Abstract
We previously showed that β2-glycoprotein I (β2GPI)–dependent lupus anticoagulants (LAs) form bivalent antigen-antibody complexes with high affinity for phospholipids; these complexes are responsible for their in vitro anticoagulant effect. We now studied the role of these bivalent complexes in arterial thrombosis in the hamster. Three monoclonal antibodies (mAbs) raised against human β2GPI were selected on the basis of their cross-reactivity with hamster β2GPI. Two of these, one with LA activity, 5H2, and one with only anticardiolipin properties, 11E8, were infused at 0 to 10 mg/kg prior to photochemically induced vessel damage. 5H2 promoted thrombus formation dose dependently, raising the thrombus size from 6.0 arbitrary units (AU) in controls (n = 9) to 65.0 AU in the high-dose group (10 mg/kg, n = 6, P = .007). The LA−mAb 11E8 and mAb 27A8, reactive with human β2GPI exclusively, did not significantly promote thrombus formation. In a second set of experiments, intact mAb 5H2 was compared to its fragments. Intact mAb 5H2 at 3.3 mg/kg and the equimolar dose of F(ab′)2 fragments (2.2 mg/kg) promoted thrombus formation equally well (55.8 AU, n = 8 and 62.5 AU, n = 7, respectively); mAb 5H2-derived Fab′ fragments were inactive. Immunohistochemical analysis showed platelet-rich thrombi, with 5H2 or its F(ab′)2fragments mainly bound to individual platelets. Our results indicate that bivalent immune complex formation plays an important role in the genesis of arterial thrombosis by certain antiphospholipid antibodies. Cellular activation via the Fc portion of these immune complexes, however, is not essential, because F(ab′)2 fragments of 5H2 still promote thrombus formation.
Introduction
Antiphospholipid antibodies (aPLs) are a heterogeneous group of immunoglobulins interacting with negatively charged phospholipids (PLs). They are found in serum or plasma of patients with rheumatic diseases, malignancies, or infections, but also in apparently healthy individuals. Persistently elevated aPL levels are associated with the occurrence of arterial and venous thrombosis, thrombocytopenia, and recurrent fetal loss.1This clinical entity, referred to as the antiphospholipid syndrome (APS), is considered secondary or primary, respectively, in subjects with or without systemic autoimmune diseases, for example, systemic lupus erythematosus (SLE).2 Paradoxically, an important subset of aPLs, termed lupus anticoagulants (LAs), prolongs in vitro plasma clotting times.3 4
The first immunoassays for the detection of aPLs made use of cardiolipin.5,6 However, the so-called anticardiolipin antibodies (aCLs) also bind to other negatively charged PLs, such as phosphatidylserine.7 Affinity purification of aCLs revealed that aCL binding to cardiolipin depends on a plasma protein, β2-glycoprotein I (β2GPI).8-10It is now generally accepted that autoimmune aPLs are directed against proteins binding to anionic PL surfaces rather than against PLs themselves, the major protein targets appearing to be β2GPI and prothrombin.11,12 LA+aPLs cross-link 2 β2GPI molecules or 2 prothrombin molecules and thereby induce the correct spatial orientation of the PL-binding domains in these proteins required for optimal binding to PLs.13-16 The dimerized β2GPI or prothrombin displays a marked gain in affinity for the PL surface and retards clotting in vitro by competing with clotting factors for the same PL surface.
Autoimmune aPLs are thought to be pathogenic because patients with aPLs not only have an increased risk for thrombosis but also show signs of a prothrombotic (hypercoagulable) state with elevated tissue factor (TF) expression17 and enhanced thrombin generation.18 The mechanisms by which these antibodies cause a prothrombotic state or promote thrombosis are still far from being elucidated. Several hypotheses have been proposed including a decreased prostacyclin formation by endothelium, inhibition of protein C activation or of activated protein C function, impairment of TF inhibition, interference with the function of antithrombin, impaired fibrinolytic potential, reduced anticoagulant potential of annexin V, and activation of platelets (for a review, see Rand19). However, none of these hypotheses explain why thrombosis can be venous as well as arterial and why LAs are more strongly associated with thrombosis than aCLs.20 21
Analogous to heparin-induced thrombocytopenia (HIT), another syndrome of antibody-mediated thrombosis, a model of prothrombotic cellular activation was proposed. Limited damage or activation of blood cells or endothelium may cause exposure of anionic PLs on the cell surface. In the presence of aPLs with LA activity, bivalent antigen-antibody complexes may form on these cell membranes enriched in anionic PLs. These complexes may then bind to cellular Fcγ receptors or activate the complement system leading to strong thrombosis-promoting cell activation via release of granule contents and of microvesicles, thromboxane A2 biosynthesis, tissue factor decryption, removal of endothelial heparan sulfate, and so forth.22 23
In the present study, this hypothesis was tested in a hamster model of arterial thrombosis,24 adapted in our laboratory to study prothrombotic phenotypes.25 A murine monoclonal antibody (mAb) against human β2GPI with clear LA activity and cross-reacting with hamster β2GPI was selected. The consequences on photochemically induced platelet-rich thrombosis in the hamster were then studied after injection of intact antibody, or its F(ab′)2, respectively, Fab′ fragments. Our findings show that aPL-associated thrombosis, in contrast to HIT, can occur independently of Fc.
Materials and methods
mAbs against β2GPI
Preparation of F(ab′)2 fragments
Purified mAb 5H2 at 3 mg/mL was dialyzed overnight at 4°C against 100 mM sodium citrate buffer, pH 3.5. Digestion was performed by addition of 5 μg pepsin beads (Sigma, St Louis, MO) per milligram mAb. After incubation for 60 minutes at 20°C, the beads were separated by centrifugation for 10 minutes at 4000 rpm and the pH of the supernatant was adjusted to 8 with 1 M Tris (tris(hydroxymethyl)aminomethane)–HCl buffer. Following dialysis against 100 mM Tris-HCl buffer, pH 8.1, intact Fc fragments and nondigested antibody were removed by protein A–Sepharose chromatography. The F(ab′)2 preparation showed a single band at 110 kDa and a doublet of bands at 25 kDa in unreduced and reduced sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining, respectively. The protein concentration was determined by absorbance (280 nm) measurements (extinction coefficient, 1.35 mL mg−1cm−1).
Preparation of Fab′ fragments
Purified mAb 5H2 at 3 mg/mL was dialyzed overnight at 4°C against 100 mM phosphate buffer, pH 7.0, after which 10 mM cysteine and 2 mM EDTA (ethylenediaminetetraacetic acid) were added. Digestion was performed by addition of 30 μg papain beads (Sigma) per milligram mAb. After incubation at 37°C for 60 minutes, the reaction was stopped by 75 mM iodoacetamide (Sigma). Following dialysis against 100 mM Tris-HCl buffer, pH 8.3, intact Fc fragments and nondigested antibody were removed by protein A–Sepharose chromatography. The Fab′ preparation showed a single band at 50 kDa and 25 kDa in unreduced and reduced SDS-PAGE and silver staining, respectively.
Reactivity of mAbs with hamster β2GPI
To assess the cross-reactivity of the antihuman β2GPI mAbs with hamster β2GPI, microtiter plates (Costar no. 3590; Corning, NY) were incubated with 50 μL/well phosphatidylserine (Sigma) dissolved in absolute ethanol (27 μg/mL) and evaporated overnight at 4°C. The plates were then blocked with 5% hamster plasma in phosphate-buffered saline (PBS), as a source of β2GPI, for 60 minutes at room temperature. Anti-β2GPI mAbs dissolved in 1% hamster plasma in PBS (0.5-10 μg/mL) were added to the plate and incubated for 120 minutes at room temperature. Plates were then washed 3 times with PBS and incubated for 120 minutes with 100 μL/well horseradish peroxidase–coupled goat antimouse immunoglobulins (GAM-HRP; Dako, Glostrup, Denmark) diluted 1:3000 in PBS, containing 1% hamster plasma. After washing, 160 μL 100 mM citrate/200 mM sodium phosphate buffer containingo-phenylenediamine (OPD; Fluka, Buchs, Switzerland) and 0.003% H2O2 was added to each well. After approximately 30 minutes at room temperature, staining was stopped with 50 μL 4 M H2SO4. Absorbance (490 nm) was then measured with a multiscan spectrophotometer (ELx808; Bio-Tek Instruments, Winooski, VT).
LA activity of anti-β2GPI mAbs in hamster plasma
Determination of LA activity was based on its effect on the dilute prothrombin time (dPT).27 Platelet-poor plasma (PPP) was prepared by double centrifugation from hamster blood collected on trisodium citrate. The anti-β2GPI mAbs were 10-fold diluted in this PPP to achieve final concentrations between 0 and 150 μg/mL and incubated for 10 minutes at 37°C prior to testing. The dPT was determined by incubating 50 μL Innovin (DADE, BEHRING, Liederbach, Germany) diluted 1:200 in Owren Veronal buffer, with 50 μL PPP containing mAb for 7 minutes at 37°C after which coagulation was initiated by adding 50 μL CaCl2 (25 mM). Coagulation times were measured using a SYSMEX CA 6000 coagulometer (TOA Medical Instruments, Kobe, Japan).
Affinity for β2GPI of intact 5H2 and its F(ab′)2 and Fab′ fragments
Microtiter plates were coated overnight at 4°C with 50 μL purified human β2GPI at 5 μg/mL. The plates were blocked with 1% fatty acid–free bovine serum albumin fraction V (BSA; Boehringer Mannheim, Mannheim, Germany) and washed 3 times with PBS containing 0.1% Tween 20 (Merck, Hohenbrunn, Germany). Intact 5H2 or its F(ab′)2 or Fab′ fragments (0-3 μg/mL PBS) were applied and incubated for 120 minutes at 20°C. After washing, antibody-β2GPI complexes were detected by adding GAM-HRP diluted 1:6000 in 0.1% BSA using OPD as substrate.
Induction of β2GPI binding to PLs by intact 5H2 and its F(ab′)2 or Fab′ fragments
Microtiter plates were incubated overnight at 4°C with 50 μL/well of a mixture containing 75% phosphatidylcholine (Sigma) and 25% phosphatidylserine (Sigma) dissolved in absolute ethanol (total concentration 27 μg/mL). The plates were blocked with 1% β2GPI-free BSA and washed 3 times with PBS. 5H2 and its F(ab′)2 or Fab′ (0-10 μg/mL), diluted in 0.2% hamster plasma, were added to the PL-coated plates for 120 minutes at 20°C. After washing, bound hamster β2GPI was detected using peroxidase-coupled rabbit polyclonal anti-β2GPI antiserum (homemade) diluted 1:3000 in 0.1% BSA.
Inhibition of intact 5H2-induced hamster β2GPI binding to PLs by Fab′ fragments
Intact 5H2, added to diluted hamster plasma at a fixed concentration of 625 ng/mL, was preincubated for 45 minutes at 20°C with its Fab′ fragments (0-20 μg/mL) and added to microtiter plates coated with phosphatidylcholine and phosphatidylserine, as indicated in the previous paragraph. After incubation for 120 minutes at 20°C, bound hamster β2GPI was detected as in the previous paragraph.
Photochemically induced thrombosis model in the hamster
All animal experiments were reviewed and approved by the Institutional Review Board of the University of Leuven and were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee. Male hamsters (Pdf gold; University of Leuven) weighing 100 to 130 g were anesthetized by an intraperitoneal injection of sodium pentobarbital (Nembutal; Sanofi Animal Care, Brussels, Belgium) at a dose of 60 mg/kg and then fixed on a thermostated operation table. A 2.5 French venous catheter (Portex, Hythe, United Kingdom) was inserted into the right jugular vein. The left carotid artery was carefully dissected from surrounding tissue and mounted on a transilluminator. Thrombus formation was induced by a photochemical reaction according to the method of Umemura et al.24 Briefly, just after injection of the dye rose-bengal (Sigma) at a dose of 20 mg/kg, the exposed artery was irradiated for 2 minutes with green light (wavelength 540 nm) from a xenon lamp (L4887; Hamamatsu Photonics, Hamamatsu, Japan) equipped with a heat-absorbing filter and a green filter. Irradiation was directed via a 3-mm–diameter optic fiber attached to a manipulator. All tested reagents were administered via an intravenous (slow) bolus injection prior to rose-bengal injection. Intact mAbs and F(ab′)2 fragments were given 15 minutes before photochemical vessel injury, whereas Fab′ and buffer injections just preceded rose-bengal injection.
Quantification of mural thrombi in the hamster carotid artery was performed as described with minor modifications.28Thrombus formation in the injured transilluminated vessel was constantly monitored for 40 minutes via a camera (CV-M70; JAI, Yokohama, Japan) mounted on a microscope. The images were digitized with an image processing software (Optimas 6.5 for Windows 95/98 and NT 4.0; Media Cybernetics, Silver Spring, MD, with a specific extension from IP Consult, Breda, The Netherlands) and constantly recorded. The transmitted light intensity versus time curve was established and thrombus formation was measured by comparing the area under the curve, expressed in arbitrary light units (AU).
Determination of the concentration of intact 5H2 and its F(ab′)2 fragments in hamster plasma
Plasma was prepared from blood drawn via the intravenous catheter just after completion of the in vivo experiments. Plasma concentrations of 5H2 or its F(ab′)2 fragments were measured by enzyme-linked immunosorbent assay (ELISA) as follows. Microtiter plates were coated overnight at 4°C with 200 μL/well polyclonal rabbit antimouse IgG (5 μg/mL), blocked with 1% BSA and washed 3 times with PBS containing 0.1% Tween 20. Hamster plasma samples, 1:2000 and 1:1000 diluted in PBS for measurement of intact 5H2 and its F(ab′)2 fragments, respectively, were added and incubated for 120 minutes at 20°C. Standard curves were constructed using 5H2 or 5H2-derived fragment solutions (0-200 μg/mL in hamster plasma), diluted in the same way as the ex vivo samples. After washing, bound mAbs were detected by GAM-HRP diluted 1:3000 in PBS, containing hamster and rabbit plasma (1:300) to adsorb goat antimouse antibodies cross-reacting with hamster and rabbit antibodies. The HRP activity was determined with OPD as a substrate.
Immunohistochemistry
Carotid arteries containing thrombi were carefully dissected, fixed overnight at 4°C in 4% formaldehyde in PBS, pH 7.0, and transferred to PBS containing 20% sucrose for 24 hours. Arteries were embedded in OTC compound (Tissue-Tec; Miles, Elkhart, IN), snap-frozen in precooled 2-methyl butane and stored at −70°C until further analysis. Then, 7-μm–thick sections were made through the whole thrombosed artery for hematoxylin-eosin staining. Immunohistochemical staining for the presence of 5H2 and its F(ab′)2 fragments was done with a GAM-HRP diluted 1:250 in TRIS-buffered saline (TBS) containing 2% BSA and preincubated for 30 minutes with 10% hamster plasma to adsorb nonspecific antibodies. Peroxidase staining was performed in 50 mM Tris-HCl buffer, pH 7.0, containing 0.06% 3,3-diaminobenzidine and 0.01% H2O2. Tissue sections were counterstained with hematoxylin.
Platelet aggregation studies
Blood for platelet aggregation studies was freshly drawn from healthy donors on 109 mM trisodium citrate and centrifuged at 150g for 15 minutes. The platelet-rich plasma (PRP) was collected and the platelet counts were adjusted to 2.5 × 105 platelets/μL with autologous PPP. Light transmission during adenosine 5′-diphosphate (ADP)–induced platelet aggregation was recorded on a 4-channel aggregometer (Chrono-log, Havertown, PA). Four minutes before stimulation with a subthreshold concentration of ADP, the PRP was incubated during 3 minutes at 37°C either with 75 μg/mL intact mAb 5H2 or 50 μg/mL of its F(ab′)2 fragments or its Fab′ fragments.
Statistical analysis
Intergroup comparison was performed with the Mann-WhitneyU test and potential correlations were evaluated using the Spearman rank order test. P < .05 were considered significant.
Results
Selection and in vitro characterization of mAbs reacting with hamster β2GPI
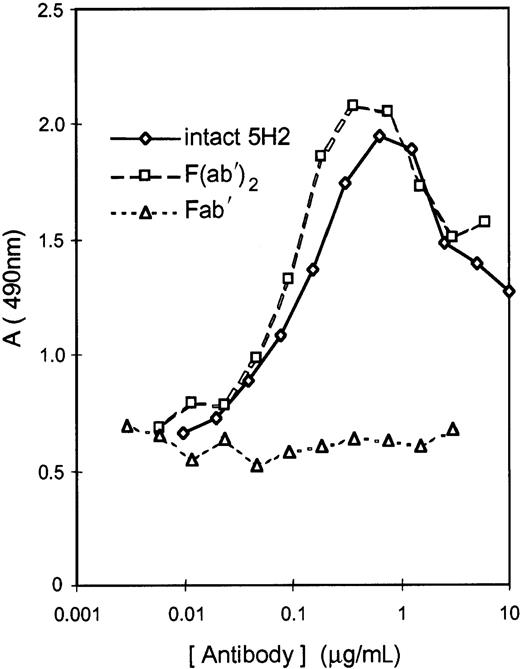
Murine mAbs previously raised against human β2GPI were selected on the basis of their cross-reactivity with hamster β2GPI. Two mAbs, 5H2 and 11E8, strongly binding to hamster β2GPI attached to phosphatidylserine, were selected. mAb 27A8, reacting with human β2GPI only, was selected as negative control. mAb 5H2 had potent LA activity in hamster plasma as documented by the concentration-dependent prolongation of the dPT. The dPT of hamster plasma spiked with 5H2 at 40 μg/mL was prolonged by a factor 1.50. mAb 11E8 did not possess LA activity. mAb 5H2 was therefore selected for the preparation of F(ab′)2and Fab′ fragments; their affinity for human β2GPI was similar to that of the intact antibody when tested in microtiter plates coated with β2GPI. Both intact 5H2 and its F(ab′)2 fragments induced hamster β2GPI binding to PLs (75% phosphatidylcholine and 25% phosphatidylserine), which was not the case for Fab′ fragments (Figure1).
Antibody-induced binding of hamster β2GPI to PLs.
Induction of β2-GPI binding to PLs (75% phosphatidylcholine and 25% phosphatidylserine) by intact 5H2 or its F(ab′)2 fragments compared to Fab′ fragments. Antibody-β2GPI complexes, attached to microtiter plate–coated PLs, were measured with a rabbit anti-β2GPI antiserum coupled to peroxidase. Data are mean of 2 determinations.
Antibody-induced binding of hamster β2GPI to PLs.
Induction of β2-GPI binding to PLs (75% phosphatidylcholine and 25% phosphatidylserine) by intact 5H2 or its F(ab′)2 fragments compared to Fab′ fragments. Antibody-β2GPI complexes, attached to microtiter plate–coated PLs, were measured with a rabbit anti-β2GPI antiserum coupled to peroxidase. Data are mean of 2 determinations.
The bell-shaped relationship between the concentration of intact 5H2, or its F(ab′)2, and β2GPI binding to the PL surface, suggests formation of bivalent antibody-β2GPI complexes on the PL surface; at very high antibody concentration, competition between monovalent and bivalent complexes causes a decrease in amount of β2GPI bound to PLs (Figure 1). The concentration-dependent inhibition of intact 5H2-induced β2GPI binding to PLs by the 5H2-derived Fab′ fragments confirms the formation of bivalent complexes between 5H2 and hamster β2GPI (data not shown).
Thrombogenicity of 5H2
Both mAbs cross-reacting with hamster β2GPI, one with (5H2) and one without LA activity (11E8) were injected intravenously at a dose ranging from 0 to 10 mg/kg prior to application of a controlled vessel injury. mAb 27A8, nonreactive with β2GPI, and buffer were selected as negative controls. 5H2 dose dependently promoted thrombus formation, enhancing the median total intensity of transilluminated light, calculated as area under the curve (AUC), from 6.0 (median value, n = 9) arbitrary light units (AU) in the controls treated with buffer to 65.0 AU in the 5H2 high-dose group (10 mg/kg, n = 6, P = .007) with a median effective dose (ED50) around 1.1 mg/kg (median 24.5 AU, n = 6; Figure 2). The LA− mAb 11E8 promoted thrombus formation only marginally (median AU at a dose of 10 mg/kg: 14.3, n = 8, P = .18; not shown). The influence of mAb 27A8 on thrombus development was negligible (median AU: 9.7, n = 6; not shown).
Photochemically induced thrombosis of hamster carotid artery.
(A) A dose-dependent effect of intact 5H2 on cumulative thrombus formation over 40 minutes expressed as total intensity of transilluminated light. (B) Comparison of thrombus light intensity in animals treated with intact 5H2 or its F(ab′)2 fragments at the indicated doses. (C) Influence of 5H2 Fab′ fragments on thrombus formation. Data are presented as median, interquartile range [IQR], and minute-max range of area under curve; P values for comparison with controls were calculated using Mann-Whitney U test. AU indicates arbitrary light units.
Photochemically induced thrombosis of hamster carotid artery.
(A) A dose-dependent effect of intact 5H2 on cumulative thrombus formation over 40 minutes expressed as total intensity of transilluminated light. (B) Comparison of thrombus light intensity in animals treated with intact 5H2 or its F(ab′)2 fragments at the indicated doses. (C) Influence of 5H2 Fab′ fragments on thrombus formation. Data are presented as median, interquartile range [IQR], and minute-max range of area under curve; P values for comparison with controls were calculated using Mann-Whitney U test. AU indicates arbitrary light units.
In a second set of experiments, 8 hamsters treated with intact 5H2 at a dose of 3.3 mg/kg were compared with animals treated with 5H2-derived F(ab′)2 fragments at a dose of 2.2 mg/kg (n = 7) and 4.5 mg/kg (n = 8) and control animals (n = 16). F(ab′)2fragments promoted thrombus formation similarly to the intact antibody both at an equimolar dose (2.2 mg/kg) and a double equimolar dose (4.5 mg/kg; Figure 2B). The median AU for the control group and for the animals receiving intact 5H2 and its F(ab′)2 fragments at an equimolar dose and a double equimolar dose were 17.9, 55.8, 62.3, and 43.2 AU, respectively. The differences between the treated groups and the control group were all statistically significant. No statistical differences were found among the 3 treated groups.
A last series of animal experiments revealed lack of thrombogenicity of the 5H2-derived Fab′ fragments administered at a dose of 2.2 mg/kg (median thrombus light intensity 3.3 AU [n = 6] versus 6.1 AU [n = 9] in the control group, P = .556; Figure 2C).
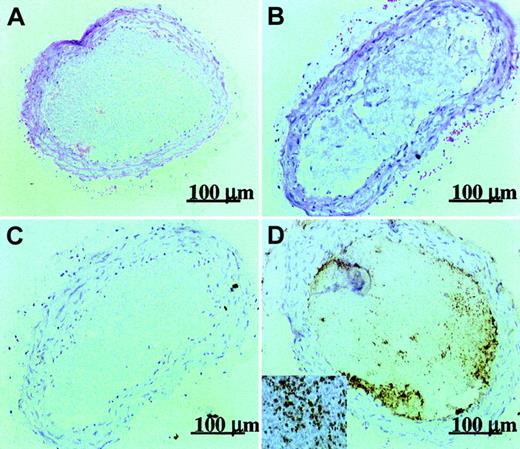
The median antibody levels measured in the plasma collected just after completion of the experiments were 37 μg/mL for the group having received intact 5H2, and 15 and 35 μg/mL for the animals treated with the lower or higher F(ab′)2 dose. No significant correlation was found between the antibody or antibody fragment concentrations and the thrombus light intensity (Spearman rank order correlations: R = 0.405, 0, and 0.309; P = .320, 1, and .456, respectively). Immunohistochemical analysis of carotid artery thrombi showed that intact 5H2 (Figure 3) and its F(ab′)2 fragments (data not shown) were mainly found in association with platelets within the platelet-rich thrombus and were to a much lesser extent bound to vascular endothelium.
Immunohistochemical evidence for the presence of intact 5H2 in the thrombus.
Hematoxylin-eosin staining of control thrombus induced by prolonged exposure (4 minutes) to xenon light (A) and thrombosis promoted by 10 mg/kg intravenous 5H2 (B). Immunohistochemical stainings of control thrombus (C) and of 5H2-promoted thrombus (D). Insert (original magnification × 16) shows association of 5H2 with individual platelets inside the thrombus.
Immunohistochemical evidence for the presence of intact 5H2 in the thrombus.
Hematoxylin-eosin staining of control thrombus induced by prolonged exposure (4 minutes) to xenon light (A) and thrombosis promoted by 10 mg/kg intravenous 5H2 (B). Immunohistochemical stainings of control thrombus (C) and of 5H2-promoted thrombus (D). Insert (original magnification × 16) shows association of 5H2 with individual platelets inside the thrombus.
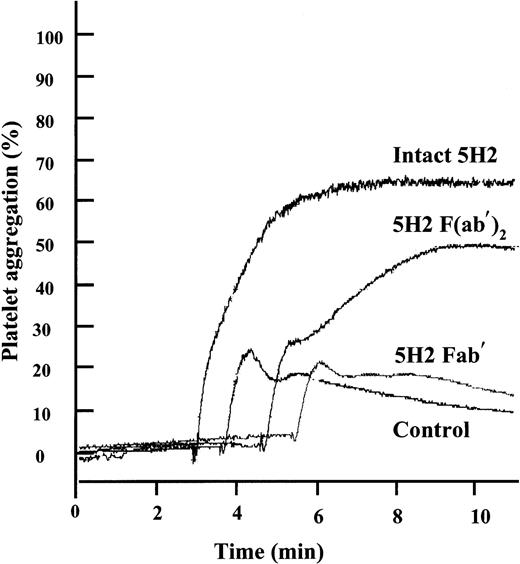
Because 5H2 and its F(ab′)2 fragments both promoted platelet-rich thrombus formation in vivo, we studied the effect of the intact mAb and its fragments on platelet aggregation in vitro using optical aggregometry. 5H2 by itself did not induce platelet aggregation, even when used at concentrations up to 200 μg/mL. However, when subthreshold concentrations of ADP, by themselves only inducing a first wave of aggregation, were added to PRP preincubated with 75 μg/mL 5H2, strong aggregation responses were observed (Figure4). Equimolar concentrations of the F(ab′)2 fragments (50 μg/mL) also promoted ADP-induced aggregation, whereas Fab′ fragments did not.
Effect of intact 5H2 and its fragments on platelet aggregation induced by low concentrations of ADP.
Platelets were incubated for 4 minutes at 37°C with 5H2 (75 μg/mL), its F(ab′)2 (50 μg/mL) or Fab′ fragments (50 μg/mL), or buffer and then stimulated with a subthreshold concentration of ADP (1.10 μM) causing by itself only a first wave of aggregation. Tracings representative of 4 different experiments are shown.
Effect of intact 5H2 and its fragments on platelet aggregation induced by low concentrations of ADP.
Platelets were incubated for 4 minutes at 37°C with 5H2 (75 μg/mL), its F(ab′)2 (50 μg/mL) or Fab′ fragments (50 μg/mL), or buffer and then stimulated with a subthreshold concentration of ADP (1.10 μM) causing by itself only a first wave of aggregation. Tracings representative of 4 different experiments are shown.
Discussion
The association between the presence of aPL and thrombosis affecting both veins and arteries is well established.1 In addition, prospective studies, showing that elevated aPL levels are a risk factor for future thrombosis, suggest that aPLs may be involved in thrombogenesis.29,30 More direct evidence for the thrombogenicity of aPLs was provided by animal models using vessel wall injury to induce thrombosis31-35 (and present study). Thus, after limited mechanical injury to the femoral vein in CD-1 mice, enhanced thrombosis was observed at the site of injury as well as slower thrombus disappearance after injection of immunoglobulin, affinity-purified aCL, and even a monoclonal IgG aCL, all from patients with APS.31-33 Similar observations were reported after active immunization with human β2GPI.34 In this experimental setting, murine monoclonal aCLs possessing LA properties were thrombogenic, whereas an anti-β2GPI antibody without LA activity had no effect.35 These studies implicate aPLs in venous thrombus formation but no direct support is available for the notion that aPLs may be involved in arterial thrombosis.
Therefore, in the present study the impact of aPLs was investigated in a model of carotid artery thrombosis in the hamster. This animal model complies with the concept of thrombosis as a “double-hit” phenomenon. Very mild thrombosis is provoked by limited photochemically induced injury to the vessel wall (“first hit”). This injury affects the entire area of the irradiated vascular segment but is confined to the endothelium. In this model, factors promoting platelet activation36 or coagulation25enhance thrombus formation (“second hit”). mAbs previously raised against human β2GPI15 were selected on the basis of their cross-reactivity with hamster β2GPI and their LA and aCL properties in hamster plasma. We found that the LA+ and aCL+ mAb 5H2, which cross-reacts with hamster β2GPI, dose dependently promoted thrombus formation (Figure 2A). This finding provides the first direct evidence that aPLs may indeed be implicated in arterial thrombosis. mAb 27A8, non–cross-reacting with hamster β2GPI and chosen as a negative control, had no clear effect. The thrombogenic effect of the aCL+ but LA− anti-β2GPI mAb 11E8 was not significant, a finding that is in line with the clinical observation that LAs are more strongly associated with thrombosis than aCLs.20 21
The availability of sufficient quantities of mAb 5H2 enabled us to prepare F(ab′)2 and Fab′ fragments from this antibody and to evaluate whether bivalent hamster β2GPI-antibody complex formation on PL surfaces and possible Fc involvement constitute the basis for the development of thrombotic complications. The F(ab′)2 and Fab′ fragments of 5H2 had similar affinity for β2GPI as the intact mAb (data not shown). The 5H2-derived F(ab′)2 fragments and the intact mAb 5H2 promoted binding of hamster β2GPI to phospholipid surfaces equally well (Figure 1). As shown previously with other LA+anti-β2GPI mAbs, this binding was concentration dependently inhibited by their Fab′ fragment that prevents bivalent β2GPI-antibody complex formation.15
An important and novel finding of this study is that F(ab′)2 fragments derived from an LA+ mAb enhance arterial thrombosis in vivo. This somewhat unexpected finding strengthens our hypothesis that the thrombogenicity of aPLs relies on cellular activation by surface-bound bivalent antigen-antibody complexes, but weakens the suggested involvement of cellular FcγR receptors or the complement system.22,23Animal models of venous thrombosis have provided evidence for Fc receptor–independent thrombotic mechanisms.37 In venous thrombosis, bivalent β2GPI-antibody complexes may reduce the anticoagulant effects of protein C and protein S by competition for the PL surface on which they function.19Immunohistochemical analysis of the arterial thrombus formed (Figure 3) localized 5H2 and its F(ab′)2 fragments mainly to platelets in certain areas of the thrombus. This allows us to propose the following scenario: following mild endothelial damage, a small platelet thrombus develops (first hit); the slightly activated platelets expose negatively charged PL; this leads to patchy deposition of bivalent β2GPI-antibody complexes; these complexes cause further platelet activation and thrombus growth (second hit). In contrast to a previous suggestion,38 binding to endothelial cells seems less involved. The possibility that bivalent β2GPI-antibody complexes might promote platelet activation in an Fc-independent manner was tested in vitro. Intact 5H2 further stimulated platelet aggregation induced by subthreshold concentrations of ADP. At least part of this proaggregatory effect was Fc independent because equimolar concentrations of F(ab′)2also potentiated ADP-induced aggregation, whereas Fab′ fragments did not. Recent work by others further strengthens the concept of platelet activation by β2GPI dimers. A chimeric recombinant protein consisting of 2 β2GPI molecules linked together through the dimerization domain (apple4) of factor XI at the amino-terminal ends of β2GPI domain I also has LA properties39 and enhances thrombus formation when added to whole blood perfused over a collagen surface.40 How β2GPI dimers increase platelet deposition in vitro40 and thrombus formation in vivo (this study) is subject to ongoing research. In conclusion, the present study has revealed that certain aPLs enhance arterial thrombosis by forming bivalent β2GPI-antibody complexes with affinity for PL and that this prothrombotic action is largely Fc independent.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-05-1310.
Supported by a grant from the Flemish Fund for medical scientific research Levenslijn 7.0032.98 and FWO G.0226.01. M.J. was supported by grant BIL098/38 from a bilateral agreement between Flanders and Poland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jef Arnout, Center for Molecular and Vascular Biology, University of Leuven, Campus Gasthuisberg O & N, Herestraat 49, B-3000 Leuven, Belgium; e-mail:jef.arnout@med.kuleuven.ac.be.

![Fig. 2. Photochemically induced thrombosis of hamster carotid artery. / (A) A dose-dependent effect of intact 5H2 on cumulative thrombus formation over 40 minutes expressed as total intensity of transilluminated light. (B) Comparison of thrombus light intensity in animals treated with intact 5H2 or its F(ab′)2 fragments at the indicated doses. (C) Influence of 5H2 Fab′ fragments on thrombus formation. Data are presented as median, interquartile range [IQR], and minute-max range of area under curve; P values for comparison with controls were calculated using Mann-Whitney U test. AU indicates arbitrary light units.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/1/10.1182_blood-2002-05-1310/6/m_h80133589002.jpeg?Expires=1769255212&Signature=RlggPzVOqNiwEvEQqzD9f1c8BQQJsVt7rcyRNpTG5FTRNKGDG5DSq2EUsOWc1yeePSo3Wf8e4SPiibsDwD9pu7sqZBknhHBoj3cS32oOdgTtSz5LVrtdZiZw3n4abd94JyXBH-Sk7C9Kd2NqnLy8csnfVb46bVvZW-Jhi~9Dk-0FYZVsqAxVq5I1kJFLYKx1-E69iXTW~4cTkx9IYRUfGjgcXumCiReHmPm8HqAGy2k-DbWxJbCFJZJGD-ZpYAzSIEwRwhwPV3UO5tCe2Hu~tRQK95o6JJyIPa2YmRIiv5eW5xG5MFuXCjT0j58x6u9fmGtZZh-JmCW1gPi1GbM02Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal