Plasma von Willebrand factor (VWF) is a multimeric protein that mediates adhesion of platelets to sites of vascular injury; however, only the very large VWF multimers are effective in promoting platelet adhesion in flowing blood. The multimeric size of VWF can be controlled by the glycoprotein, thrombospondin-1 (TSP-1), which facilitates reduction of the disulfide bonds that hold VWF multimers together. The TSP family of extracellular glycoproteins consists of 5 members in vertebrates, TSP-1 through TSP-4 and TSP-5/COMP. TSP-1 and TSP-2 are structurally similar trimeric proteins composed of disulfide-linked 150-kDa monomers. Recombinant pieces of TSP-1 and TSP-2 incorporating combinations of domains that span the entire subunit were produced in insect cells and examined for VWF reductase activity. VWF reductase activity was present in the Ca++-binding repeats and C-terminal sequence of TSP-1, but not of TSP-2. Alkylation of Cys974 in the C-terminal TSP-1 construct, which is a serine in TSP-2, ablated VWF reductase activity. These results imply that the reductase function of TSP-1 centers around Cys974 in the C-terminal sequence.
Introduction
Platelet adhesion to von Willebrand factor (VWF) in the subendothelium of a damaged blood vessel is the initial step in formation of a hemostatic plug at high shear rates (for a review, see Sadler1). VWF also acts in synergy with fibrinogen in the formation of interplatelet adhesive links to form a stable thrombus at arterial shear rates.2,3 As a carrier for procoagulant factor VIII, VWF prolongs its survival in the circulation by protecting it from inactivation by activated protein C and factor Xa. VWF is synthesized by vascular endothelial cells and megakaryocytes and circulates in blood as a series of multimers containing a variable number of about 500-kDa homodimers.4 The largest VWF multimers have a molecular mass of approximately 20 000 kDa, comparable in length to the diameter of a medium platelet (2 μM), and are released from endothelial cells following stimulation.
The assembly of VWF multimers follows a stepwise process. Pro-VWF dimers are assembled in the endoplasmic reticulum via disulfide bridges between cysteine residues located in the cysteine knotlike domains at the C-terminal ends of the pro-VWF subunits. Intersubunit disulfide bonds involve 1 or 3 of the cysteine residues at positions 2008, 2010, and 2048.5 These tail-to-tail linked pro-VWF dimers are subsequently multimerized within the Golgi apparatus by head-to-head linkage by disulfide bonds near the N-terminal domains.6 Interdimeric disulfide bonds involve Cys379 and one or more of the cysteine residues at positions 459, 462, and 464.7 After multimerization, the VWF propeptides are removed by proteolysis.6
Only large multimeric forms of VWF are hemostatically active.8 The unusually large VWF multimers secreted by endothelial cells have been shown to be more effective than the largest plasma forms in inducing platelet aggregation under conditions of high fluid shear.9 This functional importance of multimer size relates to the affinity of VWF for its ligands. Large VWF multimers bind with an approximate 100-fold greater affinity to both collagen and platelets than monomeric VWF.8 Some thrombotic disorders are characterized by altered VWF multimer size. Thrombotic thrombocytopenic purpura (TTP) is often associated with unusually large VWF multimers in the blood, which are thought to precipitate intravascular platelet clumping.10 11 Conversely, lower than average multimer size characterizes the bleeding diathesis of type IIA von Willebrand disease. Modulation of VWF multimer size is, therefore, critical to the control of its hemostatic activity.
We recently reported that the homotrimeric glycoprotein, thrombospondin-1 (TSP-1; for a review, see Lawler12), reduces the average multimer size of plasma or purified VWF both in vitro and in vivo.13 14 Incubation of TSP-1 with VWF results in formation of thiol-dependent complexes of TSP-1 and VWF, generation of new thiols in VWF, and reduction in the average multimer size of VWF. Moreover, the ratio of the concentrations of TSP-1 and VWF in plasma reflect the average multimer size of VWF. The higher the plasma TSP-1/VWF molar ratio the smaller the average VWF multimer size. These results indicate that TSP-1 regulates the multimeric size and therefore hemostatic activity of VWF. We show herein that the VWF reductase activity of TSP-1 resides in the Ca++-binding and C-terminal sequences and requires a free thiol at Cys974.
Materials and methods
Proteins and reagents
2-Nitro-5-thiocyanobenzoic acid (NTCB), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), N-ethylmaleimide (NEM), reduced glutathione (GSH), and EDTA (ethylenediaminetetraacetic acid) were from Sigma (St Louis, MO). 3-(N-maleimidylpropionyl)biocytin (MPB) was from Molecular Probes (Eugene, OR). Purified human plasma VWF and recombinant human VWF was used in this study. Plasma VWF was a gift from Dr Michael Berndt; recombinant VWF produced in baby hamster kidney cells and purified by immunoaffinity chromatography was a gift from Dr Eric Huizinga. VWF concentration was measured by BCA protein assay (Pierce, Rockford, IL). TSP-1 was purified from human platelet concentrates as described previously15 with some modifications.16 Buffers containing 0.1 mM CaCl2 were used throughout the chromatographic purification of TSP-1. TSP-1 concentration was calculated using an absorption coefficient of 10.9 for a 1% solution at 280 nm. All other reagents were of analytical grade.
TSP-1 and TSP-2 fragments
Modular pieces of TSP-1 and TSP-2 were expressed using a baculovirus expression system and purified by nickel chelate chromatography as described.17-19 Briefly, DNA encoding for TSP-1 proteins NoC-1 (residues 1-356), CP123-1 (294-529), P3E123-1 (473-673), E3CaG-1 (630-1152), and delNo-1 (294-1152) and TSP-2 proteins delNo-2 (290-1154) and E3CaG-2 (632-1154) were cloned into the baculovirus transfer vector pAcGP67.coco. The Baculogold transfection module (BD Biosciences Pharmingen, San Diego, CA) was cotransfected with a pAcGP67.coco clone into SF9 cells. Individual viral clones were isolated, and high-titer viral stocks (> 108 pfu/mL) were obtained. The TSP-derived proteins were expressed by infection of High 5 cells in SF900II serum-free media at a multiplicity of infection (MOI) of about 5 followed by growth at 27°C for 65 to 72 hours. The His-tagged proteins were purified from the conditioned media by nickel chelate chromatography.
Assays for VWF multimer size
The VWF (8 nM) was incubated with TSP-1 or TSP-1/TSP-2 fragments (0.8, 8, or 80 nM) for 1 hour at 37°C. All dilutions were made with 50 mM HEPES (N-2-hydroxyethylpiperazine-N′2-ethanesulfonic acid), 0.125 M NaCl, pH 7.4 buffer (HEPES-buffered saline) containing 0.1 mM CaCl2. Aliquots (30 μL) of the reactions were diluted 20-fold in 20 mM imidazole, 5 mM citric acid, 0.12 M NaCl, pH 7.3 buffer containing 5% bovine serum albumin and assayed for collagen-binding affinity and VWF antigen as described by Favaloro et al20 and Xie et al.13 Reactions were assayed in triplicate for collagen-binding affinity and VWF antigen and the ratio of the 2 measurements was reported. The overall error was calculated by adding the relative errors (1 SD) of each measurement. The data groups were compared using a one-way ANOVA and a Tukey post-hoc test was applied to compute significance between groups.
Citrated normal plasma was incubated with an equal volume of purified platelet TSP-1, E3CaG-1, or E3CaG-2 in HEPES-buffered saline containing 50 mM CaCl2 and 10 μM D-Phe-Pro-Arg-chloromethylketone (Calbiochem-Novabiochem, Bad Soden, Germany) for 1 hour at 37°C. The final concentrations of TSP-1, E3CaG-1, and E3CaG-2 were 40 nM, 400 nM, and 400 nM, respectively. Aliquots of the reactions (10 μL) were resolved on 1% agarose gel electrophoresis,21 transferred to polyvinylidene difluoride (PVDF) membrane (DuPont NEN, Boston, MA), blotted with 2 μg/mL peroxidase-conjugated anti-VWF polyclonal antibodies (Dako, Carpinteria, CA) and visualized using chemiluminescence (DuPont NEN).
Assay for formation of new thiols in VWF
The biotin-linked maleimide, MPB, was used to measure reduction of VWF disulfide bond(s) by TSP-1 or TSP-1/TSP-2 fragments. The protocol was essentially as described by Xie et al.13Briefly, aliquots (250 μL) of the incubation mixtures used to measure VWF multimer size were labeled with MPB (100 μM) for 10 minutes at 37°C and the unreacted MPB was quenched with GSH (200 μM) for 10 minutes at 37°C. The MPB-labeled VWF was incubated in microtiter plate wells coated with antihuman VWF polyclonal antibodies (Dako), and the biotin label was detected using StreptABComplex/HRP (Dako). The reactions were assayed in triplicate and the mean and 1 SD is reported.
Quantitation of thiols in E3CaG-1
The number of thiols in E3CaG-1 was measured using DTNB. E3CaG-1 (∼10 μM) was incubated with DTNB (∼1 mM) in 0.1 M HEPES, 0.3 M NaCl, 10 mM EDTA, pH 7.0 buffer for 10 minutes at room temperature and the TNB was measured from the absorbance at 412 nm using a Molecular Devices Thermomax Plus (Palo Alto, CA) microplate reader. The extinction coefficient for the TNB dianion at pH 7.0 is 14 150 M−1cm−1 at 412 nm.22
Alkylation of E3CaG-1 with maleimides
E3CaG-1 (0.5 mg/mL) was incubated with 10 mM NEM for 20 hours in HEPES-buffered saline containing 0.1 mM CaCl2, and the excess NEM was removed by dialysis against the same HEPES buffer. The unreacted and alkylated E3CaG-1 (10 μg/mL) was labeled with MPB (100 μM) for 30 minutes at room temperature in HEPES-buffered saline containing 10 mM EDTA. Samples of the labeled proteins (0.2 μg) were resolved on 8% to 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions, transferred to PVDF membrane, blotted with a 1:2000 dilution of streptavidin-peroxidase (Dako), and visualized using chemiluminescence.
Mapping the free thiol in E3CaG-1
NTCB specifically S-cyanylates cysteine thiols and the peptide bond on the N-terminal side of the cyanylated cysteine is then cleaved under mildly alkaline conditions.23 24E3CaG-1 (0.2 mg/mL) in 0.1 M Tris (tris(hydroxymethyl)aminomethane), pH 8.0 buffer containing either 0.1 mM or 2 mM Ca++ was incubated with NTCB (20 mM) for 60 minutes at 37°C to cyanylate the cysteine thiols. The pH of the reaction was adjusted to 9.0 using 3.0 M Tris base and incubated at 37°C for a further 60 minutes. The resulting fragments were reduced with dithiothreitol, alkylated with iodoacetamide, and resolved by 8% to 16% SDS-PAGE. The Coomassie-stained fragments were cut from the gel and analyzed by mass spectrometry.
Peptide mass fingerprinting
The SDS-PAGE gel pieces were completely destained in 1:1 acetonitrile and 25 mM NH4HCO3 (4 × 200 μL, 30 minutes), then acetonitrile (100 μL, 10 minutes) and dried in a vacuum centrifuge. The gel pieces were rehydrated in 20 μL 10 μM NH4HCO3 containing about 10 μg/mL trypsin and incubated at 37°C overnight. Aliquots (0.5 μL) of each sample were added to a matrix (1 μL 10 mg/mL 2,5-dihydroxybenzoic acid) and spotted onto a 100-well sample plate and analyzed by matrix-assisted laser desorption/ionization reflectron time of flight mass spectrometry (MALDI-rTOFMS) as described.25 The molecular mass profile of the trypsin-digested fragments was then compared with the theoretical tryptic digestion of E3CaG-1.
Results
TSP-1 and TSP-2 fragments
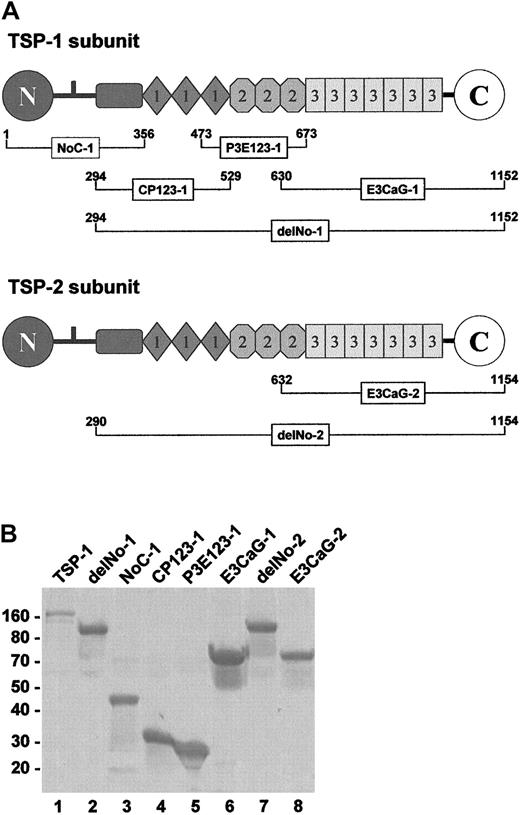
The domain structures of TSP-1 and TSP-2 and the recombinant pieces used in our experiments are shown in Figure1A. Overlapping TSP-1 constructs span the entire subunit. The TSP-2 constructs focused on the region that was active in the TSP-1 constructs. The SDS-PAGE profile of the reduced pieces are shown in Figure 1B. Without reduction, all migrated similarly except for NoC-1, which was trimeric.
TSP-1 and TSP-2 fragments.
(A) The subgroup A thrombospondins, TSP-1 and TSP-2, share a similar molecular architecture comprising a unique heparin-binding domain at the N-terminus followed by a connecting region that links 3 subunits via interchain disulfide bonds, a procollagenlike module, 3 properdinlike or type 1 modules, 3 EGF-like or type 2 modules, 7 type-3 repeats (12 unique calcium-binding loops), and a unique C-terminal sequence. The NoC-1 fragment contains the connecting region and is trimeric. The residue numbers are for the mature proteins. TSP-1 begins at Asn1, while TSP-2 begins at Gly1. (B) SDS-PAGE profile of the TSP-1 and TSP-2 fragments. Samples (4 μg) of TSP-1 (lane 1) and the TSP-1 (lanes 2-6) and TSP-2 (lanes 7 and 8) fragments were resolved on 8% to 16% SDS-PAGE under reducing conditions and stained with Coomassie blue. The positions of Mr markers are shown at left.
TSP-1 and TSP-2 fragments.
(A) The subgroup A thrombospondins, TSP-1 and TSP-2, share a similar molecular architecture comprising a unique heparin-binding domain at the N-terminus followed by a connecting region that links 3 subunits via interchain disulfide bonds, a procollagenlike module, 3 properdinlike or type 1 modules, 3 EGF-like or type 2 modules, 7 type-3 repeats (12 unique calcium-binding loops), and a unique C-terminal sequence. The NoC-1 fragment contains the connecting region and is trimeric. The residue numbers are for the mature proteins. TSP-1 begins at Asn1, while TSP-2 begins at Gly1. (B) SDS-PAGE profile of the TSP-1 and TSP-2 fragments. Samples (4 μg) of TSP-1 (lane 1) and the TSP-1 (lanes 2-6) and TSP-2 (lanes 7 and 8) fragments were resolved on 8% to 16% SDS-PAGE under reducing conditions and stained with Coomassie blue. The positions of Mr markers are shown at left.
The VWF-reducing activity of TSP-1 was contained in the E3CaG-1 fragment
Increasing concentrations of TSP-1 and TSP-1 constructs were incubated with VWF for 1 hour and VWF reductase activity was identified by the concurrent reduction of VWF multimer size and generation of new thiols in VWF. The ratio of collagen-binding affinity to VWF antigen (CBA/VWF:Ag ratio) was used as a surrogate measure of the average VWF multimer size. Reduction of disulfide bonds in VWF was measured from incorporation of the biotin-linked maleimide, MPB.
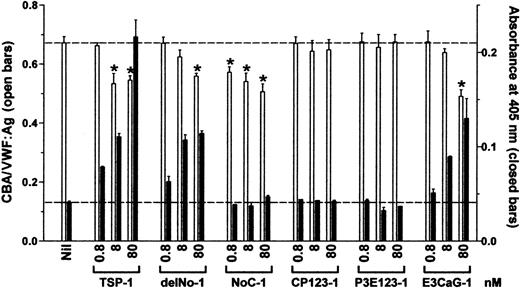
The VWF reductase activity was limited to 2 TSP-1 constructs, delNo-1 and E3CaG-1, which both contain the third type 2 repeat, the 7 type 3 domains, and the C-terminal sequence (Figure2). CP123-1 and P3E123-1 were devoid of VWF reductase activity over the same concentration range used for intact TSP-1, delNo-1, or E3CaG-1.
The VWF-reducing activity of TSP-1 is contained in the E3CaG-1 fragment.
VWF (8 nM) was incubated with 0.8, 8, or 80 nM TSP-1, delNo-1, NoC-1, CP123-1, P3E123-1, or E3CaG-1. Aliquots of the reactions were analyzed for the average VWF multimer size and for the generation of new thiols in VWF. The average VWF multimer size was estimated from the ratio of the collagen-binding activity and VWF antigen level (■). Generation of new free thiols in VWF was assessed from incorporation of the biotin-linked maleimide, MPB (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05).
The VWF-reducing activity of TSP-1 is contained in the E3CaG-1 fragment.
VWF (8 nM) was incubated with 0.8, 8, or 80 nM TSP-1, delNo-1, NoC-1, CP123-1, P3E123-1, or E3CaG-1. Aliquots of the reactions were analyzed for the average VWF multimer size and for the generation of new thiols in VWF. The average VWF multimer size was estimated from the ratio of the collagen-binding activity and VWF antigen level (■). Generation of new free thiols in VWF was assessed from incorporation of the biotin-linked maleimide, MPB (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05).
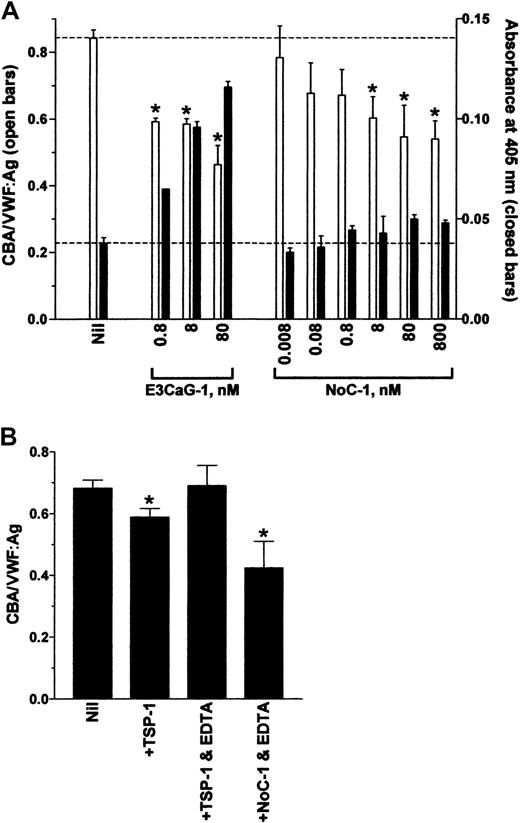
The NoC-1 fragment was unusual in that it reduced the CBA/VWF:Ag ratio but did not result in the formation of new thiols in VWF (Figure 2). This result was confirmed over a 5-log concentration range of NoC-1 (Figure 3A). Calcium ions were not required for the effect of the NoC-1 fragment on the CBA/VWF:Ag ratio, which is in contrast to the requirement for calcium ions for the VWF reductase activity of TSP-1 (Figure 3B).13 14 In addition, incubation of VWF with NoC1 did not change VWF multimer size measured by agarose gel electrophoresis (not shown). These results suggest a mechanism for the effect of NoC-1 other than the reduction of VWF multimer size. The simplest explanation is that NoC-1 competed with VWF for binding to collagen, although this would imply that the affinity of NoC-1 for collagen is significantly higher than the affinity of intact TSP-1 for collagen.
The effect of NoC-1 on the collagen binding of VWF is independent of calcium ions.
(A) VWF (8 nM) was incubated with 0.8 to 80 nM E3CaG-1 or 0.008 to 800 nM NoC-1. Aliquots of the reactions were analyzed for the average VWF multimer size (■) and for the generation of new thiols in VWF (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05). The small increase in MPB labeling (▪) at high concentrations of NoC-1 was not significantly different compared to control (P > .05). (B) VWF (8 nM) was incubated with TSP-1 or the NoC-1 fragment (80 nM) in the absence or presence of EDTA (10 mM). Aliquots of the reactions were analyzed for the collagen-binding affinity and VWF antigen level. The asterisks indicate significant difference compared to control (P < .05).
The effect of NoC-1 on the collagen binding of VWF is independent of calcium ions.
(A) VWF (8 nM) was incubated with 0.8 to 80 nM E3CaG-1 or 0.008 to 800 nM NoC-1. Aliquots of the reactions were analyzed for the average VWF multimer size (■) and for the generation of new thiols in VWF (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05). The small increase in MPB labeling (▪) at high concentrations of NoC-1 was not significantly different compared to control (P > .05). (B) VWF (8 nM) was incubated with TSP-1 or the NoC-1 fragment (80 nM) in the absence or presence of EDTA (10 mM). Aliquots of the reactions were analyzed for the collagen-binding affinity and VWF antigen level. The asterisks indicate significant difference compared to control (P < .05).
TSP-2 fragments did not contain VWF-reducing activity
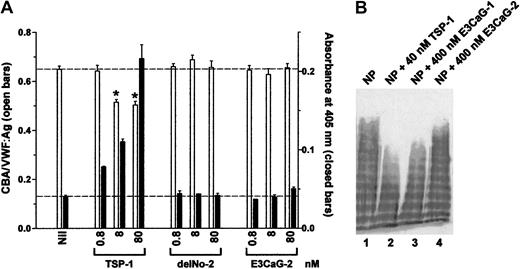
Increasing concentrations of recombinant TSP-1 or TSP-2 constructs were incubated with VWF for 1 hour and VWF reductase activity was identified by the concurrent reduction of VWF multimer size and generation of new thiols in VWF. Neither the delNo-2 nor E3CaG-2 fragments expressed VWF reductase activity (Figure4A).
TSP-2 fragments do not contain VWF-reducing activity.
(A) VWF (8 nM) was incubated with 0.8, 8, or 80 nM TSP-1, delNo-2, or E3CaG-2. Aliquots of the reactions were analyzed for the average VWF multimer size (■) and for the generation of new thiols in VWF (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05). (B) Citrated normal plasma (NP) was incubated with 40 nM TSP-1 (lane 2), 400 nM E3CaG-1 (lane 3), or 400 nM E3CaG-2 (lane 4), and aliquots of the reactions were resolved on 1% agarose gel electrophoresis. The VWF was analyzed by Western blot using peroxidase conjugated anti-VWF polyclonal antibodies.
TSP-2 fragments do not contain VWF-reducing activity.
(A) VWF (8 nM) was incubated with 0.8, 8, or 80 nM TSP-1, delNo-2, or E3CaG-2. Aliquots of the reactions were analyzed for the average VWF multimer size (■) and for the generation of new thiols in VWF (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05). (B) Citrated normal plasma (NP) was incubated with 40 nM TSP-1 (lane 2), 400 nM E3CaG-1 (lane 3), or 400 nM E3CaG-2 (lane 4), and aliquots of the reactions were resolved on 1% agarose gel electrophoresis. The VWF was analyzed by Western blot using peroxidase conjugated anti-VWF polyclonal antibodies.
The reduction of the average VWF multimer size by E3CaG-1 but not E3CaG-2 was confirmed by resolving aliquots of the reaction mixtures on 1% agarose gel electrophoresis and Western blotting for VWF (Figure4B).
Alkylation of the cysteine thiol in E3CaG-1 ablated the VWF-reducing activity
Each TSP-1 subunit contains a single free thiol at Cys974 when TSP-1 is purified in buffers containing 0.1 mM Ca++.26,27 The homologous residue in TSP-2 is serine and the cysteine in E3CaG-2 are all in disulfides.17 The number of thiols in E3CaG-1 was quantitated using DTNB and found to be 0.94 mol thiol per mol E3CaG-1. E3CaG-1, therefore, contains a single free thiol, like the intact TSP-1 subunit.
The thiol in E3CaG-1 in buffer containing 0.1 mM Ca++was alkylated with NEM, and the fragment was tested for VWF reductase activity. Extent of alkylation of E3CaG-1 was assessed by labeling with the biotin-linked maleimide, MPB, and detecting incorporation of the label by blotting with streptavidin-peroxidase. MPB labeled E3CaG-1 but not the alkylated protein (Figure 5A), which indicated that the majority of the thiols in the E3CaG-1 preparation were blocked by NEM. TSP-1 or unreacted or alkylated E3CaG-1 was incubated with VWF for 1 hour, and VWF reductase activity was identified by the concurrent reduction of VWF multimer size and generation of new thiols in VWF. Alkylation of E3CaG-1 ablated VWF reductase activity (Figure 5B).
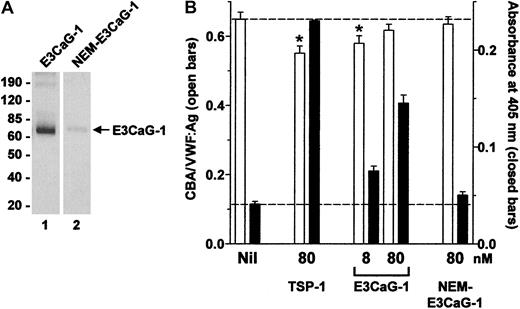
Alkylation of the cysteine thiol in E3CaG-1 ablates the VWF-reducing activity.
(A) E3CaG-1 or NEM-alkylated E3CaG-1 (NEM-E3CaG-1) was incubated with MPB, resolved on SDS-PAGE, and blotted with streptavidin-peroxidase to detect the biotin label. The positions of Mr markers are shown at left. (B) VWF (8 nM) was incubated with 80 nM TSP-1, 8 or 80 nM E3CaG-1 or 80 nM NEM-E3CaG-1. Aliquots of the reactions were analyzed for the average VWF multimer size (■) and for the generation of new thiols in VWF (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05).
Alkylation of the cysteine thiol in E3CaG-1 ablates the VWF-reducing activity.
(A) E3CaG-1 or NEM-alkylated E3CaG-1 (NEM-E3CaG-1) was incubated with MPB, resolved on SDS-PAGE, and blotted with streptavidin-peroxidase to detect the biotin label. The positions of Mr markers are shown at left. (B) VWF (8 nM) was incubated with 80 nM TSP-1, 8 or 80 nM E3CaG-1 or 80 nM NEM-E3CaG-1. Aliquots of the reactions were analyzed for the average VWF multimer size (■) and for the generation of new thiols in VWF (▪). The dotted lines represent no change in VWF multimer size (top line) or thiols in VWF (bottom line). The asterisks indicate significant difference compared to control (P < .05).
The cysteine thiol in E3CaG-1 was at position 974
Specific chemical cleavage and mass spectrometry was used to establish the position of the free thiol in the E3CaG-1 fragment.23 24 NTCB specifically S-cyanylates unpaired cysteine residues at pH 8. Cleavage of the peptide bond N-terminal to the cyanylated cysteine is achieved by transfer/migration of the cysteine residue's nitrogen to the cyano group at pH 9, forming a 2-iminothiazolidine-4-carboxylyl (ITC) peptide. This cleavage will occur at every cyanylated cysteine, and, therefore, the number of ITC peptides corresponds to the number of unpaired cysteines in the protein.
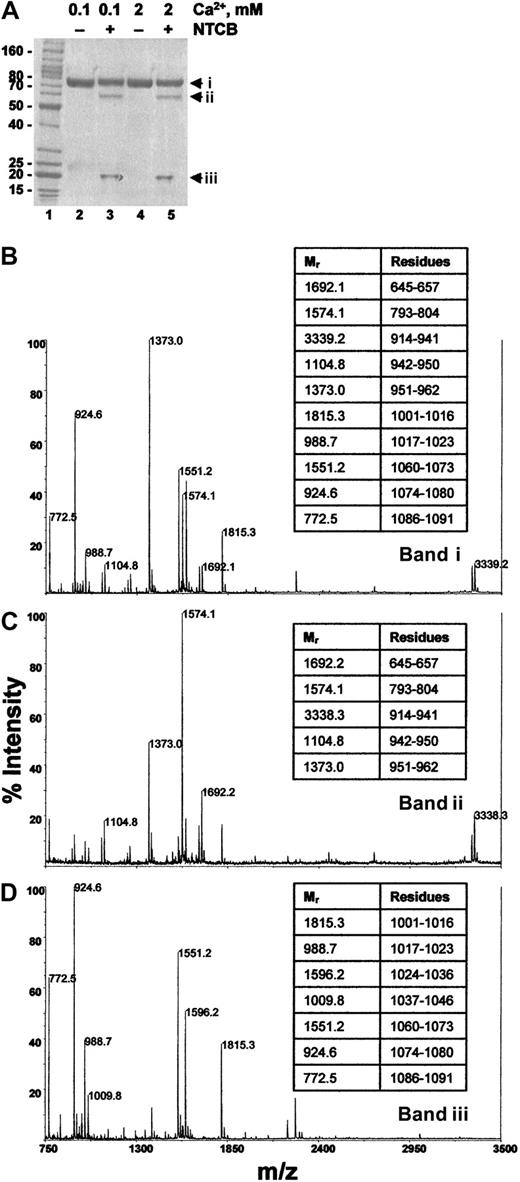
E3CaG-1 in pH 8.0 buffer containing either 0.1 mM or 2 mM Ca++ was reacted with NTCB and the peptide bond N-terminal of the cyanylated cysteine was then cleaved at pH 9. The protein was reduced and alkylated and resolved by SDS-PAGE. The E3CaG-1 fragment migrated at about 75 kDa, and NTCB cleavage in buffer containing either 0.1 mM or 2 mM Ca++ yielded peptides of about 60 and about 20 kDa (Figure 6A). The expected molecular mass of the E3CaG-1 fragment was 58 849 Da and NTCB cleavage at Cys974 should yield an N-terminal fragment of 38 278 Da and an ITC peptide of 20 726 Da. There is agreement between the expected and observed mass of the ITC peptide. The discrepancy in size on SDS-PAGE of the parent molecule and the N-terminal peptide is probably due to the high aspartate content (17%) of these fragments.
The cysteine thiol in E3CaG-1 is at position 974.
(A) E3CaG-1 in pH 8.0 buffer containing either 0.1 mM (lanes 2 and 3) or 2 mM Ca++ (lanes 4 and 5) was untreated (lanes 2 and 4) or reacted with NTCB (lanes 3 and 5). The peptide bond N-terminal of the cyanylated cysteine was then cleaved at pH 9. The protein was reduced and alkylated and resolved by SDS-PAGE. The Mr markers are shown in lane 1. (B-D) The 3 Coomassie-stained fragments were cut from the gel, digested with trypsin, and the resulting peptides analyzed by MALDI-rTOFMS. (B) Profile of band i in panel A, lane 5; (C), profile of band ii in panel A, lane 5; (D), profile of band iii in panel A, lane 5. Band i is undigested E3CaG-1, and bands ii and iii are the result of a single cleavage of band i. The Mr's of the major peptides were matched to the E3CaG-1 amino acid sequence. The amino acid residues of selected peptides are shown in the tables at the right of the profiles.
The cysteine thiol in E3CaG-1 is at position 974.
(A) E3CaG-1 in pH 8.0 buffer containing either 0.1 mM (lanes 2 and 3) or 2 mM Ca++ (lanes 4 and 5) was untreated (lanes 2 and 4) or reacted with NTCB (lanes 3 and 5). The peptide bond N-terminal of the cyanylated cysteine was then cleaved at pH 9. The protein was reduced and alkylated and resolved by SDS-PAGE. The Mr markers are shown in lane 1. (B-D) The 3 Coomassie-stained fragments were cut from the gel, digested with trypsin, and the resulting peptides analyzed by MALDI-rTOFMS. (B) Profile of band i in panel A, lane 5; (C), profile of band ii in panel A, lane 5; (D), profile of band iii in panel A, lane 5. Band i is undigested E3CaG-1, and bands ii and iii are the result of a single cleavage of band i. The Mr's of the major peptides were matched to the E3CaG-1 amino acid sequence. The amino acid residues of selected peptides are shown in the tables at the right of the profiles.
The 3 fragments were cut from the gel and digested with trypsin and the resulting peptides analyzed by MALDI-rTOFMS (Figure 6). Residues Asp914-Arg962 mapped to the approximate 60-kDa fragment. This is consistent with NTCB cleavage of E3CaG-1 at Cys974. NTCB cleavage at Cys892 or Cys928, for example, would have resulted in part or all of these amino acids being located within the ITC fragment. Furthermore, NTCB cleavage at Cys892 or Cys928 would have resulted in N-terminal/ITC peptides of 29 011/29 838 Da and 32 939/25 910 Da, respectively, which is contrary to the peptide masses resolved by SDS-PAGE. These results indicate that the free thiol in E3CaG-1 was at Cys974, which is the same position of the free thiol in intact TSP-1.27
Discussion
The TSPs are a family of extracellular glycoproteins that participate in cell-cell and cell-matrix communication. They approximate and regulate cytokines at cell surfaces and play a role in the growth and differentiation of tissues. The TSP family consists of 5 members in vertebrates, TSP-1 through TSP-428-32 and TSP-5 (also known as cartilage oligomeric matrix protein).33 A single member, dTSP, has also been identified inDrosophila.34,35 Based on their molecular architecture, the TSP gene family can be divided into 2 groups. TSP-1 and TSP-2 (subgroup A) are structurally similar trimeric proteins, composed of identical disulfide-linked 150-kDa monomers (Figure 1). The members of subgroup B, TSP-3, TSP-4, and TSP-5/COMP, are pentameric and differ from subgroup A in that they lack the procollagen and properdin modules and contain an extra epidermal growth factor (EGF)–like module (for a review, see Adams36). The aspartate-rich, Ca++-binding repeats and C-terminal sequence are common to all TSPs and have been extraordinarily well conserved. Using baculovirus-expressed recombinant overlapping constructs of TSP-1 that span the entire subunit and parallel TSP-2 constructs, we have shown that VWF reductase activity resides in the Ca++-binding repeats and C-terminal sequence of TSP-1 (E3CaG-1), but not in the parallel sequence of TSP-2 (E3CaG-2). Alkylation of the free thiol at Cys974 in the C-terminal TSP-1 fragment ablated VWF reductase activity.
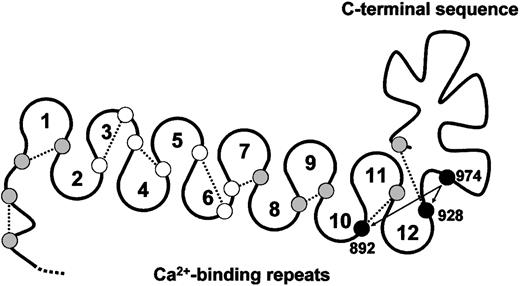
Each subunit of TSP-1 contains a free thiol.26 TSP-2, in contrast, does not contain unpaired cysteine.29,30 The TSP-1 thiol is remarkably fluid and can reside on any one of 12 different cysteines in the Ca++-binding repeats and C-terminal sequence of TSP-1 on release from platelets and chelation of Ca++ with EDTA.26 In contrast, TSP-1 purified in buffers containing 0.1 mM Ca++ is a homogeneous TSP-1 population in that the free thiol is at Cys974.27 A model of the cysteine in the Ca++-binding repeats and C-terminal sequence of TSP-1 is shown in Figure 7.
Model of the Ca++-binding loops and C-terminal sequence of TSP-1 and positions of reactive cysteine.
Small circles represent the relative positions of the 19 cysteines in the Ca++-binding loops and C-terminal sequence. Open circles, shaded circles, and solid circles represent cysteines not involved in disulfide exchange, moderately involved in disulfide exchange, and heavily involved in disulfide exchange, respectively, in Ca++-depleted TSP-1.26 In TSP-1 purified in buffers containing 0.1 mM Ca++,27 as well as in E3CaG-1, the free thiol is at Cys974. The dotted lines represent the likely disulfide bonding based on the disulfide connectivity of E3CaG-2.17 Cys974 may exchange with Cys928 or Cys892 (arrows).
Model of the Ca++-binding loops and C-terminal sequence of TSP-1 and positions of reactive cysteine.
Small circles represent the relative positions of the 19 cysteines in the Ca++-binding loops and C-terminal sequence. Open circles, shaded circles, and solid circles represent cysteines not involved in disulfide exchange, moderately involved in disulfide exchange, and heavily involved in disulfide exchange, respectively, in Ca++-depleted TSP-1.26 In TSP-1 purified in buffers containing 0.1 mM Ca++,27 as well as in E3CaG-1, the free thiol is at Cys974. The dotted lines represent the likely disulfide bonding based on the disulfide connectivity of E3CaG-2.17 Cys974 may exchange with Cys928 or Cys892 (arrows).
We have proposed that nucleophilic attack by a TSP-1 thiol on a VWF intersubunit disulfide bond results in reduction of the disulfide bond with formation of an intermediate disulfide-linked complex between TSP-1 and VWF. Attack by a second TSP-1 thiol results in release of VWF and formation of an intramolecular disulfide bond in TSP-1. We suggest that Cys974 is the TSP-1 thiol that mediates these events. This is supported by the demonstration that the free thiol in the E3CaG-1 fragment resides predominantly, if not exclusively, at Cys974 and that alkylation of this thiol ablates VWF reductase activity. It remains to be determined whether Cys974 operates in isolation or that exchange of Cys974 with Cys928 or Cys892 (Figure 7), or other TSP-1 cysteines, is important for VWF reductase activity.
Misenheimer et al17 have determined the disulfide connectivity of E3CaG-2. The disulfide pairing of the 18 cyteines in the Ca++-binding repeats and C-terminal sequence is sequential, that is, a 1-2, 3-4, 5-6, and so forth pattern. The corresponding disulfide connectivity in the Ca++-binding repeats and C-terminal sequence of TSP-1 is shown in Figure 7. It will be informative if the unpaired Cys974 in TSP-1 results in different disulfide connectivity.
TSP-3,31 TSP-4,32 TSP-5/COMP,33and dTSP34,35 each contain an unpaired cysteine, although it is not at a position equivalent to Cys974 in TSP-1 but is very close to the C-terminus. It may be that these other TSP family members also have VWF reductase activity, although their tissue distribution (cartilage, bone, ligaments, lung, and brain) does not support this function in vivo.37-39 In contrast, TSP-1 is readily demonstrable in and around blood vessels, which is where VWF acts and the regulation of its multimer size has functional relevance.
The authors thank Dr Michael Berndt and Dr Eric Huizinga for the von Willebrand factor.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-03-0770.
Supported by the National Health and Medical Research Council of Australia, the National Heart Foundation of Australia, the New South Wales Health Department, and National Institutes of Health grant HL54462.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philip Hogg, Centre for Thrombosis and Vascular Research, School of Medical Sciences, University of New South Wales, Sydney NSW 2052 Australia; e-mail: p.hogg@unsw.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal