Abstract
Because tumor progression is angiogenesis-dependent, angiogenesis density was investigated by immunohistochemistry and computed image analysis in bone marrow (BM) biopsies of 45 newly diagnosed patients with Binet stage A B-cell chronic lymphocytic leukemia (BCLL) and correlated to upstaging and progression-free survival during a 40-month follow-up period. Their microvessel areas and counts were significantly higher than those of patients with anemia due to iron or vitamin B12deficiencies. A cutoff value of 0.90 mm2 × 10−2 or greater of the microvessel area identified patients with earlier upstaging and shorter progression-free survival. When the cutoff was applied to the Rai subclassification, both Rai 0 and Rai I-II patients who upstaged and shortened the progression-free survival were classified correctly. Information of this type was not given by the microvessel counts. The cutoff did not correlate with other predictors representative of tumor mass or disease progression. The microvessel area correlated with the expression of angiogenic vascular endothelial growth factor (VEGF) by tumor tissue, and serum levels of VEGF were found to be of prognostic value. A causal relationship between risk of progression and BM angiogenesis in BCLL is suggested. A risk stratification inside Rai is proposed. The prognostic usefulness of BM angiogenesis in patients with BCLL is envisaged.
Introduction
Tumor progression in the form of growth, invasion, and metastasis depends on angiogenesis,1 whose increase is thus indicative of poor prognosis in solid tumors.2 The expression of several angiogenic growth factors, including vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2), is of comparable prognostic value.3 Knowledge of these relations in hematologic tumors, however, is circumstantial. A tumor's microvessel density predicts a risk of progression in multiple myeloma.4 It is correlated with progression stages in both B-cell non-Hodgkin lymphomas5 (NHL) and mycosis fungoides,6 and agrees with the growth of acute leukemias7 and myelodysplastic syndromes.8 9
Angiogenesis is involved in the pathogenesis of B-cell chronic lymphocytic leukemia (BCLL).10,11 VEGF, present in the patient's serum and leukemic cells,12-14 is related to poor prognosis. FGF-2 expression by leukemic cells is associated with advanced disease and resistance to fludarabine.15 Studies on 2 series of BCLL bone marrow (BM) angiogenesis carried out in limited and heterogeneous groups of patients provided conflicting results on its density and no prognostic information.11 16
This paper presents results of an investigation of microvessel density and VEGF expression in the BM of a homogeneous series of 45 patients with BCLL with early disease. Relationships between angiogenesis, VEGF, and FGF-2 serum levels and other markers of disease activity were assessed and clinical and prognostic implications were sought.
Patients, materials, and methods
Patients
Forty-five patients (25 men, 20 women) with Binet stage A17 BCLL, aged 42 to 81 years (median, 64 years) were studied. They were newly diagnosed and had no prior history of BCLL. Routine laboratory data, β2-microglobulin (β2-m), and lactic dehydrogenase (LDH) were assessed at diagnosis by standard procedures. Peripheral blood (PB) mononuclear cells were analyzed by immunophenotyping18 to establish the diagnosis of typical BCLL. Physical examination, chest x-ray, and abdominal ultrasound were always performed.
Simultaneously with Binet stage, patients were substaged according to Rai et al19: stage 0, 27 patients (60%); stage I, 5 patients (11%); stage II, 13 patients (29%). BM biopsies were performed at diagnosis, and the histology pattern (nondiffuse or diffuse) was evaluated according to Rozman et al.20Lymphocyte doubling time was assessed in 38 patients (84%) according to Montserrat et al.21 Control subjects were 7 men and 5 women, aged 48 to 82 years (median, 67 years), with anemia due to iron or vitamin B12 deficiencies.
The study was approved by the local ethics committee and all patients gave their informed consent.
Measurement of BM angiogenesis
Vessels were detected in 6-μm sections of 4% paraformaldehyde-fixed paraffin-embedded biopsies by red-staining endothelial cells with an anti–factor VIII-related antigen (FVIII-RA) rabbit antibody (Dako, Glostrup, Denmark) and a 3-layer biotin-streptavidin-peroxidase system described previously.4 Megakaryocytes also stained with FVIII-RA and were easily distinguishable by their morphology and size. Angiogenesis was measured as microvessel area and number without knowledge of the stage and substage. Microvessels (capillaries and small venules) were selected as endothelial cells, single or clustered in nests or tubes and clearly separated one from another, and either without or with a lumen not exceeding 10 μm, though larger neovessels were found in some patients. A double-headed photomicroscope (Axioplan 2, Zeiss, Oberkochen, Germany) was used in the simultaneous identification by 2 of us (A.V. and D.R.) of the microvessel, and each identification was agreed on in turn. The microvessel area was measured on 4 to 6 × 250 fields covering the whole of each of 2 sections per biopsy within a superimposed square reticle. This was drawn out by a KS-300 software (Zeiss) and formed of 22 lines per side giving 484 intersection points. At × 250 it defined an area of 12.5 × 10−2mm2 (reference area), whereas each point covered an area of 72.15 μm2.
The area occupied by microvessels was estimated by using the direct planimetric method of “point counting”22 with slight modifications for the computed image analysis (same software) as described,23 according to which the microvessel area equals the sum of point areas that hit microvessels. Because cellular areas are vascularized and noncellular areas (fat, dense connective tissue, necrotic and hemorrhagic foci, bone lamellae) are not, and because the latter hampered comparison between sections, they were always omitted from the reference area. Thus, the point areas that hit noncellular areas were subtracted from the reference area. Residual point areas defined the cellular area only and the microvessel area was measured inside it. Basically, the measurement of the microvessel and cellular areas fitted the following equations: microvessel area [y] = sum of points that hit microvessels [x] · 72.15 μm2, and cellular area = 12.5 × 10−2 mm2 − (sum of points that hit possible noncellular areas [P] · 72.15 μm2).
Values of the microvessel area were normalized to those of the cellular area by the equation: [x]/(484 −P) = y/100.
Microvessels were counted at × 250 within a computed square reticle of 12.5 × 10−2 mm2 and formed of 34 lines per side giving 1156 intersection points, which were distant 10.7 μm each other. Counts were done by the planimetric point-count method as the total number of the reticle intersection points occupied by transversely cut microvessels, whose small size (diameter ≤ 10 μm) and the sufficient distance between 2 adjacent points (10.7 μm) meant that a point could be occupied by only one microvessel.5Vessels transversely cut that touched 2 or more adjacent points (diameter > 10.7 μm) were counted as 1. Those placed on the inside or on the sides of a given small square of the reticle (ie, that did not cross the points) were not counted. Longitudinally or tangentially cut microvessels were not counted, regardless of their position. It was thus sufficiently certain that a given microvessel was counted only once, even if several of its section planes were present. The counting method makes allowances for the heterogeneous distribution of microvessels in tissue.5 Indeed, in line with others'7 and our own observations,4,5,23 BM involved by hematologic malignancies shows areas of more intense neovascularization, defined as “hot spots.” As the whole section was evaluated, microvessels not included in the hot spots were also counted. Because the whole of each of 2 sections per biopsy was evaluated, and the transversely cut microvessels hit the points randomly, the methods produced objective counts.5 23
Analysis of serial sections (n = 6-10) from 3 biopsy samples revealed an intrabiopsy variability of 10% or less (± 1.8%) in both the microvessel area and number. The variability between the investigators checking neovessels separately was 5.0% or less (± 3.2% ) for both the area and number. The area and number assessments were highly intercorrelated (Pearson r = 0.95;P < .0001). The microvessel area and number were expressed as mean ± 1 SD for each section and biopsy and groups of biopsies.
To screen for patients who likely could progress, cutoffs of microvessel area and number corresponding to the highest Youden index24 were chosen. The index combines information on sensitivity and specificity, giving equal weight to each, and measures the percentage gain in certainty of predicting the risk of progression. If microvessel area at a definite cutoff has an index of 0, it has no predictive power; if the cutoff has an index of 100%, progression is perfectly predicted. Microvessel area cutoffs set at the 25th, 50th, and 75th percentiles gave an index of 10%, 13%, and 45%, respectively. Thus, the highest cutoff was set. The microvessel number cutoff was set accordingly, because the 25th, 50th, and 75th percentiles paralleled an index of 0%, 0%, and 19%, respectively. The decision to peak 75th percentile was arbitrary. The Youden index has already been applied in patients with BCLL to compare CD38 expression with the risk of progression.25
Immunohistochemical staining of VEGF
The BM immunoreactivity to VEGF was investigated in 11 patients with a rabbit anti-VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and the immunoperoxidase staining just described. Positive controls were anti-κ plus anti-λ chains and anti-CD20 rabbit antisera (used for immunophenotyping), and the negative control was a rabbit preimmune serum (all from Santa Cruz Biotechnology). Two 6-μm sections per biopsy, adjacent to those examined for vascularity, were stained with each reagent by the same operator (L.T.).
The intensity of VEGF staining related to the degree of antigen expression. It was scored by the KS-300 software in 4 to 6 × 400 fields/section, judged to be representative of the BCLL tissue viewed from several × 160 fields. In all patients, inflammatory mononuclear cells (fibroblasts, macrophages, and polymorphs) also stained with VEGF. These cells were found as few, isolated, or clustered elements throughout the stroma and were clearly distinguishable from tumor cells both on morphologic basis and because they usually displayed a different staining intensity (in plus or minus) from tumor cells. Care was taken to recognize these inflammatory cells and omit them from the evaluation.
The red-staining intensity of BCLL tissue due to VEGF was fixed in each pixel, allocated on a gray intensity scale as density unit counts × 105, and expressed as the mean of the 4 to 6 fields/section, and finally as mean of the 2 sections. The background given by the negative control reagent in 4 to 6 × 400 fields/section was also expressed as mean of the 2 sections and subtracted. The resulting value represented the mean gray density unit counts of VEGF intensity per biopsy. The staining technique was performed 3 times, on separate days, for each biopsy, and revealed that the intrabiopsy variability in the staining intensity was 20% or less (± 3.6%) for all reagents. To overcome this variability for VEGF intensity and express it as a sole value, the final value per biopsy was the mean ± 1 SD of the 3 means per section, as described.26
Measurement of serum levels of VEGF (sVEGF) and FGF-2 (sFGF-2)
Sera from peripheral venous blood sampled at diagnosis were stored at −80°C. Their sVEGF and sFGF-2 levels were determined in duplicate by using the sandwich enzyme-linked immunosorbent assay (ELISA; Quantikine human VEGF and Quantikine human FGF-2; R & D Systems, Minneapolis, MN), according to the manufacturer's instructions. Its sensitivity was less than 5 pg/mL for VEGF and less than 3 pg/mL for FGF-2. The coefficients of variation reported by the manufacturer for interassay and intra-assay determinations vary from 6.2% to 8.8% and from 2% to 9%, respectively, for sVEGF, and from 7.4% to 9.1% and from 3% to 9.7%, respectively, for sFGF-2.
Patients were compared with 63 healthy blood donors (40 men and 23 women), aged 22 to 61 years (median, 51 years). Because measurement of sVEGF could be overestimated due to VEGF release by platelets during clotting,27 we correlated the sVEGF with the plasma VEGF in 30 randomly chosen patients and found a close interrelation (Pearsonr = 0.51, P < .0001) and correlation of both with the platelet counts (sVEGF r = 0.52; plasma VEGFr = 0.44; P < .0001).
FISH studies
Twenty-eight patients with available cytogenetic pellet at diagnosis were characterized by fluorescence in situ hybridization (FISH), using the 13q14.3 LSI D13S25 probe, the 17p13.1 LSI p53 probe, and the chromosome 12 centromeric probe CEP 12 (Vysis, distributed by Olympus, Milan, Italy), in dual-color experiments with appropriate control probes. The λ EMBL3 clones 19 and 65, spanning an area of approximately 40 kb within the middle portion of the ATMgene, were used to detect 11q22-23 deletions, as previously described.28 At least 200 interphase nuclei with well-delineated signals were counted in each slide. The FISH procedure was repeated in those slides with low hybridization efficiency (ie, < 80% cells with the expected 2 normal signals of the control probe).
Clinical studies and disease progression evaluation
The degree of BM angiogenesis was correlated with main clinical and hematologic variables, namely, Rai substages, BM histology, absolute PB lymphocytosis, lymphocyte doubling time, LDH, β2-m, cytogenetics, and with sVEGF and sFGF-2. The 75th percentile of the microvessel area and number were chosen as cutoffs, which could act as predictors of clinical outcome by using an end-point disease progression, defined as the appearance of Binet upstaging17 during the treatment-free period. The significant impact of this end-point on the overall survival of Binet A patients29 30 enables it to replace overall survival as a prognostic parameter and shortens their clinical studies. The cutoffs were chosen according to the Youden index just described.
Results
Prognostic value of enhanced BM angiogenesis
Table 1 shows the BM microvessel area and number and the cellular area in patients and control subjects. The patients' microvessel area was significantly higher. The counting gave similar results. Because of the large bias of microvessel area and number within the patient group, the 75th percentile of the area (0.90 mm2 × 10−2) and number (6/12.5 mm2 × 10−2 fields) were selected as cutoffs to discriminate between highly and poorly vascularized BM, and related to progression.
BM microvessel areas and counts
| Subjects (no.) . | Microvessel area as mm2 × 10−2 mean ± 1 SD (median, range) . | Microvessel number in 12.5 mm2 × 10−2 fields mean ± 1 SD (median, range) . | Cellular area as mm2 × 10−2 mean ± 1 SD (median, range) . |
|---|---|---|---|
| Patients (45) | 0.96 ± 0.83*,† (0.70, 0.45-4.12) | 7.39 ± 7.11* (5, 3-35) | 10.8 ± 1.7 (8.6, 8.7-11.5) |
| Controls (12) | 0.09 ± 0.01† (0.09, 0.06-0.12) | 0.66 ± 0.98 (0, 0-3) | 9.7 ± 0.9 (7.8, 6.8-10.7) |
| Subjects (no.) . | Microvessel area as mm2 × 10−2 mean ± 1 SD (median, range) . | Microvessel number in 12.5 mm2 × 10−2 fields mean ± 1 SD (median, range) . | Cellular area as mm2 × 10−2 mean ± 1 SD (median, range) . |
|---|---|---|---|
| Patients (45) | 0.96 ± 0.83*,† (0.70, 0.45-4.12) | 7.39 ± 7.11* (5, 3-35) | 10.8 ± 1.7 (8.6, 8.7-11.5) |
| Controls (12) | 0.09 ± 0.01† (0.09, 0.06-0.12) | 0.66 ± 0.98 (0, 0-3) | 9.7 ± 0.9 (7.8, 6.8-10.7) |
P < .001 (analysis of variance by Fisher and Kruskal-Wallis tests followed by paired Duncan [t], Bonferroni [t], and Wilcoxon tests), orP < .0001 (Mann-Whitney test) versus controls.
Microvessel area normalized to the cellular area: patients, 8.9% ± 7.6 (7.4%, 4.1%-35.8%); controls, 0.92% ± 0.1 (0.89%, 0.88%-1.52%).
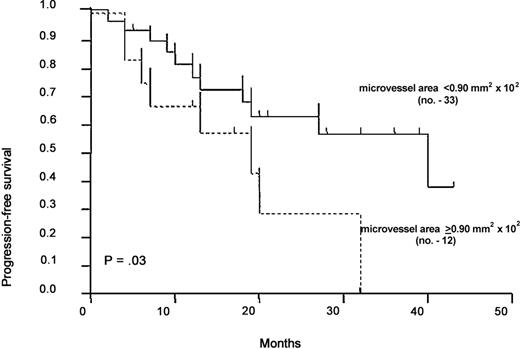
After a median follow-up of 13 months (range, 2-40 months), 18 of 45 patients (40%) progressed to a more advanced Binet stage, the risk of progression being 55.5% at 36 months. As shown in Figure1, the median duration of progression-free survival was 19 months for 12 patients with the microvessel area at least in the 75th percentile (or ≥ 0.90 mm2 × 10−2), and 40 months for those with a lower area. Neither the microvessel number at least at the 75th percentile (or ≥ 6/12.5 mm2 × 10−2) nor the 50th percentile of both parameters as cutoffs gave such a discrimination.
Progression-free survival according to the microvessel area cutoff.
Curves were plotted with the Kaplan-Meier method and statistically compared with the log-rank test (hazard risk = 0.419; 95% CI = 0.111-0.983). Number of patients is given in parentheses.
Progression-free survival according to the microvessel area cutoff.
Curves were plotted with the Kaplan-Meier method and statistically compared with the log-rank test (hazard risk = 0.419; 95% CI = 0.111-0.983). Number of patients is given in parentheses.
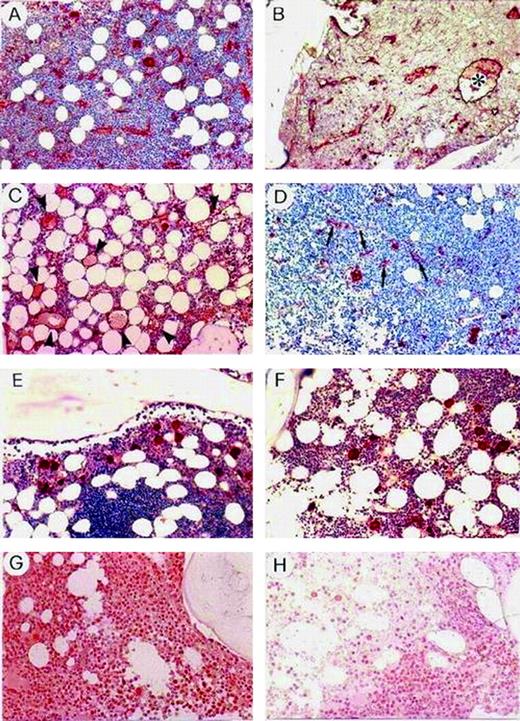
Histologically, the 12 patients with an area of at least 0.90 mm2 × 10−2 had particular microvessel configurations: 8 of them (66%) showed the microvessels widely distributed throughout the tumor tissue, as tortuous, thin, arborized tubes and single or clustered endothelial cells closely interwoven with tumor cells (Figure 2A); the remaining patients (44%) showed these microvessels coexisting with enormously dilated neovessels (Figure 2B) or displaying microaneurysmatic dilations (Figure 2C). Because all patients had a nondiffuse pattern of BM histology, a correlation between histology and each vessel configuration was not feasible. In sharp contrast with these patients, the 33 patients with a lower area uniformly showed straight, not branched, microvessels lacking dilations (Figure 2D), as did controls (Figure 2F), and tumor nodules often without microvessels (Figure 2E).
Staining of BCLL BM with FVIII-RA.
(A-C) Patients with microvessel area and number at least at the 75th percentile. Note in panel B an enormously dilated neovessel (*), and in panel C, numerous microaneurysmatic dilations of neovessels (arrowheads). (D,E) Patients with lower area and number. Note in panel D, straight, not branched neovessels (arrows), and in panel E, a tumor nodule devoid of neovessels. (F) A control subject with anemia due to vitamin B12 deficiency. In panels D, E, and F, megakaryocytes strongly stained with the FVIII-RA are recognizable. Staining of BM BCLL with VEGF (G) in the same patient as in panel B and (H) in the same patient as in panel D. Original magnification for all panels, × 250.
Staining of BCLL BM with FVIII-RA.
(A-C) Patients with microvessel area and number at least at the 75th percentile. Note in panel B an enormously dilated neovessel (*), and in panel C, numerous microaneurysmatic dilations of neovessels (arrowheads). (D,E) Patients with lower area and number. Note in panel D, straight, not branched neovessels (arrows), and in panel E, a tumor nodule devoid of neovessels. (F) A control subject with anemia due to vitamin B12 deficiency. In panels D, E, and F, megakaryocytes strongly stained with the FVIII-RA are recognizable. Staining of BM BCLL with VEGF (G) in the same patient as in panel B and (H) in the same patient as in panel D. Original magnification for all panels, × 250.
The risk of progression as progression-free survival was evaluated according to known and putative prognostic parameters. As shown in Table 2, parameters found to be significant predictors of the progression-free survival by univariate analysis were the microvessel area, BM histology, LDH, β2-m, lymphocyte doubling time, and sVEGF, whereas only LDH and β2-m provided independent prognostic information by multivariate analysis. sFGF displayed no prognostic power.
Risk of progression as progression-free survival
| Variable . | Cutoff . | Relative risk (95% CI) . | P . | |
|---|---|---|---|---|
| Univariate . | Multivariate . | |||
| Age | 65 y* | .86 | NA | |
| Sex | M/F | .49 | NA | |
| Microvessel area | 0.90 (mm2 × 10−2)† | .03 | NS | |
| Microvessel number | 6 (per 12.5 mm2 × 10−2 fields)† | .83 | NA | |
| PB lymphocytes | 21.3 (× 109/L)* | .73 | NA | |
| BM histology | Nondiffuse/diffuse | .05 | NS | |
| LDH | 478 (U/L)* | 3.30 (1.22-8.88) | .01 | .002 |
| β2-m | 2.7 (ng/mL)* | 2.57 (0.96-6.8) | .04 | .007 |
| Lymphocyte doubling time | 12 mo | .006 | NS | |
| sVEGF | 218 (pg/mL)* | .04 | NS | |
| sFGF-2 | 36.6 (pg/mL)*,‡ | .25 | NA | |
| Variable . | Cutoff . | Relative risk (95% CI) . | P . | |
|---|---|---|---|---|
| Univariate . | Multivariate . | |||
| Age | 65 y* | .86 | NA | |
| Sex | M/F | .49 | NA | |
| Microvessel area | 0.90 (mm2 × 10−2)† | .03 | NS | |
| Microvessel number | 6 (per 12.5 mm2 × 10−2 fields)† | .83 | NA | |
| PB lymphocytes | 21.3 (× 109/L)* | .73 | NA | |
| BM histology | Nondiffuse/diffuse | .05 | NS | |
| LDH | 478 (U/L)* | 3.30 (1.22-8.88) | .01 | .002 |
| β2-m | 2.7 (ng/mL)* | 2.57 (0.96-6.8) | .04 | .007 |
| Lymphocyte doubling time | 12 mo | .006 | NS | |
| sVEGF | 218 (pg/mL)* | .04 | NS | |
| sFGF-2 | 36.6 (pg/mL)*,‡ | .25 | NA | |
NA indicates not applied; NS, not significant.
The cutoffs are the median (
) or the 75th percentile (
).
The sFGF-2 levels were significantly higher than in controls (median = 2 pg/mL, range = 2-37 pg/mL;P < .00001, Mann-Whitney test). P by the Cox model.
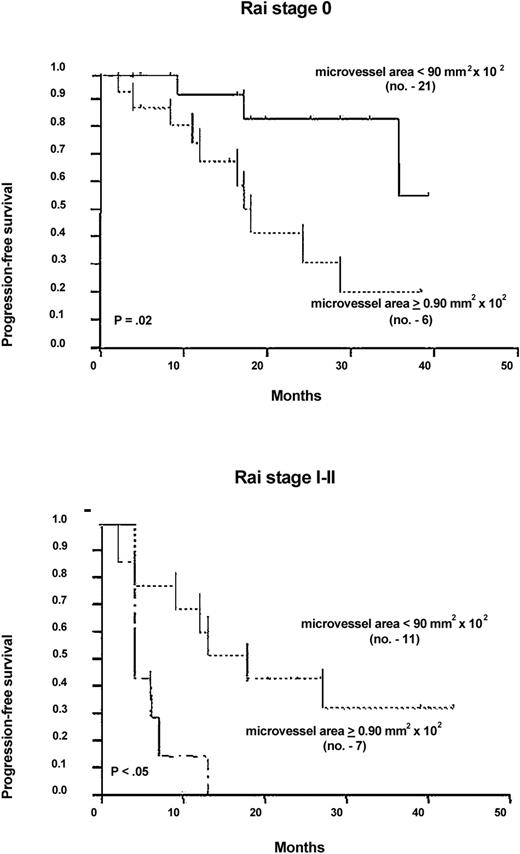
Although the microvessel area was not an independent prognostic factor in multivariate analysis (Table 2), it might be incorporated into the Rai substaging of Binet stage A patients to allow a better assessment of their risk of progression. As shown in Figure3, when Rai 0 patients were split by the cutoff area, 6 of them with at least 0.90 mm2 × 10−2 had a 20-month median progression-free survival, whereas the remaining 21 with a lower area did not reach the median at 47 months. Similarly, the cutoff discriminated 7 Rai I-II patients with 4-month median, from 11 patients with 18 months.
Progression-free survival of Rai substaged patients according to the microvessel area.
Curves were plotted with the Kaplan-Meier method and statistically compared with the log-rank test. Rai 0 curves: hazard risk = 0.22; 95% CI = 0.0181-0.740. Rai I-II curves: hazard risk = 0.373; 95% CI = 0.048-1.024. Number of patients is given in parentheses.
Progression-free survival of Rai substaged patients according to the microvessel area.
Curves were plotted with the Kaplan-Meier method and statistically compared with the log-rank test. Rai 0 curves: hazard risk = 0.22; 95% CI = 0.0181-0.740. Rai I-II curves: hazard risk = 0.373; 95% CI = 0.048-1.024. Number of patients is given in parentheses.
Serial studies were performed in 4 Rai 0 and 3 Rai I-II patients and showed a trend for the microvessel area to increase in step with progression. Specifically, 2 Rai 0 patients (of the 6 with area ≥ 0.90 mm2 × 10−2; Figure 3) had 0.98 ± 0.61 and 1.15 ± 0.84 areas at diagnosis. Values rose to 1.27 ± 0.84 (+23%) and 1.77 ± 1.1 (+36%) after 30 and 37 months in parallel with progression. The other 2 Rai 0 patients (who belonged to the group with area < 0.90; Figure 3) had 0.67 ± 0.25 and 0.52 ± 0.17 at diagnosis, and 0.49 ± 0.16 (−27%) and 0.58 ± 0.22 (+10%) after 25 and 28 months, when stable disease was recorded. Similarly, 2 Rai I-II patients (of the 7 with area ≥ 0.90; Figure 3) had 1.25 ± 1.62 and 2.21 ± 1.81 at diagnosis, and 2.62 ± 1.7 (+51%) and 2.94 ± 1.73 (+25%) after 10 and 12 months, when they progressed. The third patient with 0.77 ± 0.21 at diagnosis had 0.68 ± 0.16 (−12%) after 18 months in the presence of a stable condition. These data agree with those showing that lymph node angiogenesis in B-cell NHL enhances in step with progression.5
Another approach was to correlate the microvessel area cutoff with the prognostic parameters representative of tumor mass (Rai substages, absolute PB lymphocytosis, BM histology, LDH, β2-m), or disease progression (lymphocyte doubling time, cytogenetics).29 30 As shown in Table3, no correlations were found.
Characteristics of the patients grouped according to the extent of BM microvessel area
| Variable3-150 . | Microvessel area . | P . | |
|---|---|---|---|
| < 0.90 mm2 × 10−2 . | ≥ 0.90 mm2 × 10−2 . | ||
| Age, y | 65 (42-74) | 62 (45-81) | .724 |
| Sex, M/F | 21/12 | 8/4 | .851 |
| Rai substages | |||
| 0 | 21 | 6 | .222 |
| I-II | 12 | 6 | .320 |
| PB lymphocytes, × 109/L | 20.7 (5-100) | 22.5 (5-75) | .989 |
| BM histology, nondiffuse/diffuse | 32/1 | 12/0 | .542 |
| LDH, U/L | 431 (129-953) | 486 (36-931) | .615 |
| β2-m, ng/mL | 2.66 (1.6-8.0) | 3.20 (1.8-7.25) | .322 |
| Lymphocyte doubling time, ≥ 12 mo | 72.7% | 83.3% | .464 |
| sVEGF, pg/mL | 218 (9-1120) | 168 (36-2000) | .432 |
| sFGF-2, pg/mL | 35.9 (6-206) | 36.8 (2-142) | .912 |
| Cytogenetics | |||
| Normal karyotype | 10 | 3 | .462 |
| 13q as a sole aberration | 5 | 1 | |
| 12q trisomy | 8 | 0 | |
| 11q or 17p deletion | 2 | 0 | |
| Variable3-150 . | Microvessel area . | P . | |
|---|---|---|---|
| < 0.90 mm2 × 10−2 . | ≥ 0.90 mm2 × 10−2 . | ||
| Age, y | 65 (42-74) | 62 (45-81) | .724 |
| Sex, M/F | 21/12 | 8/4 | .851 |
| Rai substages | |||
| 0 | 21 | 6 | .222 |
| I-II | 12 | 6 | .320 |
| PB lymphocytes, × 109/L | 20.7 (5-100) | 22.5 (5-75) | .989 |
| BM histology, nondiffuse/diffuse | 32/1 | 12/0 | .542 |
| LDH, U/L | 431 (129-953) | 486 (36-931) | .615 |
| β2-m, ng/mL | 2.66 (1.6-8.0) | 3.20 (1.8-7.25) | .322 |
| Lymphocyte doubling time, ≥ 12 mo | 72.7% | 83.3% | .464 |
| sVEGF, pg/mL | 218 (9-1120) | 168 (36-2000) | .432 |
| sFGF-2, pg/mL | 35.9 (6-206) | 36.8 (2-142) | .912 |
| Cytogenetics | |||
| Normal karyotype | 10 | 3 | .462 |
| 13q as a sole aberration | 5 | 1 | |
| 12q trisomy | 8 | 0 | |
| 11q or 17p deletion | 2 | 0 | |
Ranges are presented in parentheses.
Variables were assessed in all 45 patients except those of lymphocyte doubling time and cytogenetics, which were assessed in 38 and 28 patients, respectively.
VEGF expression of the BCLL BM and sVEGF levels
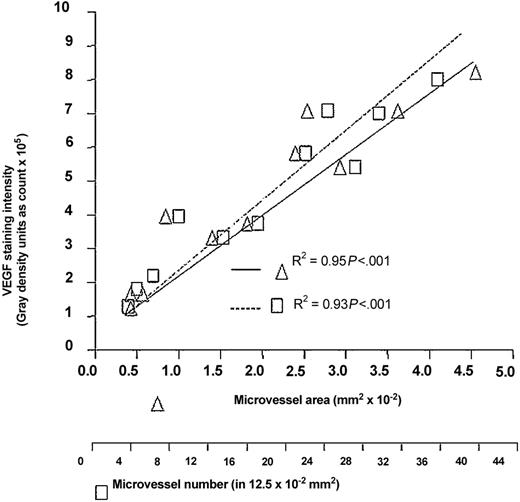
Expression of VEGF evaluated immunohistochemically as an arbitrary intensity unit in the 11 BM samples increased in proportion with both microvessel area and number (Figures 2 and4). We were unable to correlate the BM histology pattern with the intensity of VEGF expression because only one sample (the unique in our series) was diffuse and the remaining nondiffuse. The diffuse sample displayed neither the highest nor the lowest intensity, but was allocated on 4 × 105 intensity units, similarly to what was seen in 2 nondiffuse samples, which displayed 3.3 and 3.7 × 105 units, respectively. A higher number of diffuse samples is thus needed to reach safe conclusions.
BM angiogenesis and VEGF staining intensity.
BM angiogenesis, evaluated as microvessel area and number, is compared with VEGF staining intensity, evaluated as a gray score (mean value per biopsy). Significance was determined by Pearson regression analysis.
BM angiogenesis and VEGF staining intensity.
BM angiogenesis, evaluated as microvessel area and number, is compared with VEGF staining intensity, evaluated as a gray score (mean value per biopsy). Significance was determined by Pearson regression analysis.
The sVEGF was significantly higher in patients (median = 218 pg/mL, range = 9-2000 pg/mL) than controls (median = 142 pg/mL, range = 40-487 pg/mL; P < .02; Mann-Whitney test). As shown in Table 2, adoption of the median value of 218 pg/mL as a cutoff showed that sVEGF was a significant prognostic factor by univariate analysis. Because a high correlation between sVEGF and platelet counts had been previously observed (Pearson r = 0.52;P < .0001), we wondered whether the counts also had prognostic power. No association was found between the counts and the risk of disease progression (P = .09). Thus, sVEGF levels are high in Binet stage A BCLL patients and higher (≥ 218 pg/mL) in patients with poor outcome, irrespective of their platelet counts. Finally, we asked whether sVEGF could be used as an indicator of the density of BM angiogenesis. It was not, however, correlated with microvessel area and number (r = −.196,P = .200; Spearman test).
Serial evaluation of sVEGF in 6 patients showed full agreement with outcome. Three patients whose average value at diagnosis was 412, 305, and 615 pg/mL displayed 741 (+45%), 455 (+53%), and 825 (+26%) after 22, 25, and 18 months when they progressed. The others who had 278, 145, and 340 at diagnosis gave 180 (−36%), 165 (+11%), and 270 (−21%) after 9, 15, and 8 months when stable disease was assessed. These data are in line with those showing that sVEGF levels in B-cell NHL increase with progression.31
Karyotype and angiogenesis
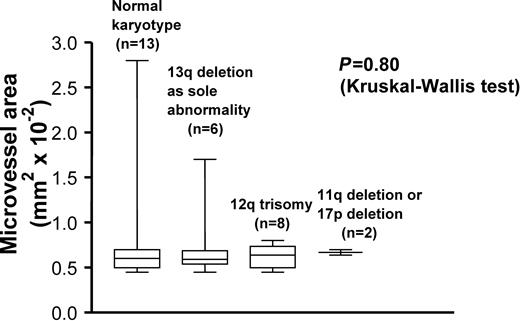
Because genomic aberrations are independent predictors of disease progression in early CLL,32 their correlation with microvessel area was sought. Twenty-eight patients were available for comparison. Thirteen patients (46.4%) had normal karyotype, whereas 15 (53.6%) had aberrations. Of these, 8 patients displayed 13q (6 had deletion as a sole aberration, and 2 had 13q plus 12q trisomy), 6 patients displayed 12q trisomy as a sole aberration, and 2 patients displayed 11q or 17p deletions. Patients were stratified into 4 groups according to major cytogenetic categories (normal karyotype, 13q as a sole aberration, 12q trisomy, 11q or 17p deletion) and aberrations were compared to microvessel area. No correlation was found (Figure5 and Table 3).
BM angiogenesis, evaluated as microvessel area, in comparison with karyotypic aberrations.
The box plots compare median values of microvessel area in 4 cytogenetic categories (P = .80 by Kruskal-Wallis test). Error bars express the 95% CI. Number of patients is given in parentheses.
BM angiogenesis, evaluated as microvessel area, in comparison with karyotypic aberrations.
The box plots compare median values of microvessel area in 4 cytogenetic categories (P = .80 by Kruskal-Wallis test). Error bars express the 95% CI. Number of patients is given in parentheses.
Discussion
Here we show a significant increase of BM angiogenesis (evaluated as FVIII-RA+ microvessel area and number) in patients with Binet stage A BCLL, compared with control subjects. Kini et al16 obtained similar results with a CD34 monoclonal antibody (another endothelial cell marker) and found that microvessel number, BM cellularity, and Binet stages were intercorrelated. In contrast, no increase in BM neovascularization and no dependence of vascularity on cellularity or stages were shown by Aguayo et al who used the FVIII-RA and counted in “hot spots.”11 A different counting system and a heterogeneous population of patients may account for this discrepancy.
We also show that the extension of microvessel area predicts the risk of progression of the Binet stage A. We calculated a cutoff at the 75th percentile or more of the area (≥ 0.90 mm2 × 10−2), at or above which patients with an increased risk of Binet upstaging were identified. Information of this type was not given by the microvessel number, suggesting that the microvessel area should be preferred for prognostic assessment. Indeed, assessment of the area implies that all neovessels, that is, those transversely, longitudinally, or tangentially cut, irrespective of their diameter are precisely quantitated as the space they occupy in the BM. In contrast, the counting system we used scores only neovessels transversely cut, which hit the intersection points of the reticle. Also, it scores both neovessels with a diameter 10 μm or less and those larger as one each. Hence, although the system provides objective counts and is a useful index of BM vascularity in BCLL (as in other tumors),2 5-7 it does not exactly quantitate the full vascular space in the tissue. Thus, the microvessel area provides better information on the degree of angiogenesis.
When the cutoff was applied to the Rai subclassification, the risk was greater for both Rai 0 and Rai I-II patients on or above the cutoff. Although such a finding concerned a relatively small series and in multivariate analysis the microvessel area was not an independent prognostic factor (different from serum β2-m and LDH), it seems of special interest for patients with early disease, because many prognostic factors such as BM histology, lymphocyte doubling time, serum CD23, β2-m, and serum thymidine kinase cannot be considered definitive markers.10,30 Also, as shown in patients with solid tumors,2 B-cell NHL,5 and multiple myeloma,4 33 evaluation of angiogenesis density in tumor sites discriminates heterogeneous clinical outcomes within apparently homogeneous (same stage) groups of patients.
On the other hand, an accurate prognostic assessment of BCLL patients with early disease is mandatory to optimize the timing of therapy and avoid exposure to the toxicity of treatment unlikely to be useful.34 One can also hypothesize that patients of this type who have enhanced BM angiogenesis (microvessel area ≥ 0.90 mm2 × 10−2 according to the present study) can be eligible for antiangiogenic treatments, such as thalidomide,35 anti-VEGF compounds,36 and 2-methoxy-estradiol,37 to delay their progression and improve prognosis.
Overall, the data could be interpreted as pointing to a threshold of BM neovascularization above which the risk of progression increases. This association is consistent with that found in solid tumors,2 and agrees with the stimulatory effect of neovessels on tumor growth, because they convey oxygen and metabolites, and endothelial cells secrete growth factors for tumor cells,1 including those of B-cell lineage.38
The level of BM angiogenesis in hematologic cancers (as in other tumors) is a complex process related to an interaction of an array of angiogenic and antiangiogenic factors released into the microenvironment and of cell populations of tumor and host stromal origin with angiogenic and antiangiogenic activities.39 It is the final product of these interactions. Our data showing that BCLL expression of VEGF was correlated with microvessel area and number suggest that tumor VEGF prevails over antiangiogenic factors/activities and is a substantial angiogenic factor in BM of BCLL. The expression of VEGF by BCLL due to activation of some oncogenes including c-myc, c-fos, ets-140-42 may switch the avascular to the vascular phase and thus contribute to disease progression and a poor prognosis. Also, some inflammatory stromal cells that stained with VEGF might be synergistic with BCLL in the induction of the vascular phase.
Studies on the correlation between tumor angiogenesis and sVEGF are circumstantial.3 We found no correlation, in agreement with studies on multiple myeloma.43 Therefore, different from the tumor expression of VEGF in the BM, sVEGF levels cannot be a marker of BM angiogenesis. The presence of many cell sources of sVEGF, such as stromal cells, platelets, and other endothelia, may account for this observation. However, much in the same way as the microvessel area, the sVEGF had prognostic significance. sFGF-2 was not of prognostic interest.
Although genomic aberrations as evaluated by the FISH technique retain prognostic value in early CLL,32 we found no correlation with the microvessel area. We consider that this correlation is probably lacking because of the limited number of patients investigated. However, it may well be that aberrations and enhanced angiogenesis reflect different biologic events that are not interrelated, thus providing a biologic basis of clinical variability of early CLL as to the disease progression.
In conclusion, our study tentatively suggests that angiogenesis is both a sizable component of the BM in BCLL and a marker of the risk of progression in early disease. Longitudinal studies are warranted to know whether the biologic evolution of early BCLL is prevascular and vascular. This will provide a rationale for the fast use of conventional therapy or antiangiogenic agents in patients with early BCLL who are likely to progress and finally die of their disease.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-01-0084.
Supported in part by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan) and from Ministero dell'Istruzione, Università e Ricerca (MIUR, cofinanced and C03 funds, Rome).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stefano Molica, Department of Hematology/Oncology, Azienda Ospedaliera “Pugliese-Ciaccio,” Viale Pio X, I-88100 Catanzaro (CZ), Italy; e-mail: smolica@libero.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal