We read with interest Mitsiades et al's recent paper1 purporting to define the intracellular factors regulating tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) activity in myeloma cells. They demonstrated quite clearly the crucial role for procaspase-8 activation in initiating TRAIL-induced apoptosis and the clear correlation between the efficiency of procaspase-8 activation and the degree of TRAIL-induced apoptosis. Furthermore, they were able to demonstrate that maneuvers capable of inhibiting antiapoptotic proteins or artificially elevating the levels of intracellular procaspase-8 enhanced the apoptosis-inducing capability of TRAIL. This preliminary data demonstrating the “sensitization” of previously TRAIL-resistant myeloma cells with novel agents are very interesting and, if it can be confirmed that these strategies do not also sensitize nonmalignant cells, may well provide a rationale for early-phase clinical trials.
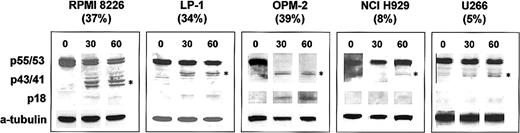
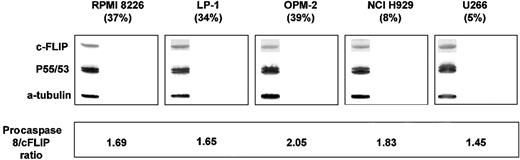
Of concern, however, is the claim that the degree of TRAIL resistance of the cell lines studied was associated with a low procaspase-8/cFLIP (FLICE inhibitory protein) ratio. No data are actually presented to support this assertion. Scrutiny of the data that are presented shows that there is no obvious correlation between the physiologic levels of cFLIP and/or procaspase-8 and the degree of TRAIL-induced apoptosis for the cell lines studied. This is clearly exemplified by the “sensitive” cell lines MM1S and RPMI showing quite different immunoblot findings, with high procaspase-8/low cFLIP ratios and low procaspase-8/high cFLIP ratios, respectively. Our own data from 5 authentic myeloma cell lines confirm the crucial relationship between the efficiency of procaspase-8 activation and TRAIL-induced apoptosis (Figure1) and also show the lack of correlation between the physiologic procaspase-8/cFLIP levels and TRAIL-induced apoptosis (Figure2).
Immunoblot analysis of procaspase-8 activation in TRAIL-treated myeloma cells.
The myeloma cells were incubated with 1 μg/mL leucine-zipper TRAIL (Immunex, Seattle, WA) and harvested at 30 and 60 minutes following incubation. The proportion of cells undergoing apoptosis, shown by the percentage in parentheses at 60 minutes, was determined by annexin-V staining. Cytosolic protein fractions (25 μg) were separated on 4% to 16% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to nitrocellulose, and probed with a caspase-8–specific goat polyclonal antibody C-20 (Santa Cruz, Santa Cruz, CA). Proteins were visualized by enhanced chemiluminescence (ECL). Membranes were stripped and reprobed with monoclonal antibody to α-tubulin (Sigma, St Louis, MO). The sensitive myeloma cell lines RPMI 8226, LP-1, and OPM-2 all show early cleavage of procaspase-8 with generation of the intermediate p43/41 product (*) and the active P18 form. Resistant cell lines NCI H929 and U266 demonstrate delayed procaspase-8 cleavage with minimal P18 generation.
Immunoblot analysis of procaspase-8 activation in TRAIL-treated myeloma cells.
The myeloma cells were incubated with 1 μg/mL leucine-zipper TRAIL (Immunex, Seattle, WA) and harvested at 30 and 60 minutes following incubation. The proportion of cells undergoing apoptosis, shown by the percentage in parentheses at 60 minutes, was determined by annexin-V staining. Cytosolic protein fractions (25 μg) were separated on 4% to 16% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to nitrocellulose, and probed with a caspase-8–specific goat polyclonal antibody C-20 (Santa Cruz, Santa Cruz, CA). Proteins were visualized by enhanced chemiluminescence (ECL). Membranes were stripped and reprobed with monoclonal antibody to α-tubulin (Sigma, St Louis, MO). The sensitive myeloma cell lines RPMI 8226, LP-1, and OPM-2 all show early cleavage of procaspase-8 with generation of the intermediate p43/41 product (*) and the active P18 form. Resistant cell lines NCI H929 and U266 demonstrate delayed procaspase-8 cleavage with minimal P18 generation.
Determination of cFLIP and procaspase-8 levels in untreated myeloma cells.
Untreated myeloma cells were lysed in whole cell lysate buffer and 80 μg protein separated on a 12% SDS-PAGE gel. Procaspase-8 was detected as described above. Membranes were then stripped and reprobed with the cFLIP monoclonal antibody G-11 (Santa Cruz). Tubulin detection was used to confirm equal protein loading. The procaspase-8/cFLIP ratios were calculated for each cell line densitometrically and do not correlate with the amount of TRAIL-induced apoptosis (r = 0.7,p = 0.19, Spearman rank correlation).
Determination of cFLIP and procaspase-8 levels in untreated myeloma cells.
Untreated myeloma cells were lysed in whole cell lysate buffer and 80 μg protein separated on a 12% SDS-PAGE gel. Procaspase-8 was detected as described above. Membranes were then stripped and reprobed with the cFLIP monoclonal antibody G-11 (Santa Cruz). Tubulin detection was used to confirm equal protein loading. The procaspase-8/cFLIP ratios were calculated for each cell line densitometrically and do not correlate with the amount of TRAIL-induced apoptosis (r = 0.7,p = 0.19, Spearman rank correlation).
There are 4 known surface receptors for TRAIL, 2 of which (TRAIL-R1 and TRAIL-R2) appear to be capable of inducing apoptosis upon binding of TRAIL.2 Our understanding of TRAIL–TRAIL receptor interactions beyond this is limited. But the earlier belief that the 2 “decoy” TRAIL receptors (TRAIL-R3 and TRAIL-R4) lacking intracellular death domains somehow protect cells from TRAIL has been shown by our group and others to be incorrect.3-5 For TRAIL to be exploited maximally as an antitumor agent, how it works must be more thoroughly understood, particularly the exact roles of the known TRAIL receptors. Acceptance of the perhaps premature assertion that myeloma-cell TRAIL sensitivity is regulated by physiologic levels of procaspase-8 and/or cFLIP will do little to improve this lack of understanding.
Resistance to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) is modulated by FLICE-inhibitory protein (FLIP) and procaspase-8 in multiple myeloma cells
At the center of the concerns raised by Dr Spencer et al is the role of FLICE-inhibitory protein (FLIP) and procaspase-8 in mediating resistance to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL, Apo2 ligand)–mediated apoptosis in myeloma cells. In our studies, TRAIL resistance was associated with underexpression of procaspase-8 and overexpression of FLIP. In support of these conclusions were experiments that demonstrated the following: (1) TRAIL-resistant myeloma cell lines tended to exhibit low levels of procaspase-8 and high levels of FLIP, as well as other antiapoptotic molecules such as cellular inhibitors of apoptosis protein–2 (cIAP-2); (2) forced overexpression of procaspase-8 in TRAIL-resistant myeloma cells increased TRAIL-sensitivity; (3) antisense oligonucleotides against FLIP increased TRAIL-sensitivity of TRAIL-resistant myeloma cells; and (4) pharmacologic modulators of FLIP expression (eg, protein kinase C [PKC] inhibitors, cycloheximide, etc) that down-regulated the expression of FLIP also led to increased TRAIL-sensitivity of TRAIL-resistant myeloma cells. Similar findings have been published by others who have studied TRAIL-mediated apoptotic pathways in other tumor cell models, and have akin to us shown that underexpression of procaspase-8 and/or overexpression of FLIP (including high caspase-8 homologue FLIP [cFLIP]/procaspase-8 levels) are associated with resistance to TRAIL.1-1-1-3 We have attempted to dissect out differences in our respective study approaches that may have led to discordant results. While Spencer et al's letter does not provide the methodology for the included experiments nor list the antibodies used to determine procaspase-8 and FLIP protein expression, there are concerns raised by the interpretation of these data in view of the cell lines and methodology previously used by these investigators. One key concern is the suboptimal level of TRAIL-induced tumor cell death noted for the RPMI 8226/S myeloma cell line in their previous study,1-4 as well as outright resistance to TRAIL for both U266 and NCI H929 myeloma cells per their letter. But in deference to their own studies, Spencer and colleagues acknowledge that NCI H929 myeloma cells are TRAIL-sensitive in their Leukemia article,1-4while describing these cells as TRAIL-resistant in their letter. One likely explanation for this discrepancy is that Spencer et al measure TRAIL-induced apoptosis by annexin V only after one hour of TRAIL treatment. This likely is a very early time point to determine the full extent of TRAIL-induced apoptosis, because as shown in our previous studies,1-5 myeloma cells become annexin V–positive after 4 to 6 hours of TRAIL treatment. This likely explains why in their letter Spencer et al have incorrectly classified the NCI H929 and U266 cell lines as TRAIL-resistant. In our hands, as well as those of many other investigators, all of the above cell lines have exhibited marked sensitivity to TRAIL,1-6-1-9 including the NCI H929 cell line, which exhibited an inhibitory concentration 50% (IC50) of below 100 ng/mL and showed TRAIL sensitivity similar to the TRAIL-sensitive LP-1 and OPM-2 myeloma cells reported by Spencer et al in Figure 1 of their letter. One possible explanation for these discordant findings is the technique used to determine induction of apoptosis by TRAIL. The authors curiously report in Leukemiathat they first undertook to remove dead cells following TRAIL treatment by density gradient centrifugation over Lymphoprep (Nycomed Pharma, Oslo, Norway) before establishing the percentage of cells that underwent apoptosis, thus potentially underestimating cell death. The rationale for taking this step remains unclear and hampers the valid interpretation of these studies since appropriately establishing TRAIL sensitivity is a prerequisite to discerning intracellular regulatory pathways.
It does remain unclear, as suggested by Spencer et al, whether the use of Epstein-Barr virus (EBV) genome containing cell lines is a confounding factor in these and other studies since modulation of both caspase-8 and FLIP have been reported as a potential mechanism of EBV tumorigenesis in Burkitt lymphoma.1-10 But a certain subset (or subsets) of multiple myeloma (MM) may be EBV-related in view of reports that have demonstrated EBV genomic presence in freshly obtained MM patient cells.1-10-1-13 Interestingly, this subset may include those patients with CD20+ disease since EBV nuclear antigen 3C (EBNA-3C) modulates the PU.1 transcription factor1-14 that is normally down-regulated in plasma cells and is responsible, along with the PU.1 interacting partner (Pip), for CD20 expression (reviewed in Treon et al1-15). This point is especially worth mentioning since the cell lines in our study that were moderately or highly TRAIL resistant were CD20+ and have been reported to be EBV+ as well.1-5

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal