Abstract
Macrophages (MΦ) play a crucial role in the development of cutaneous granulomas (CGs) initiated by foreign bodies or invasive microorganisms. However, little is known about how MΦ are recruited to sites of CG formation. To test whether mast cells (MCs) contribute to early MΦ recruitment to developing granulomas, CGs were induced in MC-deficient KitW/KitW-v mice by injection of polyacrylamide gel (PAG).KitW/KitW-v mice as well as mice deficient in the MC product TNFα exhibited markedly reduced MΦ numbers in CGs. MΦ recruitment was restored inKitW/KitW-v mice reconstituted with MCs from Kit+/+ or TNFα+/+, but not from TNFα−/− mice. MC-TNFα–dependent MΦ influx required prior recruitment of MIP-1α/β–producing neutrophils (PMNs), as PMN depletion before induction of CGs completely inhibited MΦ influx, which was restored after reconstitution with PMN supernatants. These findings indicate that MΦ recruitment to cutaneous PAG- induced granulomas is the result of a sequence of inflammatory processes initiated by MC-derived TNFα followed by PMN influx and MIP-1a/β release.
Introduction
Mononuclear phagocytes (MΦ) are crucial effector cells in the induction of protective cutaneous immune responses to infections by (1) microbes such as Leishmania major and Mycobacterium leprae1 or (2) foreign bodies such as polyacrylamide gel (PAG). MΦ-mediated protection from such intracellular microorganisms includes phagocytosis, recruitment of other proinflammatory leukocytes (ie, neutrophils [PMNs], eosinophils, and lymphocytes),1,2and, most importantly, development of cutaneous granulomas (CGs) aimed at clearing or restricting the growth of microorganisms at sites of infection.1,3 Failure to recruit MΦ and PMNs in microbial infection results in impaired granuloma formation associated with greatly impaired host defense and systemic disease.4Despite the great importance of MΦ in granulomatous inflammation,2 little is known about the mechanisms regulating their recruitment to developing cutaneous granulomas.
To better characterize the chain of inflammatory processes preceding MΦ-dependent CG formation and to elucidate potential mechanisms involved in MΦ recruitment, we have investigated PAG-induced granulomatous inflammation in the absence of mast cells (MCs). MCs are well known to initiate and orchestrate inflammatory skin responses to allergens and pathogens. We and others have shown that early activation of MCs is necessary for protective immune responses to bacterial infection in the context of acute septic peritonitis.5-8In this setting, impaired MC activation, such as in complement (C3)–deficient mice, correlates with markedly reduced (1) release of TNFα, (2) PMN recruitment, (3) clearance of bacteria, and, consequently, (4) survival.7 Furthermore, MCs have been shown to contribute to acute inflammatory monocyte recruitment by releasing MCP-1 or histamine in allergic asthma as well as after the implantation of biomaterial.9 10 However, the role of MCs in more slowly developing immune responses such as cutaneous granuloma formation has not yet been investigated.
Here, we assessed early CG formation in MC-deficient mice, using the model of PAG-induced granulomatous inflammation. We demonstrate that MCs are required to initiate a sequence of inflammatory processes resulting in MΦ recruitment to developing CGs: MC-derived TNFα is necessary to recruit PMNs, which control MΦ influx by releasing MΦ-attracting chemokines, including macrophage inflammatory protein-1 (MIP-1) α/β and MIP-2.
Materials and methods
Animals
C57BL/6 mice, genetically MC-deficient WBB6F1-KitW/KitW-v(KitW/KitW-v) mice, and congenic wild-type WBB6F1-Kit+/+(Kit+/+) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). TNFα−/− mice of a mixed 129/Sv × C57BL/6 genetic background were bred at the Department of Dermatology, Mainz.11 Mice were kept in community cages at the Animal Care Facilities at the University of Mainz. All animal care and experimentation were conducted in accordance with current federal, state, and institutional guidelines.
CG model
CGs were elicited by subcutaneous or intradermal injection of polyacrylamide gel (PAG) as previously described.12-14Briefly, PAG was prepared by addition of Biogel P-100 (BioRad, München, Germany) to phosphate buffered saline (PBS; 35 mg/mL). PAG was injected subcutaneously into the neck area in volumes of 1 mL. Initial experiments with C57BL/6 mice confirmed that the cellular infiltrate in ≤ 5-day-old granulomas consisted exclusively of PMNs and MΦ as assessed by fluorescence-activated cell-sorter (FACS) analysis.15 In some experiments, depleting anti–mouse neutrophil rat mAb clone NIMP-R14 (100 μg/mouse)16 or clone 7/4 (200 μg/mouse) (Serotec, Hamburg, Germany) or isotype control rat mAb were injected intravenously.
Phenotyping of leukocytes recovered from PAG-induced granulomas
Leukocytes were harvested from early PAG-induced granulomas and separated from PAG by filtering through a 70-μm filter in cold PBS. Cells were counted, stained for surface Ag expression,14and analyzed using a FACScan flow cytometer equipped with CellQuest software (Becton Dickinson, Heidelberg, Germany). The antibodies used were as follows: biotin-conjugated F4/80 and fluorescein isothiocyanate (FITC)–conjugated anti–neutrophil mAb (7/4) were obtained from Caltag (Hamburg, Germany); anti-CD16/CD32 (2.4G2), anti–I-Ab (2G9), anti-CD3 (145-2C4), anti-CD4 (L3T4), anti-CD8 (53-6.7), CD11b (M1/70), Gr-1 (RB6-8C5), and anti-NK1.1 (PK136) were purchased from PharMingen (Hamburg, Germany) as biotin- or phycoerythrin (PE)–modified mAb; PE-streptavidin was from Tago (Burlingame, CA).
Preparation of MCs
MC-deficient skin was reconstituted with peritoneal MCs as both peritoneal and skin MCs are connective tissue type MCs (CTMCs) and share virtually identical phenotypes.8 In brief, the peritoneal cavity was lavaged with 0.9% NaCl, and cell suspensions were stained for MCs by Kimura stain and enumerated. CTMCs were enriched to ≥ 95% purity from peritoneal lavage suspension by several gradient centrifugation steps using 23% metrizamide.17 Viability of CTMCs was ≥ 90% as assessed by trypan blue exclusion.
Cutaneous MC reconstitution ofKitW/KitW-vmice
The MC deficiency ofKitW/KitW-v mice (6-8 weeks old) was corrected selectively and locally by injection of CTMCs.8CTMCs (1 × 106 in 100 μL 0.9% NaCl) were injected intradermally, and mice were used for experiments, together with sex- and age-matched MC-deficientKitW/KitW-v andKit+/+ mice, 48 hours after adoptive transfer. MCs were injected into shaved neck skin covering an area of about 1 cm2. Reconstitution of cutaneous MC populations was confirmed by histomorphometric analysis of paraffin-embedded, Giemsa-stained sections of injected skin 48 hours after the injection.8
MC activation assays
The extent of MC degranulation in PAG-injected skin was assessed as described previously.18 Biopsies were taken 60 minutes after intradermal injection of 0.1 mL PAG, and sections were examined by an investigator blinded to the experimental design. MC degranulation was assessed by quantitative histomorphometry (at 1000×).18 A minimum of 100 MCs in ≥ 5 sections/mouse per treatment group were examined. Measurements of serotonin (5-hydroxytryptamine [5-HT]) release in vitro were performed as previously described.17 Briefly, peritoneal MC suspensions were incubated with 2 μCi (0.074 MBq)[3H]5-HT (Perkin Elmer, Freiburg, Germany) for 2 hours at 37°C, washed, stimulated with PAG or PBS for 15 minutes, and the percentage of total 5-HT release was calculated.
Reconstitution of CGs with PMN supernatants and RNAse protection assay
PMNs were isolated from 6- and 12-hour-old granulomas by filter separation. PMNs were further enriched by negative depletion of MΦ using a 2-hour plastic adherence step; > 99% of cells in PMN preparations routinely showed positive antineutrophil (clone 7/4) staining as demonstrated by FACS analysis. Chemokine production was determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Wiesbaden, Germany) in 18-hour supernatants of 2 × 106 PMN/mL RPMI/5% fetal calf serum (FCS) supplemented with glutamine, penicillin/streptomycine, and nonessential amino acids.14 PMN supernatants for reconstitution experiments were generated by plating 5 × 106 PMN/mL in RPMI/5% FCS in 6-well plates, and cell-free supernatants were harvested after 48 hours. As a control, RPMI/5% FCS was treated similarly. Supernatants were assayed by ELISA for their chemokine content and were stored at −20°C until used for reconstitution experiments. Biogel granulomas were reconstituted locally twice daily with either media alone or PMN supernatants (0.5 mL/granuloma) or PMN supernatants that had been preincubated with 10 μg/mL anti–MIP-1β and 0.1 μg/mL anti–MIP-1α (R&D Systems) for 30 minutes at 20°C (Ab amounts were about 4-fold of a concentration calculated to inhibit approximately 50% of the activity found in the supernatants).
For analysis of chemokine expression, RNA from PMNs isolated from 6- and 12-hour granulomas was generated using the High Pure RNA Isolation Kit from Roche (Mannheim, Germany). Expression of chemokines known to be potent MΦ attractants was assessed following the manufacturer's protocol using the RiboQuant RNAse Protection System and template set mCK5, both obtained from PharMingen.
Statistical analysis
All data were tested for statistical significance using the unpaired 2-tailed Student t test or χ2 test (MC degranulation in situ) and are presented as means ± SEM.
Results
Recruitment of MΦ during early granuloma formation is MC-dependent
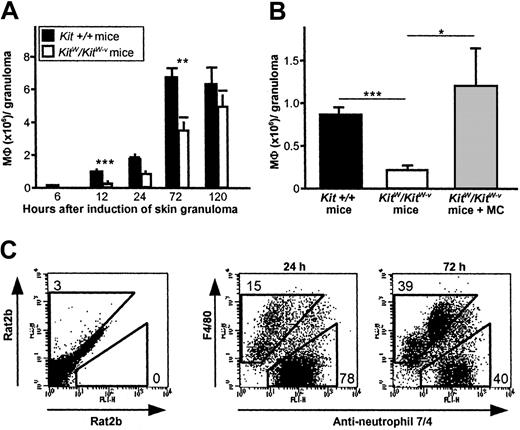
MΦ recruitment to early CGs induced by subcutaneous injection of PAG was greatly reduced in genetically MC-deficientKitW/KitW-v mice compared withKit+/+ mice during the first 72 hours following CG induction (Figure 1A). MΦ numbers in CGs of MC-deficient skin were reduced by 66% (0.3 ± 0.1 vs 0.9 ± 0.1 × 106, P = .001) and 46% (3.5 ± 0.6 vs 6.6 ± 0.7 × 106,P = .004) at 12 hours and 72 hours, respectively, compared with skin of wild-type mice (Figure 1B). To ensure that this defect is due to a lack of MCs in these mice, we reconstituted the skin ofKitW/KitW-v mice withKit+/+ CTMCs (1 × 105) prior to the induction of granulomas, which fully restored the influx of inflammatory MΦ to CGs after 12 hours (Figure 1B). These data suggest that MCs are required for normal recruitment of MΦ to sites of CG formation.
Early MΦ recruitment to PAG-induced cutaneous granulomas is MC dependent.
The absence of MCs in KitW/KitW-vmice results in impaired recruitment of MΦ to early CGs developing at sites of subcutaneous delivery of PAG. (A) Time course of MΦ recruitment to CGs after PAG injection inKit+/+ andKitW/KitW-v mice. Data were pooled from 8 or more mice per genotype and time point–tested in at least 4 independent experiments. (B) Migration of MΦ to granulomas inKitW/KitW-v mice 12 hours after induction is repaired by reconstitution of the dermis with connective tissue–type MCs obtained from Kit+/+ mice. Data were pooled from 8 or more mice per genotype in 2 independent experiments. Data in panels A and B are shown as means ± SEMs (× 106 cells/granuloma). In 1A and 1B, *P < .05, **P < .005, ***P < .001. (C) Characteristic surface staining with anti–MΦ F4/80 and anti–PMN 7/4 mAb of leukocytes recovered from 24- and 72-hour-old granulomas of normal Kit+/+ mice revealed 2 distinct populations, namely PMNs (7/4+) and MΦ (F4/80+). Numbers reflect F4/80+/7/4− cells and 7/4+/F4/80− cells in percent of total cells. One experiment representative of at least 8 independent analyses is shown.
Early MΦ recruitment to PAG-induced cutaneous granulomas is MC dependent.
The absence of MCs in KitW/KitW-vmice results in impaired recruitment of MΦ to early CGs developing at sites of subcutaneous delivery of PAG. (A) Time course of MΦ recruitment to CGs after PAG injection inKit+/+ andKitW/KitW-v mice. Data were pooled from 8 or more mice per genotype and time point–tested in at least 4 independent experiments. (B) Migration of MΦ to granulomas inKitW/KitW-v mice 12 hours after induction is repaired by reconstitution of the dermis with connective tissue–type MCs obtained from Kit+/+ mice. Data were pooled from 8 or more mice per genotype in 2 independent experiments. Data in panels A and B are shown as means ± SEMs (× 106 cells/granuloma). In 1A and 1B, *P < .05, **P < .005, ***P < .001. (C) Characteristic surface staining with anti–MΦ F4/80 and anti–PMN 7/4 mAb of leukocytes recovered from 24- and 72-hour-old granulomas of normal Kit+/+ mice revealed 2 distinct populations, namely PMNs (7/4+) and MΦ (F4/80+). Numbers reflect F4/80+/7/4− cells and 7/4+/F4/80− cells in percent of total cells. One experiment representative of at least 8 independent analyses is shown.
MΦ from developing granulomas were readily identified as F4/80+ and 7/4− cells (Figure 1C). In both MC-deficient KitW/KitW-v mice and normal Kit+/+ mice, MΦ influx to CGs was detected as early as 12 hours after PAG injection. Interestingly, the surface phenotype of infiltrating MΦ (eg, presence of major histocompatibility complex class I and II, or costimulatory molecules) was not affected by the absence of MCs (data not shown).
Recruitment of MΦ to skin granulomas is dependent on MC-derived TNFα
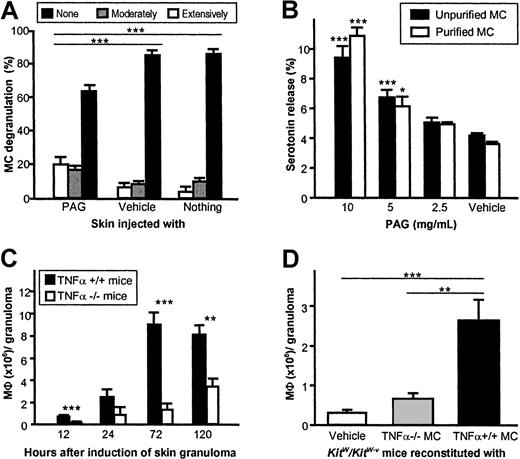
To identify the mechanisms by which MCs control MΦ recruitment to early CGs, we first determined whether CG formation after injection of PAG is associated with MC activation. PAG-injected skin exhibited significantly more extensively and moderately degranulated MCs than vehicle-treated or uninjected skin (P < .001) 1 hour after induction of granulomas in C57BL/6 mice (Figure2A). PAG was also found to induce substantial degranulation of isolated and highly purified CTMCs in vitro as assessed by serotonin release assays (Figure2B), suggesting the MCs may recruit MΦ to developing CGs by releasing preformed proinflammatory mediators.
Induction of cutaneous granuloma formation by PAG depends on release of TNFα by MCs.
(A) MCs in the direct vicinity of early PAG-induced CGs exhibit signs of profound degranulation. As assessed by quantitative histomorphometry, the extent of MC degranulation 1 hour after PAG injection in C57BL/6 mice was scored as “none” (< 10% of granules exhibited staining alterations and/or exteriorization), “moderate” (10%-50%), or “extensive” (> 50%), and expressed as means ± SEMs (data pooled from 5 mice). (B) Unpurified (purity about 1%) and purified (purity > 95%) peritoneal MCs of C57BL/6 mice release serotonin after incubation with PAG. Data were pooled from 3 independent experiments and expressed as means ± SEMs. (C) TNFα-deficient mice exhibit reduced recruitment of MΦ to sites of CG formation after injection of PAG compared with wild-type mice. Data were pooled from 5 or more mice per genotype and time point in 3 independent experiments. (D) Migration of MΦ to granulomas inKitW/KitW-v mice 12 hours after induction is repaired only by reconstitution of the skin with connective tissue–type MCs obtained from TNFα+/+ mice, but not after reconstitution with MCs from TNFα-deficient mice. Data were pooled from 8 or more mice per genotype in 2 independent experiments. Data in all panels are shown as means ± SEMs. *P < .05, **P < .005, ***P < .001.
Induction of cutaneous granuloma formation by PAG depends on release of TNFα by MCs.
(A) MCs in the direct vicinity of early PAG-induced CGs exhibit signs of profound degranulation. As assessed by quantitative histomorphometry, the extent of MC degranulation 1 hour after PAG injection in C57BL/6 mice was scored as “none” (< 10% of granules exhibited staining alterations and/or exteriorization), “moderate” (10%-50%), or “extensive” (> 50%), and expressed as means ± SEMs (data pooled from 5 mice). (B) Unpurified (purity about 1%) and purified (purity > 95%) peritoneal MCs of C57BL/6 mice release serotonin after incubation with PAG. Data were pooled from 3 independent experiments and expressed as means ± SEMs. (C) TNFα-deficient mice exhibit reduced recruitment of MΦ to sites of CG formation after injection of PAG compared with wild-type mice. Data were pooled from 5 or more mice per genotype and time point in 3 independent experiments. (D) Migration of MΦ to granulomas inKitW/KitW-v mice 12 hours after induction is repaired only by reconstitution of the skin with connective tissue–type MCs obtained from TNFα+/+ mice, but not after reconstitution with MCs from TNFα-deficient mice. Data were pooled from 8 or more mice per genotype in 2 independent experiments. Data in all panels are shown as means ± SEMs. *P < .05, **P < .005, ***P < .001.
Since one such MC product, TNFα, has been shown to regulate the recruitment of inflammatory cells, including MΦ,19-22 we assessed PAG-induced CG formation in TNFα−/− mice and TNFα+/+ mice. MΦ recruitment to CGs was dramatically impaired in TNFα−/− mice as compared to TNFα+/+ mice (Figure 2C). Notably, up to 7-fold more MΦ per granuloma were recovered in TNFα+/+ mice as compared to TNFα−/− mice (8.9 ± 1.3 vs 1.3 ± 0.7 × 106 at 72 hours, P < .001).
Since TNFα is not exclusively produced by MCs, but MCs are the only cell type known to contain preformed TNFα,19,23 we reconstituted the skin ofKitW/KitW-v mice with MCs derived from either TNFα+/+ mice or TNFα−/− mice, thus generating mice that differed solely in containing MCs that could or could not release TNFα.7 24KitW/KitW-vmice showed normal migration of MΦ to PAG-induced granulomas only after reconstitution with TNFα+/+ MCs (Figure 2D). Reconstitution with MCs derived from TNFα−/− mice did not increase MΦ influx, indicating that release of TNFα from MCs is required for normal MΦ recruitment to sites of CG formation.
MΦ influx to CGs is dependent on PMNs recruited by MC-TNFα
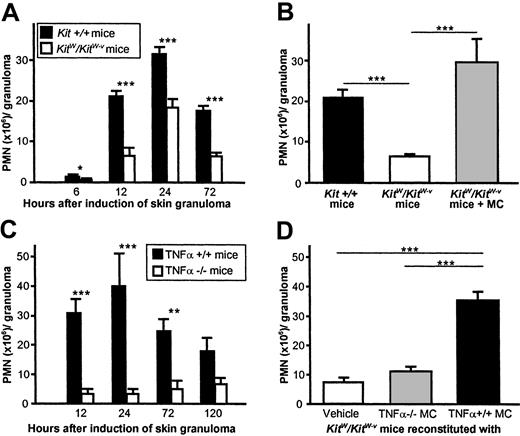
MΦ migration to inflamed skin is preceded by the influx of large amounts of PMNs, proinflammatory cells that have been shown to migrate to sites of TNFα release from MCs and to produce MΦ-recruiting chemokines.25 To assess whether MC-TNFα recruits MΦ during early CG formation directly or by inducing immigration of MΦ-attracting PMNs, we first assessed the kinetics of PMN recruitment to PAG-induced granulomas in MC-, TNFα-, or MC-TNFα–deficient skin. Genetically MC-deficientKitW/KitW-v mice exhibited markedly reduced recruitment of PMNs as compared to wild-type mice after induction of CGs by PAG injection (Figure3A). PMN numbers in 6- to 72-hour-old CGs were significantly and up to 70% lower as compared to wild-type mice at all time points studied. The absence of MCs did not affect the time course of PMN influx. Adoptive transfer of CTMCs to MC-deficient skin prior to injection of PAG restored normal recruitment of PMNs to CG (Figure 3B). PMN numbers also were greatly reduced in TNFα-deficient mice as compared to TNFα+/+ mice in up to 5-day-old granulomas (Figure 3C). PMN recruitment was completely restored inKitW/KitW-v mice reconstituted with TNFα+/+ MCs, while reconstitution with TNFα-deficient MCs did not correct impaired PMN influx in these mice. These observations indicate that MC-derived TNFα contributes not only to MΦ recruitment, but also to PMN recruitment to sites of CG formation.
Neutrophil recruitment to PAG-induced granulomas is dependent on MC-derived TNFα.
(A) Time course of PMN influx after injection of PAG inKit+/+ and KitW/KitW-vmice. Data were pooled from 10 or more mice per genotype and time point–tested in at least 7 independent experiments. (B) Impaired recruitment of PMNs to CGs inKitW/KitW-v mice is MC dependent. The dermis of KitW/KitW-v mice was reconstituted with connective tissue–type MCs from normalKit+/+ mice before PAG injection, and PMN numbers were determined 12 hours after injection of PAG. Data were pooled from 8 or more mice per genotype in at least 4 independent experiments. (C) PMN recruitment in cutaneous granuloma formation is strongly dependent on the presence of TNFα. Numbers of infiltrating PMNs were determined in PAG-injected skin of mice deficient in TNFα and in wild-type mice. Data were pooled from 5 or more mice per genotype and time point in 3 independent experiments. (D) PMN recruitment was fully restored in skin of TNF+/+ MC-reconstitutedKitW/KitW-v mice. Before PAG injection, the dermis of KitW/KitW-vmice was reconstituted with MCs from TNFα+/+ mice or TNFα-deficient mice, and numbers of PMNs were assessed 12 hours thereafter. Data were pooled from 8 or more mice per genotype in 2 independent experiments. All data in Figure 3 are shown as means ± SEMs (× 106 cells/granuloma). For all panels in Figure3, *P < .05, ***P < .005, ***P < .001.
Neutrophil recruitment to PAG-induced granulomas is dependent on MC-derived TNFα.
(A) Time course of PMN influx after injection of PAG inKit+/+ and KitW/KitW-vmice. Data were pooled from 10 or more mice per genotype and time point–tested in at least 7 independent experiments. (B) Impaired recruitment of PMNs to CGs inKitW/KitW-v mice is MC dependent. The dermis of KitW/KitW-v mice was reconstituted with connective tissue–type MCs from normalKit+/+ mice before PAG injection, and PMN numbers were determined 12 hours after injection of PAG. Data were pooled from 8 or more mice per genotype in at least 4 independent experiments. (C) PMN recruitment in cutaneous granuloma formation is strongly dependent on the presence of TNFα. Numbers of infiltrating PMNs were determined in PAG-injected skin of mice deficient in TNFα and in wild-type mice. Data were pooled from 5 or more mice per genotype and time point in 3 independent experiments. (D) PMN recruitment was fully restored in skin of TNF+/+ MC-reconstitutedKitW/KitW-v mice. Before PAG injection, the dermis of KitW/KitW-vmice was reconstituted with MCs from TNFα+/+ mice or TNFα-deficient mice, and numbers of PMNs were assessed 12 hours thereafter. Data were pooled from 8 or more mice per genotype in 2 independent experiments. All data in Figure 3 are shown as means ± SEMs (× 106 cells/granuloma). For all panels in Figure3, *P < .05, ***P < .005, ***P < .001.
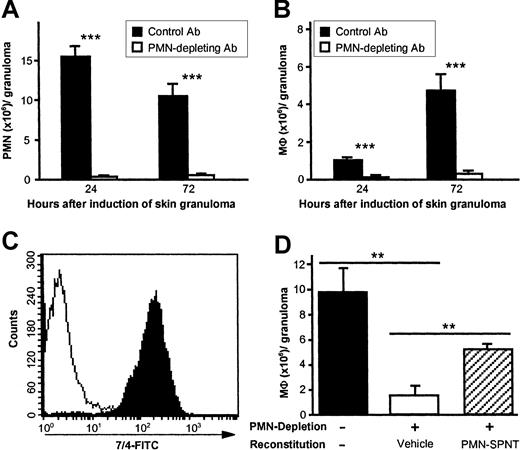
To determine if MC- and TNFα-dependent recruitment of PMNs is involved in the regulation of MΦ recruitment to CGs, C57BL/6 mice were depleted of PMNs by a single intravenous injection of the mAb NIMP-R14 or mAb 7/4 1-3 hours before induction of CG. NIMP-R14 has been shown to eliminate PMNs from the peripheral blood and bone marrow of mice for 2-3 days without affecting other leukocyte populations of the myeloid lineages.16 PMNs were found to be virtually absent in PAG-induced CGs after injection of NIMP-R14, whereas treatment with the isotype control mAb had no effect compared with untreated animals (Figure 4A). Notably, the numbers of F4/80+ MΦ were reduced by 88% and 93% at 24 hours and 72 hours, respectively, after depletion of PMN (Figure 4B), indicating that PMN recruitment is essential for normal MΦ migration to sites of CG formation.
PMN influx is required for MΦ recruitment during early cutaneous granuloma formation.
C57BL/6 mice pretreated with PMN-depleting mAb NIMP-R14 (intravenously, 100 μg, −1 hour) exhibit virtually no recruitment of MΦ to sites of PAG-induced CG formation compared with control Ab-treated mice. Numbers of PMNs (A) and MΦ (B) per CG were assessed 24 hours and 72 hours after injection of PAG. Data were pooled from 5 or more mice per treatment group and time point in 2 independent experiments. Data are shown as means ± SEMs (106 cells/granuloma). ***P < .001. (C) PMNs were isolated from CGs 12 hours after injection of biogel and were stained for anti–neutrophil Ab clone 7/4 (black area, anti–neutrophil Ab; white area, isotype control). Purity of isolated PMNs was determined to be > 99%; one experiment of at least 5 is shown. In panel D, groups of 3 C57BL/6 mice were depleted from PMNs using mAb 7/4 (100 μg intravenously, at −6 hours and +18 hours), and CGs were reconstituted locally twice daily with 0.5 mL/granuloma of either vehicle alone or PMN supernatant (PMN-SPNT). MΦ numbers were determined after 48 hours and are expressed as means ± SEMs (106 cells/granuloma). **P < .005. Data are representative of 3 independent experiments.
PMN influx is required for MΦ recruitment during early cutaneous granuloma formation.
C57BL/6 mice pretreated with PMN-depleting mAb NIMP-R14 (intravenously, 100 μg, −1 hour) exhibit virtually no recruitment of MΦ to sites of PAG-induced CG formation compared with control Ab-treated mice. Numbers of PMNs (A) and MΦ (B) per CG were assessed 24 hours and 72 hours after injection of PAG. Data were pooled from 5 or more mice per treatment group and time point in 2 independent experiments. Data are shown as means ± SEMs (106 cells/granuloma). ***P < .001. (C) PMNs were isolated from CGs 12 hours after injection of biogel and were stained for anti–neutrophil Ab clone 7/4 (black area, anti–neutrophil Ab; white area, isotype control). Purity of isolated PMNs was determined to be > 99%; one experiment of at least 5 is shown. In panel D, groups of 3 C57BL/6 mice were depleted from PMNs using mAb 7/4 (100 μg intravenously, at −6 hours and +18 hours), and CGs were reconstituted locally twice daily with 0.5 mL/granuloma of either vehicle alone or PMN supernatant (PMN-SPNT). MΦ numbers were determined after 48 hours and are expressed as means ± SEMs (106 cells/granuloma). **P < .005. Data are representative of 3 independent experiments.
Soluble mediators released by PMNs rather than structural/molecular changes induced by PMN migration may regulate MΦ recruitment to CGs. To test this hypothesis, we reconstituted CGs in PMN-depleted mice with supernatant derived from PMNs or media alone (Figure 4D). Supernatants from PMNs were generated in RPMI/5% FCS for 48 hours, and purity of isolated PMNs was determined to be > 99% using FACS analysis and staining with anti–neutrophil Ab 7/4 (Figure 4C). Local injection of PMN supernatants twice daily restored > 60% of MΦ influx to sites of CG formation, whereas vehicle alone (media + FCS) did not repair MΦ influx at all.
MIP-1α/β released by PMNs are responsible for MΦ recruitment to CGs
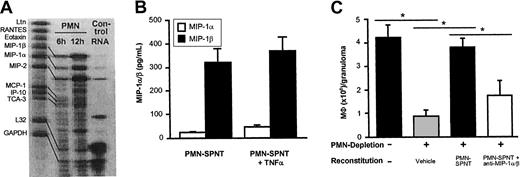
To identify the soluble mediators released by PMNs, we analyzed cytokine release and chemokine expression of PMNs isolated from either 6- or 12-hour-old granulomas (Figure 5A). While we found no significant production of TNFα or other cytokines (interleukin-4 [IL-4], IL-12p40, interferon-γ [IFNγ]) by PMNs when assessing the supernatants by ELISA (data not shown), RNAse protection assays revealed strong expression of MIP-1α, MIP-1β, and MIP-2 by PMNs. The C-C chemokines MIP-1α and MIP-1β are strong chemotactic signals for MΦ (compare Luster26), whereas MIP-2, a C-x-C chemokine and the murine homolog for IL-8, is known to recruit PMNs themselves as well as T lymphocytes.26 Therefore, our data suggest that PMN-derived MIP-1α and MIP-1β are responsible for MΦ recruitment to CGs. We next assayed 18-hour-old supernatants from PMNs for protein content by ELISA (Figure 5B). PMNs isolated from CGs predominantly released MIP-1β, although we also detected some MIP-1α activity in the supernatants. Both chemokines were up-regulated in TNFα-treated PMNs within 18 hours (Figure 5B). Finally, we attempted to inhibit the activity of MIP-1α/β in PMN-derived supernatants used for reconstitution experiments by preabsorption with neutralizing antibodies. The 48-hour PMN supernatants used for reconstitution experiments contained 3.7 ± 1.9 pg/mL MIP-1α and 197.4 ± 47 pg/mL MIP-1β, whereas RPMI/5% FCS alone was negative for the chemokines by ELISA. As demonstrated in Figure 5C, reconstitution of CGs with PMN supernatants treated with anti–MIP-1α and anti–MIP-1β inhibited about 60% of supernatant-induced recruitment of MΦ to CGs.
MIP-1α/β released by PMNs is responsible for MΦ influx into cutaneous granulomas.
(A) Expression of the chemokines MIP-1α, MIP-1β, and MIP-2 by PMNs from early CGs (6 hours and 12 hours old) was found using the RNAse protection assay (PharMingen). One of 2 experiments with similar results is shown. Lane 1 shows the template set; lanes 2 and 3, PMN RNA; lane 4, control RNA provided by the manufacturer. (B) Production of MIP-1α and MIP-1β was determined in supernatants of 2 × 106 PMNs in fully supplemented media after 18 hours. Chemokine production was enhanced in TNFα-treated PMNs (n = 5). In panel C, groups of 3 C57BL/6 mice were depleted of PMNs using mAb 7/4 as shown in Figure 4D, and CGs were reconstituted locally twice daily with either vehicle alone or PMN supernatant (PMN-SPNT) or PMN-SPNT that was pretreated with anti–MIP-1α and anti–MIP-1β for 30 minutes. MΦ numbers were determined after 48 hours and expressed as means ± SEMs (106 cells/granuloma). *P < .05. Data are pooled from 6 mice per group from 2 independent experiments.
MIP-1α/β released by PMNs is responsible for MΦ influx into cutaneous granulomas.
(A) Expression of the chemokines MIP-1α, MIP-1β, and MIP-2 by PMNs from early CGs (6 hours and 12 hours old) was found using the RNAse protection assay (PharMingen). One of 2 experiments with similar results is shown. Lane 1 shows the template set; lanes 2 and 3, PMN RNA; lane 4, control RNA provided by the manufacturer. (B) Production of MIP-1α and MIP-1β was determined in supernatants of 2 × 106 PMNs in fully supplemented media after 18 hours. Chemokine production was enhanced in TNFα-treated PMNs (n = 5). In panel C, groups of 3 C57BL/6 mice were depleted of PMNs using mAb 7/4 as shown in Figure 4D, and CGs were reconstituted locally twice daily with either vehicle alone or PMN supernatant (PMN-SPNT) or PMN-SPNT that was pretreated with anti–MIP-1α and anti–MIP-1β for 30 minutes. MΦ numbers were determined after 48 hours and expressed as means ± SEMs (106 cells/granuloma). *P < .05. Data are pooled from 6 mice per group from 2 independent experiments.
Discussion
MCs are ideally suited to initiate protective immune responses against invading pathogens because (1) MCs are preferentially located at host/environment interfaces (upper dermis in the skin, gut lamina propria, airways' subepithelium), (2) they produce a large array of proinflammatory mediators, many of which are stored within cytoplasmic granules and are released within minutes after activation, and (3) MCs have receptors that can recognize microbial molecules (including complement receptors toll-like receptors, and CD48) and can, therefore, be activated by pathogens via multiple mechanisms, including fimbrial proteins, toxins, and the proteolytic fragments of complement components.19 Indeed, MCs have been reported to be required for the induction of immediate host defense reactions in various models of acute bacterial infections.5-7 Here, we show for the first time that MCs also are required to elicit normal chronic and more slowly developing cellular responses. Indeed, the normal succession of key events in slowly developing immune responses such as CG formation is induced by and dependent on initial MC activation. Specifically, early MC degranulation is needed to elicit recruitment of MΦ, a hallmark feature of granulomatous inflammation (compare Murray3), which is required for the development of CGs. Our findings suggest that MCs facilitate normal chronic granulomatous inflammatory processes by inducing the following chain of events: release of TNFα from MCs promotes influx of PMN, which release MΦ-recruiting chemokines (such as MIP-1α/β, MIP-2), which in turn result in the recruitment of MΦ.
The early steps of granuloma development are tightly modeled in leukocyte recruitment to lesions in cutaneous leishmaniasis, where the initial inflammatory phase of granuloma development is characterized by the sequential influx of PMNs, eosinophils, and MΦ to parasite-loaded skin.2 In leishmaniasis, extensive dermal MC degranulation is found at sites of early infection,27 and MCs coincubated with Leishmania major in vitro reportedly release preformed TNFα within minutes,28 suggesting that MCs and MC-derived products may modulate the host response toLeishmania, especially during the initial phase of infection. Our data support this hypothesis by showing that MCs contribute significantly to the local inflammatory cutaneous reaction that develops at sites of CG formation in response to foreign bodies such as PAG. In future studies we will investigate in more detail if and how MCs modulate local inflammatory responses observed after intradermal delivery of Leishmania major.
Most of the leukocyte recruitment to CGs that we observed was TNFα dependent, stressing the essential role that TNFα plays in skin inflammation. To our surprise, the absence of TNFα resulted in reduced recruitment of MΦ as early as 12 hours after injection of PAG, suggesting that TNFα is, at least in part, released from preformed stores. Since MCs are the only resident skin cells shown to contain considerable amounts of preformed TNFα, it is mostly likely that most of the TNFα that accounts for the initial influx of PMNs and MΦ is released by MCs, although several other types of skin cells are capable of producing TNFα.29 At later time points after injection of PAG, the difference in inflammation between MC-deficient and normal wild-type mice was less dramatic, perhaps reflecting TNFα release or production of other factors by cells other than MCs (including dendritic cells, resident dermal MΦ, keratinocytes).
Most of the MC effect on leukocyte recruitment was TNFα-dependent and did not depend on release of other MC mediators since only reconstitution with TNFα-producing MCs fully restored inflammatory cell influx to CGs in KitW/KitW-vmice, while the adoptive transfer of TNFα-deficient MCs did not improve PMNs or MΦ recruitment in these mice. This observation is supported by a recent report showing that inflammatory skin reactions, including PMN influx in contact hypersensitivity, are impaired inKitW/KitW-v mice and that these responses were normalized only after adoptive transfer of TNFα-competent, but not TNFα-deficient, MCs.24 In addition, Zhang et al provided evidence that, in vivo, MCs produce the TNFα that augments PMN emigration in the context of acute peritoneal inflammation.30
Another remarkable finding of this study was that depletion of PMNs resulted in a strong impairment of MΦ recruitment to the site of CG formation. To ensure that the mononuclear cell population was not affected by PMN depletion, we used anti–neutrophil Ab clone NIMP-R14 for our experiments.16 In agreement with the findings of Tacchini-Cottier et al,16 we observed a similar and overlapping staining pattern of the monoclonal anti–neutrophil antibodies NIMP-R14 and 7/4 on PMNs derived from C57BL/6 mice. No expression of NIMP-R14 or 7/4 by resident or recently extravasated MΦ was detected (Figure 1C and data not shown). The sequential accumulation of first PMNs and then MΦ to tissues after initiation of inflammatory processes is a phenomenon that is observed frequently in a variety of diseases and experimental models, but the strict dependence of MΦ recruitment on prior PMN influx has not been previously demonstrated. Several reports describe impaired MΦ functions and granuloma formation in patients with neutrophil dysfunction or deficiency (such as agranulocytosis), for example, impaired granuloma formation in a patient with severe granulocytopenia led to insufficient control of fungi with severe dissemination ofAspergillus.4 In contrast, in chronic granulomatous disease, hyperactivation of neutrophils and increased release of chemoattractants for MΦ and T cells from PMNs might contribute to poorly controlled inflammatory responses and unstructured granuloma formation in affected patients.31 However, further studies are warranted to better characterize the correlation between PMNs and MΦ recruitment in cutaneous granulomatous development and to elucidate underlying mechanisms.
In summary, we show that TNFα release from activated MCs regulates PMN influx to sites of granulomatous inflammation, which in turn recruit MΦ to developing cutaneous granulomas by releasing potent MΦ-recruiting chemokines, including MIP-1α and MIP-1β. Our studies confirm and extend recently established concepts regarding the role of MCs in host-immune responses. In addition to the sentinel role in acute host-defense reactions against bacteria, MCs may also be critical effector cells responsible for the initiation and orchestration of long-lasting cell-mediated immunity.
The authors wish to thank Dr George Kollias for kindly providing TNF-deficient mice; Drs Mark C. Udey, Yasmine Belkaid, and Helmut Jonuleit for helpful discussions; Drs Michael Stassen and Edgar Schmitt for help with RNAse protection assays; Drs Kerstin Steinbrink, Karsten Mahnke, and Thomas Tüting for critically reading the manuscript; and Elena Wiese for excellent technical assistance.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-03-0921.
Parts of this work were supported by grants from the Deutsche Forschungsgemeinschaft (DFG; Ste 833/4-1 and Ma 1909/4-1) and the Mainzer Forschungsförderungsprogramm (MAIFOR) program to E. von S. and M.M.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
M. Maurer, Department of Dermatology, Johannes Gutenberg-University of Mainz, Langenbeckstrasse 1, 55131 Mainz, Germany; e-mail:maurer@hautklinik.klinik.uni-mainz.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal