Abstract
Following phagocytosis in vivo, acidification of extracellular pH (pHo) and intracellular metabolic acid generation contribute to cytosolic proton loading in neutrophils. Cytosolic pH (pHi) affects neutrophil function, although its regulation is incompletely understood. Its effect on mechanisms of neutrophil death is also uncertain. Thus, we investigated pHi regulation in Escherichia coli–exposed neutrophils, at various pathogen-to-phagocyte ratios (0:1-50:1), under conditions simulating the inflammatory milieu in vivo and correlated pHi changes with mechanisms of neutrophil death. Following phagocytosis, proton extrusion was dominated early by passive proton conductance channels, and later by Na+/H+ exchange (NHE). H+-translocating adenosine triphosphatase (V-ATPase) pHi regulation was evident primarily at lower bacterial densities. At physiologic pHo, lower pathogen-to-phagocyte ratios alkalinized pHi and inhibited apoptosis, whereas higher ratios acidified pHi (despite proton extrusive mechanisms) and promoted apoptosis. Necrosis was induced by high-density bacterial exposure at reduced pHo. Following phagocytosis, targeted inhibition of NHEs, proton conductance channels, or V-ATPases (amiloride, ZnCl2, or bafilomycin, respectively) moderately hyperacidified pHi and accelerated apoptosis. However, in combination they profoundly acidified pHi and induced necrosis. Proinflammatory mediators in vivo might affect both pHi regulation and cell death, so we tested the effects of bronchoalveolar lavage (BAL) fluid from patients with cystic fibrosis (CF) and healthy subjects. Only CF BAL fluid alkalinized pHi and suppressed apoptosis at physiologic pHo, but failed to prevent necrosis following phagocytosis at low pHo. Thus, a precarious balance between cytosolic proton loading and extrusion after phagocytosis dictates the mode of neutrophil cell death. pHi/pHo might be therapeutically targeted to limit neutrophil necrosis and protect host tissues during necrotizing infections.
Introduction
Neutrophil phagocytosis is an essential and generally effective host response to microbial invasion. However, during necrotizing infections and in abscesses, neutrophils are ineffective at killing bacteria and instead contribute to host tissue destruction. Both intracellular and extracellular factors might contribute to dysregulation of neutrophilic inflammation in such situations, although these are not well characterized. pH may be important in this regard because it has the capacity to modulate neutrophil function. Thus, we investigated the mechanisms of intracellular pH (pHi) regulation following phagocytosis, under conditions simulating the inflammatory microenvironment, and assessed how pHi might contribute to the regulation or dysregulation of acute inflammation by altering mechanisms of neutrophil death.
pHi homeostasis is uniquely challenged in phagocytosing neutrophils because cellular activation generates sufficient acid equivalents to lethally acidify the cell, and extracellular pH (pHo) at inflammatory sites is often decreased.1,2 The balance between cytosolic proton loading and extrusion is important because many neutrophil functions, including microbicidal behavior, cell migration, intracellular oxidant generation, tumor cell cytotoxicity, and azurophil granule exocytosis, are pH dependent.3-8 Mechanisms potentially contributing to pHi regulation in neutrophils include Na+/H+ exchangers (NHEs), proton-translocating adenosine triphosphatases (V-ATPases), and reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–associated proton conductance channels.9-11 Although NHEs are activated following phagocytosis in neutrophils,12 it is not known whether additional mechanisms also contribute to proton extrusion, or indeed whether they are collectively sufficient to preserve pHiclose to physiologic levels, under conditions of increased proton loading. Further, the combined effect on pHi regulation of mediators in the inflammatory milieu could also be important in vivo, although it is poorly characterized. Although some soluble inflammatory mediators activate proton extrusion in vitro,13-16others, such as lipopolysaccharide (LPS), may impair it.17Thus, in the first series of experiments reported here, we investigated which pumps and channels are involved in pHi regulation after phagocytosis and tested how their function is affected by proinflammatory mediators from the lungs of cystic fibrosis (CF) patients and healthy subjects. CF epithelial lining fluid (ELF) is an attractive model of an inflammatory microenvironment for such studies because its pulmonary phenotype is characterized by intense, persistent inflammation and neutrophil-mediated parenchymal destruction.
pHi and the inflammatory microenvironment may also affect survival of phagocytosing neutrophils by modulating rates of apoptotic and necrotic cell death, both of which may occur in response to bacterial exposure in vitro.18-20 This distinction is important because the former is a protective, orderly involutionary process, whereas the latter likely contributes to disease by exposing host tissues to destructive neutrophil contents.21pHi and pHo directly affect apoptosis in many cell types,22-30 whereas severely acidic pHimight nonspecifically injure cells and thereby promote necrosis.31 Because phagocytosing neutrophils exhibit marked alterations in metabolic acid generation and are subject to variable pHo, it is surprising that the relationship between pHi and cell death in neutrophils has not been characterized more rigorously. The literature in this regard is, indeed, conflicting, as different studies have implicated acidic pHi in both stimulation and inhibition of neutrophil or myeloid cell apoptosis.6,13,32 33 Thus, we addressed the consequences of altered pHi and pHo on cell survival in neutrophils exposed to increasing bacterial loads. We focused on pHi changes over the first 90 minutes to probe early metabolic factors implicated in triggering these processes. Further, because soluble inflammatory mediators affect granulocyte apoptosis, we also evaluated the effect of bronchoalveolar lavage (BAL) fluid from CF patients and healthy subjects on neutrophil survival.
We show that pHi following phagocytosis is not preserved by active pHi regulatory mechanisms when neutrophils are exposed to a high-density bacterial challenge, or when neutrophils are cultured at low pHo, although proton extrusion is elevated by mediators present in the inflammatory milieu. Further, we demonstrate that changes in pHi/pHo and the inflammatory microenvironment determine mechanisms of cell death in phagocytosing neutrophils. Our results illustrate that a precarious balance exists in vivo between cytosolic proton loading and extrusion, and that the resultant change in pHi is a significant determinant of cell survival and mode of death.
Materials and methods
Isolation of neutrophils and measurement of pHi
The institution's ethical review board approved the studies. Subjects gave informed consent (14 healthy individuals). Neutrophils were isolated from heparinized peripheral venous blood by density gradient centrifugation, as previously described.34 The neutrophil population was at least 96% pure by morphologic assessment (Cytospin preparations and Giemsa staining). Viability was at least 99% by trypan blue dye exclusion (light microscopy) and propidium iodide (PI) exclusion (flow cytometry). Cells were cultured in RPMI-1640 containing 25 mM NaHCO3 (Sigma Cell Culture; Poole Dorset) and freshly supplemented withl-glutamine 0.3 g/L (pH 7.35; Sigma Cell Culture). In additional experiments, cells were cultured under HCO-free conditions in a HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered medium with pH adjusted to 5.8 with HCl (Sigma Cell Culture). pHi was measured by flow cytometry (FACScan; Becton Dickinson, Mountain View, CA) using the pH-sensitive fluorescent dye carboxy-SNARF, as previously described.34Briefly, carboxy-SNARF–loaded cells were stimulated with argon laser light (488 nm), and the ratio of emitted fluorescence at 580 nm and 630 nm was measured. pH was extrapolated following calibration based on the nigericin/high K+ method, which was carried out on cells from all subjects. Calibration was repeated in stimulated cells to ensure that activation did not affect accuracy of the pH measurements. During sample acquisition, gating according to characteristic side and forward scatter characteristics confined the analysis to neutrophils, excluding clusters and debris.
Cellular activation and determination of relative roles of proton extrusive mechanisms
During experimentation, cells were exposed to heat-killed, human serum–opsonized Escherichia coli (Orpegen Pharma, Heidelberg, Germany) across a range of pathogen-to-phagocyte ratios from 0:1 to 50:1. After addition of E coli, the cells were agitated at moderate speed to promote adherence, and pHiwas measured. Some pathogen-to-phagocyte ratios induced both alkalinization and acidification in the same cells over time, compared with pHi in “non–bacteria-exposed neutrophils.” To quantify these complex pH changes, we used commercial software (GraphPad) to calculate the additional area “above” (alkalinization) or “below” (acidification) the pH trace recorded in bacteria-free cells that was induced by exposure to varying densities of E coli, and expressed this area in arbitrary units. This is effectively a measure of the net change in pHi. Positive values indicate net alkalinization, whereas negative values indicate net acidification.
To assess the roles of individual mechanisms of proton extrusion, we exposed phagocytosing cells to specific inhibitors of pHiregulatory pumps and channels, including proton-translocating ATPases (bafilomycin A1, 100 nM), Na+/H+exchange (amiloride, 0.2 mM), and NADPH oxidase passive proton conductance–associated channels (ZnCl2, 50 μM). Amiloride, bafilomycin A1, and ZnCl2 were obtained from Sigma.
Determination of intracellular oxidant generation
Neutrophil suspensions were incubated with normal saline or human serum–opsonized E coli (pathogen-to-phagocyte ratio 0:1-50:1) at 37°C in the presence of the oxidant-sensitive probe 123-dihydrorhodamine (Orpegen Pharma),35 after which they were immediately placed on ice. Cells were partially fixed for 20 minutes with a solution containing formaldehyde and less than 50% diethylene glycol (FACS lysing solution; Becton Dickinson, Cowley, Oxford, United Kingdom) before washing in phosphate-buffered saline (PBS). Finally, the samples were exposed to a cytoplasmic membrane permeabilizing solution containing PI (Permeabilizing Solution; Becton Dickinson) to stain DNA. At least 10 000 cells in each sample were analyzed by flow cytometry (FACScan; Becton Dickinson). Neutrophils were identified by characteristic size and granularity, as indicated by low-angle forward-scattering and right-angle side-scattering properties of argon laser light (488 nm). Mean emitted fluorescence intensity (mean channel fluorescence; MCF) at 520 nm, expressed on a 4-decade logarithmic scale with constant photomultiplier gain values, was quantified as a measure of intracellular oxidant generation. Analysis of the emitted fluorescence of PI at 520 nm differentiated viable cells from bacterial clumps on the basis of their DNA content. Only cells with the DNA binding characteristics of mammalian cells were included in the analysis. In all cases, background emitted fluorescence at 520 nm in unstained cells was subtracted from fluorescence in stained cells.
Quantification of phagocytosis in isolated neutrophils
Neutrophils were gently stirred while being incubated with fluorescein isothiocyanate (FITC)–labeled human serum–opsonizedE coli (Orpegen Pharma; pathogen-to-phagocyte ratio 0:1-50:1) for 15 minutes at 37°C. The cells were then placed on ice to terminate phagocytosis, and a quenching solution containingN-ethyl maleimide (Becton Dickinson) was added. After sedimentation (500g 10 minutes at 4°C), cells were washed in PBS and sedimented again before fixation, as described above. Following fixation, cells were treated with a permeabilizing solution containing PI to stain DNA, and at least 10 000 neutrophils in each sample were identified as described above and analyzed by flow cytometry. Cells were stimulated with an argon laser at 488 nm. Mean emitted fluorescence intensity (MCF) at 520 nm was expressed on a 4-decade logarithmic scale with constant photomultiplier gain values and was quantified as a measure of bacterial ingestion. Analysis of the emitted fluorescence of PI at 580 nm differentiated cells from bacterial clumps on the basis of their DNA content. Only cells with the DNA binding characteristics of mammalian cells were included in the analysis. In all cases, background emitted fluorescence at 520 nm in unstained cells was subtracted from fluorescence in stained cells.
Measurement of viability and cell death in phagocytosing neutrophils
Assessment of cell membrane integrity.
Cell membrane integrity is preserved until the late phase of apoptosis. Thus, we exploited the intracellular staining with the vital dyes (trypan blue via light microscopy and PI via flow cytometry) to identify necrotic cells. Aliquots of cell suspension were centrifuged and resuspended in a solution containing either trypan blue or PI. In the former case, 200 cells were examined under light microscopy, and the percentage of dye-excluding cells was calculated. In the latter case, the PI-stained solution was assessed by flow cytometry. The cell suspension was excited with argon laser light, and emitted light at 520 nm was quantified in at least 10 000 cells. The percentage of cells with PI staining of DNA (necrotic cells) was quantified. Both techniques were in close agreement (r2 = 0.93). Data are thus reported interchangeably.
Assessment of cellular morphology.
Neutrophils were cytocentrifuged and stained with Diff-Quick. Slides were mounted and viewed under oil immersion microscopy. At least 500 cells per slide were inspected by an observer who was blinded to the experimental condition. Features that distinguished normal from apoptotic morphology (nuclear pyknosis, chromatin condensation, and cytoplasmic vacuolation) were sought.
Assessment of neutrophil apoptosis, annexin-V binding, and DNA fragmentation.
An alternative and independent method for quantification of neutrophil apoptosis was also employed: the detection of phosphatidylserine expression on the external surface of the cytoplasmic membrane. Briefly, following washing in PBS, 1 × 106 neutrophils were gently resuspended in a solution containing FITC-labeled recombinant human annexin V in a high-Ca++ binding buffer (Boerhinger-Mannheim, Europe). Following incubation in the dark at room temperature for 15 minutes, the binding buffer was diluted and the samples were immediately analyzed by flow cytometry. At least 10 000 cells were excited with argon laser light (488 nm), and emitted fluorescence at 520 nm minus background fluorescence was quantified. Because “annexin positivity” could be due to apoptosis-induced externalization of phosphatidylserine or annexin-V binding to intracellular phosphatidylserine in necrotic cells, additional experiments were always performed to quantify rates of necrosis, and the appropriate correction was made. Reproducibility of this method compared favorably with apoptosis detection by morphologic assessment at 16 hours. To further validate this approach for the detection of apoptosis, we (favorably) compared our results with those of a commercially available enzyme-linked immunosorbent assay of intracellular histone-associated DNA fragmentation. The percentage apoptosis reported is a mean of the results obtained by morphology and annexin-V binding.
Effect of CF ELF on pH regulation and cell death in phagocytosing neutrophils
Normal and CF epithelial lining fluid (ELF) was derived from bronchoalveolar lavage performed on healthy volunteers or CF patients (exacerbation free for 6 weeks). In brief, during fiberoptic bronchoscopy, 3 × 1 mL/kg body weight aliquots of sterile saline at 37°C were injected into a division of the right middle lobe of the lung and reaspirated immediately.36 BAL fluid was filtered through sterile gauze and centrifuged at 1100 rpm for 10 minutes, and the supernatant was stored at −80°C. The volume of ELF was calculated according to the urea dilution method,37and the lavage was concentrated using Centricon filter devices (Millipore, Bedford, MA). Similar volumes (40 μL/mL) of saline or concentrated normal ELF or CF ELF were added to suspensions of freshly isolated normal neutrophils. We assessed the immediate effects on pHi, as well as the effects on pHi in E coli–exposed neutrophils after preincubation (1 hour).
Data presentation and statistical analysis
All data are mean ± standard error (SE) of the mean. Statistical analysis was performed using GraphPad Prism software or Statistica software. Linear regression analysis was used to test linearity of the correlation between variables. Differences over time within individual groups were analyzed using repeated-measures analysis of variance (ANOVA) and Bonferroni post hoc correction. Differences over time between groups were compared using either multivariate analysis of variance (MANOVA) or 2-way ANOVA and Bonferroni post hoc correction as appropriate.
Results
pHi in neutrophils following phagocytosis as a function of bacterial load
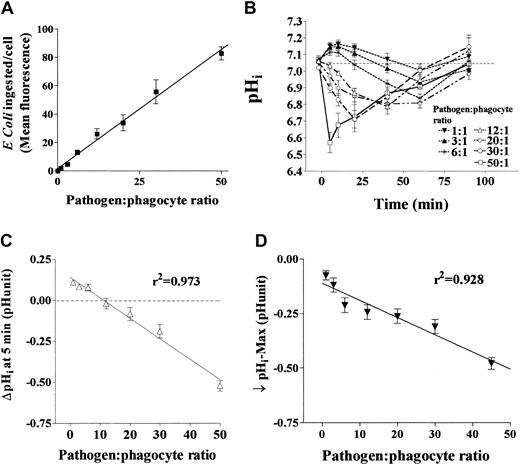
Using FITC-labeled E coli, we confirmed that increasing bacterial density led to a linear increase in the mean number of bacteria ingested over the range of pathogen-to-phagocyte ratios we studied (Figure 1A). We then investigated how increasing the number of bacteria ingested affected pHi. Following exposure to E coli, pHi exhibited striking dependence on the bacterial load (Figure 1B). The lowest pathogen-to-phagocyte ratios predominantly increased pHi over 90 minutes. Somewhat higher ratios induced transient alkalinization and later acidification of pHi. In contrast, marked and rapid declines of pHi were evident at the highest pathogen-to-phagocyte ratios studied. At all concentrations of E coli, pHi returned toward baseline by 90 minutes. Pathogen-to-phagocyte ratios correlated strongly with pHiat 5 minutes and the lowest pHi value recorded over the first 90 minutes (r2 = 0.97 and 0.928, respectively, by linear regression analysis; Figure 1C,D). Viability at 90 minutes (by vital dye exclusion) was preserved at all densities ofE coli exposure at physiologic pHo (data not shown).
pHi in phagocytosing neutrophils is determined by bacterial load.
Isolated human peripheral blood neutrophils were exposed to increasing densities of heat-killed opsonized E coli while intracellular pH (pHi) was monitored by flow cytometry using cytosolic pH-sensitive dyes and, in separate experiments, phagocytosis of fluorescently labeled bacteria was measured by flow cytometry. (A) The correlation between increasing pathogen-to-phagocyte ratio and mean number of bacteria ingested (mean channel fluorescence) by neutrophils (4 replicates each from 5 healthy donors, coefficient of determination r2 = 0.96). (B) The effect of increasing pathogen-to-phagocyte ratios and thus mean number of bacteria ingested (0:1-50:1) on pHi over time in normal neutrophils (4 replicates each from 10 healthy donors). pHiresponses at each pathogen-to-phagocyte ratio differed significantly from the others (by MANOVA, P < .01). (C,D) The correlation between bacterial load and the change in pHi after 5 minutes and the lowest pH recorded. All data mean ± SEM.
pHi in phagocytosing neutrophils is determined by bacterial load.
Isolated human peripheral blood neutrophils were exposed to increasing densities of heat-killed opsonized E coli while intracellular pH (pHi) was monitored by flow cytometry using cytosolic pH-sensitive dyes and, in separate experiments, phagocytosis of fluorescently labeled bacteria was measured by flow cytometry. (A) The correlation between increasing pathogen-to-phagocyte ratio and mean number of bacteria ingested (mean channel fluorescence) by neutrophils (4 replicates each from 5 healthy donors, coefficient of determination r2 = 0.96). (B) The effect of increasing pathogen-to-phagocyte ratios and thus mean number of bacteria ingested (0:1-50:1) on pHi over time in normal neutrophils (4 replicates each from 10 healthy donors). pHiresponses at each pathogen-to-phagocyte ratio differed significantly from the others (by MANOVA, P < .01). (C,D) The correlation between bacterial load and the change in pHi after 5 minutes and the lowest pH recorded. All data mean ± SEM.
Contribution of NHE, V-ATPases, and ZnCl2-sensitive proton channels to pHiregulation in phagocytosing neutrophils
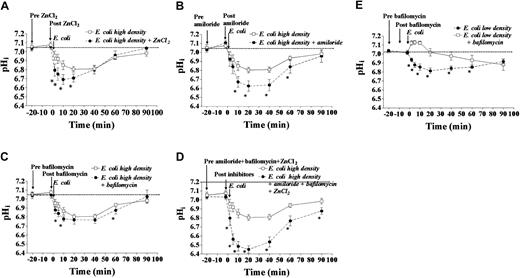
We examined the effect of specific inhibitors of proton extrusive mechanisms on pHi following phagocytosis. As can be seen in Figure 2, the presence of each inhibitor singly and in combination for 20 minutes did not significantly affect pHi, which suggests that the proton extrusive mechanisms that they inhibit are inactive under basal conditions. We studied cells exposed to a pathogen-to-phagocyte ratio of 20:1, having established that this caused sustained cytoplasmic acidification and therefore was likely to have stimulated proton extrusion. Inhibition of NADPH-associated passive proton conductance channels (ZnCl2, 50 μM) enhanced acidification during the first 20 minutes following phagocytosis (Figure 2A). Amiloride (0.2 mM) strongly affected pHi after phagocytosis, between 10 and 60 minutes (Figure 2B). Amiloride (but not ZnCl2) treatment reduced pHi at lower pathogen-to-phagocyte ratios (data not shown). At a pathogen-to-phagocyte ratio of 20:1, inhibition of V-ATPases with bafilomycin had a small (but significant) effect on pHi(Figure 2C). A combination of ZnCl2, amiloride, and bafilomycin led to more profound acidification, suggesting that the effects of these 3 proton extrusive mechanisms are additive (Figure2D). We were surprised that bafilomycin did not have a more pronounced effect on pHi regulation. Because V-ATPases are trafficked to the cytoplasmic membrane on cellular activation,11 we speculated that they may also be rapidly reinternalized at high pathogen-to-phagocyte ratios (due to infolding of the plasma membrane during continuing phagocytosis). Therefore, we investigated the effect of bafilomycin on pHi regulation at a lower pathogen-to-phagocyte ratio of 3:1. Bafilomycin (100 nM) did induce significant acidification of pHi under these conditions (Figure 2E). As can be discerned in Figure 2, amiloride, bafilomycin, or ZnCl2, either alone or in combination, did not affect pHi before E coli exposure. Further experiments demonstrated that pHi was not altered in the presence of these inhibitors in the absence of bacteria over 120 minutes (data not shown).
Mechanisms of pHiregulation in phagocytosing neutrophils.
The individual and collective contributions of several putative mechanisms of proton extrusion were investigated in isolated human peripheral blood neutrophils. Neutrophils (4 replicates each from 10 healthy donors) were exposed to opsonized heat-killed E coli at a pathogen-to-phagocyte ratio of 20:1 in the presence or absence of inhibitors. (A) The significant effect on pHifollowing phagocytosis of inhibition of passive proton conductance channels (ZnCl2, 50 μM, P < .01 versusE coli alone by 2-way ANOVA). (B) The significant effect on pHi following phagocytosis of inhibition of Na+/H+ exchange (amiloride, 0.2 mM,P < .001 by 2-way ANOVA). (C) The small but significant effect on pHi following phagocytosis of inhibition of V-ATPases (bafilomycin, 100 nM, P < .05 by 2-way ANOVA). (D) The significant effect on pHi following phagocytosis of inhibition of passive proton conductance channels, Na+/H+ exchange, and V-ATPases (P < .0001 by 2-way ANOVA). (E) At a lower pathogen-to-phagocyte ratio (3:1), inhibition of V-ATPases with bafilomycin (100 nM) has a more significant effect on pHi following phagocytosis (*P < .001 by 2-way ANOVA). All data mean ± SEM.
Mechanisms of pHiregulation in phagocytosing neutrophils.
The individual and collective contributions of several putative mechanisms of proton extrusion were investigated in isolated human peripheral blood neutrophils. Neutrophils (4 replicates each from 10 healthy donors) were exposed to opsonized heat-killed E coli at a pathogen-to-phagocyte ratio of 20:1 in the presence or absence of inhibitors. (A) The significant effect on pHifollowing phagocytosis of inhibition of passive proton conductance channels (ZnCl2, 50 μM, P < .01 versusE coli alone by 2-way ANOVA). (B) The significant effect on pHi following phagocytosis of inhibition of Na+/H+ exchange (amiloride, 0.2 mM,P < .001 by 2-way ANOVA). (C) The small but significant effect on pHi following phagocytosis of inhibition of V-ATPases (bafilomycin, 100 nM, P < .05 by 2-way ANOVA). (D) The significant effect on pHi following phagocytosis of inhibition of passive proton conductance channels, Na+/H+ exchange, and V-ATPases (P < .0001 by 2-way ANOVA). (E) At a lower pathogen-to-phagocyte ratio (3:1), inhibition of V-ATPases with bafilomycin (100 nM) has a more significant effect on pHi following phagocytosis (*P < .001 by 2-way ANOVA). All data mean ± SEM.
Effect of low extracellular pH on pHi following ingestion of bacteria
We investigated pHi regulation in phagocytosing neutrophils at reduced pHo (5.8). Compared with physiologic pHo, bacterial ingestion at acidic pHo led to significantly more acidic pHi (Figure3), even though lower numbers of bacteria were ingested at acidic pHo (bacterial ingestion, MCF: pH 7.4, 33 ± 4.3 versus pH 5.8, 22 ± 6.1;P < .05). pHi failed to return to basal levels over 90 minutes. Under these conditions, a subpopulation of neutrophils with pH similar to that of the extracellular milieu was noted after 30 minutes, potentially representing necrosing cells with impaired membrane integrity (data not shown).
The effect of an acidic extracellular milieu on pHi in phagocytosing neutrophils.
Isolated human peripheral blood neutrophils (4 replicates from 8 healthy donors) were exposed to opsonized heat-killed E coli(pathogen-to-phagocyte ratio 20:1) in standard HCO-buffered RPMI medium (pH 7.45) and a bicarbonate-free HEPES-buffered medium (pH 5.8). pHi was monitored during bacterial ingestion. Cytosolic acidification was significantly greater following phagocytosis in the acidic extracellular medium (P < .001 by 2-way ANOVA). All data mean ± SEM.
The effect of an acidic extracellular milieu on pHi in phagocytosing neutrophils.
Isolated human peripheral blood neutrophils (4 replicates from 8 healthy donors) were exposed to opsonized heat-killed E coli(pathogen-to-phagocyte ratio 20:1) in standard HCO-buffered RPMI medium (pH 7.45) and a bicarbonate-free HEPES-buffered medium (pH 5.8). pHi was monitored during bacterial ingestion. Cytosolic acidification was significantly greater following phagocytosis in the acidic extracellular medium (P < .001 by 2-way ANOVA). All data mean ± SEM.
Effect of ELF from CF patients and healthy controls on pHi in phagocytosing neutrophils
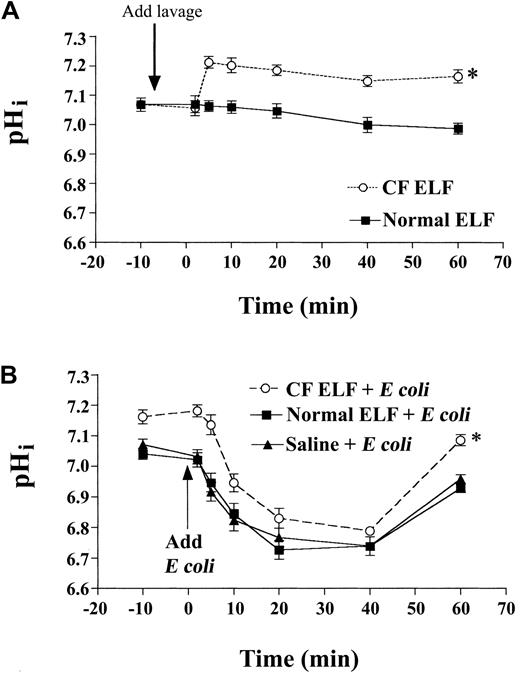
Neutrophils were incubated with saline, normal ELF, or CF ELF. Normal ELF did not affect resting pHi compared with saline vehicle, but CF ELF induced a sustained alkalinization (Figure4A). CF ELF, but not normal ELF, also increased intracellular oxidant generation in response to bacterial exposure (intracellular oxidant generation, MCF: normal lavage, 400 ± 24.5 versus CF lavage, 550 ± 36.7; P < .05) and efficiency of phagocytosis in neutrophils (bacterial ingestion, MCF: normal lavage, 30 ± 3.6 versus CF lavage, 44 ± 4.2;P < .05). To test the effects of ELF on pHi following E coli challenge, we incubated neutrophils for 1 hour with equivalent volumes of saline, CF ELF, or normal ELF, and then exposed them to bacteria. Upon E colichallenge, pHi in normal ELF–treated cells was similar to that in cells treated with vehicle, whereas pHi remained significantly higher in cells pre-exposed to CF but not normal ELF (Figure 4B), suggesting that inflammatory mediators limit acidification in phagocytosing cells.
The effect of the inflammatory milieu from the lungs of CF patients on baseline pHi and pHi in phagocytosing neutrophils.
Isolated human peripheral blood neutrophils were exposed to CF ELF (concentrated from BAL fluid from stable CF patients or healthy controls), and pHi was measured. (A) The acute alkalinizing effect on pHi of the addition of CF ELF (40 μL/mL) to neutrophils in culture. (B) The effect of preincubation with CF ELF (30 minutes, 30 μL/mL) on pHi after exposure to opsonized heat-killed E coli (EC; pathogen-to-phagocyte ratio 20:1) (*P < .02, CF versus normal ELF by 2-way ANOVA). All data mean ± SEM.
The effect of the inflammatory milieu from the lungs of CF patients on baseline pHi and pHi in phagocytosing neutrophils.
Isolated human peripheral blood neutrophils were exposed to CF ELF (concentrated from BAL fluid from stable CF patients or healthy controls), and pHi was measured. (A) The acute alkalinizing effect on pHi of the addition of CF ELF (40 μL/mL) to neutrophils in culture. (B) The effect of preincubation with CF ELF (30 minutes, 30 μL/mL) on pHi after exposure to opsonized heat-killed E coli (EC; pathogen-to-phagocyte ratio 20:1) (*P < .02, CF versus normal ELF by 2-way ANOVA). All data mean ± SEM.
Effect of low- and high-level bacterial exposure on neutrophil apoptosis at physiologic pHo, and its correlation with pHi and intracellular oxidant production
At low pathogen-to-phagocyte ratios, E coli ingestion significantly delayed apoptosis, whereas higher ratios accelerated it (Figure 5A). It is puzzling that a quantitatively dissimilar but qualitatively similar signal produced opposite effects on apoptosis. We speculated that this might be the result of differential effects on pHi, and thus correlated apoptosis with pHi changes and also with intracellular oxidant generation following phagocytosis, because the latter has previously been implicated in neutrophil apoptosis. Apoptosis correlated significantly with the lowest recorded pHifollowing E coli exposure (r2 = 0.872) and with pHi 5 minutes after exposure to E coli(r2 = 0.902). Net change in pHi(calculated by measuring the curve area above and below pHiin non–bacteria-exposed neutrophils; see “Materials and methods”) over 90 minutes correlated even more closely with rates of apoptosis (r2 = 0.95; Figure 5B). Low pathogen-to-phagocyte ratios alkalinized pHi and inhibited apoptosis, whereas higher ratios acidified pHi and activated apoptosis. Intracellular oxidant generation correlated less significantly with rates of apoptosis (Figure 5C). Of note, at low pathogen-to-phagocyte ratios, intracellular oxidant generation was modestly increased, whereas apoptosis was suppressed rather than stimulated.
Correlation of phagocytosis-induced neutrophil apoptosis with changes in pHi and intracellular oxidant generation.
Isolated peripheral blood neutrophils were exposed to varying pathogen-to-phagocyte ratios, and early changes in pHi, intracellular oxidant production, and apoptosis were measured after 16 hours. (A) Neutrophil apoptosis is inhibited at lower pathogen-to-phagocyte ratios (*P < .01 by 2-way ANOVA), but is activated at higher ratios (**P < .005 by 2-way ANOVA). (B) Linear correlation between ΔpHi over 90 minutes and Δ% apoptosis at varying pathogen-to-phagocyte ratios. Net ΔpHi is based on a calculation of the phagocytosis-induced change in net pHi curve area above (net alkalinization) and below (net acidification) baseline pHi (baseline pHi curve is that of non–bacteria-exposed neutrophils) over time. (C) Linear correlation between intracellular oxidant generation over 90 minutes and Δ% apoptosis at varying pathogen-to-phagocyte ratios. All data mean ± SEM; r2 indicates coefficient of determination.
Correlation of phagocytosis-induced neutrophil apoptosis with changes in pHi and intracellular oxidant generation.
Isolated peripheral blood neutrophils were exposed to varying pathogen-to-phagocyte ratios, and early changes in pHi, intracellular oxidant production, and apoptosis were measured after 16 hours. (A) Neutrophil apoptosis is inhibited at lower pathogen-to-phagocyte ratios (*P < .01 by 2-way ANOVA), but is activated at higher ratios (**P < .005 by 2-way ANOVA). (B) Linear correlation between ΔpHi over 90 minutes and Δ% apoptosis at varying pathogen-to-phagocyte ratios. Net ΔpHi is based on a calculation of the phagocytosis-induced change in net pHi curve area above (net alkalinization) and below (net acidification) baseline pHi (baseline pHi curve is that of non–bacteria-exposed neutrophils) over time. (C) Linear correlation between intracellular oxidant generation over 90 minutes and Δ% apoptosis at varying pathogen-to-phagocyte ratios. All data mean ± SEM; r2 indicates coefficient of determination.
Effect of manipulation of intracellular pH and CF ELF on apoptosis and necrosis in phagocytosing neutrophils
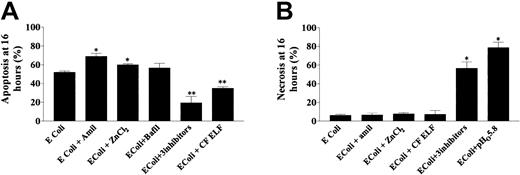
The correlation between pHi and apoptosis may be an epiphenomenon. To probe a direct link between pHi and apoptosis, we manipulated pHi in phagocytosing neutrophils by inhibition of one or more proton extrusive mechanisms and assessed the effect on the mechanism of cell death. Either amiloride or ZnCl2 accelerated apoptosis in neutrophils exposed toE coli at a pathogen-to-phagocyte ratio of 20:1 (Figure6A). Bafilomycin did not alter rates of apoptosis at a pathogen-to-phagocyte ratio of 20:1, but at lower pathogen-to-phagocyte ratios (3:1), at which we had established that bafilomycin more clearly affected pHi, apoptosis was promoted (apoptosis at 16 hours, mean ± SE: E coli, 18.9% ± 4.2% versus E coli + bafilomycin, 31% ± 4.8%; P < .05). At lower pathogen-to-phagocyte ratios (3:1), amiloride and ZnCl2 acidified pHiand accelerated apoptosis to a modest degree (apoptosis at 16 hours, mean ± SE: E coli (3:1), 18.9% ± 4.2% versusE coli (3:1) + amiloride, 25.7% ± 3.9%;P < .05). The presence of ZnCl2 at this lower pathogen-to-phagocyte ratio, which had only a minor effect on pHi, did not affect apoptotic rates significantly (apoptosis at 16 hours, mean ± SE: E coli (3:1), 18.9% ± 4.2% versus E coli (3:1) + ZnCl2, 21.7% ± 4.8%; P = .17). Exposure of neutrophils to these inhibitors in the absence of E colidid not affect rates of apoptosis, suggesting that they do not exert significant pHi-independent effects on the cell's apoptotic machinery (apoptosis, mean ± SE: control, 38.5% ± 4.3%, amiloride, 37.7% ± 6.3%, bafilomycin, 40.5% ± 4.0%, ZnCl2, 43.5% ± 6.1%;P = .3 by ANOVA).
Perturbations of pHi and the inflammatory milieu alter mechanisms of cell death in phagocytosing neutrophils.
(A) The effects of amiloride (0.2 mM), ZnCl2 (50 μM), bafilomycin (100 nM), and CF ELF (40 μL/mL) on rates of apoptosis at 16 hours in phagocytosing neutrophils (4 replicates from 5 healthy donors) exposed to E coli at a pathogen-to-phagocyte ratio of 20:1. Inhibitors or ELF was added 10 minutes after bacteria. *P < .05; % apoptosis greater than E colialone; **P < .05; % apoptosis less than E coli. Note that bafilomycin did affect apoptosis in neutrophils exposed to lower pathogen-to-phagocyte ratios (see text). (B) The effects of amiloride, ZnCl2, CF ELF, a combination of amiloride plus ZnCl2 plus bafilomycin, and lowering of the extracellular pH on rates of necrosis in the same cells (*P < .005 versus E coli alone).
Perturbations of pHi and the inflammatory milieu alter mechanisms of cell death in phagocytosing neutrophils.
(A) The effects of amiloride (0.2 mM), ZnCl2 (50 μM), bafilomycin (100 nM), and CF ELF (40 μL/mL) on rates of apoptosis at 16 hours in phagocytosing neutrophils (4 replicates from 5 healthy donors) exposed to E coli at a pathogen-to-phagocyte ratio of 20:1. Inhibitors or ELF was added 10 minutes after bacteria. *P < .05; % apoptosis greater than E colialone; **P < .05; % apoptosis less than E coli. Note that bafilomycin did affect apoptosis in neutrophils exposed to lower pathogen-to-phagocyte ratios (see text). (B) The effects of amiloride, ZnCl2, CF ELF, a combination of amiloride plus ZnCl2 plus bafilomycin, and lowering of the extracellular pH on rates of necrosis in the same cells (*P < .005 versus E coli alone).
Of note, all 3 inhibitors together at high bacterial density did not increase apoptosis, but instead promoted necrosis (Figure 6B). In the absence of inhibitors and at physiologic pHo, little necrosis was induced following high-density bacterial exposure (Figure6B), suggesting efficient function of apoptotic machinery even when bacteria are numerous. However, high-density bacterial exposure when pHo was low (5.8) induced significant necrosis (Figure 6B), suggesting that excessive declines in pHi interfere with the inception or perpetuation of apoptosis.
Because the inflammatory milieu may contain many proapoptotic and antiapoptotic substances (eg, proapoptotic inflammatory mediators soluble FAS ligand (CD95) and tumor necrosis factor–α; antiapoptotic inflammatory mediators LPS, N-formyl-methionyl-leucyl-phenylalanine [FMLP], and interleukin-8 [IL-8]), we monitored apoptosis following preincubation with CF ELF and normal ELF. Normal ELF did not affect apoptosis (data not shown). However, CF ELF delayed apoptosis in phagocytosing neutrophils, without inducing necrosis (Figure 6A,B). CF ELF also delayed constitutive apoptosis (apoptosis: control, 39.4% ± 4.2% versus CF ELF treated, 31.0% ± 4.2%; P < .05). However, when neutrophils were exposed to high bacterial densities at low extracellular pH (5.8), pretreatment with CF ELF did not prevent necrosis (necrosis: control, 76.4% ± 9.2% versus CF ELF, 67.8% ± 10.2%;P = .56).
Discussion
These studies were designed first to elucidate the capacity and mechanisms of pHi regulation in phagocytosing neutrophils under conditions simulating the inflammatory environment in vivo, and second to establish whether pHi has a role in regulating neutrophil death. Our results define the relative contributions of NHEs, proton conductance channels, and V-ATPases in neutrophil pHi regulation following bacterial ingestion and show that cytosolic pH is not preserved when many bacteria are ingested or when extracellular pH is reduced, although proton extrusive capacity may be augmented by components of the inflammatory milieu. Furthermore, they illustrate that early changes in pHi dictate the fate of the phagocytosing cell. Acidification of pHi following phagocytosis accelerates apoptosis, whereas more marked decrements in pHi induce necrosis.
Our results confirm previous observations that NHEs are implicated in pHi regulation following phagocytosis12 and extend those observations by demonstrating that Zn++-sensitive passive proton conductance channels also participate, being relatively more important in combating the early phase of acidification after phagocytosis. V-ATPases are implicated predominantly upon low-level bacterial exposure. These latter pumps are stored internally and are trafficked to the plasma membrane upon cellular activation.11 They have been shown to be reinternalized and incorporated into the phagocytic vacuoles as bacteria are ingested, where they reduce phagosomal pH. Their proportionately lesser role in pHi regulation when bacterial density is high (and the cell ingests more bacteria) may reflect their rapid reinternalization under these circumstances. It is important to note that all 3 proton extrusive mechanisms acting in combination did not maintain pHi near resting levels at high-density bacterial exposures. The simplest explanation for this is that increasing bacterial loads trigger metabolic acid production that simply exceeds the cell's maximal capacity to extrude protons. Alkalinizing and acidifying effects of low and high bacterial density thus reflect a scenario where phagocytosis triggers signal transduction events that maximally activate proton extrusive mechanisms. It is, however, also conceivable that early acidification of pHi, induced by high bacterial density, reflects active inhibition of proton extrusion as a prerequisite for neutrophil apoptosis. Consistent with this postulate, H2O2 and caspase-8 (which is activated upstream of mitochondrial changes during apoptosis) induce cytosolic acidification by inhibiting proton extrusion in the course of apoptosis in HL60 cells and MCF-7 cells, respectively.25,27 This is also consistent with our unpublished observations that phorbol-12-myristate-13-acetate (PMA) is more potent than bacterial ingestion as an activator of NADPH oxidase (the major source of metabolically generated protons in activated neutrophils38), but yet does not induce the same rapid acidification of pHi (May 1998). This lack of a rapid fall in pHi is also consistent with the observation that, although PMA induces some features of apoptosis in neutrophils, it does not appear to stimulate caspase-mediated DNA fragmentation,39 which may require a decline in pHi for its promotion.
These studies implicate pHi in the regulation of neutrophil apoptosis following phagocytosis. There are few previous studies in this regard relating to neutrophils and related cells, and their results are conflicting.6,13,32,33 The role of cytosolic acidification in promoting the apoptotic cascade appears to be more established in other cell types. Critical for the inception of mitochondria-dependent apoptosis, which has been described in neutrophils,40 is activation of the proapoptotic caspase-3 by mitochondrial cytochrome C (cytC). This is maximal at pH values less than 6.8 but minimal at physiologic pH.22 This finding is consistent with the pHi values that we detected in neutrophils under conditions in which apoptosis was either activated or suppressed. In our study, significant acceleration of apoptosis occurred only when pHi during the first hour fell below 6.8, and even higher rates of apoptosis accompanied more profound and sustained falls in pHi (high bacterial loading), whereas increases in pHi (low bacterial loading) were paralleled by inhibition of apoptosis. Our studies suggest that pHiactively modulates apoptosis, rather than simply paralleling it, because manipulation of pHi (by targeted inhibition of NHEs, V-ATPases, or proton conductance channels) after phagocytosis acidified pHi and accelerated apoptosis. The relationship between pHi and apoptosis is likely to be complex. Reports that extracellular acidification inhibits constitutive neutrophil apoptosis may reflect differential pH sensitivity of constitutive versus phagocytosis-associated apoptotic pathways. For example, the insertion of the proapoptotic Bax protein into the mitochondrial cell membrane of neutrophils (an event occurring upstream of cytC release) is favored under alkaline conditions and appears to be inhibited at acidic pHi.41 Phagocytosis may induce signals that overcome the pH dependence of Bax insertion, leading to a cascade of events downstream with an alternative pH sensitivity. This scenario would be teleologically advantageous because acidification of pHi in an acidic milieu would protect neutrophils from constitutive apoptosis (before they have usefully ingested bacteria), but accelerate apoptosis after successful bacterial ingestion (in cells that have exhausted their useful capacity, but pose a threat to host tissues if they undergo necrosis). Alternatively, it has been suggested that constitutive neutrophil apoptosis is independent of mitochondria, in which case lack of a proapoptotic effect of cytosolic and extracellular acidification would be an anticipated finding.40 Our use of pharmacologic inhibition of individual mechanisms of proton extrusion might be problematic because these inhibitors may have pH-independent effects on apoptotic pathways. However, the weight of evidence from our experiments, particularly the close correlation between pHi and apoptosis in the absence of these pharmacologic inhibitors, favors a role for pHi in regulating apoptosis in neutrophils when considered in toto.
pHi is likely to be an important modulator, rather than an initiator, of agonist-mediated neutrophil apoptosis. Reactive oxygen intermediates have been implicated in the instigation of neutrophil apoptosis.42,43 However, this relationship is apparently not a simple one because oxidants do not appear to be involved in neutrophil apoptosis in some situations,44 and proinflammatory signals, such as FMLP and IL-8, activate the generation of reactive oxygen intermediates but also inhibit apoptosis. Oxidants may be an initial signal for cytC release, with downstream events modulated significantly by pHi. This is consistent with our observation that low-density bacterial exposure increased the generation of reactive oxygen intermediates but alkalinized pHi and suppressed apoptosis.
How might our findings be integrated into a model of neutrophil behavior in inflammatory microenvironments? Optimally, neutrophil survival at inflammatory sites should be lengthy enough to facilitate ingestion of the maximum number of bacteria before an orderly process of cell death is instigated, thus restricting harm to host tissues. Although neutrophils were reported to undergo apoptosis after E coli ingestion,18 other studies suggested inhibition of apoptosis at low pathogen-to-phagocyte ratios, but induction of necrosis at higher ratios.19,20 This report refines previous observations on the effects of bacterial load on neutrophil apoptosis, suggesting that neutrophil survival is promoted by ingestion of relatively few bacteria. Where bacterial density in vivo is low (in mild or early infection, for instance), neutrophil lifespan would be prolonged, increasing the probability of useful subsequent phagocytosis if bacterial density subsequently increases. Because the capacity of neutrophils to kill bacteria is limited by their inability to regenerate intracellular microbicidal substances (such as lysosomal granule contents), it is desirable that apoptosis is triggered when the ingestion of a larger number of bacteria exhausts these stores. Because many diseases involve apparently dysregulated neutrophil inflammation, a limited ability of homeostatic mechanisms to support a favorable mode of cell death under all pathologic conditions is hardly surprising. In this respect, although we found that these processes were efficient at physiologic extracellular pH, they were compromised in acidic environments (pH 5.8), where significant necrosis (instead of apoptosis) occurred on exposure to bacteria at high density. Although the exceptional buffering capacity of the plasma compartment means that the tissue interstitium will generally not acidify to this degree (even allowing for anticipated local proton production due to immune-cellular and bacterial respiration), situations may arise in which microenvironmental pH does fall significantly. It has been established that serious and poorly resolving bacterial infections (eg, lung abscesses and pulmonary empyemas) are associated with very low pH, and that low pH under these circumstances is a marker of tissue destruction.1 The inability of neutrophils to regulate pHi while ingesting bacteria in such a milieu may contribute to the poorly resolving and necrotizing nature of these infections by promoting neutrophil necrosis. How the host manipulates micro-milieu pH in response to infection and inflammation is largely unknown and merits scrutiny, particularly in relation to sites that are not in continuity with the well-buffered plasma and interstitial compartments, such as the luminal epithelial surfaces of the pulmonary, gastrointestinal, and urogenital tracts. Regulation of the surface liquid pH on these epithelia may have significant effects on neutrophil function and survival. This may be particularly pertinent in the case of CF. Lung disease in this condition is characterized by severe and unremitting bacterial infection and parenchymal lung destruction, which are mediated in large part by neutrophil-derived proteolytic and oxidant species. Lack of a CFTR (cystic fibrosis transmembrane conductance regulator)–dependent apical epithelial bicarbonate conductance has been hypothesized to cause hyperacidified airway surface liquid.45 Our results predict that in this abnormally acidic milieu, bacterial ingestion may induce neutrophil necrosis, rather than apoptosis, and thus promote lung parenchymal degradation. Although we demonstrated that the net effect of CF ELF was to increase proton extrusion and inhibit apoptosis in phagocytosing neutrophils, this effect was insufficient to prevent significant necrosis of neutrophils when pHo was low and bacterial density was high, a circumstance that likely replicates the situation in vivo. This would contrast with the situation in the non-CF lung, where the antiapoptotic effect of the inflammatory microenvironment coupled with the ability of normal epithelia to alkalinize surface liquid may protect neutrophils therein from the deleterious effects of low pH. In keeping with this notion, apoptosis is suppressed in lung neutrophils from patients with pneumonia.21 Manipulation of the inflammatory microenvironmental pH may therefore provide a means of attenuating tissue destruction in necrotizing infections.
In conclusion, we have demonstrated that following phagocytosis in vivo, a precarious balance between cytosolic proton loading and extrusion likely exists. The resultant changes in pHimodulate cell death in neutrophils in a manner likely to be relevant in disease pathology and potentially amenable to therapeutic manipulation.
We thank Dr Barbara Grubb for her useful suggestions with regard to this manuscript.
Supported by the Higher Education Authority of Ireland, the Royal College of Surgeons in Ireland, the Health Research Board of Ireland, and the Irish Lung Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Raymond J. Coakley, University of North Carolina at Chapel Hill, The CF/Pulmonary Research and Treatment Center, Room 7013 Thurston-Bowles Building, CB #7248, Chapel Hill, NC 27516-7248; e-mail: ray_coakley@med.unc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal