The T-cell receptor ζ (TCR-ζ) and FcR-γ chains play a critical role in mediating signal transduction. We have previously described HIV glycoprotein 120 (gp120)–specific chimeric immune receptors (CIRs) in which the extracellular domain of CD4 is linked to the signaling domain of ζ (CD4ζ) or γ (CD4γ). Such CIRs are efficiently expressed following retroviral transduction of mature T cells and specifically redirect effector functions toward HIV-infected targets. In this report, we examine development of CD4ζ- or CD4γ-expressing T cells from retrovirally transduced hematopoietic stem cells following bone marrow transplantation. Although CD4ζ/γ-expressing myeloid, NK, and B cells were efficiently reconstituted, parallel development of CD4ζ/γ-expressing T cells was blocked prior to the CD25+CD44+prothymocyte stage. In contrast, T cells expressing a signaling-defective CIR were efficiently generated. When major histocompatibility complex (MHC) class II–deficient mice were used as transplant recipients, development of CD4ζ/γ-expressing T cells was restored. We conclude that CD4ζ/γ signaling generated following engagement of MHC class II selectively arrests T-lineage development.
Introduction
We have previously described HIV glycoprotein 120 (gp120)–specific chimeric immune receptors (CIRs) in which the cytoplasmic domain of either the T-cell receptor ζ (TCR-ζ) or the FcR-γ chain is linked to the extracellular and transmembrane domains of the human CD4 receptor.1-5 Mature CD4ζ-expressing T lymphocytes generated ex vivo by retroviral transduction are capable of highly efficient and specific cytolysis of both HIV-infected primary cells and HIVgp120-expressing tumors in vitro,1,4 and clinical trials involving adoptive transfer of autologous CD4ζ-transduced T cells in HIV-infected patients have been undertaken.6 7
In vivo development of CIR-modified T cells from transplanted hematopoietic stem cells (HSCs) may have some advantages over adoptive transfer of ex vivo-transduced mature T cells, because the former bypasses the need for extensive ex vivo cell expansion and may improve T-cell function in vivo. Our previous studies have shown that severe combined immunodeficient (SCID) mice (which lack T and B cells) rapidly reconstitute CD4ζ-expressing myeloid and natural killer (NK) cells following transplantation with retrovirally transduced syngeneic bone marrow.3 Furthermore, such SCID mice that received CD4ζ transplants are protected from a lethal dose of HIVgp120-expressing leukemia cells.3 One of the many potential barriers to implementation of a bone marrow transplantation (BMT) approach for generating functional CD4ζ T cells is the impact, if any, of retroviral-driven CIR expression early in hematopoiesis on T-cell development. The affinity of the human CD4 receptor for murine and human major histocompatibility complex class II (MHCII) is insufficient for activation of CD4ζ/γ-expressing T cells.1,2 8-11 However, the consequences of such low-affinity interactions on lymphoid development have not been examined.
T cells develop from HSC-derived progenitor cells that migrate to the thymus. Subsequent thymocyte development is regulated by sequential expression of the pre-TCR12,13 and the mature αβTCR,14,15 both of which are multimeric complexes that rely on the associated invariant CD3 and ζ chains for transmitting essential survival, differentiation, and proliferation signals. The most immature thymocytes reside within the CD4−CD8−CD3− triple-negative (TN) population, which comprises only about 1% to 2% of total thymocytes. The TN population itself can be divided into the sequential subsets TN1 (CD44+25−), TN2 (CD44+25+), TN3 (CD44−25+), and TN4 (CD44−25−).12 13 The pre-TCR permits progression from TN3 through to the CD4+CD8+ double-positive (DP) stage, whereas the αβTCR drives subsequent selection to mature CD4+and CD8+ single-positive cells. The studies described in this report were designed to determine the impact, if any, of CD4ζ/γ CIR expression on thymocyte development in the setting of BMT.
Study design
Vectors
Mice
The MHCII-deficient (MHCII−) mice possess a disruption of the MHC class II Ab β gene (B6.129-Abbtm1; Taconic, Germantown, MD) and therefore do not express surface MHCII in the C57BL/6 background. Wild-type C57BL/6 mice (Taconic) were used as MHCII+recipients in experiments involving donor marrow from MHCII− mice.
Flow cytometry
Antibodies (Pharmingen, San Diego, CA) used to stain peripheral blood and thymocytes are described in the figure legends. Stained cells were analyzed on a FACScan cytometer (Becton Dickinson, San Jose, CA).
Results and discussion
Bone marrow was isolated from C3H mice and retrovirally transduced with CD4ζ, CD4γ, or a signaling-defective CIR (CD4del) as previously described.3,5 Surface CIR expression on transduced bone marrow cells before infusion is shown in Figure 1A. Although the percentage of cells expressing CD4ζ and CD4γ was similar, the mean intensity of expression was considerably higher for the CD4ζ and CD4del receptors as seen in previous studies.5 The transduced bone marrow was subsequently transplanted into sublethally irradiated C3H mice via tail-vein injection.
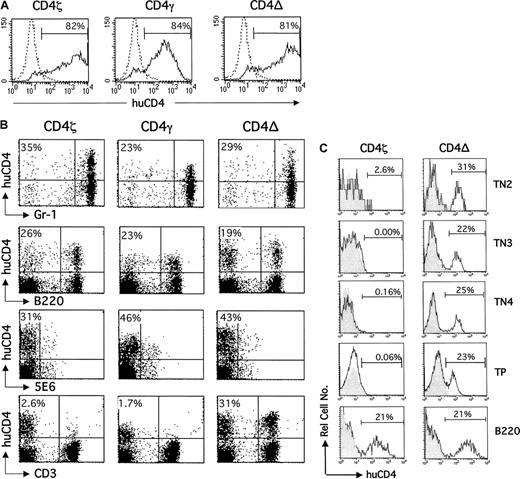
Development of CD4ζ/γ-expressing T cells, but not CD4ζ/γ-expressing myeloid, NK, and B cells is blocked following BMT.
(A) Bone marrow cells isolated from C3H mice were exposed to retroviral supernatant encoding CD4ζ, CD4γ, or CD4del (CD4Δ) in the presence of polybrene for 4 hours. Twenty-four hours later, cells were stained with antihuman CD4-phycoerythrin (CD4-PE; solid line) or isotype-matched control monoclonal antibody (dotted line) and analyzed by fluorescence-activated cell sorting (FACS) to determine the efficiency of transduction. Histograms for control cells stained with antihuman CD4-PE or isotype control were indistinguishable (data not shown). The percentage of human CD4-expressing cells over background is indicated. (B) The transduced bone marrow cells shown in panel A were infused into sublethally irradiated C3H mice. Six weeks after transplantation, peripheral blood was isolated and analyzed by 2-color flow cytometry using PE-conjugated antihuman CD4 and FITC-conjugated anti–Gr-1, anti-B220, anti-5E6, or anti-CD3. Forward and side-scatter properties were used to determine gates for myeloid-specific (Gr-1) and lymphoid-specific (B220, 5E6, and CD3) markers. The percentage of lineage-positive cells expressing human CD4 is shown in the top left hand corner of each dot plot. Control cells stained with antihuman CD4-PE or isotype control yielded indistinguishable results (data not shown). Results are representative of at least 15 additional mice. (C) Thymocytes were isolated from mice that received transplants of CD4ζ or CD4del (CD4−) 6 weeks after transplantation, stained with allophycocyanin (APC)–conjugated anti-CD3/anti-CD4/anti-CD8, PE-conjugated anti-CD25, fluorescein isothiocyanate (FITC)–conjugated anti-CD44, and energy-coupled dye (ECD)–conjugated antihuman CD4, and analyzed by 4-color flow cytometry. APC− (ie, TN cells) were subsequently analyzed for CD44 and CD25 expression to define TN subsets. The level of human CD4 expression (solid line) for CD25+CD44+(TN2), CD25+CD44− (TN3), and CD25−CD44− (TN4) cells is shown. APC+ TP (ie, CD3+CD4+CD8+) thymocytes were also analyzed for human CD4 expression (solid line). The percentage of peripheral blood–derived B cells (B220) expressing human CD4 in the same animals is also shown for comparison. Shaded histograms represent cells from control animals that received transplants of unmodified bone marrow in each case. The percentage of TN2, TN3, TN4, TP, or B cells (B220) expressing human CD4 is indicated in each histogram.
Development of CD4ζ/γ-expressing T cells, but not CD4ζ/γ-expressing myeloid, NK, and B cells is blocked following BMT.
(A) Bone marrow cells isolated from C3H mice were exposed to retroviral supernatant encoding CD4ζ, CD4γ, or CD4del (CD4Δ) in the presence of polybrene for 4 hours. Twenty-four hours later, cells were stained with antihuman CD4-phycoerythrin (CD4-PE; solid line) or isotype-matched control monoclonal antibody (dotted line) and analyzed by fluorescence-activated cell sorting (FACS) to determine the efficiency of transduction. Histograms for control cells stained with antihuman CD4-PE or isotype control were indistinguishable (data not shown). The percentage of human CD4-expressing cells over background is indicated. (B) The transduced bone marrow cells shown in panel A were infused into sublethally irradiated C3H mice. Six weeks after transplantation, peripheral blood was isolated and analyzed by 2-color flow cytometry using PE-conjugated antihuman CD4 and FITC-conjugated anti–Gr-1, anti-B220, anti-5E6, or anti-CD3. Forward and side-scatter properties were used to determine gates for myeloid-specific (Gr-1) and lymphoid-specific (B220, 5E6, and CD3) markers. The percentage of lineage-positive cells expressing human CD4 is shown in the top left hand corner of each dot plot. Control cells stained with antihuman CD4-PE or isotype control yielded indistinguishable results (data not shown). Results are representative of at least 15 additional mice. (C) Thymocytes were isolated from mice that received transplants of CD4ζ or CD4del (CD4−) 6 weeks after transplantation, stained with allophycocyanin (APC)–conjugated anti-CD3/anti-CD4/anti-CD8, PE-conjugated anti-CD25, fluorescein isothiocyanate (FITC)–conjugated anti-CD44, and energy-coupled dye (ECD)–conjugated antihuman CD4, and analyzed by 4-color flow cytometry. APC− (ie, TN cells) were subsequently analyzed for CD44 and CD25 expression to define TN subsets. The level of human CD4 expression (solid line) for CD25+CD44+(TN2), CD25+CD44− (TN3), and CD25−CD44− (TN4) cells is shown. APC+ TP (ie, CD3+CD4+CD8+) thymocytes were also analyzed for human CD4 expression (solid line). The percentage of peripheral blood–derived B cells (B220) expressing human CD4 in the same animals is also shown for comparison. Shaded histograms represent cells from control animals that received transplants of unmodified bone marrow in each case. The percentage of TN2, TN3, TN4, TP, or B cells (B220) expressing human CD4 is indicated in each histogram.
Peripheral blood was isolated from reconstituted animals at approximately 6 weeks after transplantation and analyzed by 2-color flow cytometry using monoclonal antibodies against human CD4 and the following mouse cell lineage markers: GR-1 (granulocytes); B220 (B cells); 5E6 (NK cells), or CD3 (T cells; Figure 1B). CD4ζ, CD4γ, and CD4del were expressed on granulocytes and NK cells at similar frequencies, as previously described for SCID mice following BMT.5 Although similar levels of expression were observed in B cells for all 3 receptors, T cells expressing CD4ζ or CD4γ were not detected. In contrast, expression of the signaling-defective CIR, CD4del, was retained on this lineage.
The differential expression of CD4del and CD4ζ/γ in mature T lymphocytes prompted us to examine CIR expression during thymocyte development. Thymocytes were harvested from mice that had received either CD4ζ- or CD4del-transduced bone marrow 6 weeks before, and TN2, TN3, TN4, and TP (triple-positive) CD3+CD4+CD8+ populations were examined for human CD4 expression by flow cytometry as described in Figure 1C. Low levels of TN1 cells precluded analysis of this particular subset. Whereas CD4del expression was observed at similarly high levels at every stage of thymocyte development examined, CD4ζ expression was not detected at any stage. Similar results were observed for CD4γ (data not shown). In contrast to the T-cell lineage, CD4ζ expression was detected at comparable levels to that of CD4del in B, myeloid, and NK cells (Figure 1B-C) in peripheral blood samples isolated from the same CD4ζ-marrow animal that underwent transplantation.
To determine whether engagement of CD4ζ or CD4γ (CD4ζ/γ) by MHCII was indeed responsible for the failure of CD4ζ/γ-bearing thymocyte development, immune reconstitution was examined in mice lacking MHCII expression. Specifically, bone marrow derived from C57BL/6 mice lacking expression of MHCII (MHCII−) was transduced with CD4ζ, CD4γ, or CD4del, and transplanted into syngeneic (MHCII−) or congenic (MHCII+) wild-type recipients. As before, peripheral blood was harvested from reconstituted mice approximately 6 weeks after transplantation, and B- and T-cell lineages examined for human CD4 expression by flow cytometry (Figure 2A). Analysis of peripheral blood isolated from MHCII+ or MHCII− recipients revealed expression of each of the 3 receptors on B cells (Figure 2A), myeloid cells, and NK cells (data not shown) as expected. Consistent with the experiment summarized in Figure 1 using C3H mice, T cells expressing the CD4del receptor, but not CD4ζ or CD4γ, were present in reconstituted MHCII+ recipients. In striking contrast, T cells from MHCII− recipients expressed either CD4ζ or CD4γ receptors at levels comparable to those of the CD4del receptor. Flow cytometric analysis of thymocytes isolated from the MHCII− recipient mice in Figure 2B revealed that CD4ζ-expressing TN2, TN3, TN4, and TP thymocytes could now be detected at equivalent frequencies to the CD4del subsets (Figure 2C).
Development of CD4ζ/γ-expressing thymocytes and T cells is rescued in MHCII− mice.
Bone marrow cells isolated from MHCII− mice were exposed to retroviral supernatant encoding CD4ζ, CD4, or CD4del (CD4−), and then infused into sublethally irradiated wild-type C57BL/6 (A) or MHCII− C57BL/6 (B) mice. Six weeks after transplantation, peripheral blood was isolated and analyzed by 2-color flow cytometry using PE-conjugated antihuman CD4 and FITC-conjugated anti-B220 or anti-CD3. The percentage of lineage positive cells expressing human CD4 is shown in the top left hand corner of each dot plot. Control cells stained with antihuman CD4-PE or isotype controls yielded indistinguishable results (data not shown). Results are representative of at least 8 additional mice. (C) Thymocytes were isolated from MHCII− mice in panel B, which had received CD4ζ- or CD4del (CD4−)–transduced bone marrow, and were analyzed by 4-color flow cytometry as described in the legend to Figure 1. The percentage of human CD4-expressing cells (solid line) in each TN and TP subset is indicated in each histogram. Shaded histograms represent cells from control animals that received transplants of unmodified bone marrow in each case. ND indicates insufficient cells to analyze.
Development of CD4ζ/γ-expressing thymocytes and T cells is rescued in MHCII− mice.
Bone marrow cells isolated from MHCII− mice were exposed to retroviral supernatant encoding CD4ζ, CD4, or CD4del (CD4−), and then infused into sublethally irradiated wild-type C57BL/6 (A) or MHCII− C57BL/6 (B) mice. Six weeks after transplantation, peripheral blood was isolated and analyzed by 2-color flow cytometry using PE-conjugated antihuman CD4 and FITC-conjugated anti-B220 or anti-CD3. The percentage of lineage positive cells expressing human CD4 is shown in the top left hand corner of each dot plot. Control cells stained with antihuman CD4-PE or isotype controls yielded indistinguishable results (data not shown). Results are representative of at least 8 additional mice. (C) Thymocytes were isolated from MHCII− mice in panel B, which had received CD4ζ- or CD4del (CD4−)–transduced bone marrow, and were analyzed by 4-color flow cytometry as described in the legend to Figure 1. The percentage of human CD4-expressing cells (solid line) in each TN and TP subset is indicated in each histogram. Shaded histograms represent cells from control animals that received transplants of unmodified bone marrow in each case. ND indicates insufficient cells to analyze.
In summary, we have shown that development of CD4ζ/γ-bearing T cells from transplanted HSCs is arrested prior to the TN2 (CD44+CD25+) stage in normal mice. The developmental block is specific for the T lineage because myeloid, NK, and B cells are unaffected. In addition, we have shown that T-lineage arrest is dependent on the signaling domain of either ζ or γ (because CD4del-expressing T cells develop normally), and on expression of the CD4 ligand, MHCII. The process by which HSCs commit to the T-lymphoid lineage is poorly understood, particularly during adult steady-state hematopoiesis. Several studies suggest that TN1 cells in the thymus arise from, or are equivalent to, common lymphocyte precursors (CLPs) found in bone marrow.17,18 TN1 cells (and CLPs) have lost myeloid potential, but can still develop into lymphocytes (T, B, and NK cells).17,18 By the TN2 stage, however, the potential to develop into B and NK cells has also been lost. Our current hypothesis is that ζ/γ-mediated signal transduction occurs in response to CD4ζ/γ-MHCII engagement during the CLP/TN1→TN2 transition. The TN3→TN4 transition is mediated by pre-TCR signaling via the associated immunoreceptor tyrosine-based activation motif (ITAM)–bearing CD3/ζ chains.19-23 Presumably, premature or aberrant ITAM-mediated signal transduction by CD4ζ/γ during the CLP/TN1→TN2 transition arrests T-lineage commitment or differentiation of committed T-cell precursors. This hypothesis is consistent with recent data from our laboratory showing that CD4ζ is expressed at high levels on TN thymocytes when under the transcriptional control of the CD2 enhancer instead of the constitutively active pgk promoter of the retroviral vector (manuscript in preparation). Despite the presence of only a single ITAM, CD4γ is as effective as CD4ζ in mediating this developmental defect. The FcR-γ chain is known to be expressed in normal DN thymocytes and has been implicated in the development of certain T-cell subsets.24 25 The mechanisms driving lineage commitment during the CLP/TN1→TN2 transition remain obscure. Studies are now underway to determine the specific step in this complex pathway at which CD4ζ/γ-induced arrest occurs, and to further dissect the process of T-lineage determination.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-02-0428.
Supported in part by research funding from Cell Genesys to M.R.R. and W.Y.L. and from the University of Virginia to M.R.R.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Margo R. Roberts, Department of Microbiology, University of Virginia, PO Box 800734, UVA Health System, Charlottesville, VA 22908; e-mail: mroberts@virginia.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal