular vein. The peptide was allowed to circulate for 10 to 15 minutes before the administration of a bolus of IRBCs. Rolling and adhesion of IRBCs in 3 animals treated with PpMC-179 were compared to that observed in 4 untreated animals. The results show that firm adhesion was inhibited from a baseline of 1.4 ± 0.24 to 0.18 ± 0.13 IRBC/100 μm for parasite isolate P00-24 (P < .01) and 1.94 ± 0.13 to 0.28 ± 0.13 IRBC/100 μm for P99-14 (P < .001; Figure3A). The drop in adhesion represented an inhibition of 87.1% and 85.5%, respectively. However, the effect of PpMC-179 on the rolling flux fraction was different between the 2 parasites isolates. Isolate P00-24 showed a significant increase of the rolling flux fraction from 1.46 ± 0.77 to 6.02 ± 2.0 (P < .05), whereas the rolling of P99-14 did not appear to be affected (Figure 3B). The lack of effect of CD36 blockade on the rolling of P99-14 is consistent with the comparatively low interaction of P99-14 with ICAM-1 (Table 2).
Receptor profile of clinical parasite isolates studied
| Parasite isolate . | No. adherent IRBC/1000 target cells* . | |||
|---|---|---|---|---|
| L-cells . | CD36 . | ICAM-1 . | CD36/ICAM-1 . | |
| P94-59 | 2 ± 2 | 478 ± 38 | 42 ± 12 | 11 |
| P98-10 | 1 ± 0.5 | 756 ± 164 | 326 ± 58 | 2 |
| P99-14 | 0 | 113 ± 9 | 7 ± 3 | 16 |
| P99-26 | 5 ± 7 | 194 ± 20 | 30 ± 4 | 6 |
| P99-34 | 2 ± 1 | 246 ± 38 | 56 ± 8 | 4 |
| P00-24 | 0 | 197 ± 49 | 27 ± 8 | 7 |
| P00-27 | 0 | 598 ± 54 | 60 ± 9 | 10 |
| P00-28 | 5 ± 2 | 470 ± 23 | 55 ± 18 | 9 |
| Parasite isolate . | No. adherent IRBC/1000 target cells* . | |||
|---|---|---|---|---|
| L-cells . | CD36 . | ICAM-1 . | CD36/ICAM-1 . | |
| P94-59 | 2 ± 2 | 478 ± 38 | 42 ± 12 | 11 |
| P98-10 | 1 ± 0.5 | 756 ± 164 | 326 ± 58 | 2 |
| P99-14 | 0 | 113 ± 9 | 7 ± 3 | 16 |
| P99-26 | 5 ± 7 | 194 ± 20 | 30 ± 4 | 6 |
| P99-34 | 2 ± 1 | 246 ± 38 | 56 ± 8 | 4 |
| P00-24 | 0 | 197 ± 49 | 27 ± 8 | 7 |
| P00-27 | 0 | 598 ± 54 | 60 ± 9 | 10 |
| P00-28 | 5 ± 2 | 470 ± 23 | 55 ± 18 | 9 |
Values are mean ± SD of duplicates.
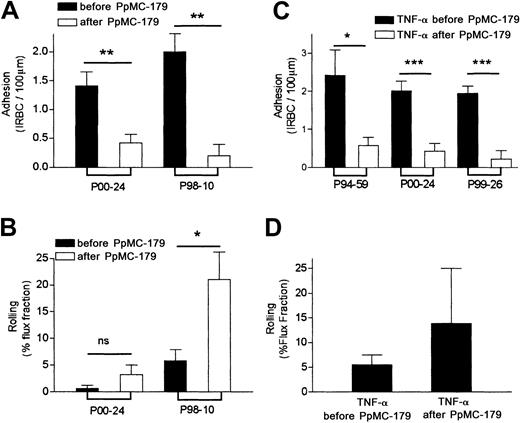
Inhibition of cytoadherence in resting and stimulated skin grafts by PpMC-179 in vivo.
PpMC-179 (2 μM) in a volume of 100 μL was administered intravenously into the SCID mouse with an unstimulated or TNF-α–stimulated (100 ng intradermally, 4 hours) human skin graft. The peptide was allowed to circulate for 10 minutes before IRBCs were injected. (A) PpMC-179 significantly inhibited the adhesion of parasite isolate P00-24 (n = 13 for baseline and 8 with PpMC-179) and P99-14 (n = 6 for baseline and 5 with PpMC-179) in unstimulated skin grafts. **P < .01 and ***P < .001 compared to baseline. (B) Rolling flux fraction of parasite isolate P00-24 was increased but not P99-14. *P < .05 compared to baseline. (C) In TNF-α–stimulated skin grafts, there was a dramatic inhibition of IRBC adhesion of both parasite isolate P00-24 (n = 14 for TNF-α alone and 10 for TNF-α + PpMC-179) and P99-14 (n = 3 for TNF-α alone and 5 for TNF-α + PpMC-179) . ***P < .001 compared to TNF-α. (D) The reduction in adhesion was associated with an increase in rolling flux fraction for parasite isolate P00-24. **P < .01 compared to TNF-α.
Inhibition of cytoadherence in resting and stimulated skin grafts by PpMC-179 in vivo.
PpMC-179 (2 μM) in a volume of 100 μL was administered intravenously into the SCID mouse with an unstimulated or TNF-α–stimulated (100 ng intradermally, 4 hours) human skin graft. The peptide was allowed to circulate for 10 minutes before IRBCs were injected. (A) PpMC-179 significantly inhibited the adhesion of parasite isolate P00-24 (n = 13 for baseline and 8 with PpMC-179) and P99-14 (n = 6 for baseline and 5 with PpMC-179) in unstimulated skin grafts. **P < .01 and ***P < .001 compared to baseline. (B) Rolling flux fraction of parasite isolate P00-24 was increased but not P99-14. *P < .05 compared to baseline. (C) In TNF-α–stimulated skin grafts, there was a dramatic inhibition of IRBC adhesion of both parasite isolate P00-24 (n = 14 for TNF-α alone and 10 for TNF-α + PpMC-179) and P99-14 (n = 3 for TNF-α alone and 5 for TNF-α + PpMC-179) . ***P < .001 compared to TNF-α. (D) The reduction in adhesion was associated with an increase in rolling flux fraction for parasite isolate P00-24. **P < .01 compared to TNF-α.
The peptide PpMC-179 was next tested for its ability to inhibit cytoadherence in TNF-α–stimulated microvasculature, simulating the clinical situation in severe falciparum malaria where there is a marked elevation of proinflammatory cytokines.26 Human TNF-α (100 ng in 50 μL sterile PBS) was injected intradermally at the edge of the human skin graft and experiments were conducted 4 hours later. Data were collected from 3 untreated and 3 stimulated skin grafts. Compared to baseline values, PpMC-179 reduced adhesion dramatically from 2.0 ± 0.25 to 0.15 ± 0.05 IRBC/100 μm (P < .001) and 3.44 ± 0.4 to 0.63 ± 0.27 IRBC/100 μm (P < .001) for P00-24 and P99-14, respectively (Figure 3C). Thus, preincubation with PpMC-179 inhibited the adhesion of parasite isolate P00-24 by 87.5% and parasite isolate P99-14 by 81.7%. The reduction in adhesion was associated with a significant increase in the rolling flux fraction for both parasite isolates (Figure 3D). Interestingly, the rolling of isolate P99-14 that was not affected by PpMC-179 in nonstimulated grafts (Figure 3B) was markedly increased in TNF-α–stimulated vessels (Figure 3D). This suggests that stimulation of the endothelium has a direct effect on parasite rolling, presumably by a marked increase in ICAM-1 expression.
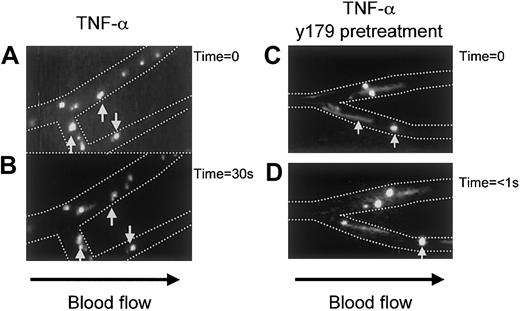
Videotaped images depicting the rolling and adhesion of IRBCs on human microvessels in the absence or presence of PpMC-179 are shown in Figure4. Adherent and rolling cells appeared round, whereas fast rolling and noninteracting cells appeared as streaks. Figure 4, panels A and B, show a number of adherent IRBCs that remained stationary for at least 30 seconds (indicated by arrows), whereas several others rolled for short distances in that duration. The situation was quite different when PpMC-179 was administered 10 to 15 minutes before the bolus of IRBCs (Figure 4C,D). The IRBCs were no longer able to adhere, although some of them still rolled, whereas others became noninteracting.
Videotaped images of the inhibition of cytoadherence by PpMC-179 on TNF-α–stimulated human microvasculature in vivo.
Panel A shows rolling and adherent IRBCs as distinct bright circles in the postcapillary venules. Panel B shows the same vessel 30 seconds later, with the arrows indicating adherent IRBCs. Panels C and D, show the appearance of postcapillary venules when IRBCs were injected 10 to 15 minutes after 2 μm PpMC-179. The IRBCs were no longer adherent and were either rolling or became completely noninteracting (streaks).
Videotaped images of the inhibition of cytoadherence by PpMC-179 on TNF-α–stimulated human microvasculature in vivo.
Panel A shows rolling and adherent IRBCs as distinct bright circles in the postcapillary venules. Panel B shows the same vessel 30 seconds later, with the arrows indicating adherent IRBCs. Panels C and D, show the appearance of postcapillary venules when IRBCs were injected 10 to 15 minutes after 2 μm PpMC-179. The IRBCs were no longer adherent and were either rolling or became completely noninteracting (streaks).
Reversal of IRBC cytoadherence in human microvasculature by recombinant PpMC-179 in vivo
To determine if PpMC-179 was able to detach IRBCs that were already adherent, a bolus of IRBCs was allowed to circulate and adhere within the human microvasculature for 5 to 10 minutes prior to the administration of 2 μM PpMC-179. In control animals, the number of circulating IRBCs gradually decreased over 15 to 20 minutes due to IRBC adhesion within the human microvasculature, as well as trapping in the mouse liver and spleen (M.H. et al, unpublished observation, 2000). In contrast, in 3 animals that received PpMC-179 after IRBCs were allowed to adhere, the number of adherent IRBCs dropped within 5 minutes (Figure 5A) from 1.4 ± 0.2 to 0.4 ± 0.2 IRBC/100 μm for P00-24 (P < .01) and from 2.0 ± 0.3 to 0.2 ± 0.2 IRBC/100 μm for P98-10 (P < .01). This represents a 71.4% and a 90.0% reversal of cytoadherence, respectively, and an average reversal of 80.7% (Figure 5A). As IRBCs became detached, the rolling flux fraction increased markedly (P < .05) for P98-10 but not for isolate P00-24 (Figure 5B). Interestingly, P98-10 had the highest binding of the 5 clinical isolates tested to ICAM-1 transfectants under static conditions (Table 2).
Reversal of cytoadherence in resting and stimulated skin grafts by PpMC-179 in vivo.
IRBCs were allowed to firmly adhere to human microvessels in unstimulated and TNF-α–stimulated skin grafts for 5 to 10 minutes. Then, 2 μM PpMC-179 was administered intravenously. (A) A significant number of adherent IRBCs from P00-24 (n = 13 for before PpMC-179 and 12 for after PpMC-179) and P98-10 (n = 5 for before PpMC-179 and 5 for after PpMC-179) became detached from the human microvessels in unstimulated skin grafts within the first 5 minutes of PpMC-179 administration. **P < .01 compared to baseline. (B) The reversal of IRBC adhesion was associated with an increase in the rolling flux fraction of parasite isolate P98-10. *P < .05 compared to baseline. (C) In TNF-α–stimulated skin grafts, IRBC adhesion of parasite isolates P94-59 (n = 4 for before PpMC-179 and n = 4 for after PpMC-179), P00-24 (n = 14 for before PpMC-179 and 7 for after PpMC-179), and P99-26 (n = 7 for before PpMC-179 and n = 3 for after PpMC-179) was significantly reversed. *P < .05 and ***P < .001 compared to TNF-α. (D) An increase in the mean rolling flux fraction of the 3 isolates was also observed, but it was not statistically significant.
Reversal of cytoadherence in resting and stimulated skin grafts by PpMC-179 in vivo.
IRBCs were allowed to firmly adhere to human microvessels in unstimulated and TNF-α–stimulated skin grafts for 5 to 10 minutes. Then, 2 μM PpMC-179 was administered intravenously. (A) A significant number of adherent IRBCs from P00-24 (n = 13 for before PpMC-179 and 12 for after PpMC-179) and P98-10 (n = 5 for before PpMC-179 and 5 for after PpMC-179) became detached from the human microvessels in unstimulated skin grafts within the first 5 minutes of PpMC-179 administration. **P < .01 compared to baseline. (B) The reversal of IRBC adhesion was associated with an increase in the rolling flux fraction of parasite isolate P98-10. *P < .05 compared to baseline. (C) In TNF-α–stimulated skin grafts, IRBC adhesion of parasite isolates P94-59 (n = 4 for before PpMC-179 and n = 4 for after PpMC-179), P00-24 (n = 14 for before PpMC-179 and 7 for after PpMC-179), and P99-26 (n = 7 for before PpMC-179 and n = 3 for after PpMC-179) was significantly reversed. *P < .05 and ***P < .001 compared to TNF-α. (D) An increase in the mean rolling flux fraction of the 3 isolates was also observed, but it was not statistically significant.
Reversal experiments were also conducted in skin grafts treated with TNF-α. Adhesion of P94-59 was reversed from 2.4 ± 0.7 to 0.6 ± 0.2 IRBC/100 μm, P00-24 from 2.0 ± 0.3 to 0.4 ± 0.2, and P99-26 from 2.0 ± 0.2 to 0.2 ± 0.2 (P < .001; Figure 5C). These results represent reversal of 75%, 85 %, and 90% with a mean reversal of 83.3%. The detachment of IRBCs from the blood vessel wall was associated with an increase in the number of rolling cells, but this difference did not reach statistical significance (Figure 5D). When a second bolus of IRBCs was injected 25 minutes after PpMC-179 administration, no further adhesion of IRBCs was observed for a further 15 minutes. This observation indicates a relatively long-term effect of PpMC-179 and suggests that the protein persisted in vivo bound to CD36 for at least 45 minutes.
Discussion
Antimalarial drugs are the mainstay of treatment for P falciparum infections. However, for some patients this intervention may come too late to save their lives. This is particularly true for comatose patients and for those developing life-threatening syndromes before antimalarial agents become effective. The spread of drug-resistant parasites increases the likelihood of such events and requires the development of novel adjunct therapies to rapidly halt the disease process. In the past few years, anticytokine therapy in the form of anti–TNF-α antibodies27,28 and pentoxifylline29 that inhibits TNF-α production has been tried with limited clinical improvement. Exchange transfusion is used to correct hyperparasitemia,30 but this therapy is difficult to administer in developing countries and carries obvious risks in transmission of diseases.
With increasing understanding of the molecular mechanisms of cytoadherence, an alternative therapeutic modality for severe falciparum malaria might be antiadhesive therapy. This type of therapy has been shown to be efficacious in inflammatory diseases such as rheumatoid arthritis in which the recruitment of lymphocytes to the synovium is successfully inhibited by antileukocyte function associated antigen 1 (anti–LFA-1) antibody.31 Soluble ICAM-1 has also been used successfully to inhibit the binding of rhinovirus, the causative agent of the common cold, to nasal epithelium.32There is also precedent in the use of antiadhesive therapy in the treatment of malaria in animal models. In Aotus and Saimiri monkeys infected with P falciparum, passive transfer of hyperimmune sera resulted in parasite detachment and subsequent clearance by the spleen.33,34 In addition, it has been reported that soluble chondroitin sulfate A (CSA) was able to inhibit and reverse adhesion of CSA-adherent parasites in splenectomized Saimiri monkeys.35 However, the degree of detachment in these studies was not established.
Among the various host receptors, adhesion to CD36 appears to be a vital component for parasite sequestration in microvasculature beds. With few exceptions, all nonplacental parasites, including those from patients with cerebral malaria, exhibit binding to CD36. This interaction is mediated by the M2 region of the CIDR1 domain of PfEMP1.36 In this study, we show that a 179–amino acid peptide from the CIDR domain of MCvar1 PfEMP1 inhibited and reversed the adhesion of IRBCs from multiple clinical parasite isolates to HDMECs under flow conditions in vitro and to human microvessels in skin grafts on SCID mice in vivo. This is the first demonstration that cytoadherence of diverse wild-type parasite strains on a physiologic substratum can be inhibited by a single parasite protein under shear stress. On HDMEC monolayers in vitro, the peptide was effective in inhibiting IRBC adhesion on resting endothelium and acted in concert with an anti–ICAM-1 antibody on endothelium that had been stimulated with TNF-α, which results in an augmentation of ICAM-1 expression. These results are similar to our previous observations using OKM5, the mAb against CD36.5 6 In human microvessels, PpMC-179 alone was able to inhibit approximately 80% to 85% of the adhesion under both resting and stimulated conditions. The inhibition of adhesion was associated to a variable degree with an increase in rolling flux fraction, which suggests that PpMC-179 specifically affects stationary binding to CD36 but not the interaction with other host receptors involved with IRBC rolling. Indeed, large numbers of IRBCs were observed to remain circulating, whereas they generally disappeared from the circulation within 15 to 20 minutes in control animals. The inhibitory effect of PpMC-179 lasted for the duration of the experiments that was at least 30 to 40 minutes. Thus, PpMC-179 is stable in vivo (at least when bound to CD36) for a significant length of time, an important factor for a potential therapeutic agent.
More striking was the observation that PpMC-179 could rapidly detach already adherent IRBCs in vivo, suggesting that the peptide can have both a preventive and curative role in relation to cytoadherence. No further IRBC adhesion occurred once they were displaced, even when a second bolus of IRBCs was administered after the peptide. The rapid reversal within 5 minutes was different from previous results from an in vitro flow chamber assay in which perfusion of recombinant peptide for over 2 hours was needed for reversal.16 We believe that the human microvasculature in the human/SCID mouse chimera used in this study represents a much more physiologic model for cytoadherence than immobilized CD36 protein in a flow chamber. Nevertheless, we recognize that even the human/SCID mouse model has its limitations. Reversal of adhesion was substantial but never complete and may not be sufficient for altering the course of disease progression. In addition, questions remain regarding the importance of CD36 binding in adhesion to cerebral microvascular endothelium that expresses very little CD36 by immunohistochemical staining.37 On the other hand, a low level of CD36 expression might well be sufficient to mediate cytoadherence to brain microvasculature through the synergistic interactions with other adhesion molecules. This possibility is consistent with our observation that IRBC binds preferentially to CD36 on TNF-α–stimulated HDMECs although the mean fluorescent intensity of CD36 on flow cytometric analysis is almost 1 log lower than that of ICAM-1.5
In conclusion, we demonstrated that it is possible to inhibit and reverse parasite sequestration with a small functional peptide from PfEMP1 in vivo. The peptide has an inhibitory effect on all parasite isolates tested. The finding strongly suggests that antiadhesive therapy based on PpMC-179 is feasible and will offer a therapeutic intervention with immediate effect.
The authors are grateful to Dr Robert Lindsay, Department of Surgery, Foothills Hospital and Dr Caroline Lane, Valley View Family Practice Clinic, Calgary, AB, Canada for providing the skin specimens.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-06-1725.
Supported by a group grant from the Canadian Institutes of Health Research, the Alberta Heritage Foundation for Medical Research, AB, Canada, and a Faculty of Medicine Endowment Award, University of Calgary, Calgary, AB, Canada. P.K. is a scientist of the Canadian Institutes of Health Research and holds a Canada Research Chair in Immunology, M.H. is a Senior Scholar of the Alberta Heritage Foundation for Medical Research (AHFMR), and B.G.Y. is supported by a studentship from AHFMR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
May Ho, Department of Microbiology and Infectious Diseases, 3330 Hospital Dr NW, Calgary, AB, Canada T2N 4N1; e-mail: mho@ucalgary.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal