Abstract
Donor-lymphocyte infusion (DLI) before transplantation can lead to specific tolerance to allografts in mice, nonhuman primates, and humans. We and others have demonstrated a role for regulatory T cells in DLI-induced, donor-specific transplantation tolerance, but it is not known how regulatory T cells are activated and where they execute their function. In this study, we observed, in both transgenic and normal mice, that DLI before transplantation is required for activation of αβ-T-cell-receptor–positive, CD3+CD4−CD8− double-negative (DN) regulatory T cells in the periphery of recipient mice. More interestingly, DLI induced DN regulatory T cells to migrate preferentially to donor-specific allogeneic skin grafts and to form a majority of graft-infiltrating T cells in accepted skin allografts. Furthermore, both recipient-derived peripheral and graft-infiltrating DN T cells were able to suppress and kill antidonor CD8+ T cells in an antigen-specific manner. These data indicate that DLI may induce donor-specific transplantation tolerance by activating recipient DN regulatory T cells in the periphery and by promoting migration of regulatory T cells to donor-specific allogeneic skin grafts. Our results also show that DN regulatory T cells can eliminate antidonor T cells both systemically and locally, a finding suggesting that graft-infiltrating T cells can be beneficial to graft survival.
Introduction
Induction of tolerance to an allogeneic graft without the need for nonspecific immunosuppression is a major goal of transplantation therapy. There are many experimental models in which tolerance can be induced by donor-lymphocyte infusion (DLI) before transplantation,1-7 and the effects of DLI have also been observed clinically in recipients of renal, cardiac, and bone marrow transplants.8-14 Several mechanisms have been postulated to explain DLI-induced tolerance, including mixed allogeneic chimerism,15-18 deletion of donor-reactive T cells,19-23 induction of clonal anergy,24immune deviation,24-26 and regulatory T cells.4,27-32 Regulatory T cells have also been found to play an important role in preventing autoimmune diseases33-43 and allograft rejection.27-29,32,44 45
We previously showed that pretransplantation infusion of lymphocytes from Ld or bm1 donors with a single major histocompatibility complex (MHC) class I locus mismatch led to permanent or significantly prolonged survival of donor-specific but not third-party skin allografts in both transgenic and normal mice.14,32,46,47 We have also identified and cloned a novel αβ-T-cell-receptor (TCR)–positive, CD3+CD4−CD8−, double-negative (DN) regulatory T cell from mice that permanently accepted donor-specific skin allografts after one dose of a single class I locus–mismatched DLI.31,32,48 We found that infusion of DN regulatory T-cell clones into syngeneic naive animals led to a significantly prolonged survival of allogeneic skin grafts in a dose-dependent and antigen-specific manner.32 We also reported data indicating that adoptive transfer of DLI-activated DN T cells can significantly prolong skin-graft survival in a model with a single class II mismatch.49 These studies indicate the importance of DN regulatory T cells in preventing allograft rejection. However, the mechanism by which DLI induces donor-specific transplantation tolerance remains elusive. The nature of the relationship between DLI and regulatory T cells is not known, nor is the location at which regulatory T cells mediate their function in vivo.
The goal of the current study was to determine whether DLI is required for activation of antigen-specific DN regulatory T cells and where these DN regulatory T cells execute their function in vivo. Our results constitute the first direct in vivo evidence that DLI promotes the activation and function of recipient peripheral DN regulatory T cells. The activated DN regulatory T cells preferentially infiltrated donor-specific skin allografts and were cytotoxic to antidonor CD8+ T cells. The presence of graft-infiltrating cells has traditionally been used as a criterion for the diagnosis of graft rejection.50 Our findings show that graft-infiltrating T cells can be beneficial to graft survival and highlight the importance of determining the identity of graft-infiltrating cells when using their existence as an indication of graft rejection.
Materials and methods
Mice
C57BL/6 (B6, H-2b), SJL (H-2s), B6 × BALB/c first filial generation (F1) (H-2b/d, Ld+), and BALB/c H-2-dm2 (dm2, a BALB/c Ld-loss mutant, H-2 Dd+, Kd+, Ld−) mice were purchased from Jackson Laboratories (Bar Harbor, ME). A breeding stock of 2C transgenic mice (on B6 background) was provided by Dr Dennis Y. Loh.51 The 2C (H-2b/b) transgenic mice carry functionally rearranged TCR α-chain (one copy) and β-chain (8 copies) transgenes from a cytotoxic T-cell clone (2C) that is specific for Ld MHC class I antigen.51 The 2C clonotypic TCR is recognized by the monoclonal antibody (mAb) 1B2 (hybridoma provided by Dr Herman Eisen, Massachusetts Institute of Technology). The 2C mice were bred with dm2 mice to obtain (2C × dm2)F1 (H-2b/d, Ld−, 1B2+) mice.
DLI and tail-skin grafting
The (2C × dm2)F1 and (B6 × dm2)F1mice (both Ld−) were used as recipients and given intravenous infusions of 4 × 107 lymphocytes from sex-matched Ld+ (B6 × BALB/c)F1 mice as described previously.32 Seven days after DLI, each recipient mouse received 2 sex-matched skin grafts from (B6 × BALB/c)F1 (Ld+; donor-specific) and SJL (H-2s; third-party control) mice.52 A piece of donor tail skin about 1 × 0.5 cm2 and with a thickness including the epidermis and most of the dermis was removed with a sharp scalpel and transferred to the sides of the recipient's tail, from which an equivalent amount of skin had been removed. The grafts were covered with a clear spray bandage (New-Skin; Dedtech Labs, Jackson, WY) and further protected with a light, loosely fitted transparent glass tube. Grafts were monitored by visual inspection daily for the first 2 weeks and twice a week thereafter. A graft was considered rejected when more than 90% was necrotic.
Histologic analysis
Twenty-one and 120 days after skin grafting, the accepted skin allografts from (2C × dm2)F1 mice were harvested, fixed in 10% buffered formalin, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin and examined under light microscopy. The accepted syngeneic skin grafts were treated in the same way and used as controls.
Isolation of graft-infiltrating cells
Skin allografts were cut into small pieces and incubated in a solution of 0.2 U/mL collagenase (Sigma, St Louis, MO) and 0.2 U/mL dispase (ICN Biomedicals, Costa Mesa, CA) in α-minimum essential medium at 37°C for 30 minutes. After incubation, the cells were gently pressed through a stainless steel mesh and then filtered. Cell suspensions were washed twice with phosphate-buffered saline (PBS) and then either stained for surface markers or used for in vitro cytotoxicity and suppression assays.
Cell-surface marker staining
Spleen, lymph node, or graft-infiltrating cells were stained with fluorescein isothiocyanate–conjugated 1B2 or anti-CD3 mAb, CyChrome-conjugated anti-CD8 mAb, and either phycoerythrin (PE)–conjugated anti-CD4 mAb, PE-conjugated anti-CD11b mAb, PE-conjugated anti-NK1.1, or PE-conjugated anti-γδ TCR mAb (all from Pharmingen, San Diego, CA). Data were acquired and analyzed using an EPICS XL-MCL flow cytometer (Coulter, Miami, FL). Statistical analysis was performed with the Student t test.
Enrichment and purification of DN T cells
Lymph node, spleen, or graft-infiltrating cell suspensions were incubated for 30 minutes with RL172-4 (anti-CD4 depleting mAb) and 3.168 (anti-CD8 depleting mAb) at 4°C, washed, and incubated for 45 minutes with rabbit C′ (Cedarlane Laboratories, Hornby, ON, Canada) at 37°C. The cells were washed 3 times and used in suppression or cytotoxicity assays. The suspension contained less than 1% CD4+ and CD8+ T cells after depletion, according to fluorescence-activated cell-sorter scanning analysis. To purify DN T cells, cell suspensions were additionally stained with biotin-labeled anti-CD3 mAb (Pharmingen), washed with 0.5% PBS–bovine serum albumin, and labeled with magnetically activated cell-sorter antibiotin microbeads (Miltenyi Biotec, Auburn, CA) for 15 minutes. The cells were washed, and the CD3+ T cells were purified on an LS column (Miltenyi Biotech).
Suppression assays
Cell suspensions from spleen, lymph nodes, or graft-infiltrating cells were collected, stained to determine the proportion of DN T cells, and used as putative suppressor cells. Splenocytes from naive (2C × dm2)F1 or (B6 × dm2)F1 mice were used as responders (1000 1B2+CD8+ cells/well or 104 (B6 × dm2)F1 CD8+ T cells/well) and were stimulated with irradiated (20 Gy) (B6 × BALB/c)F1 or SJL spleen cells (105cells/well) in the presence of 50 U/mL recombinant interleukin 2 (rIL-2) and 30 U/mL rIL-4. After 3 days, cells were labeled with 1 μCi (0.037 MBq)/well tritium-thymidine, harvested 18 hours later, and counted in a scintillation counter (TopCount; Packard, Meriden, CT). Suppression was calculated by using the following equation: percentage of suppression = 1−(E/R), where E is the counts/minute of each well and R (responders) is the counts/minute of the responders alone.
Cytotoxicity assay
Cell suspensions from spleen, lymph nodes, or graft-infiltrating cells were prepared as described above, stimulated overnight in the presence of IL-2, IL-4, and irradiated (B6 × BALB/c)F1(Ld+) cells, and used as effectors. The (B6 × dm2)F1 splenocytes were stimulated with either irradiated (20 Gy) (B6 × BALB/c)F1 (anti-Ld) or SJL (anti-H-2s) spleen cells. Four days later, the activated Ld-specific or H-2s–specific CD8+ T cells or BW5147 (H-2k) tumor cells were labeled with 10 μCi/mL (0.37 MBq) tritium-thymidine at 37°C overnight and used as targets (104 cells/well). After coculture of the effector cells and targets at 37°C for 18 hours in the presence of fresh irradiated allogeneic splenocytes, the cells were harvested and counted in a scintillation counter (TopCount). Specific cell lysis was calculated by using the following equation: percentage of specific killing = (S−E)/S × 100, where E (experimental) is the counts/minute of retained DNA in the presence of effector cells and S (spontaneous) is the counts/minute of retained DNA in the absence of effector cells.
Results
DLI is required for activation of recipient-derived antigen-specific DN regulatory T cells
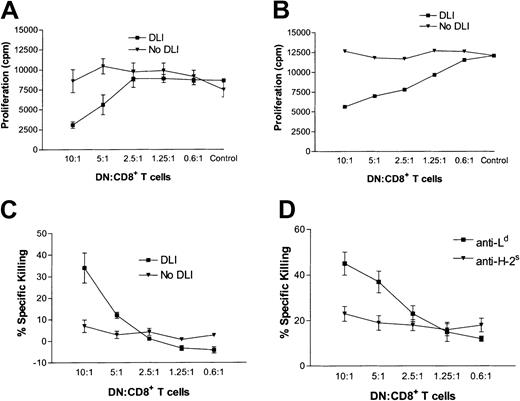
We previously found that pretransplantation infusion of single class I locus Ld–mismatched splenocytes led to permanent survival of Ld+ skin allografts and normal rejection of third-party skin allografts.31,46 We also showed that DN T-cell clones generated in vitro can specifically suppress and kill antidonor T cells in vitro.32 It is not known, however, whether DLI directly activates recipient-derived, antigen-specific DN regulatory T cells or protects donor-specific allografts from rejection by another mechanism. To address this issue, groups of mice were given DLI or left untreated and then given skin allografts. One week after transplantation, the ability of peripheral DN T cells in the spleens and lymph nodes of recipient mice to suppress the proliferation of antidonor CD8+ T cells was compared in untreated mice and mice treated with DLI. We found that only the lymphocytes in DLI-treated mice were able to suppress the proliferation of antidonor CD8+ T cells, in a dose-dependent manner, whereas DN T cells from recipient mice not treated with DLI showed no suppressive activity (Figure 1A and 1B). Furthermore, peripheral DN T cells isolated from DLI-treated mice, but not untreated mice, were able to kill activated syngeneic 1B2+CD8+ T cells, in a dose-dependent manner (Figure 1C), and the killing mediated by DN T cells purified from DLI-treated mice was antigen specific, since CD8+ T cells activated by third-party SJL alloantigens (H-2s) were not killed (Figure 1D). These data confirm the results with DN T-cell clones. More importantly, these findings show clearly that DLI is required for activation of recipient-derived, antigen-specific DN immunoregulatory T cells, an observation that provides a novel explanation for how DLI induces donor-specific transplantation tolerance.
DN T cells from DLI-treated mice can suppress antidonor CD8+ T cells.
(A,B) A group of (2C × dm2)F1 mice were given DLI (▪) or left untreated (▾) and then given transplants. At 1 week after transplantation, splenocytes (A) or lymph node cells (B) were collected from recipient mice and the proportion of DN T cells was determined by flow cytometry. Various numbers of lymphocytes (up to 7.0 × 104 total cells) containing the indicated numbers of DN T cells were used as putative suppressor cells and cultured with 1000 naive 1B2+CD8+ T-cell–responder cells and irradiated splenocytes from (B6 × BALB/c)F1 mice. Three days later, 1 μCi (0.037 MBq) tritium-thymidine was added to each well. The plate was incubated overnight, and cells were harvested 18 hours later. Shown is the mean proliferation ± SD in counts/minute for 3 replicates in 2 independent experiments. (C) Naive 1B2+CD8+ T cells were stimulated for 4 days with irradiated (B6 × BALB/c)F1 cells, labeled overnight with 10 μCi (0.37 MBq) tritium-thymidine, and used as target cells. Various numbers of lymph node cells from DLI-treated (▪) and untreated (▾) mice were used as effector cells. Shown is the mean percentage of specific killing ± SD for 3 replicates in 2 independent experiments. (D) DN T cells were purified from the lymph nodes of DLI-treated mice 3 days after transplantation and used as effector cells. Activated anti-Ld+ (▪) or anti-H-2s (▾) CD8+ T cells were used as targets at the ratios indicated. Shown is the mean percentage of specific killing ± SD for 3 replicates in 2 independent experiments.
DN T cells from DLI-treated mice can suppress antidonor CD8+ T cells.
(A,B) A group of (2C × dm2)F1 mice were given DLI (▪) or left untreated (▾) and then given transplants. At 1 week after transplantation, splenocytes (A) or lymph node cells (B) were collected from recipient mice and the proportion of DN T cells was determined by flow cytometry. Various numbers of lymphocytes (up to 7.0 × 104 total cells) containing the indicated numbers of DN T cells were used as putative suppressor cells and cultured with 1000 naive 1B2+CD8+ T-cell–responder cells and irradiated splenocytes from (B6 × BALB/c)F1 mice. Three days later, 1 μCi (0.037 MBq) tritium-thymidine was added to each well. The plate was incubated overnight, and cells were harvested 18 hours later. Shown is the mean proliferation ± SD in counts/minute for 3 replicates in 2 independent experiments. (C) Naive 1B2+CD8+ T cells were stimulated for 4 days with irradiated (B6 × BALB/c)F1 cells, labeled overnight with 10 μCi (0.37 MBq) tritium-thymidine, and used as target cells. Various numbers of lymph node cells from DLI-treated (▪) and untreated (▾) mice were used as effector cells. Shown is the mean percentage of specific killing ± SD for 3 replicates in 2 independent experiments. (D) DN T cells were purified from the lymph nodes of DLI-treated mice 3 days after transplantation and used as effector cells. Activated anti-Ld+ (▪) or anti-H-2s (▾) CD8+ T cells were used as targets at the ratios indicated. Shown is the mean percentage of specific killing ± SD for 3 replicates in 2 independent experiments.
DN T cells form the majority of graft-infiltrating cells in Ld+ skin allografts from DLI-treated mice
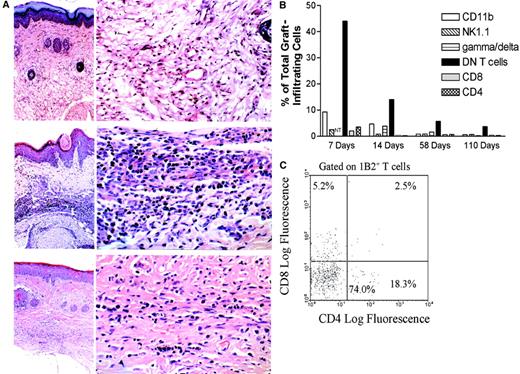
Although extensive studies have shown that regulatory T cells can down-regulate immune responses to self-antigens and alloantigens systemically, whether these regulatory cells can also inhibit immune responses and prevent tissue damage locally remains unknown. Because cellular infiltrates have been observed in nonrejected grafts as well as in tissues protected from autoimmune destruction,26 53-57 we hypothesized that DN regulatory T cells might be able to migrate to skin allografts and execute their function locally. To test this novel hypothesis, (2C × dm2)F1 mice were given DLI from Ld-mismatched (B6 × BALB/c)F1 mice and a transplant of both a (B6 × BALB/c)F1 and a syngeneic skin graft 1 week later. At 21 and 120 days after transplantation, both grafts were harvested and stained with hematoxylin and eosin. As shown in Figure 2A, a dense mononuclear cell infiltration in accepted Ld-mismatched (B6 × BALB/c)F1 skin grafts was found at 21 days after transplantation (middle panel). The number of graft-infiltrating cells, although markedly reduced at 120 days (lower panel), was still significantly higher than the number in the syngeneic skin grafts (upper panel).
DN T cells infiltrate donor-specific skin allografts and form the dominant subset of graft-infiltrating T cells.
(A) A group of (2C × dm2)F1 mice were given DLI followed by transplantation of both (B6 × Balb/c)F1allogeneic and (B6 × dm2)F1 syngeneic skin grafts. At 21 days (top and middle panels) and 120 days (bottom panel) after transplantation, the accepted skin grafts were harvested and stained with hematoxylin and eosin. In each instance, an overview picture of the skin-graft site is shown in the left panel (× 50) and a close-up view of the deep dermis is shown in the right (× 400). The top panel shows a syngeneic graft at 21 days after transplantation. The epidermis and dermis are normal, and dermal appendages are present (left). In the deep dermis, there is no cellular infiltrate and blood vessels and collagen fibers are normal. The middle panel shows an allograft at 21 days after transplantation. The epidermis shows acanthosis, hyperkeratosis, and focal keratotic plugging. The dermis is edematous and there is a heavy cellular infiltrate in the middle and deep dermis (left). The infiltrate in the deep dermis is predominantly lymphocytic (right). The bottom panel shows an allograft at 120 days after transplantation. The skin structure is normal, except in the deep dermis, where there is a mild cellular infiltrate and fibrosis (left). The deep dermis shows an increase in collagen fibers and is less vascular than normal skin. The infiltrate cells are much less numerous than what was observed at 21 days and are predominantly lymphocytes. (B) Graft-infiltrating cells were collected from the accepted (B6 × BALB/c)F1 skin grafts 7, 14, 58, and 110 days after transplantation. The cells were analyzed by flow cytometry for CD11b+, NK1.1+, γδ-TCR+, CD8+, CD4+, and 1B2+DN T cells. Shown are representative proportions of positive graft-infiltrating cells from at least 3 mice. NT indicates not tested. (C) Graft-infiltrating cells were collected from the DLI-treated (B6 × BALB/c)F1 skin grafts of (2C × dm2)F1 mice 1 week after transplantation. The cells were analyzed by flow cytometry for expression of 1B2, CD4, and CD8. The histogram is gated on the 1B2+ T-cell population.
DN T cells infiltrate donor-specific skin allografts and form the dominant subset of graft-infiltrating T cells.
(A) A group of (2C × dm2)F1 mice were given DLI followed by transplantation of both (B6 × Balb/c)F1allogeneic and (B6 × dm2)F1 syngeneic skin grafts. At 21 days (top and middle panels) and 120 days (bottom panel) after transplantation, the accepted skin grafts were harvested and stained with hematoxylin and eosin. In each instance, an overview picture of the skin-graft site is shown in the left panel (× 50) and a close-up view of the deep dermis is shown in the right (× 400). The top panel shows a syngeneic graft at 21 days after transplantation. The epidermis and dermis are normal, and dermal appendages are present (left). In the deep dermis, there is no cellular infiltrate and blood vessels and collagen fibers are normal. The middle panel shows an allograft at 21 days after transplantation. The epidermis shows acanthosis, hyperkeratosis, and focal keratotic plugging. The dermis is edematous and there is a heavy cellular infiltrate in the middle and deep dermis (left). The infiltrate in the deep dermis is predominantly lymphocytic (right). The bottom panel shows an allograft at 120 days after transplantation. The skin structure is normal, except in the deep dermis, where there is a mild cellular infiltrate and fibrosis (left). The deep dermis shows an increase in collagen fibers and is less vascular than normal skin. The infiltrate cells are much less numerous than what was observed at 21 days and are predominantly lymphocytes. (B) Graft-infiltrating cells were collected from the accepted (B6 × BALB/c)F1 skin grafts 7, 14, 58, and 110 days after transplantation. The cells were analyzed by flow cytometry for CD11b+, NK1.1+, γδ-TCR+, CD8+, CD4+, and 1B2+DN T cells. Shown are representative proportions of positive graft-infiltrating cells from at least 3 mice. NT indicates not tested. (C) Graft-infiltrating cells were collected from the DLI-treated (B6 × BALB/c)F1 skin grafts of (2C × dm2)F1 mice 1 week after transplantation. The cells were analyzed by flow cytometry for expression of 1B2, CD4, and CD8. The histogram is gated on the 1B2+ T-cell population.
To analyze the phenotype of graft-infiltrating cells, cells were collected from donor-specific (B6 × BALB/c)F1 skin allografts of DLI-treated mice at 1, 2, 8, and 15 weeks after transplantation and stained with a variety of mAbs, including 1B2, anti-CD4, anti-CD8, anti-CD11b, anti-NK1.1, and anti-γδ-TCR. As shown in Figure 2B, almost half of the graft-infiltrating cells were 1B2+DN T cells at 1 week after skin grafting, and the proportion of DN T cells was higher than that of other cell types at all time points. We further examined the subsets of donor-specific T cells in Ld+ skin grafts and found that DN T cells comprised more than 70% of 1B2+ T cells. Approximately 5% of 1B2+ T cells were CD8+, and less than 20% were CD4+ (Figure 2C). These data show that DN T cells formed the majority of infiltrating T cells in accepted donor-specific skin grafts of DLI-treated recipients.
DLI promotes migration of DN T cells to donor-specific skin allografts
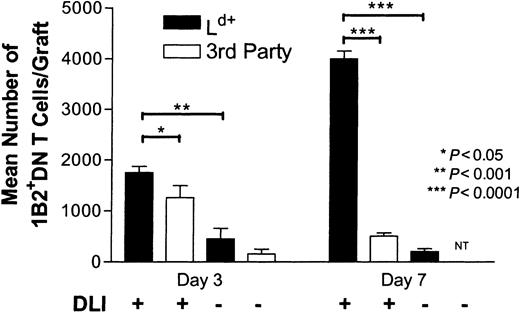
On the basis of the findings that DLI led to activation of antigen-specific DN regulatory T cells in recipients (Figure 1) and that the DN T cells accumulated in accepted donor-specific skin allografts (Figure 2), we reasoned that DLI may induce donor-specific transplantation tolerance by promoting DN regulatory T-cell migration to donor-specific skin allografts. Therefore, we compared the number of infiltrating DN T cells in untreated mice and DLI-treated mice after transplantation. As shown in Figure 3, the number of DN T cells in Ld+ skin grafts of DLI-treated mice was significantly higher than that in untreated mice (4-fold increase on day 3 [P = .0006] and 20-fold increase on day 7 [P < .0001]). To further assess whether DN T cells migrated to allografts in an antigen-specific fashion, we compared the number of graft-infiltrating DN T cells in Ld+and third-party SJL skin allografts. We found that although DN T cells infiltrated both Ld+ and third-party skin grafts of DLI-treated mice at 3 days after transplantation, the number of DN T cells continued to increase in Ld+ skin grafts but decreased in third-party skin grafts. At 7 days after transplantation, the number of DN T cells in Ld+ skin grafts was 8 times higher than that in third-party skin grafts (Figure 3). These data show that DLI before transplantation promoted migration of DN T cells to skin allografts and persistence of these cells in donor-specific but not third-party skin allografts.
DN T cells accumulate in donor-specific but not third-party skin grafts.
Graft-infiltrating cells from Ld+ (dark bars) and third-party (light bars) allografts of DLI-treated and untreated mice were collected on days 3 and 7 after transplantation and analyzed as described for Figure 2C. The number of 1B2+DN T cells in skin grafts of DLI-treated and untreated recipients was determined by multiplying the number of total graft-infiltrating cells by the proportion of 1B2+DN T cells determined by flow cytometry. Shown are mean pooled results ± SD from at least 4 skin grafts.
DN T cells accumulate in donor-specific but not third-party skin grafts.
Graft-infiltrating cells from Ld+ (dark bars) and third-party (light bars) allografts of DLI-treated and untreated mice were collected on days 3 and 7 after transplantation and analyzed as described for Figure 2C. The number of 1B2+DN T cells in skin grafts of DLI-treated and untreated recipients was determined by multiplying the number of total graft-infiltrating cells by the proportion of 1B2+DN T cells determined by flow cytometry. Shown are mean pooled results ± SD from at least 4 skin grafts.
Infiltrating 1B2+DN T cells from accepted skin grafts are able to specifically kill activated antidonor T cells
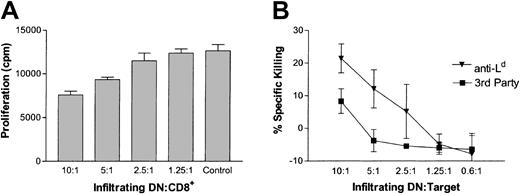
Our findings that DLI activated peripheral antigen-specific DN regulatory T cells (Figure 1) and that DN T cells were the predominant cell type in accepted Ld+ skin allografts of DLI-treated mice (Figure 2) suggested that DN T cells may migrate to the donor-specific allografts to down-regulate antidonor T-cell responses locally. To test this hypothesis, infiltrating cells from Ld+ (B6 × BALB/c)F1 skin grafts of DLI-treated mice were harvested and their ability to suppress the proliferation of anti-Ld CD8+ T cells was assessed. As shown in Figure 4A, graft-infiltrating cells were able to suppress the proliferation of anti-Ld CD8+ T cells, in a dose-dependent manner. To further investigate whether graft-infiltrating 1B2+DN T cells can specifically kill anti-LdCD8+ T cells, 1B2+DN T cells were collected from Ld+(B6 × BALB/c)F1 skin allografts of DLI-treated mice 1 week after transplantation and used as effector cells. Both activated Ld-specific 1B2+CD8+ T cells and third-party allogeneic (H-2k) tumor cells were used as targets in a JAM cytotoxicity assay.58 Graft-infiltrating 1B2+DN T cells were able to kill activated anti-Ld T cells more effectively than third-party allogeneic tumor cells (Figure 4B). These data indicate that DN regulatory T cells from DLI-treated recipients were able not only to specifically migrate to Ld+ skin grafts but also to retain their antigen-specific regulatory function. This finding suggests that these graft-infiltrating DN regulatory T cells may protect skin allografts from rejection by eliminating antigraft T cells locally.
Graft-infiltrating DN T cells can suppress and kill antidonor CD8+ T cells.
(A) Graft-infiltrating cells were collected from 14 Ld+skin grafts of DLI-treated mice, stained to determine the proportion of DN T cells, and used as suppressor cells. Naive 1B2+CD8+ T cells were used as responders. Shown is the mean proliferation in counts/minute for 3 replicates. (B) Graft-infiltrating cells were collected as described for panel A and were depleted of CD4+ and CD8+ T cells. The enriched DN T cells were stimulated overnight and used as effector cells in a cytotoxicity assay. Activated 1B2+CD8+ (anti-Ld; ▾) and BW5147 (third-party; ▪) cells were used as targets. Shown is the mean percentage of specific killing ± SD of target cells for 3 replicates.
Graft-infiltrating DN T cells can suppress and kill antidonor CD8+ T cells.
(A) Graft-infiltrating cells were collected from 14 Ld+skin grafts of DLI-treated mice, stained to determine the proportion of DN T cells, and used as suppressor cells. Naive 1B2+CD8+ T cells were used as responders. Shown is the mean proliferation in counts/minute for 3 replicates. (B) Graft-infiltrating cells were collected as described for panel A and were depleted of CD4+ and CD8+ T cells. The enriched DN T cells were stimulated overnight and used as effector cells in a cytotoxicity assay. Activated 1B2+CD8+ (anti-Ld; ▾) and BW5147 (third-party; ▪) cells were used as targets. Shown is the mean percentage of specific killing ± SD of target cells for 3 replicates.
Validation of the effect of DLI on activation and migration of DN regulatory T cells in normal mice
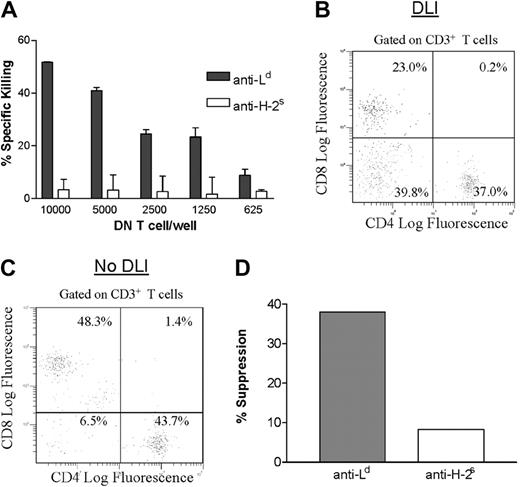
The results reported above showed, in (2C × dm2)F1transgenic mice, that administration of an Ld-mismatched DLI before transplantation activated recipient DN T cells that could migrate to donor-specific skin allografts and kill antidonor T cells. Because (2C × dm2)F1 transgenic mice have a higher-than-average proportion of DN T cells in the periphery, it was important to verify these findings in normal mice. First, we addressed the question whether DLI can also activate antigen-specific DN T cells in the periphery in nontransgenic mice. Thus, (B6 × dm2)F1 mice were given DLI from (B6 × BALB/c)F1 mice. Because dm2 is a BALB/c Ld-loss mutant, the only mismatch between donor and recipient in this situation is Ld, which mimics the transgenic model used in the above studies. Using DLI-treated (B6 × dm2)F1 mice, we previously found that (B6 × BALB/c)F1 grafts survived indefinitely but third-party SJL skin grafts were rejected.46 To determine whether DLI leads to activation of peripheral DN regulatory T cells in nontransgenic mice, DN T cells were purified from the spleens of DLI-treated (B6 × dm2)F1 mice 7 days after transplantation and assessed for their ability to kill antidonor CD8+ T cells. As was observed in (2C × dm2)F1 transgenic mice, purified splenic DN T cells from DLI-treated mice were able to kill anti-Ld but not anti-H-2s CD8+ T cells (Figure5A).
DLI activates functional DN T cells in nontransgenic mice.
(A) A group of (B6 × dm2)F1 mice were given DLI and transplants. At 1 week after transplantation, spleen cells were harvested from 4 mice and DN T cells were purified. Activated anti-Ld (dark bars) or anti-H-2s (light bars) CD8+ T cells were used as targets at the effector-to-target ratios indicated. Shown is the mean percentage of specific killing ± SD for 3 replicates. (B,C) A group of B6 × dm2F1 mice were given DLI (B) or left untreated (C) and then given transplants. At 1 week after transplantation, graft-infiltrating cells were harvested and stained by using anti-CD3, anti-CD4, and anti-CD8 mAbs. Data shown are gated on CD3+ T cells and represent pooled results from 4 mice. (D) Graft-infiltrating T cells were purified from the Ld+ graft of DLI-treated (B6 × dm2)F1 mice 1 week after skin grafting. DN T cells were purified and used as putative suppressor cells (5000 cells/well). Splenocytes from naive (B6 × dm2)F1 mice were used as responder cells and were stimulated with either (B6 × BALB/c)F1 (Ld+) or SJL (H-2s) irradiated splenocytes as indicated. Shown is the percentage of inhibition of proliferation of CD8+ responder cells (pooled results from 4 mice).
DLI activates functional DN T cells in nontransgenic mice.
(A) A group of (B6 × dm2)F1 mice were given DLI and transplants. At 1 week after transplantation, spleen cells were harvested from 4 mice and DN T cells were purified. Activated anti-Ld (dark bars) or anti-H-2s (light bars) CD8+ T cells were used as targets at the effector-to-target ratios indicated. Shown is the mean percentage of specific killing ± SD for 3 replicates. (B,C) A group of B6 × dm2F1 mice were given DLI (B) or left untreated (C) and then given transplants. At 1 week after transplantation, graft-infiltrating cells were harvested and stained by using anti-CD3, anti-CD4, and anti-CD8 mAbs. Data shown are gated on CD3+ T cells and represent pooled results from 4 mice. (D) Graft-infiltrating T cells were purified from the Ld+ graft of DLI-treated (B6 × dm2)F1 mice 1 week after skin grafting. DN T cells were purified and used as putative suppressor cells (5000 cells/well). Splenocytes from naive (B6 × dm2)F1 mice were used as responder cells and were stimulated with either (B6 × BALB/c)F1 (Ld+) or SJL (H-2s) irradiated splenocytes as indicated. Shown is the percentage of inhibition of proliferation of CD8+ responder cells (pooled results from 4 mice).
Next, we studied whether DLI promotes migration of DN T cells to donor-specific skin allografts. Thus, (B6 × dm2)F1 mice were given an Ld-mismatched DLI from (B6 × BALB/c)F1 mice, followed by donor-specific skin grafting 7 days later. As controls, a group of (B6 × dm2)F1 mice were given transplants of (B6 × BALB/c)F1 skin grafts without DLI before transplantation. One week after transplantation, graft-infiltrating cells were harvested from skin allografts of both DLI-treated and untreated mice and stained by using anti-CD3, anti-CD4, and anti-CD8 mAbs. The percentages of graft-infiltrating CD4+, CD8+, and DN T cells in DLI-treated mice were compared with those in untreated mice. As shown in Figure 5B, nearly 40% of the graft-infiltrating T cells were DN T cells in Ld+ skin grafts of DLI-treated mice, whereas only 6.5% of DN T cells were found in skin grafts from untreated mice. Furthermore, the percentage of CD8+ T cells in skin grafts of DLI-treated mice was lower than that in Ld skin grafts from untreated mice (Figure5C). These data are consistent with the findings in (2C × dm2)F1 transgenic mice and show that DLI promoted migration of DN T cells into skin allografts in nontransgenic (B6 × dm2)F1 mice.
The finding that the skin allografts from DLI-treated mice had a higher number of DN T cells and a lower number of CD8+ T cells indicated the possibility that the graft-infiltrating DN T cells may be able to suppress antidonor CD8+ T cells. To determine the function of graft-infiltrating DN T cells, these cells were isolated from Ld+ (B6 × BALB/c)F1 skin grafts from DLI-treated mice and tested for their ability to suppress anti-Ld and third-party anti-SJL CD8+ T cells. As shown in Figure 5D, graft-infiltrating DN T cells were able to suppress the proliferation of anti-Ld (donor-specific) but not anti-H-2s (third-party) CD8+ T cells in vitro. Together, these data corroborate the previous findings in (2C × dm2)F1 transgenic mice and show that pretransplantation DLI led to activation of antigen-specific DN regulatory T cells in normal mice and that these cells were able to infiltrate skin allografts and specifically suppress antidonor CD8+ T cells after transplantation.
Discussion
It was previously established that pretransplantation DLI can enhance donor-specific allograft survival in rodents,1,2,4-7 nonhuman primates,59,60 and humans.9 However, the mechanism by which DLI promotes donor-specific allograft survival remains elusive. Various types of regulatory T cells, including CD4+, CD8+, and DN T cells, have been shown to be involved in DLI-induced tolerance.4,27-32 Roelen et al prolonged graft survival by adoptively transferring CD4+ regulatory T cells that were induced by donor-specific transfusion.29 Using a model of donor-specific transfusion and heart-allograft transplantation, Douillard et al showed that a subset of CD8+ T cells (Vβ18-Dβ1-Jβ2.7) may function as regulatory T cells,30 since administration of an anti-TCR–specific DNA vaccination to eliminate Vβ18-Dβ1-Jβ2.7 CD8+ T cells abolished heart-allograft tolerance.28 Similarly, data reported by Iwakoshi et al27 indicate that CD4+ regulatory T cells are activated in mice treated with a donor-specific transfusion together with anti-CD154 mAb, since depletion of CD4+ T cells abolished long-term survival of skin grafts. Here, we extended our previous studies using DN T-cell clones generated in vitro32 and found that DN regulatory T cells from DLI-treated mouse recipients can specifically down-regulate antidonor immune responses.
Despite the demonstration of the involvement of regulatory T cells in DLI-induced transplantation tolerance, it is not clear how DLI promotes allograft survival through regulatory T cells. In this study, we observed, in both transgenic and normal mice, that DLI before transplantation can activate recipient-derived DN regulatory T cells and promote migration of these regulatory T cells to donor-specific skin allografts. We also found that both peripheral and graft-infiltrating DN T cells from DLI-treated mice can specifically kill antidonor CD8+ T cells (Figures 1C, 1D, and 5A), whereas an equivalent number of DN T cells from untreated mice had no suppressive activity (Figure 1A and 1B). These data provide the first direct evidence that DLI can enhance donor-specific survival of skin grafts through promoting activation and migration of antigen-specific DN regulatory T cells, which can in turn down-regulate antidonor responses both systemically and locally.
We previously demonstrated that DLI can lead to permanent acceptance of donor-specific but not third-party skin allografts.31 46Although similar numbers of DN T cells were observed in donor-specific Ld+ (B6 × BALB/c)F1 and third-party SJL skin grafts 3 days after transplantation, at 7 days, the number of DN T cells had increased substantially in (B6 × BALB/c)F1skin grafts and decreased markedly in SJL skin grafts (Figure 3). These data indicate that although the migration of DN T cells to third-party skin grafts at 3 days after transplantation may be the result of nonspecific inflammatory responses, DN T cells are able to accumulate only in donor-specific skin grafts. Moreover, cytotoxicity assays using DN T cells from DLI-treated transgenic and normal mice found that DN T-cell–mediated killing was antigen specific (Figures 1D, 4B, and 5A). Together, these findings help to explain why donor-specific grafts are protected, whereas third-party skin grafts are rejected.
The finding that DN regulatory T cells migrate to and compose the majority of graft-infiltrating T cells is intriguing. Graft-infiltrating cells in general are considered detrimental to graft survival, and the presence of infiltrating cytotoxic T cells in grafts has been correlated with graft rejection.61,62 The presence of graft-infiltrating lymphocytes is one of several criteria for the diagnosis of graft rejection.50 On the other hand, several studies have observed infiltrating lymphocytes in well-functioning grafts26,30,53-56,63 and some studies have suggested that subsets of graft-infiltrating lymphocytes are regulatory cells.30,55 In particular, CD8+ regulatory T cells have been found in cardiac allografts after DLI treatment28,30 and in kidney allografts after oral administration of allogeneic splenocytes.55 In this study, we observed a 20-fold increase in the number of graft-infiltrating DN regulatory T cells in DLI-treated transgenic mice (Figure 3) and a 6-fold increase in normal mice (Figure 5B and 5C) after skin transplantation compared with the number in controls not given DLI. Furthermore, graft-infiltrating DN T cells isolated directly from skin grafts of DLI-treated mice could suppress and specifically kill antidonor T cells (Figure 5A and 5D) in both transgenic and nontransgenic mice. These data show that not only peripheral but also graft-infiltrating DN T cells from DLI-treated mice have a regulatory function. Our findings support the novel concept that graft-infiltrating T cells can be beneficial to graft survival and indicate the importance of determining the identity of infiltrating cells in the diagnosis of graft rejection.
In previous studies, we showed that DN regulatory T cells have the capacity to kill activated syngeneic CD8+ T cells that carry the same TCR through Fas–Fas ligand (FasL) interactions.32 The data from the current study, together with our previous findings, support the following model of induction by DLI of donor-specific skin-graft protection through DN regulatory T cells. DLI leads to activation of recipient DN regulatory T cells that carry TCRs recognizing alloantigens expressed on the donor lymphocytes. After transplantation, the activated DN regulatory T cells migrate to the skin grafts, along with antidonor T cells. Antidonor CD8+ T cells are then killed by DN regulatory T cells through Fas-FasL interactions. Because DN regulatory T cells are unable to kill T cells that carry a different TCR, third-party skin grafts are not protected. This model is consistent with previous studies showing that intragraft FasL expression can confer immune privilege64 and with a study suggesting that Fas-FasL is necessary for DLI-induced tolerance in H-Y mice.65
Although the results in the current study focus on the beneficial effect of DLI through DN regulatory T cells, it is possible that other cells may participate in tolerance induction, for example, by supplying cytokines to help DN regulatory T cells. We previously found that DN regulatory T cells require both antigen stimulation and cytokines in order to expand and function in vitro.32 Furthermore, we have observed that DLI also leads to an increase in the concentration of IL-4 in the serum of treated animals.31 Because DN T cells do not produce IL-4, it is likely that this cytokine (and perhaps others) is produced by accessory cells. It is therefore possible that in addition to activating regulatory T cells, DLI may stimulate accessory cells or production of cytokines that are required for DN regulatory T-cell activation and function.
In conclusion, we found in this study that DLI leads to activation of antigen-specific DN regulatory T cells in the periphery of recipient mice. These activated DN regulatory T cells can migrate to donor-specific skin allografts after transplantation. Both peripheral and graft-infiltrating DN regulatory T cells can kill antidonor CD8+ T cells in an antigen-specific manner. These findings show that DLI-activated DN T cells can execute their function both systemically and locally. Our data also suggest that graft-infiltrating T cells can be beneficial and highlight the importance of determining the identity of graft-infiltrating cells in the diagnosis of graft rejection.
We thank H. Eilson for the 1B2 hybridoma and D.Y. Loh for permission to use 2C transgenic mice.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-01-0235.
Supported by the Canadian Institutes of Health Research (L.Z. [MOP 14431]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Li Zhang, CCRW 2-809, 101 College St, Toronto, ON, Canada M5G 2C4; e-mail: lzhang@transplantunit.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal