Abstract
A major drawback of the current antineoplastic treatments is their lack of specificity toward cancer cells, because they are most often cytotoxic to normal cells, thus creating related side effects. Hence, the identification of new apoptosis-inducing agents, specifically targeting malignant cells while sparing their normal counterparts, is of crucial interest. We show here that monoglycerides, a family of lipids consisting of a single fatty acid attached to a glycerol backbone, induce cell death in several human leukemic cell lines. Importantly, treatment of primary leukemic cells, obtained from B-cell chronic lymphocytic leukemia patients, resulted in rapid apoptosis. In striking contrast, resting or activated human peripheral blood mononuclear cells from healthy individuals were resistant to the same treatment. Therefore, these compounds could represent potential antileukemic drugs or could allow for the design of novel therapeutic agents applied to leukemia.
Introduction
Several therapeutic approaches exploited thus far to eradicate malignant cells are limited by their lack of cellular specificity.1 Therefore, new compounds displaying specific killing of neoplastic cells need to be identified. Monoglycerides, a family of lipids consisting of a single saturated or unsaturated fatty acid moiety bound to a glycerol backbone, are found in mammalian tissues (eg, plasma, spleen) and frequently in a variety of foods (eg, low-calorie fat spreads, breads, peanut butter, ice cream).2,3 A study by Kato et al reported an apparent antitumor activity for monoglycerides in vivo without damage to the animal host.4 However, whether monoglycerides acted directly or indirectly on malignant cells was not addressed. We have demonstrated that several monoglycerides induced rapid dose-dependent apoptosis in murine cells.5 Thus, the objective of this work was to evaluate the death-inducing potential of monoglycerides on neoplastic and normal human cells. Using 1-C18:1 monoglyceride as a prototype, we provide evidence that monoglycerides can trigger death in various human leukemic cell lines. We also assessed the killing efficacy of monoglycerides on leukemic cells from B-cell chronic lymphocytic leukemia (B-CLL) patients. B-CLL, distinguished by the accumulation of CD5+ B cells, represents the most common hematologic malignancy in Western countries and is presently an incurable disease.6-8 Interestingly, monoglycerides triggered dose-dependent death in leukemic cells from B-CLL patients while sparing peripheral blood mononuclear cells (PBMCs) from healthy individuals.
Study design
Cell culture and blood sample preparation
Cell lines used were prototypes of T-cell (Jurkat and DU-528),9 promyelocytic (HL-60), myelocytic-basophilic (KU-812),10 promonocytic (U-937), or monocytic (THP-1) leukemias; erythroleukemia (K-562); Burkitt B-cell lymphoma (Raji); or mammary (MCF-7), prostate (PC-3), and endometrial (HEC-1A) adenocarcinomas (American Type Culture Collection, Rockville, MD). Nonadherent cell lines were cultured in RPMI 1640 medium with 5% fetal calf serum (FCS). MCF-7 cells were cultured in RPMI–5% FCS with 5 μg/mL insulin and 10 ng/mL epidermal growth factor; PC-3 in Ham's F-12 medium with 5% FCS; and HEC-1A in RPMI–5% FCS with 1 mM sodium pyruvate.
PBMCs were from healthy donors. B-CLL cells were from patients diagnosed on the basis of clinical examination and peripheral blood counts as previously described.11 The median age of the patients was 67 years (range, 56-73 years). Two patients were Binet stage A, 1 was stage B, and 2 were stage C. Three patients were untreated and 2 received conventional therapy. Mononuclear cells were isolated in normal or B-CLL samples by Ficoll-Hypaque density gradient centrifugation. Mononuclear cells from B-CLL patients were further depleted of T cells11 and contained more than 98% B cells. For activation experiments, normal PBMCs were incubated for 48 hours with either 5 μg/mL phytohemagglutinin (Sigma-Aldrich, Oakville, ON, Canada) or 25 μg/mL lipopolysaccharides (Sigma-Aldrich) to activate T or B cells, respectively. Following activation, dead cells were removed by Ficoll-Hypaque. The use of human samples was approved by PROCREA BioSciences's independent ethics review board.
Drug treatment, antibodies, and apoptosis
Fresh PBMCs or B-CLL cells were plated at 4 × 106/mL in RPMI–10% FCS. Nonadherent and adherent cell lines were plated at 8 × 105/mL and 4 × 105/mL in RPMI–5% FCS, respectively. The agents used were glycerol, oleic acid, 1-monooleoyl-glycerol (1-C18:1), 1-monopalmitoleyl-glycerol (1-C16:1), and 3-monopalmitoyl-glycerol (3-C16:0), which were purchased and prepared as previously described,5 and N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD-fmk; Kamiya Biomedical, Seattle, WA). Unless specified, “monoglycerides” refers to 1-C18:1 monoglyceride.
Phycoerythrin (PE)–conjugated anti-CD8 (SK1) and fluorescein isothiocyanate (FITC)–conjugated anti-CD69 (L78) were from BD BioSciences, Oakville, ON, Canada. Phycoerythrin–Texas Red–x (ECD)–conjugated anti-CD4 (SFCI12T4D11), anti-CD3–ECD (HIT3A), anti-CD56–PE (N901), anti-CD5–FITC (BL1A), anti-CD20–PE (H299), and anti-CD14–PE (116) were from Beckman Coulter, Ville St-Laurent, QC. Staining procedures were performed according to the manufacturer's recommendations.
As described previously,5 12 assays for apoptosis were the following: loosening of membrane phospholipids, measured with merocyanin-540 (MC540; Molecular Probes, Eugene, OR); phosphatidylserine exposure, assayed by FITC- or biotin-conjugated annexin V followed by Quantum Red–conjugated streptavidin (BD BioSciences); retention of vital dyes, detected with propidium iodide (PI; Sigma-Aldrich); and DNA degradation, measured with the TdT-dependent dUTP-biotin nick end-labeling (TUNEL) assay.
Results and discussion
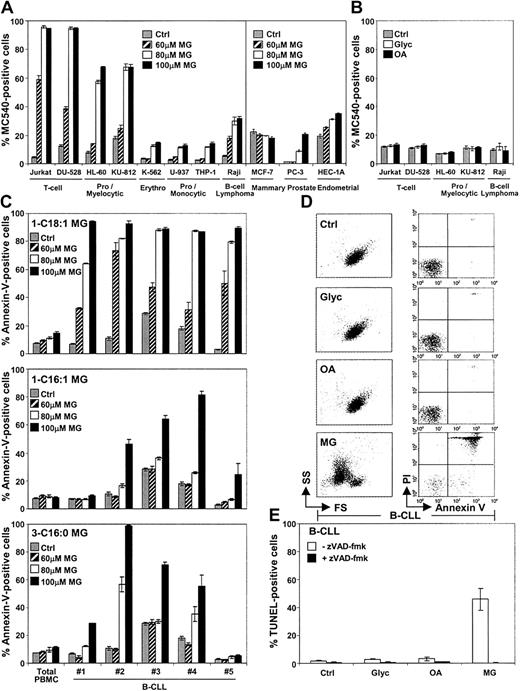
We have shown that monoglycerides induce apoptosis in murine cells5 and wanted to examine the cytotoxicity of monoglycerides on malignant and normal human cells. We first evaluated the death-inducing potential of monoglycerides in a panel of human leukemic cell lines (Figure 1A). While monoglycerides were indeed able to induce cell death in human leukemic cell lines, albeit with different potencies, following a 24-hour (Figure 1A, left panel) or a 3-hour stimulation (not shown), the glycerol backbone or the fatty acid alone was ineffective (Figure 1B). In contrast to leukemic cells, epithelial cells from mammary, prostate, and endometrial tumors were quite resistant to monoglyceride treatment, even after a 48-hour incubation (Figure 1A, right panel). Hence, these results indicate that monoglycerides efficiently kill some human leukemic cell lines while being relatively ineffective against the adenocarcinomas tested.
Monoglycerides induce cell death in various leukemic cell lines and in primary leukemic B-CLL cells.
(A) Several human cancer cell lines corresponding to either T-cell leukemia (Jurkat and DU-528), promyelocytic (HL-60) or myelocytic (KU-812) leukemia, erythroleukemia (K-562), promonocytic (U-937) or monocytic (THP-1) leukemia, B-cell lymphoma (Raji), mammary adenocarcinoma (MCF-7), prostate adenocarcinoma (PC-3), or endometrial adenocarcinoma (HEC-1A) were incubated with the vehicle alone (1% ethanol; Ctrl; gray bars), 60 μM (hatched bars), 80 μM (white bars), or 100 μM (black bars) 1-C18:1 monoglyceride (MG). After 24-hour (left panel) or 48-hour (right panel) stimulation, cells were stained with MC540. Results are shown as mean percentages of MC540-positive cells ± SD obtained from triplicates. (B) The most susceptible cell lines from panel A were stimulated with the vehicle (Ctrl; gray bars), 100 μM glycerol (Glyc; white bars), or 100 μM oleic acid (OA; black bars) for 24 hours and stained with MC540. Results are shown as mean percentages of MC540-positive cells ± SD obtained from triplicates. Noteworthy is the fact that controls with the vehicle (1% ethanol) gave similar background as medium alone (not shown). (C) Purified B cells obtained from B-CLL patients or total normal PBMCs obtained from a healthy donor were incubated with the vehicle (Ctrl; gray bars), 60 μM (hatched bars), 80 μM (white bars), or 100 μM (black bars) 1-C18:1 monoglyceride (MG; top panel), 1-C16:1 monoglyceride (middle panel), or 3-C16:0 monoglyceride (bottom panel). Following a 3-hour treatment, cells were stained with annexin V. Results are shown as mean percentages of annexin V–positive cells ± SD from triplicates. Similar results were obtained for normal PBMCs from 3 different healthy individuals. (D) B-CLL cells obtained from patient no. 5 were incubated for 3 hours with the vehicle (Ctrl), 100 μM glycerol (Glyc), 80 μM oleic acid (OA), or 80 μM 1-C18:1 monoglyceride (MG). The cells were labeled with annexin V and PI. Flow cytometric analysis of forward scatter (FS) versus side scatter (SS) and annexin V versus PI profiles are shown for each condition. Each profile is a representative from triplicates. (E) B-CLL cells from patient no. 5 were incubated for 6 hours with the vehicle (Ctrl), 100 μM glycerol (Glyc), 80 μM oleic acid (OA), or 80 μM 1-C18:1 monoglyceride (MG), either in the absence (white bars) or the presence (black bars) of 50 μM zVAD-fmk and labeled by the TUNEL assay. Results are shown as mean percentages of TUNEL-positive cells ± SD from triplicates.
Monoglycerides induce cell death in various leukemic cell lines and in primary leukemic B-CLL cells.
(A) Several human cancer cell lines corresponding to either T-cell leukemia (Jurkat and DU-528), promyelocytic (HL-60) or myelocytic (KU-812) leukemia, erythroleukemia (K-562), promonocytic (U-937) or monocytic (THP-1) leukemia, B-cell lymphoma (Raji), mammary adenocarcinoma (MCF-7), prostate adenocarcinoma (PC-3), or endometrial adenocarcinoma (HEC-1A) were incubated with the vehicle alone (1% ethanol; Ctrl; gray bars), 60 μM (hatched bars), 80 μM (white bars), or 100 μM (black bars) 1-C18:1 monoglyceride (MG). After 24-hour (left panel) or 48-hour (right panel) stimulation, cells were stained with MC540. Results are shown as mean percentages of MC540-positive cells ± SD obtained from triplicates. (B) The most susceptible cell lines from panel A were stimulated with the vehicle (Ctrl; gray bars), 100 μM glycerol (Glyc; white bars), or 100 μM oleic acid (OA; black bars) for 24 hours and stained with MC540. Results are shown as mean percentages of MC540-positive cells ± SD obtained from triplicates. Noteworthy is the fact that controls with the vehicle (1% ethanol) gave similar background as medium alone (not shown). (C) Purified B cells obtained from B-CLL patients or total normal PBMCs obtained from a healthy donor were incubated with the vehicle (Ctrl; gray bars), 60 μM (hatched bars), 80 μM (white bars), or 100 μM (black bars) 1-C18:1 monoglyceride (MG; top panel), 1-C16:1 monoglyceride (middle panel), or 3-C16:0 monoglyceride (bottom panel). Following a 3-hour treatment, cells were stained with annexin V. Results are shown as mean percentages of annexin V–positive cells ± SD from triplicates. Similar results were obtained for normal PBMCs from 3 different healthy individuals. (D) B-CLL cells obtained from patient no. 5 were incubated for 3 hours with the vehicle (Ctrl), 100 μM glycerol (Glyc), 80 μM oleic acid (OA), or 80 μM 1-C18:1 monoglyceride (MG). The cells were labeled with annexin V and PI. Flow cytometric analysis of forward scatter (FS) versus side scatter (SS) and annexin V versus PI profiles are shown for each condition. Each profile is a representative from triplicates. (E) B-CLL cells from patient no. 5 were incubated for 6 hours with the vehicle (Ctrl), 100 μM glycerol (Glyc), 80 μM oleic acid (OA), or 80 μM 1-C18:1 monoglyceride (MG), either in the absence (white bars) or the presence (black bars) of 50 μM zVAD-fmk and labeled by the TUNEL assay. Results are shown as mean percentages of TUNEL-positive cells ± SD from triplicates.
The potent activity of monoglycerides toward leukemic cell lines prompted us to examine whether these compounds would be as efficient in primary leukemic cells, such as leukemic B cells from patients suffering from B-CLL. Notably, monoglycerides triggered dose-dependent death in B cells from B-CLL patients, whereas PBMCs from healthy individuals treated with the same concentrations remained unaffected (Figure 1C, top panel), even after a 24-hour incubation (not shown). Monoglyceride-induced apoptosis in B-CLL cells is characterized by phosphatidylserine exposure, retention of vital dyes (ie, PI), cell shrinkage, DNA fragmentation, and was inhibited by the broad inhibitor of caspases, zVAD-fmk (Figure 1D,E). Again, the effect of monoglycerides was specific, because the glycerol or the free fatty acid was inactive (Figure 1D,E). Although unsaturated 1-C16:1 and saturated 3-C16:0 monoglycerides are not as potent as 1-C18:1 monoglyceride, they nevertheless can kill B-CLL cells while sparing normal PBMCs under the same doses and conditions (Figure 1C, middle and bottom panels). Together, these findings illustrate that primary leukemic B-CLL cells undergo apoptosis upon exposure to monoglycerides.
The cytotoxic activity of monoglycerides toward selected populations of normal PBMCs was also evaluated. As previously shown, the total population of PBMCs remained resistant to monoglyceride treatment (Figures 1C and 2A). Among normal PBMCs, T-helper cells, T-cytotoxic cells, conventional (CD5−) and B-1a (CD5+) B cells, natural killer cells, and monocytes remained unaffected by monoglycerides (Figure 2A). Similarly, activated T and B cells, obtained from healthy donors, were refractory to monoglyceride treatment (Figure 2B). Therefore, the data show that, under the conditions tested, monoglycerides can have a direct cytotoxic effect in vitro on leukemic cells while sparing normal resting and activated human PBMCs. However, because we only tested major leukocyte subsets, we cannot exclude the possibility that other blood cells, especially in the bone marrow, could be susceptible to monoglycerides. Nevertheless, the mechanism of monoglyceride action is uncertain and remains to be investigated.
Monoglycerides do not induce cell death in total PBMCs, in major leukocyte populations, or in activated PBMC subsets from healthy individuals.
(A) (Left panel) T-leukemic Jurkat cells or total normal PBMCs were incubated for 3 hours with the vehicle (Ctrl; white bars) or 80 μM 1-C18:1 monoglyceride (MG; black bars). After treatment, cells were stained with annexin V. (Right panel) Normal PBMCs were stimulated with the vehicle (Ctrl; white bars) or 80 μM 1-C18:1 monoglyceride (MG; black bars). After a 3-hour incubation period, the samples were stained for cell surface molecules (anti-CD4 and anti-CD8, anti-CD20 and anti-CD5, anti-CD56 and anti-CD3, or anti-CD14) and annexin V and analyzed by flow cytometry. Electronic gating was performed to determine the percentage of dying cells in distinct leukocyte populations: namely, T helper cells (Th; CD4high), T cytotoxic cells (Tc; CD8+), conventional B cells (Bc; CD20+CD5−), B-1a B cells (BB-1a; CD20+CD5+), natural killer cells (NK; CD56+CD3−), or monocytes (Mono; CD14+). (B) Normal PBMCs were stimulated for 48 hours with either phytohemagglutinin (left panel) or lipopolysaccharides (right panel). Fresh and activated PBMCs were cultured for 3 hours with the vehicle (Ctrl; gray and hatched bars, respectively) or 80 μM 1-C18:1 monoglyceride (MG; white and black bars, respectively). (Left panel) A 4-color staining was performed with anti-CD69 (early activation marker), anti-CD8, anti-CD4, and annexin V. Using electronic gating, sensitivity to monoglyceride-induced cell death was assessed in resting (total CD69− PBMCs, CD4highTh, and CD8+ Tc; gray and white bars) and activated (total CD69+ PBMCs, CD4highTh, and CD8+ Tc; hatched and black bars) populations. (Right panel) A 3-color staining was performed with anti-CD69, anti-CD20, and annexin V to detect cell death among resting (total CD69− PBMCs and Bc[CD20+]; gray and white bars) and activated (total CD69+ PBMCs and Bc [CD20+]; hatched and black bars) cells. Results are shown as mean percentages of annexin V–positive cells ± SD from triplicates. Similar results were obtained from 3 different healthy donors.
Monoglycerides do not induce cell death in total PBMCs, in major leukocyte populations, or in activated PBMC subsets from healthy individuals.
(A) (Left panel) T-leukemic Jurkat cells or total normal PBMCs were incubated for 3 hours with the vehicle (Ctrl; white bars) or 80 μM 1-C18:1 monoglyceride (MG; black bars). After treatment, cells were stained with annexin V. (Right panel) Normal PBMCs were stimulated with the vehicle (Ctrl; white bars) or 80 μM 1-C18:1 monoglyceride (MG; black bars). After a 3-hour incubation period, the samples were stained for cell surface molecules (anti-CD4 and anti-CD8, anti-CD20 and anti-CD5, anti-CD56 and anti-CD3, or anti-CD14) and annexin V and analyzed by flow cytometry. Electronic gating was performed to determine the percentage of dying cells in distinct leukocyte populations: namely, T helper cells (Th; CD4high), T cytotoxic cells (Tc; CD8+), conventional B cells (Bc; CD20+CD5−), B-1a B cells (BB-1a; CD20+CD5+), natural killer cells (NK; CD56+CD3−), or monocytes (Mono; CD14+). (B) Normal PBMCs were stimulated for 48 hours with either phytohemagglutinin (left panel) or lipopolysaccharides (right panel). Fresh and activated PBMCs were cultured for 3 hours with the vehicle (Ctrl; gray and hatched bars, respectively) or 80 μM 1-C18:1 monoglyceride (MG; white and black bars, respectively). (Left panel) A 4-color staining was performed with anti-CD69 (early activation marker), anti-CD8, anti-CD4, and annexin V. Using electronic gating, sensitivity to monoglyceride-induced cell death was assessed in resting (total CD69− PBMCs, CD4highTh, and CD8+ Tc; gray and white bars) and activated (total CD69+ PBMCs, CD4highTh, and CD8+ Tc; hatched and black bars) populations. (Right panel) A 3-color staining was performed with anti-CD69, anti-CD20, and annexin V to detect cell death among resting (total CD69− PBMCs and Bc[CD20+]; gray and white bars) and activated (total CD69+ PBMCs and Bc [CD20+]; hatched and black bars) cells. Results are shown as mean percentages of annexin V–positive cells ± SD from triplicates. Similar results were obtained from 3 different healthy donors.
Altogether, our study demonstrates that monoglycerides, used in vitro at similar concentrations as other classes of proapoptotic lipids,13-16 represent a novel family of apoptotic compounds that appear to specifically kill leukemic cells. Clearly, further studies are still required before considering monoglycerides as potential antileukemic agents. For instance, elucidation of the mechanism involved in monoglyceride-induced death would shed some light on the molecular basis for the relative selectivity toward leukemic cells displayed by these compounds.
We thank D. Gagné for helpful discussions; Drs P. Duplay, D. Gosselin, T. Hoang, P. Jolicoeur, and G. Sauvageau for sharing their reagents; and Dr M. Ratcliffe for stimulating discussions.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood- 2002-03-0894.
Supported by PROCREA BioSciences. F.P. is a recipient of a studentship from the Fonds de la Recherche en Santé du Québec/Fonds pour la formation de chercheurs et l'aide àla recherche.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ann-Muriel Steff, PROCREA BioSciences, Division of Research and Development, 6100 Royalmount, Montréal, QC, Canada H4P 2R2; e-mail: asteff@procrea.com.

![Fig. 2. Monoglycerides do not induce cell death in total PBMCs, in major leukocyte populations, or in activated PBMC subsets from healthy individuals. / (A) (Left panel) T-leukemic Jurkat cells or total normal PBMCs were incubated for 3 hours with the vehicle (Ctrl; white bars) or 80 μM 1-C18:1 monoglyceride (MG; black bars). After treatment, cells were stained with annexin V. (Right panel) Normal PBMCs were stimulated with the vehicle (Ctrl; white bars) or 80 μM 1-C18:1 monoglyceride (MG; black bars). After a 3-hour incubation period, the samples were stained for cell surface molecules (anti-CD4 and anti-CD8, anti-CD20 and anti-CD5, anti-CD56 and anti-CD3, or anti-CD14) and annexin V and analyzed by flow cytometry. Electronic gating was performed to determine the percentage of dying cells in distinct leukocyte populations: namely, T helper cells (Th; CD4high), T cytotoxic cells (Tc; CD8+), conventional B cells (Bc; CD20+CD5−), B-1a B cells (BB-1a; CD20+CD5+), natural killer cells (NK; CD56+CD3−), or monocytes (Mono; CD14+). (B) Normal PBMCs were stimulated for 48 hours with either phytohemagglutinin (left panel) or lipopolysaccharides (right panel). Fresh and activated PBMCs were cultured for 3 hours with the vehicle (Ctrl; gray and hatched bars, respectively) or 80 μM 1-C18:1 monoglyceride (MG; white and black bars, respectively). (Left panel) A 4-color staining was performed with anti-CD69 (early activation marker), anti-CD8, anti-CD4, and annexin V. Using electronic gating, sensitivity to monoglyceride-induced cell death was assessed in resting (total CD69− PBMCs, CD4highTh, and CD8+ Tc; gray and white bars) and activated (total CD69+ PBMCs, CD4highTh, and CD8+ Tc; hatched and black bars) populations. (Right panel) A 3-color staining was performed with anti-CD69, anti-CD20, and annexin V to detect cell death among resting (total CD69− PBMCs and Bc[CD20+]; gray and white bars) and activated (total CD69+ PBMCs and Bc [CD20+]; hatched and black bars) cells. Results are shown as mean percentages of annexin V–positive cells ± SD from triplicates. Similar results were obtained from 3 different healthy donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/1/10.1182_blood-2002-03-0894/6/m_h80133642002.jpeg?Expires=1767818850&Signature=hvNrobERi6GneHO9KNa4yC8ikkoKbaBUjmbKIXtVoQfMglqySZEN8GjoTxWnQeGQYaLM~PfcLATXXXbyLOH-pLRw689nyWF9tnOgtxF07YcrVaPPysxMzQnkPad0cGUgMRqzCVKSYigV2kcG6SekI09ALh4WXyK8dLslJDkJgAAoAmST4Umkpy1IKShCG4mU48b1y-e501A~vOQJ8jsgr1OkJK8gEBS3okySljbNRidd~R~rVVkH5jOtc3OQEuFCDUF0Zgr45cRFaEVfCqRlEXi~fKZE4pw9WGgaS19I1~30kAdH66AScq-QLUoFaZold0cOgJJTFSFnUkKvuh-~xg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal