Abstract
Calcium entry into mature erythrocytes (red blood cells; RBCs) is associated with multiple changes in cell properties. At low intracellular Ca2+, efflux of potassium and water predominates, leading to changes in erythrocyte rheology. At higher Ca2+ content, activation of kinases and phosphatases, rupture of membrane-to-skeleton bridges, stimulation of a phospholipid scramblase and phospholipase C, and induction of transglutaminase-mediated protein cross-linking are also observed. Because the physiologic relevance of these latter responses depends partially on whether Ca2+ entry involves a regulated channel or nonspecific leak, we explored mechanisms that initiate controlled Ca2+ influx. Protein kinase C (PKC) was considered a prime candidate for the pathway regulator, and phorbol-12 myristate-13 acetate (PMA), a stimulator of PKC, was examined for its influence on erythrocyte Ca2+. PMA was found to stimulate a rapid, dose-dependent influx of calcium, as demonstrated by the increased fluorescence of an entrapped Ca2+-sensitive dye, Fluo-3/am. The PMA-induced entry was inhibited by staurosporine and the PKC-selective inhibitor chelerythrine chloride, but was activated by the phosphatase inhibitors okadaic acid and calyculin A. The PMA-promoted calcium influx was also inhibited by ω-agatoxin-TK, a calcium channel blocker specific for Cav2.1 channels. To confirm that a Cav2.1-like calcium channel exists in the mature erythrocyte membrane, RBC membrane preparations were immunoblotted with antiserum against the α1A subunit of the channel. A polypeptide of the expected molecular weight (190 kDa) was visualized. These studies indicate that an ω-agatoxin-TK–sensitive, Cav2.1-like calcium permeability pathway is present in the RBC membrane and that it may function under the control of kinases and phosphatases.
Introduction
Although erythrocytes (red blood cells; RBCs) have historically been considered inert to regulatory signals from other cells, RBCs are surprisingly well equipped with the machinery required for intercellular communication. Thus, in addition to a plethora of hormone receptors, mature RBCs contain substantial numbers of cyclases, phospholipases, kinases, phosphatases, and both ligand-gated and mechanically activated ion channels.1 There is even mounting evidence that RBCs play an active role in regulating both blood rheology2-5 and hemostasis,6-10responding to such diverse stimuli as β-adrenergic agonists, nitric oxide, oxygen tension, platelet releasates, and hypertensive agents in their participation in the functions of the circulatory system. Not surprisingly, modulation of RBC properties has become a viable pharmacologic target for the treatment of certain circulatory diseases.11-14
Although the responsiveness of RBCs to regulators of circulatory health and homeostasis is becoming more apparent, the signaling pathways that mediate the changes in RBC properties remain unresolved. One of the more common intermediates implicated in regulating RBC behavior is an influx of extracellular calcium. Although activation of the Gardos channel and the consequent loss of cell water have been the only responses demonstrated to occur in vivo,15,16 there is still abundant in vitro evidence to suggest that elevated intracellular calcium can do the following: (1) activate a scramblase that translocates phosphatidylserine to the outer leaflet,17,18(2) induce changes in membrane skeletal architecture via association with calmodulin,19-21 (3) promote protein cross-linking by activation of a transglutaminase,22 (4) stimulate membrane phospholipases C23,24 and A,25 (5) activate various protein kinases and phosphatases,26,27 and (6) stimulate calpains that can cleave membrane proteins.22 Although the magnitudes and frequencies of these latter responses to calcium entry remain unresolved, calcium clearly constitutes a reasonable candidate for mediating communication between erythrocytes and other cells of the circulatory system.
Because mature RBCs lack intracellular calcium stores, elevation in intracellular calcium must stem from calcium influx. Although increased cytosolic calcium has been commonly ascribed to breaches in the permeability barrier of the membrane, calcium fluxes that mediate signal transduction would seem more likely to derive from a controlled pathway for Ca2+ entry. Pharmacologic evidence, in fact, already suggests that some type of calcium channel may operate in the mature RBC membrane,28-33 but its identity has never been established. We report here the identification of an ω-agatoxin-TK–sensitive, Cav2.1-like (P/Q-type) calcium channel in the RBC membrane that functions under the control of intracellular kinases and phosphatases. We further propose that this route of calcium influx might constitute a signaling intermediate that could participate in promoting an erythrocyte response to signals from other blood cells.
Materials and methods
Materials
Fluo-3/am, staurosporine, K252A, chelerythrine chloride, okadaic acid, calyculin A, ω-agatoxin-TK, and goat antirabbit (GAR)–horseradish peroxidase (HRP) were purchased from Calbiochem (La Jolla, CA). Nifedipine, verapamil, phorbol-12 myristate-13 acetate (PMA), A23187, dimethyl sulfoxide (DMSO), Tris-OH, bovine serum albumin (BSA), and Tween-20 were obtained from Sigma (St Louis, MO). The α1A (P/Q-type) calcium channel subunit antibody and peptide were purchased from Alomone Labs (Jerusalem, Israel). Enhanced chemiluminescence (ECL) Western blotting detection reagents were obtained from Amersham Life Science (Buckinghamshire, England).
Red blood cells
Fresh venous blood from healthy human volunteers was collected into Becton Dickinson (Franklin Lakes, NJ) Vacutainer acid/citrate/dextrose anticoagulant tubes. Approval was obtained from the institutional review board at Purdue University for these studies, and informed consent was provided according to the Declaration of Helsinki. Donors were free of any medication for at least 14 days before venipuncture. All experiments used fresh whole blood that was less than 2 hours old, unless otherwise specified. Whole anticoagulated blood was centrifuged on a swinging bucket rotor and washed 2 times in phosphate-buffered saline (PBS) (NaCl 125 mM, KCl 2.7 mM, Na2HPO4 8 mM, K2H2PO4 1.5 mM, pH 7.4) or PBS-glucose (PBS-G) (PBS, glucose 10 mM, pH 7.4). Residual white blood cells were removed from the RBC preparations by either of the following methods: (1) passing blood down a 1:1 column of α-cellulose/Sigmacell 50 (Sigma), eluting the column with PBS, and then washing eluted RBCs 3 times with 20 volumes of PBS; or (2) carefully removing the plasma and buffy coat from the RBCs after each of 3 washes in PBS-G.
Preparation of Fluo-3/am–loaded RBCs
Fluo-3/am was loaded into RBCs according to an established protocol34 using one of the following buffer solutions: PBS-G (NaCl 125 mM, KCl 2.7 mM, Na2HPO4 8 mM, K2H2PO4 1.5 mM, glucose 10 mM, pyruvate 10 mM, pH 7.4) or HEPES-G (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–glucose) (NaCl 123 mM, KCl 5 mM, MgCl2 1 mM, CaCl2 2 mM, HEPES 25 mM, glucose 10 mM, pyruvate 10 mM, pH 7.4). Briefly, 20 μL of packed RBCs (hematocrit [HCT] approximately 80%) were placed in 10 mL HEPES-G buffer (0.16% HCT) in a 50-mL conical tube and mixed gently by hand. The tube containing the RBCs was then covered in aluminum foil to exclude light, and 10 μL of 2.0 mM Fluo-3/am was added to the dilute RBC suspension. The RBC–Fluo-3/am suspension was then incubated at 37°C for 15 minutes with vigorous shaking. An additional 10 μL Fluo-3/am (2.0 mM stock) was added, with incubation carried out for an additional 25 minutes (final Fluo-3/amconcentration 3.5 μM). The shaking was then decreased to a gentle motion for an additional 20 minutes. All subsequent manipulations were performed with the Fluo-3/am–loaded RBCs carefully protected from light. Fluo-3/am–loaded RBCs were centrifuged at 1000g for 3 minutes at 22°C, and the incubation buffer was carefully removed. The Fluo-3/am–loaded packed RBCs were then washed 2 times with PBS-G plus 0.5% BSA (Sigma) and one time in HEPES buffer.
Fluorescence spectroscopy
The Fluo-3/am–loaded RBCs were resuspended in 2 mL HEPES-G buffer and allowed to equilibrate for 15 minutes at 22°C. One hundred microliters of Fluo-3/am–loaded RBCs (0.8% HCT) was added to 1400 μL HEPES-G buffer in a stirred quartz cuvette in a fluorimeter (Aminco Bowman Series 2 Luminescence Spectrometer; SLM-AMINCO, Rochester, NY). Kinase and phosphatase inhibitors, when desired, were added individually to the diluted Fluo-3/am–loaded RBCs and incubated for 10 minutes at 22°C before transfer to the spectrofluorimeter. Fifty seconds of baseline fluorescence was initially recorded, and then PMA (dissolved in DMSO) was added at the desired final concentration. The same concentration of DMSO was used in all control experiments, with the final DMSO concentration never exceeding 0.1% of the total volume. RBCs used in the phosphatase inhibition experiments were diluted in PBS-G instead of HEPES buffer to avoid the phosphatase inhibitor activity present in the HEPES.35Fluo-3/am–loaded cells were excited at 506 nm with a 4-nm bandpass, and the emission was set at 530 nm with an 8-nm bandpass. Calcium influx was monitored by recording the change in fluorescence intensity over time.
Flow cytometry
For flow cytometry, Fluo-3/am–loaded RBCs were resuspended in 5 mL HEPES buffer (0.32% HCT) and allowed to equilibrate for 15 minutes at 22°C. PMA was then added, and the cells were incubated for an additional 3 minutes before analysis. For calcium inhibition experiments, Fluo-3/am–loaded RBCs (50 μL) were added to 450 μL HEPES buffer and incubated for approximately 10 minutes with the calcium channel antagonists or control solvents. These antagonist-pretreated, Fluo-3/am–loaded RBCs were then stimulated with PMA or A23187 and placed in a Coulter ELITE Flow Cytometer for evaluation of fluorescence (Beckman Coulter, Hiahleah, FL). The excitation source for Fluo-3/am was a 488-nm air-cooled argon laser, and the emission was measured using a 525-nm bandpass filter.
Western blotting
Washed RBCs were lysed using 30 volumes of ice-cold lysing buffer (Na2HPO4, 5 mM; ethylenediaminetetraacetic acid [EDTA], 1 mM; pepstatin A, leupeptin, and aprotinin, 1 μg/mL; phenylmethylsulfonyl fluoride, 0.2 mM; benzamidine, 0.1 mg/mL; and calpain inhibitor I and II, 8 μg/mL each; pH 8.0) and then washed in the same buffer until the membranes were white. The protein concentration of treated ghosts was determined using a premixed bicinchonicic acid (BCA) assay (Pierce, Rockford, IL). RBC membranes (50-80 μg) were loaded onto an 8% gel and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Membranes were then transferred to nitrocellulose for 2 hours at 4°C using 200 mV. The nitrocellulose membranes were blocked for a minimum of 2 hours at 22°C with 10% powdered skim milk in PBS plus 0.2% Tween-20. The primary α1A antibody (produced in rabbits against rat calcium channel α1A peptide; accession number P54282; sequence SSPERAPGREGPYGRE) was incubated at 1:200 dilution for 2 hours at 22°C. For competition of the α1A antibody with the above specific peptide on Western blots, 10 μg of the α1A antibody was preincubated with 10 μg peptide in Tris (tris[hydroxymethyl]aminomethane)-buffered saline (TBS)/Tween-20/BSA buffer (20 mM Tris-OH, 137 mM NaCl, 0.05% Tween-20, 2% BSA, pH 7.6) for 1 hour at 22°C and then centrifuged at 10 000g for 5 minutes. The antigen–peptide solution was then diluted 1:200 in the same TBS/Tween-20/BSA buffer and incubated for 2 hours at 22°C. The secondary antibody (GAR-HRP) was diluted 1:10 000 and incubated for 1 hour at 22°C. Reactive bands were revealed by chemiluminescence using ECL Western blot detection reagents on Kodak X-OMAT film.
Osmotic fragility test
Buffer A (125 mM NaCl, 3 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 16 mM HEPES, 10 mM glucose, pH 7.4) was mixed with buffer B (99 mL 2 mM CaCl2 plus 1 mL 120 mM NaHPO4, pH 7.2-7.4) to generate solutions of 0, 30, 60, 75, 90, 105, 120, 135, 150, and 300 mOsm. Nine hundred microliters of each osmotic solution was then placed in a 1.5-mL Eppendorf tube and cooled to 4°C. Fresh, washed RBCs (HCT 10% in buffer A) were incubated with 3 μM PMA (or DMSO as control) for 40 minutes at 37°C in a shaking water bath. These samples were staggered at 20-minute intervals to allow time for processing. After the appropriate incubation period, 100 μL of RBCs was added to the first 10 Eppendorf tubes, mixed, and incubated at 4°C for 5 minutes. The cells were then centrifuged for 5 minutes. Five hundred microliters of supernatant was immediately placed in 500 μL Drabkin solution (Sigma), and absorbance at 540 nm was recorded using an ultraviolet spectrophotometer.
Forward light scattering detected by flow cytometry
Forward light scattering data of PMA-treated RBCs were obtained by flow cytometry as a means to detect changes in RBC size on an individual cell basis. Fresh whole blood was collected and washed as described above, and 20 μL of packed RBCs was loaded with Fluo-3/am. An additional 20 μL of packed RBCs were subjected to the same experimental conditions as the Fluo-3/am loading without any Fluo-3/am present to compare size changes in RBCs loaded or not loaded with Fluo-3/am. The Fluo-3/am–loaded and nonloaded cells were equilibrated in HEPES buffer and treated with 3 μM PMA, 2 μM A23187, or 5 μM 1,2-didecanoyl-rac-glycerol (or DMSO as control) for 40 minutes at 22°C. Forward light scattering data of RBCs treated with PMA, DMSO, 1,2-didecanoyl-rac-glycerol, and A23187 were collected using a Coulter Elite Flow Cytometer.
Results
Activation of protein kinase C induces a rapid influx of calcium into RBCs
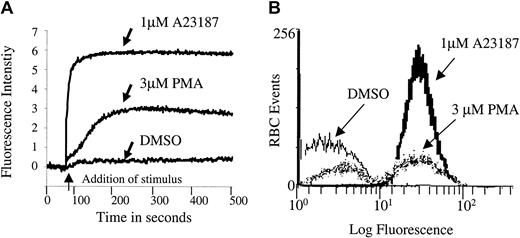
After considering a variety of potential signaling components, we selected protein kinase C (PKC) as a leading candidate for regulation of erythrocyte Ca2+ because of the following: (1) It is known to modulate Ca2+ homeostasis in other cells36-39; (2) it is highly active in mature human erythrocytes40,41; and (3) it influences both erythrocyte morphology and ion transport.40-44 To test directly whether PKC can modulate erythrocyte calcium homeostasis, we stimulated RBCs with PMA, a well-known activator of PKC, and examined them for changes in cytosolic Ca2+ using the Ca2+-sensitive fluorescent dye, Fluo-3/am, as a reporter molecule. As shown in Figure 1, RBCs treated with 3 μM PMA displayed a Ca2+ influx that was slower than that catalyzed by the Ca2+ ionophore, A23187, but more rapid than that promoted by the PMA solvent DMSO. The influx was concentration dependent, and it reached a maximum near 3 μM (data not shown).
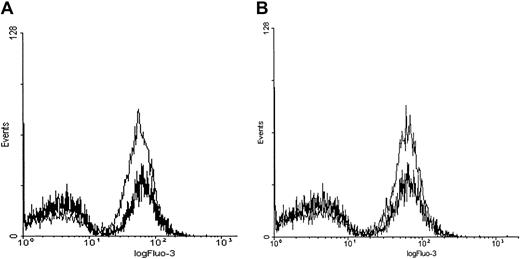
PMA induces a rapid calcium influx in Fluo-3/am–loaded erythrocytes.
Cell suspensions of Fluo-3/am–loaded RBCs at 0.8% HCT were treated with 3 μM PMA, 1 μM A23187, or DMSO (negative control). Calcium influx was measured by both (A) fluorescence spectroscopy and (B) flow cytometry. Tracings representative of 11 different donors are shown.
PMA induces a rapid calcium influx in Fluo-3/am–loaded erythrocytes.
Cell suspensions of Fluo-3/am–loaded RBCs at 0.8% HCT were treated with 3 μM PMA, 1 μM A23187, or DMSO (negative control). Calcium influx was measured by both (A) fluorescence spectroscopy and (B) flow cytometry. Tracings representative of 11 different donors are shown.
Although Fluo-3/am is commonly used to monitor changes in cell calcium and although Fluo-3/am becomes stably entrapped within a cell following its hydrolysis to the free acid, 2 artifacts can still arise that require additional controls. First, there was concern that the Ca2+-sensitive dye might have leaked out of the cell and become fluorescent as it encountered extracellular calcium. Therefore, calcium influx was monitored by flow cytometry because the flow cytometer can be adjusted to detect fluorescence only from intact cells. As seen in Figure 1B, PMA indeed stimulated calcium influx into intact erythrocytes. However, the cation is found to enter only a subpopulation of PMA-treated cells. Thus, incubation with 3 μM PMA for more than 3 minutes at 22°C induced approximately 47% ± 18% (n = 11, P < .001) of the RBCs to take up Ca2+ in an “all or none” fashion. In contrast, incubation with 1 μM A23187, as expected, caused approximately 99% (n = 11, P < .001) of the cells to exhibit increased fluorescence. Further, RBCs treated with the solvent DMSO as a negative control predictably displayed only background numbers of RBCs with equivalent fluorescent intensity (3.9% ± 4.7%, n = 11).
Second, intracellular hydrolysis of acetoxymethyl esters (eg, Fluo-3/am) releases formaldehyde, which can block glycolysis if present in sufficient quantities.45 46Because blockade of glycolysis can lead to depletion of adenosine triphosphate (ATP) and a concomitant passive influx of extracellular calcium, we decided to measure cellular ATP content at multiple time points during loading of Fluo-3/am. It is important to note that erythrocyte ATP concentration was found to decrease by no more than 10%, even at the longest incubation times (data not shown). These data dismiss the possibility that ATP depletion was responsible for the PMA-stimulated Ca2+ fluxes. The observation that the Ca2+ influx occurred within a minute of PMA stimulation (Figure 1, also confirmed by flow cytometry) also argues that its entry was not a consequence of ATP depletion.
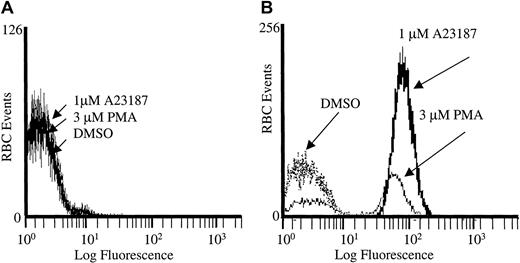
To ensure that the PMA-stimulated rise in Fluo-3 fluorescence was directly caused by calcium entry, we also treated the Fluo-3/am–loaded RBCs with A23187, PMA, or DMSO in the absence of calcium (Figure 2). Under these conditions, no increase in Fluo-3/am fluorescence was detected. However, when calcium was reintroduced into the extracellular buffer of the same cell suspensions 5 minutes after PMA stimulation, the previously identified calcium influx was once again observed in the A23187- and PMA-treated RBCs. Thus, PMA opens a calcium influx pathway whether calcium is present or not, and the activated Ca2+ channel remains open for several minutes.
PMA-induced calcium influx is dependent on extracellular calcium.
(A) Cell suspensions of Fluo-3/am–loaded RBCs were treated with 3 μM PMA, 1 μM A23187, or DMSO in the absence of Ca2+ and examined on an individual cell basis by flow cytometry. (B) Alternatively, the cells were restored to normal extracellular Ca2+ levels (2 mM) 5 minutes after stimulation with PMA, A23187, or DMSO, as described above. Representative tracing from one donor is shown; tracing was performed in duplicate.
PMA-induced calcium influx is dependent on extracellular calcium.
(A) Cell suspensions of Fluo-3/am–loaded RBCs were treated with 3 μM PMA, 1 μM A23187, or DMSO in the absence of Ca2+ and examined on an individual cell basis by flow cytometry. (B) Alternatively, the cells were restored to normal extracellular Ca2+ levels (2 mM) 5 minutes after stimulation with PMA, A23187, or DMSO, as described above. Representative tracing from one donor is shown; tracing was performed in duplicate.
PKC regulates calcium channel activity in PMA-treated RBCs
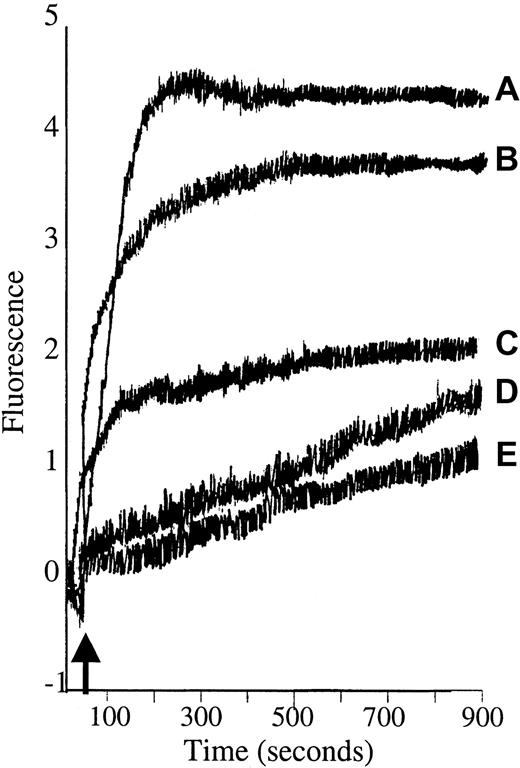
Diacylglycerol (DAG) is well established as the natural activator of PKC, although it is weaker and shorter acting than PMA.47 Synthetic DAG isoforms such as 1,2-didecanoyl-rac-glycerol also activate PKC,48 49 and because they are more water soluble than DAG, they were used in these studies to explore whether the calcium influx seen in PMA-treated RBCs could be replicated by a physiologic activator. For this purpose, Fluo-3/am–loaded RBCs were stimulated with either 5 μM or 10 μM 1,2-didecanoyl-rac-glycerol, and fluorescence intensity was monitored as a function of time using a fluorescence spectrophotometer. The results showed that 1,2-didecanoyl-rac-glycerol stimulated calcium influx into Fluo-3/am–loaded RBCs similar to PMA, although the magnitude of the fluorescence change was somewhat less (Figure 3). It is important to note that 4α-phorbol, an inactive analog of PMA, induced a Ca2+ influx no different from the solvent control, DMSO. These data demonstrate that the PMA-promoted calcium influx requires the correct stereochemistry of the PMA–PKC interaction.
1,2-didecanoyl-rac-glycerol (DAG) stimulates calcium influx into Fluo-3/am–loaded RBCs similar to PMA.
Cell suspensions of Fluo-3/am–loaded RBCs were stimulated with (A) 3 μM PMA or (B) 10 μM or (C) 5 μM 1,2-didecanoyl-rac-glycerol dissolved in DMSO, and fluorescence was monitored by fluorescence spectroscopy. Negative controls included (D) 4α-phorbol and (E) DMSO. Tracings representative of 3 different donors are shown. Arrow designates time of addition of stimulus.
1,2-didecanoyl-rac-glycerol (DAG) stimulates calcium influx into Fluo-3/am–loaded RBCs similar to PMA.
Cell suspensions of Fluo-3/am–loaded RBCs were stimulated with (A) 3 μM PMA or (B) 10 μM or (C) 5 μM 1,2-didecanoyl-rac-glycerol dissolved in DMSO, and fluorescence was monitored by fluorescence spectroscopy. Negative controls included (D) 4α-phorbol and (E) DMSO. Tracings representative of 3 different donors are shown. Arrow designates time of addition of stimulus.
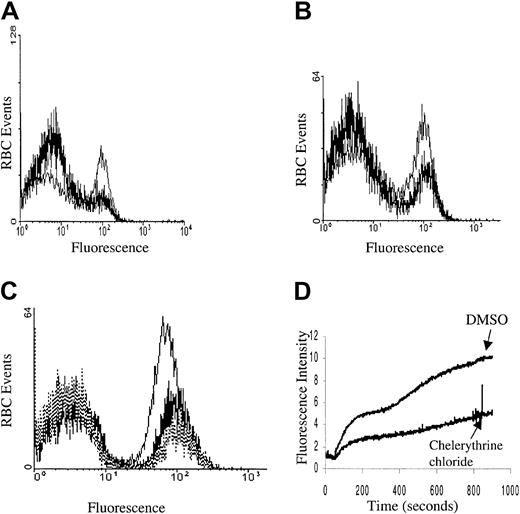
Although calcium influx was shown to be stimulated by both PMA and 1,2 didecanoyl-rac-glycerol, it was still necessary to establish directly the possible participation of PKC in this signaling pathway. In this effort, we initially confirmed that PKC-α was present, that it translocated to the membrane, and that it was catalytically activated upon PMA treatment by blotting the treated membranes with anti–PKC-α antibodies and by monitoring the change in PKC activity using a PKC-specific substrate and radiolabeled ATP (PKC enzyme assay system RPN77; Amersham Life Sciences, Cleveland, OH; data not shown). We then sought to determine whether the PMA-stimulated Ca2+influx could be prevented by PKC inhibitors. As seen in Figure4, Ca2+ entry was indeed partly inhibited by both the nonspecific PKC inhibitors staurosporine and K252A, and also by the PKC-specific inhibitor chelerythrine chloride. Thus, following preincubation of erythrocytes with inhibitory concentrations of these inhibitors, roughly half of the normally responsive population of PMA-stimulated RBCs failed to take up Ca2+. Whether the inhibitor-resistant fraction of cells represents erythrocytes with nonspecific leaks or simply an inhibitor-resistant influx pathway cannot be ascertained from the data. However, the fact that many of the RBCs do not respond to PMA following the addition of PKC inhibitors suggests that at least part of the PMA-stimulated Ca2+ influx is mediated by PKC.
Kinase inhibition of PMA-induced calcium influx.
Cell suspensions of Fluo-3/am–loaded RBCs were pretreated with (A) 3 μM staurosporine (thick line) or DMSO (thin line), (B) 1 μM K252A (thick line) or DMSO (thin line), and (C) 5 μM chelerythrine chloride (thick line), 10 μM chelerythrine chloride (dotted line), or DMSO (thin line) and then stimulated with 3 μM PMA and assayed for Fluo-3 fluorescence by flow cytometry. All preincubations were carried out for 10 minutes at 22°C. (D) Alternatively, Fluo-3/am–loaded RBCs were pretreated with 10 μM chelerythrine chloride or DMSO for 10 minutes at 22°C and then stimulated with 3 μM PMA and monitored in the fluorescence spectrophotometer as a function of time. Tracings representative of 4 different donors are shown.
Kinase inhibition of PMA-induced calcium influx.
Cell suspensions of Fluo-3/am–loaded RBCs were pretreated with (A) 3 μM staurosporine (thick line) or DMSO (thin line), (B) 1 μM K252A (thick line) or DMSO (thin line), and (C) 5 μM chelerythrine chloride (thick line), 10 μM chelerythrine chloride (dotted line), or DMSO (thin line) and then stimulated with 3 μM PMA and assayed for Fluo-3 fluorescence by flow cytometry. All preincubations were carried out for 10 minutes at 22°C. (D) Alternatively, Fluo-3/am–loaded RBCs were pretreated with 10 μM chelerythrine chloride or DMSO for 10 minutes at 22°C and then stimulated with 3 μM PMA and monitored in the fluorescence spectrophotometer as a function of time. Tracings representative of 4 different donors are shown.
Because phosphatases generally provide the “off” switch for proteins activated by PKC phosphorylation, we hypothesized that the PMA-induced calcium influx might be augmented in response to one or more phosphatase inhibitors. As seen in Figure5, both okadaic acid and calyculin A enhanced the calcium uptake by PMA-treated RBCs.
Phosphatase inhibitors enhance calcium influx in PMA-treated RBCs.
Cell suspensions of Fluo-3/am–loaded RBCs were pretreated with (A) 1 μM okadaic acid (thin line) or DMSO (thick line) for 10 minutes or (B) 0.4 μM calyculin A (thin line) or DMSO (thick line) for 2.5 minutes and then stimulated with 3 μM PMA. Calcium influx was monitored by flow cytometry. For maximum effect, RBCs were suspended in PBS-G buffer because the intrinsic phosphatase inhibitor activity associated with HEPES tended to reduce the magnitude of the inhibitor stimulation.35 Tracings representative of 3 different donors are shown.
Phosphatase inhibitors enhance calcium influx in PMA-treated RBCs.
Cell suspensions of Fluo-3/am–loaded RBCs were pretreated with (A) 1 μM okadaic acid (thin line) or DMSO (thick line) for 10 minutes or (B) 0.4 μM calyculin A (thin line) or DMSO (thick line) for 2.5 minutes and then stimulated with 3 μM PMA. Calcium influx was monitored by flow cytometry. For maximum effect, RBCs were suspended in PBS-G buffer because the intrinsic phosphatase inhibitor activity associated with HEPES tended to reduce the magnitude of the inhibitor stimulation.35 Tracings representative of 3 different donors are shown.
PMA-mediated calcium influx occurs through an ω-agatoxin-TK–sensitive, Cav2.1-like calcium channel
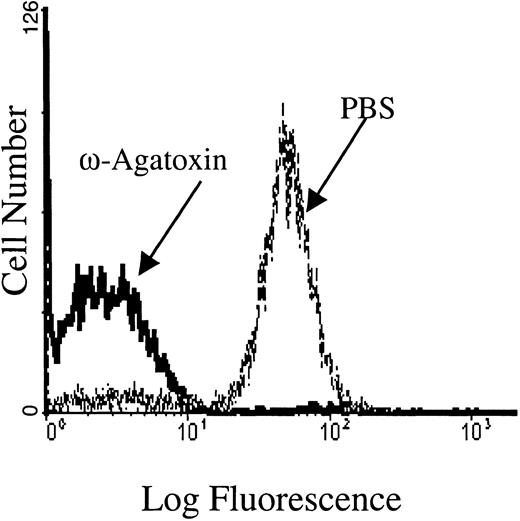
To confirm that the PKC-regulated Ca2+ influx occurred from Ca2+ entry through a channel rather than a leak, we attempted to identify the channel subtype operating in the PMA-stimulated erythrocytes. Pharmacologic inhibitors of various calcium channels were therefore examined for their abilities to inhibit the Ca2+ influx. Notably, ω-agatoxin-TK, a specific P-type calcium channel blocker,50 was found to inhibit the PMA-induced influx in a dose-dependent manner. In fact, RBCs incubated with 1 μM ω-agatoxin-TK before PKC stimulation displayed more than 95% inhibition of Ca2+ entry (Figure6). Even at a lower ω-agatoxin-TK concentration of 0.5 μM, only background levels of PMA-treated RBCs responded with increased fluorescence, compared with approximately 70% of control RBCs from the same donor. In a dose-response study, the 50% inhibitory concentration (IC50) of ω-agatoxin-TK was determined to be 100 nM. Inhibition of the PMA-mediated calcium influx was minimally detectable at 10 nM. These inhibitory concentrations of ω-agatoxin are similar to those used to inhibit calcium influx in native P-type calcium channels found in rat neurons.51 It is important to note that ω-agatoxin-TK did not inhibit A23187-induced calcium influx (data not shown).
The PMA-induced calcium influx is inhibited by ω-agatoxin-TK, a P-type calcium channel blocker.
Flow cytometry tracings are shown for Fluo-3/am–loaded RBC cell suspensions pretreated with 1 μM ω-agatoxin-TK and then stimulated with 3 μM PMA. Pretreatment of Fluo-3/am–loaded RBCs with PBS (the agatoxin-TK solvent) showed no inhibition of calcium uptake. Tracings representative of 4 different donors are shown.
The PMA-induced calcium influx is inhibited by ω-agatoxin-TK, a P-type calcium channel blocker.
Flow cytometry tracings are shown for Fluo-3/am–loaded RBC cell suspensions pretreated with 1 μM ω-agatoxin-TK and then stimulated with 3 μM PMA. Pretreatment of Fluo-3/am–loaded RBCs with PBS (the agatoxin-TK solvent) showed no inhibition of calcium uptake. Tracings representative of 4 different donors are shown.
L-type calcium channel blockers were also examined for their inhibition of the PMA-induced calcium influx. However, only at concentrations well above levels that normally inhibit L-type calcium channels in classic electrically excitable cells52 were these inhibitors able to reduce influx (data not shown). Thus, IC50 values for blockade of PMA-induced calcium entry into erythrocytes were determined to be approximately 100 μM for both nifedipine and verapamil.
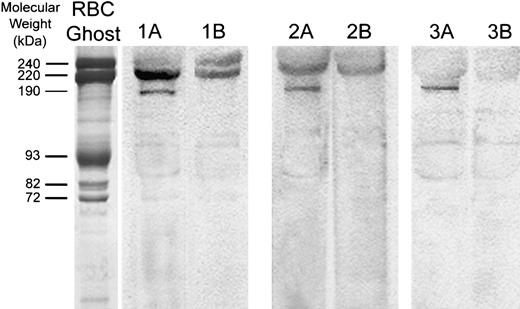
Because pharmacologic evidence suggested that a Cav2.1 (P/Q-type) calcium channel may function in the RBC membrane, we further explored the specific identity of the calcium channel. Erythrocyte membranes separated by SDS-PAGE and transferred to nitrocellulose were blotted with a commercial anti-α1A (anti-P/Q type) calcium channel–specific antibody raised against residues 865 to 881 of the differentially processed calcium channel polypeptide in the rat (accession no. Rat P54282; (C)PSSPERAPGREGPYGRES). The rat peptide sequence used to generate this antibody is 100% identical to the corresponding amino acid sequence from the human α1Asubunit over the last 9 amino acids (accession no. Human AAB61613; AGSQEAELSREGPYGRES) and 80% homologous over the entire length of the protein. Consistent with their presence in the erythrocyte membrane, polypeptides with Mr of approximately 190 kDa and 220 kDa were readily visualized by ECL staining (Figure7). Polypeptides of similar molecular weights have been visualized by others in membrane preparations from rat brain.53 Competitive inhibition of RBC membrane immunostaining with the above antigenic peptide blocked detection of the 190-kDa band, confirming that staining of the 190-kDa polypeptide is antigen specific. Nonspecific binding of the α1Aantibody to spectrin and other proteins in the band-3 region was not prevented by the antigenic peptide. However, after competitive inhibition with the peptide, a band of approximately 220 kDa was also decreased in intensity, but not entirely eliminated. Purification of the calcium channel from the RBC membrane cytoskeleton will be necessary to evaluate fully the higher molecular-weight forms of the Cav2.1 calcium channel.
Western blot analysis of the α1A subunit of voltage-gated calcium channels in human erythrocyte ghosts from 3 different donors.
Red cell membrane proteins were separated electrophoretically at high protein loads (80 μg). After electrophoretic transfer to nitrocellulose paper, blots were stained with antibodies directed against residues 865 to 881 of the α1A subunit of the rat brain voltage-gated calcium channel (lanes 1A, 2A, 3A). Because several nonspecific bands were also visualized, competition of Cav2.1 antibody staining using its specific peptide was also performed. For this purpose, 10 μg of the Cav2.1 antibody was preincubated with 10 μg of antibody-specific peptide for 1 hour at 22°C and then further incubated with the blot for 2 hours at 22°C (lanes 1B, 2B, 3B). Polypeptides with Mr of approximately 190 000 and 220 000 are characteristic of the major splicing variants of the α1A subunit of Cav2.1 in the brain. An SDS-PAGE Coomassie blue–stained gel of RBC membranes (left lane; “RBC Ghost”) serves as an approximate molecular weight marker.
Western blot analysis of the α1A subunit of voltage-gated calcium channels in human erythrocyte ghosts from 3 different donors.
Red cell membrane proteins were separated electrophoretically at high protein loads (80 μg). After electrophoretic transfer to nitrocellulose paper, blots were stained with antibodies directed against residues 865 to 881 of the α1A subunit of the rat brain voltage-gated calcium channel (lanes 1A, 2A, 3A). Because several nonspecific bands were also visualized, competition of Cav2.1 antibody staining using its specific peptide was also performed. For this purpose, 10 μg of the Cav2.1 antibody was preincubated with 10 μg of antibody-specific peptide for 1 hour at 22°C and then further incubated with the blot for 2 hours at 22°C (lanes 1B, 2B, 3B). Polypeptides with Mr of approximately 190 000 and 220 000 are characteristic of the major splicing variants of the α1A subunit of Cav2.1 in the brain. An SDS-PAGE Coomassie blue–stained gel of RBC membranes (left lane; “RBC Ghost”) serves as an approximate molecular weight marker.
Physiologic changes in PMA-stimulated RBCs
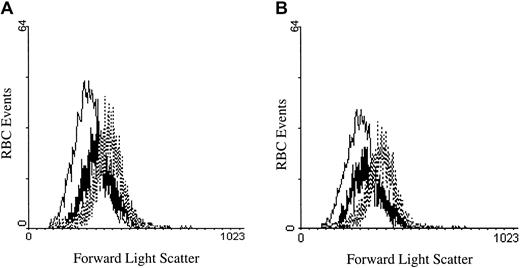
To determine whether activation of PKC might affect erythrocytes in a physiologically meaningful manner, we investigated the impact of PMA-initiated calcium fluxes on erythrocyte volume. The mechanistic motivation for this study stems from the fact that one of the earliest consequences of Ca2+ entry is activation of the Gardos channel and the resulting efflux of cell water. To test whether PKC stimulation indeed leads to cell shrinkage, we incubated PMA (3 μM)–treated RBCs for 40 minutes at 37°C and evaluated them for a change in volume using forward scatter flow cytometry. As seen in Figure 8A, PMA-treated RBCs were significantly smaller than DMSO-treated negative controls, although not as small as A23187-treated positive controls.54 Similarly, erythrocytes stimulated with 1,2-didecanoyl-rac-glycerol showed an analogous decline in cell size (Figure 8B). Further, chelation of intracellular calcium by Fluo-3 had no effect on the size change of the treated RBCs (data not shown). These data suggest that PMA can induce influx of sufficient calcium to promote a change in cell volume.
PMA- and DAG-treated RBCs decrease in cell size after treatment.
(A) RBCs treated with 2 μM A23187 (thin line), 3 μM PMA (thick line), or DMSO (control; dotted line) were incubated for 40 minutes at 37°C and compared for changes in cell volume by forward light scattering using a flow cytometer. (B) The same experiment as in (A) was performed, except that the DAG analog 1,2-didecanoyl-rac-glycerol (thick line) was incubated at 5 μM. Tracings representative of 3 different donors are shown.
PMA- and DAG-treated RBCs decrease in cell size after treatment.
(A) RBCs treated with 2 μM A23187 (thin line), 3 μM PMA (thick line), or DMSO (control; dotted line) were incubated for 40 minutes at 37°C and compared for changes in cell volume by forward light scattering using a flow cytometer. (B) The same experiment as in (A) was performed, except that the DAG analog 1,2-didecanoyl-rac-glycerol (thick line) was incubated at 5 μM. Tracings representative of 3 different donors are shown.
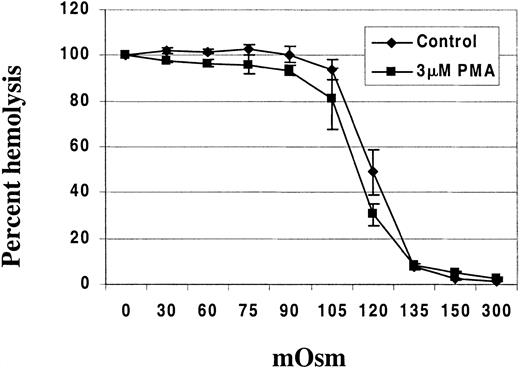
To further document this change in surface-to-volume ratio, we suspended PMA-treated RBCs in salt solutions of varying tonicities ranging from 0 to 300 mOsm to determine their resistance to hypotonic lysis. PMA-treated RBCs exhibited increased resistance to hypotonic lysis when compared with DMSO-treated controls in osmotic fragility tests (Figure 9).
PMA-treated RBCs exhibit resistance to hypotonic lysis compared with DMSO (control)-treated RBCs.
RBCs (HCT 10%) were incubated with either 3 μM PMA or DMSO as control for 40 minutes at 37°C in a shaking water bath. Osmotic fragility was then assessed using solutions of 0, 30, 60, 75, 90, 105, 120, 135, 150, and 300 mOsm. The hemoglobin concentration in the supernatant from each test solution was determined using Drabkin reagent (Sigma, St Louis, MO). The data points represent the mean ± SD from 2 different donors with 3 replicates per person.
PMA-treated RBCs exhibit resistance to hypotonic lysis compared with DMSO (control)-treated RBCs.
RBCs (HCT 10%) were incubated with either 3 μM PMA or DMSO as control for 40 minutes at 37°C in a shaking water bath. Osmotic fragility was then assessed using solutions of 0, 30, 60, 75, 90, 105, 120, 135, 150, and 300 mOsm. The hemoglobin concentration in the supernatant from each test solution was determined using Drabkin reagent (Sigma, St Louis, MO). The data points represent the mean ± SD from 2 different donors with 3 replicates per person.
Discussion
We have presented 3 lines of evidence to demonstrate that a regulatable channel can mediate the influx of Ca2+ across the human erythrocyte membrane. First, both phorbol myristate acetate (PMA) and diacylglycerol, 2 activators of protein kinase C (PKC), were found to stimulate calcium entry into RBCs, whereas 4α-phorbol ester, an inactive PMA analog, did not. Second, calcium influx into PMA-stimulated cells was partially inhibited by the addition of various kinase inhibitors, but enhanced by treatment with phosphatase inhibitors, suggesting that channel activity is controlled by protein phosphorylation. Third, the PMA-stimulated calcium influx was potently inhibited by nanomolar quantities of ω-agatoxin-TK, a calcium channel blocker from the funnel web spider.55,56 Because this toxic peptide is reported to be specific for Cav2.1-type channels, these data also suggest that the responsible protein is a Cav2.1-like (P/Q-related) channel. Immunostaining of erythrocyte membranes with antibodies raised against a subunit of this channel, in fact, confirmed the presence of this class of polypeptides in the erythrocyte membrane. Although P/Q-type Ca2+channels are best characterized in neurons,55-58 there is evidence that the channels are also present in other cells of the body.59 An isoform of the Cav2.1-type channel now appears to be present also in the mature human erythrocyte.
Cav2.1 calcium channels from brain cells are well known to be voltage gated, activating at membrane potentials of −45 to −35 mV and reaching a maximum probability of being open at greater than 0 mV.55,57 Human erythrocytes, however, are not believed to undergo significant changes in membrane potential. Thus, the usual resting potential of a mature RBC is normally −12 mV,60 a value that would seem poorly positioned for significant depolarization, and no action potentials have ever been observed in human erythrocytes. Nevertheless, RBCs have already been reported to contain a nonspecific, voltage-gated cation channel that favors monovalent cations, but can transport Ca2+ as well.28,33 60 Whether the channel we have characterized is related to this nonspecific channel will be an important area of future investigation. It will also be important to examine whether PMA or DAG stimulates membrane depolarization in human erythrocytes and whether the above PKC activators promote the permeability of other cations as well.
In a related study, Lijnen et al61 observed that PMA activates the human erythrocyte Na+/H+exchanger. During their studies, these authors also noted that PMA stimulated calcium influx into the treated cells, in close agreement with our observations. Although activation of the 2 influx pathways may arise independently, it is also conceivable that activation of Na+/H+ exchange and stimulation of Ca2+ influx might be mechanistically related. Thus, PMA activation of PKC could trigger stimulation of Na+ influx via the Na+/H+ exchanger, and the elevation of intracellular Na+ could promote Ca2+ entry via induction of Na+/Ca2+ exchange. Further analysis of the linkage between Ca2+ influx and Na+/Ca2+ exchange activity will obviously be necessary before this hypothesis can be adequately evaluated.
Earlier, our laboratory reported a calcium influx pathway initiated by lysophosphatidic acid (LPA), a product of platelet activation.34 Although there are clear similarities between the influx pathways, differences also exist to suggest that 2 separate signal transduction pathways might be converging on a common calcium permeability pathway. Thus, the LPA influx pathway was not inhibited by the specific PKC inhibitor chelerythrine chloride, whereas the PMA-mediated pathway was. Further, PMA was observed to activate roughly twice as many cells to influx anywhere from 2- to 10-fold more calcium per cell than LPA, assuming that Fluo-3/am fluorescence intensity is proportional to intracellular calcium concentration. Nevertheless, the 2 calcium permeability pathways are both blocked by ω-agatoxin-TK. Taken together, the data suggest that related or identical calcium channels can respond to multiple regulatory signals and trigger an influx of extracellular calcium.
Finally, calcium entry into RBCs is of more than academic interest because a variety of diseases are now characterized by elevated erythrocyte Ca2+. Although in vitro experiments have documented a number of changes in RBCs with increased intracellular calcium, the physiologic consequences of such calcium entry have not been demonstrated in vivo other than in sickle cells,62,63where at least part of the dehydration appears to be a consequence of Ca2+ activation of the Gardos channel.64-67 We suggest that the Cav2.1-like calcium permeability pathway described here might contribute to this Ca2+-stimulated process. Further, because activation of PKC by PMA causes RBCs to expose phosphatidylserine on their outer leaflets,68Ca2+ entry could enhance the thrombotic tendency of sickle cells by enriching their cell surfaces as an established procoagulant. Elevated erythrocyte calcium has also been associated with essential hypertension,69,70 primary hyperparathyroidism,71 idiopathic hypercalciuria,72 diabetes,73 and sepsis.74 75 Although these are clearly syndromes that are primarily manifested in other tissues, the impact of elevated cytosolic Ca2+ on erythrocyte structure and rheology may contribute to the pathologies of the diseases. And where such linkages become established, pharmacologic inhibition of RBC Ca2+permeability may contribute to an eventually optimal therapy.
We thank Kathy Ragheb of the Purdue University Flow Cytometer Laboratory for her advice and expertise with the flow cytometer and Dr Jiazhen Wang for her excellent technical assistance in Western blotting.
Supported in part by National Institutes of Health grants GM24417 and K08HL03350.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dina A. Andrews, Department of Veterinary Pathobiology, Purdue University, 1243 Veterinary Pathology Bldg, West Lafayette, IN 47907-1243; e-mail: andrews@purdue.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal