Antigen-presenting cells are localized in essentially every tissue, where they operate at the interface of innate and acquired immunity by capturing pathogens and presenting pathogen-derived peptides to T cells. C-type lectins are important pathogen recognition receptors and the C-type lectin, dendritic cell–specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN), is unique in that, in addition to pathogen capture, it regulates adhesion processes such as DC trafficking and T-cell synapse formation. We have isolated a murine homologue of DC-SIGN that is identical to the previously reported murine homologue mSIGNR1. mSIGNR1 is more closely related to the human DC-SIGN homologue L-SIGN than to DC-SIGN itself because mSIGNR1 is specifically expressed by liver sinusoidal endothelial cells, similar to L-SIGN, and not by DCs. Moreover, mSIGNR1 is also expressed by medullary and subcapsular macrophages in lymph nodes and by marginal zone macrophages (MZMs) in the spleen. Strikingly, these MZMs are in direct contact with the bloodstream and efficiently capture specific polysaccharide antigens present on the surface of encapsulated bacteria. We have investigated the in vivo function of mSIGNR1 on MZMs in spleen. We demonstrate here that mSIGNR1 functions in vivo as a pathogen recognition receptor on MZMs that capture blood-borne antigens, which are rapidly internalized and targeted to lysosomes for processing. Moreover, the antigen capture is completely blocked in vivo by the blocking mSIGNR1-specific antibodies. Thus, mSIGNR1, a murine homologue of DC-SIGN, is important in the defense against pathogens and this study will facilitate further investigations into the in vivo function of DC-SIGN and its homologues.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that sample foreign pathogens in the periphery and migrate to the lymphoid tissues where they present processed antigens to naive T cells, initiating an immune response.1 DCs express a repertoire of specialized receptors to perform their unique functions. We have identified the human DC-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN; CD209), which mediates several key functions throughout the lifetime of a DC; DC-SIGN binding to its endothelial ligand intercellular adhesion molecule 2 (ICAM-2) mediates transendothelial migration of DCs,2 and DC-SIGN enables primary immune responses by initiating transient DC–T-cell interactions through binding of ICAM-3 on naive T cells.3Recently, we demonstrated that DC-SIGN also functions as an antigen receptor by rapidly internalizing soluble ligands that are targeted to late endosomes/lysosomes; here the internalized antigen is efficiently processed and subsequently presented to CD4+ T cells.4 Besides playing an important role in the initiation of immune responses, DC-SIGN also functions as a novel HIV-1trans-receptor important in the dissemination of HIV-1 from sites of infection.5 Resident mucosal DCs capture HIV-1 through the interaction of DC-SIGN with the HIV-1 envelope glycoprotein, gp120, and subsequently migrate to the lymphoid tissues where DC-SIGN facilitates the transmission of HIV-1 to T cells.5 The DC-specific expression of human DC-SIGN (hDC-SIGN) in situ and in vitro correlates well with both its immunologic functions3 and its role in HIV-1 dissemination.5
The DC-SIGN homologue, L-SIGN,6 which has also been called DC-SIGN related (DC-SIGNR),7 has a similar activity to DC-SIGN, but is not expressed by DCs.6 Human L-SIGN (hL-SIGN) is specifically expressed by liver sinusoidal endothelial cells (LSECs), which are liver resident APCs of monocyte origin, and in lymph nodes.6,8 LSECs interact strongly with leukocytes and appear to constitute a central mechanism of peripheral immune tolerance in the liver.9 The tissue location and ligand-binding properties of hL-SIGN strongly implicate a physiologic role for this receptor in antigen clearance,6 as has been reported for other C-type lectins10 11 as well as in LSEC-leukocyte adhesion.
Functional studies into the in vivo function of DC-SIGN have been hampered by the lack of murine homologues. Therefore, we set out to identify and isolate the murine homologue of DC-SIGN. We have isolated a cDNA clone OtB7, which is homologous to hDC-SIGN and is identical to murine SIGNR1 (mSIGNR1), a recently reported murine homologue of DC-SIGN.12 Here we describe the cell-specific expression and in vivo function of this murine homologue of DC-SIGN. We generated antibodies against this homologue and demonstrated that its cell-specific expression is similar to that of hL-SIGN. mSIGNR1 has a similar binding activity in vitro to both hDC-SIGN and hL-SIGN. Its high affinity for murine ICAM-2, and its localization at sites that are in close contact with blood, suggest a role for mSIGNR1 in lymphocyte migration from the blood into tissues. Moreover, we demonstrate that marginal zone macrophages (MZMs) in the spleen efficiently capture blood-borne antigens in vivo through mSIGNR1. mSIGNR1 rapidly internalizes blood-borne antigens, which are targeted to lysosomes. These results demonstrate that a murine homologue of DC-SIGN is expressed by highly phagocytic macrophages in the spleen and functions in vivo as an pathogen recognition receptor.
Materials and methods
Antibodies
The following antibodies were used: N148 (CD11c), anti–ICAM-2 (BD Pharmingen, San Diego, CA), NLDC-40 (anti–DEC-205), M1/70 (CD11b), MECA-32, MOMA-1, ER-TR9.13Polyclonal antisera were raised in rabbits against 2 mSIGNR1-specific peptides, KTPNTERQKEQEKILQ and SRFQKYWNRGEPNNI (single-letter amino acid codes; Eurogentec, Seraing, Belgium).
Mice
BALB/c mice were bred and maintained in the laboratory animal facilities at the Faculty of Medicine of the Vrije Universiteit Medical Center Amsterdam under conventional conditions. Mice were used at ages between 8 and 15 weeks.
Characterization of the cDNA encoding the murine homologues of hDC-SIGN
The cDNA encoding mSIGNR1 was amplified by reverse transcription–polymerase chain reaction (RT-PCR) on total RNA derived from lung. The PCR primers used for isolation of mSIGNR1 were as follows: forward primer, ATG AGT GAC TCC ACA GAA GCC AAG ATG CAG; reverse primer, AAG AAG AAT CCC AGA GCC TTT TTC ACG ATC C. The PCR product was sequenced and subsequently cloned into the eukaryotic expression vector pRc/CMV. The homologue was expressed either by transient transfections of 293T cells or by stable transfections in the erythroleukemic cell line K562. We have submitted the full mSIGNR1 cDNA sequence to GenBank under accession number AF422108.
Generation of murine ICAM-2 and mSIGNR1 fusion proteins
The extracellular domain of murine ICAM-2 was isolated by RT-PCR on total liver RNA using primers based on the murine ICAM-2 cDNA sequence.14 The primers were as follows: forward primer CTA AGC TTC CCC ACC TGA GAT GTC TTC TTT TGC; reverse primer CGG ATC CGC CAC GGG ACG TGC CCG AAA G. The identity of the cDNA was confirmed by sequencing, and the cDNA encoding murine ICAM-2 was cloned into the Sig-pIgG1-Fc vector.15 The resulting ICAM-2 Fc chimera contains the extracellular domain of ICAM-2 fused to the IgG1 Fc domain. ICAM-2-Fc was produced in COS cells by cotransfection of ICAM-2-Sig-pIgG1-Fc and the pEE14 vector. ICAM-2-Fc concentrations in the supernatant were determined by an anti-IgG1 enzyme-linked immunosorbent assay (ELISA).
mSIGNR1 Fc consists of the extracellular portion of mSIGNR1 (amino acid residues 75-225) fused at the C-terminus to a human IgG1-Fc fragment. mSIGNR1 Fc was produced in COS cells similarly as described for mICAM-2 Fc.
mSIGNR1-specific ELISA
mSIGNR1-specific antibody ER-TR9 (5 μg/mL) was coated in ELISA plates for 18 hours at 4°C, followed by blocking with 1% bovine serum albumin (BSA) for 30 minutes at 4°C. Soluble mSIGNR1 Fc (1 μg) was added and incubated for 2 hours at 4°C. Unbound protein was washed away and mSIGNR1 was detected by the mSIGNR1-specific antiserum 698 (1:200) 1 hour at 4°C, followed by peroxidase-conjugated goat-antirabbit IgG.
Fluorescent bead adhesion assay
Carboxylate-modified TransFluorSpheres (488/645 nm, 1.0 μm; Molecular Probes, Eugene, OR) were coated with ICAM-2-Fc, ICAM-3-Fc, and HIV-1 gp120MN as described previously.3,5 The fluorescent bead adhesion assay was performed as described.3 Briefly, 5 × 106cells in adhesion buffer (20 mM Tris [tris(hydroxymethyl)aminomethane)]-HCl, pH 8.0, 150 mM NaCl, 1 mM CaCl2, 2 mM MgCl2, 0.5% BSA) were preincubated with 20 μg/mL monoclonal antibody (mAb) for 10 minutes at room temperature. Ligand-coated fluorescent beads (20 beads/cell) were added and the suspension was incubated for 30 minutes at 37°C. Adhesion was determined using flow cytometry (FACScan, Becton Dickinson, Oxnard, CA) by measuring the percentage of cells, which had bound fluorescent beads.
Bone marrow–derived DCs
The DCs were generated as described previously,16with modifications. Briefly, femurs were dissected, placed in 70% alcohol for 1 minute, and washed with phosphate-buffered saline (PBS). Marrow was flushed and passed through nylon mesh to remove debris. After washing, lymphocytes, granulocytes, and I-A+ cells were removed by immunomagnetic depletion against the CD45R, CD4, CD8, and I-A antigens. Remaining cells were cultured overnight, and the nonadherent cells were seeded at 2 × 105 cells/mL and 4 mL/well in the presence of 20 ng/mL recombinant murine granulocyte macrophage colony-stimulating factor (rmGM-CSF) and 20 ng/mL recombinant murine interleukin 4 (rmIL-4) in 6-well plates (Costar, Badhoevedorp, The Netherlands). On day 4, the cultures were refreshed by adding 1 mL culture medium supplemented with GM-CSF and IL-4 (both at 10 ng/mL). At day 7, nonadherent and loosely adherent proliferating DC aggregates were collected and replated in fresh medium with cytokines (1 × 106 cells/mL).
CD11c+ DC isolation
Spleens were digested with collagenase IV for 40 minutes at 37°C, followed by erythrocyte lysis. Splenic CD11c+ DCs were isolated using MACS CD11c microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol.
Macrophage depletion in vivo
Systemic depletion of macrophages was performed by treatment with clodronate-filled liposomes, as described previously.17 Mice received a single intravenous injection of 0.2 mL multilamellar liposomes in PBS, containing 2 mg liposome-encapsulated clodronate (dichloromethylene biphosphonate, a kind gift from Roche Diagnostics, Mannheim, Germany). Depletion of macrophages from the spleen was assessed by immunofluorescence analysis 2 days after liposome injection.
Antigen capture
For studying the interaction of antigens with MZMs, 200 μL fluorescein isothiocyanate (FITC)–dextran (1 mg/mL, 500-kDa dextran; Molecular Probes) was injected intravenously into the tail veins of naive mice. Where appropriate, mice were treated 30 minutes before dextran administration with either 200 μL mannan (1 mg/mL) or 200 μL ER-TR9 by intravenous injection into the tail vein. Mice were killed 40 minutes after dextran injection by immediate CO2asphyxiation. The spleen was rapidly removed and directly frozen in Tissue-Tek (Sakura Finetek, Torrance, CA).
Immunofluorescence analysis
Tissues were collected and frozen in liquid nitrogen. Cryosections (7 μm) of the tissues were fixed in 100% acetone (10 minutes), washed with PBS, and incubated with the first antibody (10 μg/mL) for 60 minutes at 37°C. The final staining was performed with secondary antibodies labeled with either FITC or Texas red for 30 minutes at room temperature.
Results
Isolation of a murine homologue of DC-SIGN
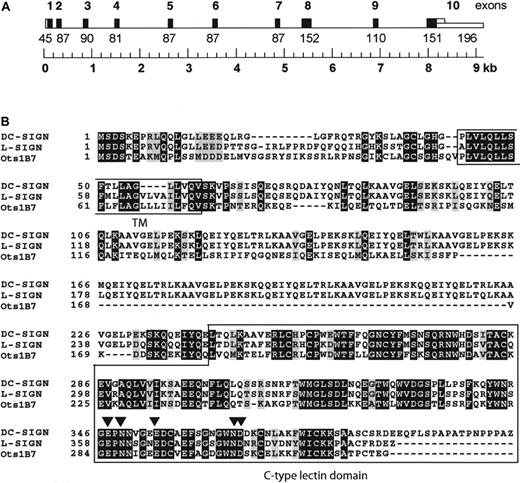
A 415-nucleotide–long cDNA fragment, called OtB7, was isolated by differential gene expression from a mouse model of allergic asthma, using lung-draining lymph node mRNA obtained from control and ovalbumin-challenged mice, which display phenotypic characteristics resembling human asthma.18 The cDNA fragment was homologous to hDC-SIGN and to 2 mouse genomic sequences present in the high-throughput division of GenBank (accession nos. AC073804.1 andAC073706.2). Using a combination of BLAST searches and multiple alignment analyses, we were able to construct a contiguous 19 619-bp–long genomic sequence composed of overlapping sequences present in both GenBank entries (Figure1A). A gene with homology to hDC-SIGN was predicted, and the 1101-bp cDNA encoding the predicted mDC-SIGN homologue (GenBank entry AF422108) was isolated by RT-PCR from lymph node mRNA. The OtB7 cDNA encodes a type II transmembrane C-type lectin consisting of 325 amino acid residues. The C-type lectin domain of the murine homologue has 74% similarity with that of hDC-SIGN, and the amino acid residues essential for ligand as well as Ca++binding are identical to those of hDC-SIGN19 (Figure 1B), suggesting a similar ligand specificity. The predicted transmembrane-spanning region is 85% identical to that of both hDC-SIGN and hL-SIGN. Comparison of the cytoplasmic region of OtB7 with that of the human homologues demonstrates that OtB7 lacks the LL sequence motif that is important for internalization of hDC-SIGN.4,20 The C-type lectin domain of hDC-SIGN is separated from the cell membrane by a stalk of 8 repeats, which form an α helix,21 whereas several splice variants of hL-SIGN exist, differing in the number of repeats from 3 to 9.6The stalk of the murine DC-SIGN homologue OtB7 is considerably shorter and lacks these repeats; however, the region contains regularly spaced hydrophobic sequences that have been observed in other α helices. The predicted amino acid sequence of OtB7 is as homologous to hL-SIGN as it is to hDC-SIGN.
Clone OtB7 is identical to the murine DC-SIGN homologue SIGNR1.
(A) Schematic representation of the genomic structure of clone OtB7. Exons are numbered and depicted as black bars. The genomic sequence was constructed by a combination of BLAST searches and multiple alignment analyses and is composed of overlapping sequences present in the GenBank entries (AC073804.1 and AC073706.2). (B) Clone OtB7 is homologous to both human DC-SIGN and L-SIGN as demonstrated by the amino acid sequence alignments of clone OtB7 (GenBank AF422108) with human DC-SIGN (AAK20997) and L-SIGN (AAK20998). Amino acid residues involved in direct ligand binding, as was shown for DC-SIGN,19 are denoted by filled arrowheads.
Clone OtB7 is identical to the murine DC-SIGN homologue SIGNR1.
(A) Schematic representation of the genomic structure of clone OtB7. Exons are numbered and depicted as black bars. The genomic sequence was constructed by a combination of BLAST searches and multiple alignment analyses and is composed of overlapping sequences present in the GenBank entries (AC073804.1 and AC073706.2). (B) Clone OtB7 is homologous to both human DC-SIGN and L-SIGN as demonstrated by the amino acid sequence alignments of clone OtB7 (GenBank AF422108) with human DC-SIGN (AAK20997) and L-SIGN (AAK20998). Amino acid residues involved in direct ligand binding, as was shown for DC-SIGN,19 are denoted by filled arrowheads.
mSIGNR1 binds both murine ICAM-2 and HIV-1 gp120
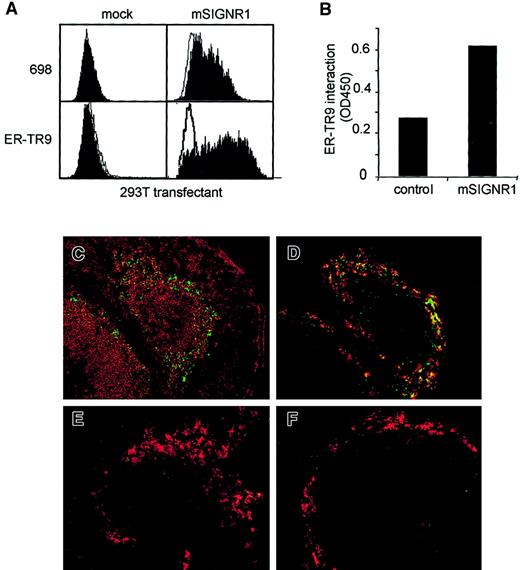
We raised polyclonal antibodies against 2 peptides present in the C-terminal region of mSIGNR1 to investigate the expression of this putative DC-SIGN homologue in different tissues. The human kidney 293T cell line was transfected with mSIGNR1 cDNA and mSIGNR1 was detected on the cell surface by the mSIGNR1-specific polyclonal antibody 698 (Figure 2A). Thus, as predicted, mSIGNR1 is a type II transmembrane protein because the antibodies, raised against the C-terminal region, stain intact 293T cells transfected with mSIGNR1 (Figure 2A). The antibodies stained neither mock-transfected nor hDC-SIGN–transfected cells (results not shown). The mAbs against hDC-SIGN (AZN-D1, -D2, and -D3) and hL-SIGN (AZN-D2 and -D3) did not cross-react with the murine homologue mSIGNR1 (results not shown).
mSIGNR1 binds both mouse ICAM-2 and HIV-1 gp120, similar to both hDC-SIGN and hL-SIGN.
(A) Polyclonal antibodies against mSIGNR1 stain 293T cells transfected with mSIGNR1 cDNA. 293T cells were transfected with different cDNAs and subsequently stained with antibodies against hDC-SIGN (AZN-D1; filled histogram) and mSIGNR1 (698; filled histogram). Isotype controls are shown as open histograms. One representative experiment of 3 is shown. (B) mSIGNR1 binds human ICAM-2, ICAM-3, and HIV-1 gp120 but not human ICAM-1, similar to hDC-SIGN and hL-SIGN. The adhesion of 293T cells transfected with mSIGNR1 to the ligands was determined using the fluorescent bead adhesion assay. Specificity was determined in the presence of blocking antibodies (AZN-D2, 20 μg/mL), mannan (5 μg/mL), or EGTA (5 mM). Cells were also preincubated with the polyclonal antibody against mSIGNR1 (1:10). One representative experiment of 3 is shown. (C) mSIGNR1 binds murine ICAM-2 with high affinity, similar to hDC-SIGN and hL-SIGN. The adhesion assay was performed as described in panel B. One representative experiment of 3 is shown.
mSIGNR1 binds both mouse ICAM-2 and HIV-1 gp120, similar to both hDC-SIGN and hL-SIGN.
(A) Polyclonal antibodies against mSIGNR1 stain 293T cells transfected with mSIGNR1 cDNA. 293T cells were transfected with different cDNAs and subsequently stained with antibodies against hDC-SIGN (AZN-D1; filled histogram) and mSIGNR1 (698; filled histogram). Isotype controls are shown as open histograms. One representative experiment of 3 is shown. (B) mSIGNR1 binds human ICAM-2, ICAM-3, and HIV-1 gp120 but not human ICAM-1, similar to hDC-SIGN and hL-SIGN. The adhesion of 293T cells transfected with mSIGNR1 to the ligands was determined using the fluorescent bead adhesion assay. Specificity was determined in the presence of blocking antibodies (AZN-D2, 20 μg/mL), mannan (5 μg/mL), or EGTA (5 mM). Cells were also preincubated with the polyclonal antibody against mSIGNR1 (1:10). One representative experiment of 3 is shown. (C) mSIGNR1 binds murine ICAM-2 with high affinity, similar to hDC-SIGN and hL-SIGN. The adhesion assay was performed as described in panel B. One representative experiment of 3 is shown.
The C-type lectin domain of mSIGNR1 contains the amino acid residues that have been shown to be essential in both ligand and Ca++ binding by hDC-SIGN.19 Therefore, we investigated whether mSIGNR1 was functionally similar to both hDC-SIGN and hL-SIGN. 293T cells transfected with mSIGNR1 cDNA interacted with human ICAM-2, ICAM-3, and HIV-1 gp120, but not with human ICAM-1, as was also observed for both hDC-SIGN– and hL-SIGN–expressing cells (Figure 2B). Thus, as predicted by the high homology between C-type lectin domains, mSIGNR1 has a similar ligand-binding activity to both of these human homologues.
Next we investigated whether mSIGNR1 is able to interact with murine ICAM-2, because mice do not express a homologue of human ICAM-3. Indeed, mSIGNR1-expressing cells interacted strongly with murine ICAM-2, in contrast to mock-transfected cells (Figure 2C). The adhesion was similar to that observed for both hDC-SIGN– and hL-SIGN–transfected cells (Figure 2C). Moreover, the adhesion was inhibited by both the Ca++ chelator EGTA (ethyleneglycoltetraacetic acid) and the polycarbohydrate mannan, demonstrating that mSIGNR1 functions as a mannose-binding C-type lectin (Figure 2C). The binding site on ICAM-2 for DC-SIGN and its homologues is conserved between species because both hL-SIGN and hDC-SIGN interact with murine ICAM-2 (Figure 2C), and mSIGNR1 binds to human ICAM-2 (Figure 2B). These data demonstrate that mSIGNR1 functions as an adhesion molecule with similar specificity to hDC-SIGN and hL-SIGN.
The expression pattern of mSIGNR1 is similar to that of hL-SIGN
Next, we used our polyclonal antibodies to investigate whether DCs express mSIGNR1, because mRNA encoding mSIGNR1 has been detected by RT-PCR from bone marrow–derived DCs22 and splenic CD11c+ DCs.12 Strikingly, no mSIGNR1 was detected on the cell surface of either bone marrow–derived DCs or splenic CD11c+ DCs (Figure3A), demonstrating that mSIGNR1, in contrast to hDC-SIGN, is not expressed by either in vitro– or in vivo–derived murine DCs. Immunofluorescence analysis demonstrated that mSIGNR1 is expressed by cells that line the sinusoids in the liver (Figure 3B). The mSIGNR1 staining was similar to that observed for hL-SIGN6 and demonstrates that mSIGRN1 is expressed by LSECs. This is further confirmed by the absence of mSIGNR1 expression from Kupffer cells, which were stained with the marker F4/80 (Figure3C).
The expression pattern of mSIGNR1 is similar to that of hL-SIGN.
(A) mSIGNR1 is not expressed by bone marrow–derived DCs or by splenic CD11c+ DCs. Bone marrow–derived DCs and splenic CD11c+ DCs were stained with the anti-mSIGNR1 antibody 698. Bone marrow DCs were more than 90% pure as determined by CD11c staining (data not shown). Dotted and open histograms represent isotype and mSIGNR1 staining, respectively. Splenic DCs were double-stained with CD11c and the mSIGNR1-specific antibody 698. (B,C) Immunofluorescence staining of murine liver tissue sections with the polyclonal antibody against mSIGNR1 698 (green, B and C) and double staining with F4/80 (red, C). Original magnification × 200. (D-G) Immunofluorescence double staining of murine lymph node tissue sections with the polyclonal antibody against mSIGNR1 698 (green) and in red CD11c (D), DEC-205 (E), SER-4 (F), MOMA-1 (G). Original magnification × 200.
The expression pattern of mSIGNR1 is similar to that of hL-SIGN.
(A) mSIGNR1 is not expressed by bone marrow–derived DCs or by splenic CD11c+ DCs. Bone marrow–derived DCs and splenic CD11c+ DCs were stained with the anti-mSIGNR1 antibody 698. Bone marrow DCs were more than 90% pure as determined by CD11c staining (data not shown). Dotted and open histograms represent isotype and mSIGNR1 staining, respectively. Splenic DCs were double-stained with CD11c and the mSIGNR1-specific antibody 698. (B,C) Immunofluorescence staining of murine liver tissue sections with the polyclonal antibody against mSIGNR1 698 (green, B and C) and double staining with F4/80 (red, C). Original magnification × 200. (D-G) Immunofluorescence double staining of murine lymph node tissue sections with the polyclonal antibody against mSIGNR1 698 (green) and in red CD11c (D), DEC-205 (E), SER-4 (F), MOMA-1 (G). Original magnification × 200.
Both hDC-SIGN and hL-SIGN are expressed in lymph nodes, and staining of murine lymph node tissue sections demonstrated that mSIGNR1 is also highly expressed in lymph nodes (Figure 3D-G), but not by lymph node–resident DCs because neither CD11c nor DEC205 colocalize with mSIGNR1 expression (Figure 3D-E). The mSIGNR1+ cells coexpress the antigens for SER-4 (Figure 3F) and MOMA-1 (Figure 3G), markers for both medullary and subcapsular macrophages in lymph nodes.23 Thus, mSIGNR1 is specifically expressed by medullary and subcapsular macrophages in lymph nodes. These data demonstrate that the cell-specific expression of mSIGNR1 is similar to that of hL-SIGN and this indicates mSIGNR1 is more closely related to hL-SIGN than to hDC-SIGN.
mSIGNR1 is specifically expressed by MZMs and is recognized by the MZM-specific antibody ER-TR9
We next investigated the expression of mSIGNR1 in the spleen, because mSIGNR1 mRNA has been detected in spleen (results not shown and Park et al12). We performed in situ immunofluorescence analysis using the polyclonal antibody 698 to identify those spleen cells expressing mSIGNR1, because no mSIGNR1 was expressed on the cell surface of splenic CD11c+ DCs (Figure 3A). mSIGNR1 is highly expressed by cells present in the marginal zone bordering the white pulp (Figure 4A-D). These mSIGNR1+ cells are not DCs because they do not express the DC markers CD11c and DEC-205 (Figure 4A-B). However, the mSIGNR1+ cells are specifically stained with the MZM-specific antibody ER-TR9 (Figure 4C). Thus, mSIGNR1 is specifically expressed by MZMs in the spleen and these cells are present in the marginal zone bordering the white pulp (Figure 4D). MZMs are highly phagocytotic cells whose dendrites are in direct contact with the arterial bloodstream (for a review, see Kraal24), and they can be effectively killed by a single intravenous injection of liposomes containing dichloromethylene diphosphonate.25Indeed, all MZMs were depleted from the spleen after injection of the liposomes, because no staining of the MZM marker ER-TR9 was observed (Figure 4E). Moreover, no staining was observed with the polyclonal antibodies against mSIGNR1, following MZM depletion from the spleen, confirming that mSIGNR1 is MZM specific (Figure 4F).
mSIGNR1 is specifically expressed by splenic MZMs.
(A-D) Immunofluorescence double staining of murine spleen tissue sections with the polyclonal antibody against mSIGNR1 698 (green) and in red CD11c (A), DEC-205 (B), ER-TR9 (C), B220 (D). Original magnification × 200). (E,F) Immunofluorescence of double staining of murine spleen tissue sections after depletion with dichloromethylene diphosphonate liposomes. Double staining with (E) CD11c (red) and ER-TR9 (green); and (F) CD11b (red) and polyclonal mSIGNR1 antibody 698 (green). Original magnification × 200.
mSIGNR1 is specifically expressed by splenic MZMs.
(A-D) Immunofluorescence double staining of murine spleen tissue sections with the polyclonal antibody against mSIGNR1 698 (green) and in red CD11c (A), DEC-205 (B), ER-TR9 (C), B220 (D). Original magnification × 200). (E,F) Immunofluorescence of double staining of murine spleen tissue sections after depletion with dichloromethylene diphosphonate liposomes. Double staining with (E) CD11c (red) and ER-TR9 (green); and (F) CD11b (red) and polyclonal mSIGNR1 antibody 698 (green). Original magnification × 200.
The antigen of the MZM-specific antibody ER-TR9 has not been identified to date. The similar expression patterns of the polyclonal antibody 698 against mSIGNR1 and the MZM-specific antibody ER-TR9 in spleen (Figure4), liver, and lymph node (results not shown) led us to investigate whether mSIGNR1 is the antigen for this antibody. Indeed, 293T cells transfected with mSIGNR1 were stained with the monoclonal antibody ER-TR9, whereas mock-transfected 293T cells were not (Figure5A). Thus, the receptor recognized by ER-TR9 on MZMs is mSIGNR1. Moreover, this was further confirmed by the specific recognition of the recombinant mSIGNR1 fusion protein by ER-TR9, in a mSIGNR1-specific sandwich ELISA (Figure 5B). Comparison of the ER-TR9–specific expression pattern in spleen, lymph node, and liver with that of the polyclonal antibody 689 against mSIGNR1 demonstrates that ER-TR9 specifically recognizes mSIGNR1 but not the other murine homologues of DC-SIGN (results not shown and Figure 4).
mSIGNR1 efficiently captures polysaccharide antigens in vivo.
(A) The MZM-specific antibody ER-TR9 stains 293T cells transfected with mSIGNR1 cDNA. 293T cells were transfected with mSIGNR1 cDNA and subsequently stained either with the mSIGNR1-specific antibodies 698 or ER-TR9 (filled histograms). Isotype controls are represented by open histograms. (B) Recombinant mSIGNR1 is specifically detected by ER-TR9 using a sandwich ELISA. ER-TR9–coated wells are incubated with recombinant mSIGNR1 and binding is detected by the mSIGNR1-specific antibody 698. SD is less than 0.05. (C,D) MZMs efficiently capture dextran-FITC in vivo. Naive mice were injected intravenously with FITC-dextran. Spleens were isolated and immunofluorescence analyses of murine spleen tissue sections were performed. Double stainings with FITC-dextran (green) and B220 (red, C) or mSIGNR1-specific antibody 698 (red, D) are shown. Original magnification × 200. (E,F) Dextran capture by MZMs in vivo is blocked by the mSIGNR1-specific antibody ER-TR9 and mannan. Naive mice were treated with either purified ER-TR9 (E) or the yeast-derived polysaccharide mannan (F) prior to intravenous injection with FITC-dextran. Double stainings were performed with FITC-dextran (green) and the mSIGNR1-specific antibody 698 (red). Original magnification × 200.
mSIGNR1 efficiently captures polysaccharide antigens in vivo.
(A) The MZM-specific antibody ER-TR9 stains 293T cells transfected with mSIGNR1 cDNA. 293T cells were transfected with mSIGNR1 cDNA and subsequently stained either with the mSIGNR1-specific antibodies 698 or ER-TR9 (filled histograms). Isotype controls are represented by open histograms. (B) Recombinant mSIGNR1 is specifically detected by ER-TR9 using a sandwich ELISA. ER-TR9–coated wells are incubated with recombinant mSIGNR1 and binding is detected by the mSIGNR1-specific antibody 698. SD is less than 0.05. (C,D) MZMs efficiently capture dextran-FITC in vivo. Naive mice were injected intravenously with FITC-dextran. Spleens were isolated and immunofluorescence analyses of murine spleen tissue sections were performed. Double stainings with FITC-dextran (green) and B220 (red, C) or mSIGNR1-specific antibody 698 (red, D) are shown. Original magnification × 200. (E,F) Dextran capture by MZMs in vivo is blocked by the mSIGNR1-specific antibody ER-TR9 and mannan. Naive mice were treated with either purified ER-TR9 (E) or the yeast-derived polysaccharide mannan (F) prior to intravenous injection with FITC-dextran. Double stainings were performed with FITC-dextran (green) and the mSIGNR1-specific antibody 698 (red). Original magnification × 200.
mSIGNR1 captures blood-borne antigens in vivo
The spleen plays an important role in the defense against pathogens and MZMs are important in this defense (for a review, see Kraal24). MZMs are in direct contact with the bloodstream and are highly phagocytotic cells. These cells have been demonstrated to bind specific polysaccharide antigens present at the surface of encapsulated bacteria.26,27 We examined the molecular basis for this specificity by determining the mechanisms by which these antigens preferentially targeted to MZMs. Polysaccharide antigens rapidly localize to the MZMs as demonstrated by intravenous injection of naive wild-type mice with the model polysaccharide antigen FITC-dextran (Figure 5C-D). The FITC-dextran staining is localized in the marginal zone bordering the white pulp (Figure 5C) and colocalizes with the anti-mSIGNR1 polyclonal antibody 698 (Figure 5D). Because mSIGNR1 contains a C-type lectin domain similar to hDC-SIGN, which has been shown to bind polycarbohydrates,3 28 we next determined whether mSIGNR1 was integral to this dextran capture by MZMs. Naive mice were injected with purified ER-TR9 and after 40 minutes FITC-dextran was injected intravenously. In these mice, the FITC-dextran was not retained in the spleen by MZMs (Figure 5E) because no FITC-dextran was detected in the marginal zone. This demonstrates that mSIGNR1 is responsible for the efficient capture of dextran by MZMs. These results were further confirmed by injecting naive mice with the yeast-derived polysaccharide mannan prior to FITC-dextran injection. Strikingly, MZMs were then no longer able to capture FITC-dextran (Figure 5F) because mannan is efficiently bound by mSIGNR1 and blocks its function (Figure 2B-C). Thus, the well-known function of MZMs to filter blood-borne antigens from blood is mediated by mSIGNR1, and this mSIGNR1 function is inhibited by ER-TR9, and other polycarbohydrates such as mannan.
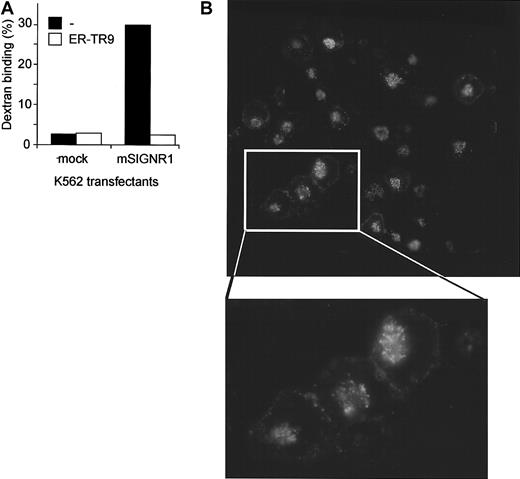
The MZMs rapidly internalize antigens on binding and we investigated whether mSIGNR1 also mediates the internalization of ligands. K562 transfectants expressing mSIGNR1 have a high affinity for FITC-dextran, whereas mock-transfected cells do not (Figure6A), confirming the in vivo function of mSIGNR1. The dextran interaction with mSIGNR1 in vitro was specifically blocked by the ER-TR9 antibody (Figure 6A). The fate of the bound dextran was followed by immunofluorescence analysis. FITC-dextran was rapidly internalized and targeted to the lysosomes (Figure 6B), demonstrating that mSIGNR1 is responsible for both capture and internalization of dextran by MZMs, and suggesting that mSIGNR1 captures antigens from blood for degradation and antigen processing.
mSIGNR1 mediates rapid internalization of captured dextran.
(A) Dextran is efficiently captured by mSIGNR1. K562 cells expressing mSIGNR1 and mock-transfected K562 cells were incubated with FITC-dextran (1 μg/mL) at 37°C and unbound dextran was washed away after 45 minutes. Specificity was determined in the presence of the antibody ER-TR9. Binding was analyzed by flow cytometry. SD was less than 5%. (B) Dextran is rapidly internalized by SIGNR1. The fate of bound FITC-dextran was followed by analyzing FITC-staining of K562 cells expressing mSIGNR1 after incubation with FITC-dextran using immunofluorescence microscopy. Original magnification × 200.
mSIGNR1 mediates rapid internalization of captured dextran.
(A) Dextran is efficiently captured by mSIGNR1. K562 cells expressing mSIGNR1 and mock-transfected K562 cells were incubated with FITC-dextran (1 μg/mL) at 37°C and unbound dextran was washed away after 45 minutes. Specificity was determined in the presence of the antibody ER-TR9. Binding was analyzed by flow cytometry. SD was less than 5%. (B) Dextran is rapidly internalized by SIGNR1. The fate of bound FITC-dextran was followed by analyzing FITC-staining of K562 cells expressing mSIGNR1 after incubation with FITC-dextran using immunofluorescence microscopy. Original magnification × 200.
Discussion
Antigen-presenting cells are present in essentially every tissue where they operate at the interface of innate and acquired immunity by recognizing pathogens and presenting pathogen-derived peptides to T cells. C-type lectins play an important role as pathogen recognition receptors that capture and destroy pathogens for antigen processing to permit major histocompatibility complex (MHC) class II–restricted presentation. It is becoming clear that not all C-type lectins serve as antigen receptors recognizing pathogens through carbohydrate structures. The DC-specific C-type lectin hDC-SIGN is unique in that it regulates adhesion processes, such as DC trafficking through ICAM-2, and T-cell synapse formation through ICAM-3, as well as antigen capture.3,4 Moreover, even though several C-type lectins have been shown to bind HIV-1, hDC-SIGN not only captures HIV-1, but also protects it in early endosomes allowing HIV-1 transport by peripheral DCs to the lymphoid tissues, where it enhancestrans-infection of T cells.5
However, functional studies into the in vivo function of DC-SIGN have been hampered by the lack of murine homologues. Here we report the identification and isolation of a cDNA called OtB7, which encodes a murine protein that is homologous to both hDC-SIGN and hL-SIGN (Figure1B). During the preparation of this manuscript, 5 murine DC-SIGN homologues were identified by RT-PCR from CD11c+ DCs although only one, called murine DC-SIGN (mDC-SIGN), was expressed at high mRNA levels in CD11c+ DCs.12 In contrast the other cDNAs, designated mSIGNR1-4, were hardly detectable in DC, but were detected at various mRNA levels in B and T cells by RT-PCR.12 The OtB7 clone that we have isolated from lymph node cDNA is identical to the DC-SIGN homologue mSIGNR1. We generated polyclonal antibodies against mSIGNR1 to investigate the cell-specific expression of this hDC-SIGN homologue, because of the complex mRNA expression patterns of the reported DC-SIGN homologues. mSIGNR1 is expressed neither by in vitro–generated DCs nor by in situ CD11c+ DCs in spleen and lymph nodes (Figures 3 and 4). However, mSIGNR1 is expressed by LSECs in liver and by medullary and subcapsular macrophages in the lymph nodes (Figure 3). This specific expression is very similar to that of hL-SIGN, which is expressed by LSECs6 8 and by a specific subpopulation of nonendothelial macrophagelike cells in lymph nodes (A. Engering, personal communication, November 2001), indicating that, based on expression, mSIGNR1 is more closely related to hL-SIGN than to hDC-SIGN.
Intermediate mRNA levels of mSIGNR1 were detected in murine spleen by RNA hybridization (data not shown and Park et al12). Here, we demonstrated using mSIGNR1-specific antibodies that mSIGNR1 is specifically expressed by ER-TR9+ MZMs in the spleen (Figure 4). These data were further confirmed by the intravenous injection of liposomes containing dichloromethylene diphosphonate, which systemically depletes macrophages from the red pulp and marginal zone.25 Injection of the liposomes depleted the spleen of MZMs as shown by the absence of ER-TR9 staining (Figure 4E). No mSIGNR1 was present in MZM-depleted spleens confirming the specific expression of mSIGNR1 by MZMs (Figure 4F). Interestingly, hDC-SIGN is expressed in spleen in contrast to hL-SIGN (T.B.H.G., unpublished results, October 2000). Thus, the expression pattern of mSIGNR1 is similar but not identical to that of hL-SIGN, and mSIGNR1 has attributes of both hDC-SIGN and hL-SIGN suggesting that the distinction between hL-SIGN and hDC-SIGN may be different and far less defined in the murine system.
The C-type lectin domain of mSIGNR1 has 74% similarity with that of hDC-SIGN, and the amino acid residues important for both Ca++ and ligand binding19 are conserved in the murine homologue. Indeed, mSIGNR1 has a similar binding activity for hICAM-2, hICAM-3, and HIV-1 gp120 to both hDC-SIGN and hL-SIGN (Figure2). Thus, mSIGNR1 can function as an adhesion receptor. However, mice do not express an ICAM-3 homologue and therefore binding of mSIGNR1 to ICAM-3 is not physiologic. mSIGNR1 also interacts with murine ICAM-2, which is widely expressed on murine lymphocytes,29 and could therefore function as the leukocyte ligand for mSIGNR1 mediating contact between mSIGNR1+ cells and leukocytes in mice. Strikingly, mSIGNR1 is abundantly expressed in liver and spleen by LSECs and MZMs, respectively. Both cell types are in direct contact with the blood and are important in lymphocyte interactions. LSEC-leukocyte interactions appear to constitute a central mechanism of peripheral immune surveillance in the liver.30 The expression and ligand specificity of mSIGNR1 suggests a physiologic role for this receptor in LSEC-leukocyte adhesion through binding of ICAM-2 on lymphocytes, similar to hL-SIGN.6 The strategic localization of MZMs in the spleen resembles that of LSECs in the liver, and the splenic marginal zone is important as a gateway for migrating lymphocytes (for a review, see Kraal24). MZMs interact with incoming lymphocytes and antigens in the marginal sinus because part of the arterial blood supply opens into the wider space of the marginal sinus resulting in a blood flow with lower resistance. The only entry into the white pulp is via the marginal zone, and lymphocytes entering the marginal zone are either directed into the white pulp or will leave the marginal zone through the red pulp side. Little is known about the cellular interactions important for this lymphocyte migration but carbohydrate interactions are known to be important in this migration to the white pulp because polysaccharides such as mannan inhibit this process.26,31,32 Strikingly, here we demonstrate that the mSIGNR1-dependent binding of ICAM-2 is inhibited by mannan, suggesting that mSIGNR1 provides the interactions that enable lymphocyte migration into the white pulp. This hypothesis is further supported by the clustering of MZMs with lymphocytes26 and the high expression of ICAM-2 on murine lymphocytes.29
Besides being a gateway for migrating lymphocytes, the splenic marginal zone is also important as a defense against pathogens. Because of their position adjacent to the marginal sinuses, MZMs are among the first cells that can interact with blood-borne antigens and are presumed to have a critical role in host defense against bacterial pathogens (for a review, see Kraal24). MZMs are specifically stained by the monoclonal antibody ER-TR913and although its antigen had not been identified, results suggested that the ER-TR9 antigen is involved in antigen capture. Strikingly, mSIGNR1 is specifically expressed by MZMs and we demonstrate here that mSIGNR1 is the antigen of ER-TR9 (Figure 5A-B). Thus, the C-type lectin mSIGNR1 may have a function as a pathogen recognition receptor. C-type lectins play an important role as pathogen recognition receptors that capture and destroy pathogens for antigen processing to permit MHC class II–restricted presentation. Recently, we have demonstrated that hDC-SIGN functions as a pathogen recognition receptor in vitro that rapidly internalizes captured antigen into lysosomal compartments for processing and antigen presentation.4 Therefore, we investigated whether mSIGNR1 is involved in pathogen recognition and capture in vivo. MZMs are highly phagocytotic cells as demonstrated by their depletion after injection of liposomes containing dichloromethylene diphosphonate (Figure 4) and their capacity to filter pathogens from blood.27 Indeed, bacterial antigens rapidly localize to the MZMs as demonstrated by intravenous injection of naive wild-type mice with the model antigen, FITC-dextran (Figure 5C). The FITC-dextran is specifically captured by the MZMs in the marginal zone and the FITC staining colocalizes with antibodies against mSIGNR1 (Figure 5D). Treatment of naive mice with purified anti-SIGNR1 antibody ER-TR9 prior to the FITC-dextran injection completely abrogates dextran binding by MZMs (Figure 5E), demonstrating that mSIGNR1 mediates the dextran capture by MZMs. The anti-SIGNR1 antibody ER-TR9 also inhibits the in vitro binding of mSIGNR1 to dextran-FITC (Figure 6A), confirming that ER-TR9 indeed inhibits the antigen uptake by mSIGNR1 observed in vivo. Injection of the yeast-derived polysaccharide mannan prior to FITC-dextran injection also prevents dextran binding by MZMs, demonstrating that mSIGNR1 also binds mannan in vivo (Figure 5F) and that mannan blocks the in vivo function of mSIGNR1. These data demonstrate that mSIGNR1 is involved in pathogen recognition by MZMs in vivo. Moreover, antigens captured by mSIGNR1 are rapidly internalized and targeted to lysosomes for degradation (Figure 6). Rapid internalization of mSIGNR1 on ligand binding (Figure 6B) suggests that ER-TR9 blocks binding in vivo through down-regulation of mSIGNR1 on the surface of MZMs. The cytoplasmic region of mSIGNR1 lacks the di-leucine motif present in hDC-SIGN, which mediates the internalization of hDC-SIGN. However, the murine receptor contains a tri-acid cluster (DDD) in its cytoplasmic region that functions as an internalization motif in DEC-205 where it is responsible for targeting to lysosomes for antigen processing.33 The observed lysosomal targeting of internalized ligand-mSIGNR1 complexes is likely be due to this tri-acidic cluster. Thus, mSIGNR1 is important in the defense against pathogens by capturing pathogens from the blood, which are then rapidly internalized into lysosomes and processed. MZMs do not express MHC class II molecules and it is thought that pathogen degradation products are shed from the cell surface and taken up by marginal zone B cells after opsonization by complement. The marginal zone B cells migrate into the follicle and present the captured antigens to elicit an immune response.34 Thus, the function of mSIGNR1 links innate immunity with adaptive immunity. Furthermore, the affinity of mSIGNR1 for HIV-1 gp120 suggests that mSIGNR1 may be able to interact with both pathogens containing dextranlike and mannanlike structures, and viruses containing viral components with structures similar to gp120.
In conclusion, we have isolated a murine homologue of hDC-SIGN, mSIGNR1, and investigated its cell-specific protein expression using antibodies against mSIGNR1. We have demonstrated that mSIGNR1 functions as a pathogen recognition receptor in vivo and thus is important in the defense against pathogens. This in vivo model and the identification of blocking anti-mSIGNR1 antibodies will facilitate studies into the in vivo function of DC-SIGN.
We are grateful to E. van Kesteren-Hendrix and N. van Rooijen for liposome-depleted tissues. We thank J. Samsom, W. Jansen, and R. Mebius for helpful discussions and providing antibodies, and L. Colledge for suggestions and editing of the manuscript.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-04-1044.
P.C.G. and M.A.N. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Teunis B. H. Geijtenbeek, Department of Molecular Cell Biology, Vrije Universiteit Medical Center Amsterdam, v.d. Boechorststraat 7, 1081 BT Amsterdam, The Netherlands; e-mail:t.geijtenbeek.cell@med.vu.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal