T-cell depletion (TCD) and immunosuppressive medications (ISTs) are 2 methods used for graft-versus-host disease (GVHD) prophylaxis in unrelated donor (URD) transplantation. However, comparisons of the clinical outcomes including quality of life and direct medical costs associated with each type of procedure have not been reported. We reviewed 48 TCD and 98 IST procedures performed from 1/1/97 to 12/31/99 at the Dana-Farber Cancer Institute, Boston, MA. With a median follow-up of 1.5 years for survivors, no differences were seen in relapse, acute GVHD, and overall survival between TCD and IST patients. Multivariable Cox modeling showed that age of 50 years or less (P = .002) and low-risk disease (P = .001) predicted survival, but method of GVHD prophylaxis (P = .6) and degree of human leukocyte antigen (HLA) matching (P = .8) did not. A subset of patients (53%) completed quality of life surveys prior to and at 6 and 12 months after transplantation; participation in the quality of life study was not associated with clinical characteristics and outcomes. No differences were seen in quality of life scores prior to transplantation, and changes over time were similar between groups. Costs ($113 000 vs $155 000, P < .0001) and total hospital days (34 vs 46, P = .0006) were significantly lower for patients undergoing TCD procedures. As prospective, randomized studies comparing methods of GVHD prophylaxis are performed, assessment of quality of life and costs should be included to fully understand the overall impact of each intervention.
Introduction
Almost 30 years after the first successful stem cell transplantation from an unrelated donor, acute graft-versus-host disease (GVHD) remains a major barrier to transplantation success.1 Prophylaxis against acute GVHD is broadly divided into 2 categories: reduction in T-cell type or number (T-cell depletion [TCD]) and immunosuppressive medications (ISTs) that interfere with T-cell function or survival. Observational studies of unrelated donor (URD) marrow recipients suggest that long-term survival and disease-free survival are similar using either TCD or IST approaches to acute GVHD prophylaxis.2-6 However, many of the same studies also show that TCD is associated with less acute and chronic GVHD.2,3 Furthermore, TCD appears to be associated with reduced peritransplantation complications such as hepatic veno-occlusive disease and pulmonary dysfunction,6-8suggesting that costs may be lower and quality of life better with TCD. In the absence of a clear survival or clinical advantage, these considerations may influence choice of GVHD prophylaxis. However, no published studies thus far have compared the 2 major methods of GVHD prophylaxis along these parameters.
We reviewed our experience with 146 URD transplants for hematologic diseases performed over 3 years to compare clinical outcomes, quality of life, and costs associated with TCD and IST approaches to acute GVHD prophylaxis.
Patients and methods
Subjects
We reviewed 146 consecutive patients undergoing URD transplantation for hematologic diseases from January 1997 through December 1999. Approval was obtained from the institutional review board at Dana-Farber Cancer Institute (Boston, MA) for these studies. Informed consent for transplantation was provided according to the Declaration of Helsinki. Disease status was categorized prior to transplantation into 2 groups. Low-risk patients were those with acute leukemia in first complete remission or stable-phase chronic myelogenous leukemia (CML). High-risk patients had all other disease types and stages.
Choice of GVHD prophylaxis (TCD vs IST) was based on attending physician recommendation and patient preferences after a discussion of the risks and benefits of each method. Patients were treated on a variety of clinical protocols. Human leukocyte antigen (HLA) matching was performed using both serologic and molecular methods. Marrow was harvested without growth factor treatment of donors. The day of marrow infusion was designated day 0. Conditioning regimens consisted primarily of cyclophosphamide (TCD 60 mg/kg per day × 2, IST 1800 mg/m2 per day × 2) and total body irradiation (14 Gy), although busulfan and cytoxan were delivered if radiation was contraindicated by prior radiotherapy. In addition, TCD patients received total lymphoid irradiation at a dose of 4.5 Gy or 7.5 Gy. TCD was accomplished using an anti-CD6 antibody and rabbit complement resulting in 1 to 2 logs depletion but sparing B and natural killer cells.9 TCD patients received filgrastim (5 mcg/kg starting day +1) after marrow infusion until engraftment but did not receive any additional immunosuppressive medications unless they required treatment for GVHD. GVHD prophylaxis for IST patients included combinations of methotrexate (15 mg/m2 day 1, 10 mg/m2 on days +3, +6, +11) and cyclosporine (2.5 mg/kg twice daily, starting day −3) or tacrolimus (0.02 mg/kg 4 times a day, 24 hours by continuous infusion).
Routine clinical care for TCD and IST patients after transplantation was delivered in the same outpatient facility using similar algorithms for infectious disease prophylaxis and immune-suppressive medication tapers. Our transplant program practices a primary physician outpatient model so that all pretransplantation and posttransplantation care is delivered by the same physician. Although the composition of individual practices varied, all physicians cared for both TCD and IST patients. Most patients are local and receive their posttransplantation care and any necessary hospitalizations at our institution.
Data collection
Clinical data.
Clinical information on survival, relapse, acute and chronic GVHD, veno-occlusive disease, organ toxicity, and causes of death were obtained from the clinical transplant database. Relapse was diagnosed based on hematologic parameters, tissue biopsy, bone marrow biopsy findings, and cytogenetic or molecular methods according to particular disease presentations. The consensus conference was used to grade acute GVHD,10 and chronic GVHD was graded according to published criteria.11 Patients' overall and event-free survival follow-up information reflects data in the clinical database as of July 2001. Causes of death attributed to relapse, pulmonary complications (including interstitial pneumonitis, diffuse alveolar hemorrhage, and respiratory failure), infections, and all other causes were recorded. All severe, life-threatening or fatal pulmonary, renal/bladder, and infectious complications were collected.
Quality of life data.
Fifty-three percent of both TCD and IST patients participated in a prospective quality of life study. Patients were surveyed by mail prior to transplantation and at 6 and 12 months after the procedure using the Medical Outcomes Study Short Form 36 (SF-36), the Quality of Life Index (QLI), and a rating scale (RS) question. No patient was purposefully excluded from the quality of life study. Nonparticipation resulted from inability to contact the patient by telephone, failure to return the baseline survey, or patient inability to communicate in English.
The SF-36 is a 36-item, multidimensional quality of life instrument with 8 domains: physical functioning, role limitations due to physical or emotional problems, bodily pain, general health perceptions, vitality, social functioning, and emotional well-being, as well as 2 composite scales: physical and mental functioning. Median reliability is 0.80, except for the 2-item social functioning scales (0.76).12 Scores are converted to a 0 to 100 scale and the physical and mental composite scores are normalized so that 50 indicates average functioning with a standard deviation of 10. Higher scores indicate better functioning. It has been suggested that a 7- to 10-point change represents a clinically meaningful difference in a stem cell transplant population.13 The QLI is a 5-item validated instrument assessing the domains of activity, daily living, general health, social support, and outlook.14 Using multiattribute utility scaling, patient utilities for different health states were calculated on a scale from 0 (dead) to 1.00 (perfect health). The RS asks for a rating of overall health on a scale from 0 (dead) to 100 (perfect health).
Baseline questionnaires elicited information on sociodemographics (age, sex, marital status, race, education, and work status [including school and homemaking]) and general health status (excellent, very good, good, fair, poor). Follow-up surveys collected information on return to work, hospital admissions, and perception of overall health (excellent, very good, good, fair, poor). Follow-up surveys were identical and addressed recovery issues. A copy of the survey may be obtained by contacting the corresponding author.
Cost data.
Inpatient charges and total hospital days for the first year after transplantation were retrieved from the administrative database. Charges were converted to costs using departmental ratios of charges to costs, and adjusted to the year 2000 using the medical care component of the consumer price index.15 Professional charges (eg, attending billings) were not captured, but are expected to mirror direct inpatient costs. Outpatient direct medical costs (eg, clinic visits), patient time costs (eg, time spent in clinic), productivity costs (eg, time off work to recover from transplantation), and direct nonmedical costs (eg, transportation, local lodging) were not captured, but are expected to be low compared with direct medical inpatient costs.16
Biostatistical analysis
Associations between categorical variables and method of GVHD prophylaxis (TCD vs IST) were assessed by a Fisher exact or chi-square test, as appropriate. The Wilcoxon rank-sum test was used for testing differences between continuous variables. No statistical adjustment was made for performing multiple tests, but a P value of more than .01 should be interpreted with care. All probability values are 2-sided.
Patients' overall and event-free survival reflects follow-up information in the database as of July 2001. For event-free survival, relapses and deaths were considered events. The method of Kaplan and Meier was used to estimate survival curves and the log-rank test was used to compare the curves. Cumulative incidences of acute GVHD and relapse are reported separately with death as a competing risk. Cumulative incidence of extensive chronic GVHD is reported with both death and relapse as competing risks.17
We compared overall survival between the 2 GVHD prophylaxes, TCD and IST, in a Cox regression analysis adjusting for patient age (≤ 50 years vs > 50 years), sex matching (female donor and male patient vs all other combinations), degree of HLA match (matched vs mismatched), patient cytomegalovirus (CMV) serostatus (seronegative vs seropositive), and disease risk (low risk vs high risk). The estimates of relative risks and 95% confidence intervals (CIs) are reported at a median follow-up of 18 months.
Quality of life scores were calculated using algorithms and guidelines for handling missing data recommended by the developers. Results are presented as medians, ranges, and where applicable, 25th to 75th percentiles. The Wilcoxon rank-sum test was used to compare baseline variables. A mixed model assuming a variance/covariance structure with constant variance and covariance was used to compare TCD and IST patients over time and at specific time points. A mixed model uses all available data while allowing for the correlation between observations attributable to within subject (time) and between subject (type of GVHD prophylaxis) factors.18
We analyzed heterogeneity of costs and length of stay between the 2 methods of GVHD prophylaxis by analysis of variance (ANOVA), adjusting for patient age (≤ 50 years vs > 50 years), sex matching (female donor and male patient vs all other combinations), degree of HLA match (matched vs mismatched), patient CMV serostatus (seronegative vs seropositive), and disease risk (low risk vs high risk). The SAS type III sums-of-squares, where each test of significance is adjusted for the other factors, was used in ascertaining statistically significant differences.19 These tests used the natural logarithm of the cost and length of stay data, because this transformation approximated a normal distribution and was effective in stabilizing the variances. However, all estimates and confidence intervals presented have been transformed back to the original scales (dollars and days) and thus correspond to ratios.
Results
Patient characteristics
Table 1 shows the patient characteristics. Briefly, 48 (33%) received TCD while 98 (67%) received IST. The groups were similar in age, patient-donor sex matching, patient-donor CMV status, and use of total body irradiation (TBI)–based conditioning. However, TCD patients were more likely to have better disease status (46% vs 24% low risk, respectively, P = .01) and less likely to have a mismatched donor (2% [one HLA-A mismatch] vs 16% [4 HLA-A, 8 HLA-B, 3 HLA-DR mismatches], P = .01). In addition, the spectrum of diseases differed with a higher proportion of acute leukemia in the TCD group, and more patients with chronic myelogenous leukemia and myelodysplastic syndrome in the IST group.
Patient characteristics
| Variable . | T-cell depletion . | Immunosuppressive medication . | P . |
|---|---|---|---|
| N (%) | 48 (33) | 98 (67) | |
| Age, median years (range) | 48 (21-60) | 42 (20-61) | — |
| 50 y or younger (%) | 30 (62) | 71 (72) | .25 |
| Older than 50 y (%) | 18 (38) | 27 (28) | |
| Sex match (%) | |||
| M-M | 19 (39) | 31 (32) | .39 |
| M-F | 11 (23) | 21 (21) | |
| F-M | 10 (21) | 17 (17) | |
| F-F | 8 (17) | 29 (30) | |
| Mismatched, N (%) | 1 (2) | 16 (16) | .01 |
| CMV, patient/donor (%) | |||
| Neg/Neg | 20 (42) | 32 (33) | .43 |
| Pos/Neg | 6 (12) | 22 (22) | |
| Neg/Pos | 13 (27) | 29 (30) | |
| Pos/Pos | 9 (19) | 15 (15) | |
| Conditioning regimen (%) | |||
| Cyclophosphamide/total body irradiation | 46 (96) | 95 (97) | .66 |
| Busulfan/cyclophosphamide | 2 (4) | 3 (3) | — |
| Total lymphoid irradiation* | |||
| 4.5 Gy | 25 (57) | N/A | |
| 7.5 Gy | 19 (43) | N/A | |
| Disease risk (%) | |||
| Low risk | 22 (46) | 24 (24) | .01 |
| High risk | 26 (54) | 74 (76) | |
| Disease (%) | |||
| CML | 10 (21) | 45 (46) | < .01 |
| AML | 15 (31) | 20 (20) | |
| MDS | 4 (8) | 13 (13) | |
| CLL | 0 (0) | 1 (1) | |
| MM | 1 (2) | 0 (0) | |
| NHL | 4 (8) | 5 (5) | |
| ALL | 14 (29) | 9 (9) | |
| Other (HL, other leukemia, other) | 0 (0) | 5 (5) | |
| Median follow-up, mo (range) for alive patients | 14.8 (6.1-36.5) | 18.7 (8.4-39.2) | .25 |
| Variable . | T-cell depletion . | Immunosuppressive medication . | P . |
|---|---|---|---|
| N (%) | 48 (33) | 98 (67) | |
| Age, median years (range) | 48 (21-60) | 42 (20-61) | — |
| 50 y or younger (%) | 30 (62) | 71 (72) | .25 |
| Older than 50 y (%) | 18 (38) | 27 (28) | |
| Sex match (%) | |||
| M-M | 19 (39) | 31 (32) | .39 |
| M-F | 11 (23) | 21 (21) | |
| F-M | 10 (21) | 17 (17) | |
| F-F | 8 (17) | 29 (30) | |
| Mismatched, N (%) | 1 (2) | 16 (16) | .01 |
| CMV, patient/donor (%) | |||
| Neg/Neg | 20 (42) | 32 (33) | .43 |
| Pos/Neg | 6 (12) | 22 (22) | |
| Neg/Pos | 13 (27) | 29 (30) | |
| Pos/Pos | 9 (19) | 15 (15) | |
| Conditioning regimen (%) | |||
| Cyclophosphamide/total body irradiation | 46 (96) | 95 (97) | .66 |
| Busulfan/cyclophosphamide | 2 (4) | 3 (3) | — |
| Total lymphoid irradiation* | |||
| 4.5 Gy | 25 (57) | N/A | |
| 7.5 Gy | 19 (43) | N/A | |
| Disease risk (%) | |||
| Low risk | 22 (46) | 24 (24) | .01 |
| High risk | 26 (54) | 74 (76) | |
| Disease (%) | |||
| CML | 10 (21) | 45 (46) | < .01 |
| AML | 15 (31) | 20 (20) | |
| MDS | 4 (8) | 13 (13) | |
| CLL | 0 (0) | 1 (1) | |
| MM | 1 (2) | 0 (0) | |
| NHL | 4 (8) | 5 (5) | |
| ALL | 14 (29) | 9 (9) | |
| Other (HL, other leukemia, other) | 0 (0) | 5 (5) | |
| Median follow-up, mo (range) for alive patients | 14.8 (6.1-36.5) | 18.7 (8.4-39.2) | .25 |
CMV indicates cytomegalovirus; CML, chronic myelogenous leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; ALL, acute lymphocytic leukemia; and HL, Hodgkin lymphoma.
Of 48 TCD patients, 44 (92%) also had total lymphoid irradiation.
Clinical outcomes
Table 2 summarizes clinical outcomes. TCD and IST patients were observed to have a similar cumulative incidence of relapse, grades II to IV acute GVHD and extensive chronic GVHD. The rate of grades III to IV GVHD (TCD 21% vs IST 17%), and overall and disease-free survival were similar. No differences could be detected in the incidence of severe, life-threatening, and fatal pulmonary, renal/bladder, and infectious complications, but the incidence of veno-occlusive disease was higher (22% vs 8%, P = .04) in the IST patients. The spectrum of contributory causes of death was similar between the groups. Median time to engraftment was much shorter for TCD patients (12 days [range, 9-45 days] vs 24 days [range, 15-41 days], P < .0001); these results exclude 8 IST patients who failed to engraft prior to death.
Clinical outcomes and costs
| Variable . | T-cell depletion . | Immunosuppressive medication . | P . |
|---|---|---|---|
| N | 48 | 98 | |
| Cumulative incidence of relapse at 1 y | 16% ± 5% | 15% ± 4% | .84 |
| Cumulative incidence of grades II-IV acute graft-versus-host disease at 100 d | 46% ± 7% | 36% ± 5% | .15 |
| Cumulative incidence of extensive chronic graft-versus-host disease at 1 y | 9% ± 4% | 9% ± 3% | .69 |
| Event-free survival at 1 y | 42% ± 8% | 47% ± 5% | .48 |
| Survival at 1 y | 46% ± 8% | 49% ± 5% | .25 |
| Organ toxicity (%) | |||
| Pulmonary | 7 (15) | 27 (28) | .10 |
| Veno-occlusive disease | 4 (8) | 22 (22) | .04 |
| Renal/bladder | 4 (8) | 10 (10) | .99 |
| Infectious | 4 (8) | 11 (11) | .77 |
| Cause of death* (%) | |||
| Relapse | 4 (16) | 19 (31) | .19 |
| Graft-versus-host disease | 3 (12) | 11 (18) | .75 |
| Pulmonary | 11 (44) | 24 (39) | .81 |
| Infection | 8 (32) | 14 (23) | .42 |
| Other | 6 (24) | 15 (24) | .99 |
| Hospital days within first year, median (range) | 34 (19-127) | 46 (18-176) | .0006 |
| Length of initial hospital stay, d (range) | 26 (19-68) | 39 (18-84) | < .0001 |
| Cost of first year, 2000; $ × 1000 median (range) | 113 (44-390) | 155 (67-479) | < .0001 |
| Cost of initial hospitalization, 2000; $ × 1000 median (range) | 86 (37-259) | 139 (56-479) | < .0001 |
| Variable . | T-cell depletion . | Immunosuppressive medication . | P . |
|---|---|---|---|
| N | 48 | 98 | |
| Cumulative incidence of relapse at 1 y | 16% ± 5% | 15% ± 4% | .84 |
| Cumulative incidence of grades II-IV acute graft-versus-host disease at 100 d | 46% ± 7% | 36% ± 5% | .15 |
| Cumulative incidence of extensive chronic graft-versus-host disease at 1 y | 9% ± 4% | 9% ± 3% | .69 |
| Event-free survival at 1 y | 42% ± 8% | 47% ± 5% | .48 |
| Survival at 1 y | 46% ± 8% | 49% ± 5% | .25 |
| Organ toxicity (%) | |||
| Pulmonary | 7 (15) | 27 (28) | .10 |
| Veno-occlusive disease | 4 (8) | 22 (22) | .04 |
| Renal/bladder | 4 (8) | 10 (10) | .99 |
| Infectious | 4 (8) | 11 (11) | .77 |
| Cause of death* (%) | |||
| Relapse | 4 (16) | 19 (31) | .19 |
| Graft-versus-host disease | 3 (12) | 11 (18) | .75 |
| Pulmonary | 11 (44) | 24 (39) | .81 |
| Infection | 8 (32) | 14 (23) | .42 |
| Other | 6 (24) | 15 (24) | .99 |
| Hospital days within first year, median (range) | 34 (19-127) | 46 (18-176) | .0006 |
| Length of initial hospital stay, d (range) | 26 (19-68) | 39 (18-84) | < .0001 |
| Cost of first year, 2000; $ × 1000 median (range) | 113 (44-390) | 155 (67-479) | < .0001 |
| Cost of initial hospitalization, 2000; $ × 1000 median (range) | 86 (37-259) | 139 (56-479) | < .0001 |
All listed causes of death, so total percent exceeds 100%.
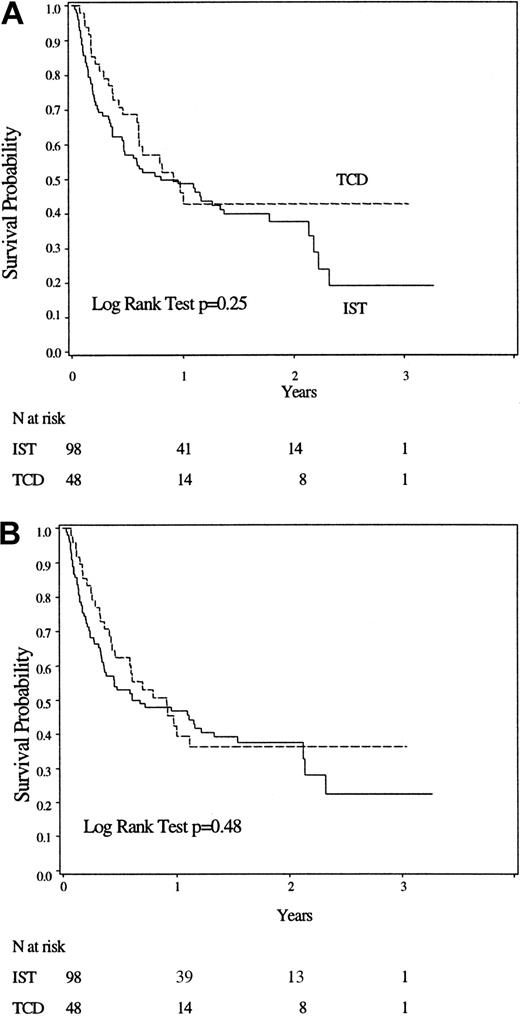
Figure 1 shows overall and disease-free survival for 48 TCD and 98 IST patients. Modeling of survival considering method of GVHD prophylaxis, patient age, patient-donor sex matching, patient CMV serologic status, degree of HLA-matching, and disease stage showed that age greater than 50 years (hazard ratio [HR] 2.07, 95% CI 1.32-3.25, P = .002) and high risk disease (HR 2.27, 95% CI 1.34-3.85, P = .002) were significantly associated with a worse outcome. Method of GVHD prophylaxis (TCD or IST) was not associated with survival (HR 0.88, 95% CI 0.54-1.44, P = .60). (Table3).
Overall and disease-free survival of the 2 cohorts.
(A) Overall survival. (B) Event-free survival.
Overall and disease-free survival of the 2 cohorts.
(A) Overall survival. (B) Event-free survival.
Cox regression analysis for overall survival
| Variable3-150 . | HR . | 95% CI . | P . |
|---|---|---|---|
| T-cell depletion vs immunosuppressive medications | 0.88 | 0.54 -1.44 | .60 |
| Age, older than 50 y vs 50 y or younger | 2.07 | 1.32 -3.25 | .002 |
| High-risk disease vs low-risk disease | 2.27 | 1.34 -3.85 | .002 |
| Male patient/female donor vs other combinations | 1.00 | 0.57 -1.74 | .99 |
| HLA-mismatched vs HLA-matched | 0.83 | 0.42 -1.63 | .83 |
| Patient CMV serologic status negative vs positive | 0.70 | 0.45 -1.09 | .70 |
| Variable3-150 . | HR . | 95% CI . | P . |
|---|---|---|---|
| T-cell depletion vs immunosuppressive medications | 0.88 | 0.54 -1.44 | .60 |
| Age, older than 50 y vs 50 y or younger | 2.07 | 1.32 -3.25 | .002 |
| High-risk disease vs low-risk disease | 2.27 | 1.34 -3.85 | .002 |
| Male patient/female donor vs other combinations | 1.00 | 0.57 -1.74 | .99 |
| HLA-mismatched vs HLA-matched | 0.83 | 0.42 -1.63 | .83 |
| Patient CMV serologic status negative vs positive | 0.70 | 0.45 -1.09 | .70 |
HR indicates hazard ratio; CI, confidence interval; HLA, human leukocyte antigen; and CMV, cytomegalovirus.
Reference groups: immunosuppressive medications, age ≤ 50 years, low-risk disease, other than male patient/female donor, HLA-matched, patient CMV serologic status positive.
Quality of life
There were 25 (52%) TCD and 53 (54%) IST patients who filled out baseline quality of life surveys. Patients who did or did not provide quality of life data were similar regarding patient, disease, and treatment characteristics, and outcomes including survival, disease-free survival, acute GVHD, and relapse (P > .12). There was a trend for patients without quality of life data to be more likely mismatched (17% vs 7%, P = .06).
Comparison of patients who did or did not provide quality of life data within the TCD and IST populations showed that rates of relapse, event-free survival, and overall survival were not different (data not shown). For the patients who did return baseline surveys, quality of life was not different between TCD and IST patients in any of the domains of the SF-36, the utility calculated from the QLI scores, or the rating scale. In Table 4, results of the mixed model showed that quality of life changed over time, but both TCD and IST groups reported similar changes as measured by the physical and mental composite scales of the SF-36, the QLI-calculated utility, and the rating scale.
Patient sociodemographics and quality of life prior to transplantation
| Variable . | T-cell depletion . | Immunosuppressive medication . | P . |
|---|---|---|---|
| N | 25 | 53 | |
| Participation rate (%) | 25/48 (52) | 53/98 (54) | |
| Married/living with partner, N (%) | 20 (80) | 40 (75) | .66 |
| White, N (%) | 24 (96) | 47 (89) | .29 |
| College or postgraduate degree, N (%) | 14 (56) | 29 (55) | .92 |
| Working, in school, homemaking (%) | 24 (96) | 51 (96) | .96 |
| General health very good or excellent (%) | 17 (68) | 36 (68) | .99 |
| Severe or very severe side effects from prior therapy (%) | 8 (32) | 9 (17) | .13 |
| Quality of life scales4-150 | |||
| Physical composite score | 47 (16-64) | 46 (20-61) | .19 |
| Mental composite score | 53 (24-63) | 52 (28-61) | .95 |
| Physical | 85 (15-100) | 80 (5-100) | .37 |
| Role physical | 50 (0-100) | 75 (0-100) | .61 |
| Pain | 100 (41-100) | 74 (10-100) | .03 |
| General health | 62 (15-100) | 70 (15-100) | .64 |
| Vitality | 55 (0-100) | 50 (5-90) | .26 |
| Social functioning | 88 (13-100) | 75 (25-100) | .23 |
| Role emotional | 100 (0-100) | 100 (0-100) | .37 |
| Mental health | 76 (16-100) | 78 (36-100) | .74 |
| Quality of life index (QLI) | 0.92 (0.49-1.00) | 0.91 (0.61-1.00) | .50 |
| Rating scale | 86 (50-100) | 80 (40-100) | .12 |
| Variable . | T-cell depletion . | Immunosuppressive medication . | P . |
|---|---|---|---|
| N | 25 | 53 | |
| Participation rate (%) | 25/48 (52) | 53/98 (54) | |
| Married/living with partner, N (%) | 20 (80) | 40 (75) | .66 |
| White, N (%) | 24 (96) | 47 (89) | .29 |
| College or postgraduate degree, N (%) | 14 (56) | 29 (55) | .92 |
| Working, in school, homemaking (%) | 24 (96) | 51 (96) | .96 |
| General health very good or excellent (%) | 17 (68) | 36 (68) | .99 |
| Severe or very severe side effects from prior therapy (%) | 8 (32) | 9 (17) | .13 |
| Quality of life scales4-150 | |||
| Physical composite score | 47 (16-64) | 46 (20-61) | .19 |
| Mental composite score | 53 (24-63) | 52 (28-61) | .95 |
| Physical | 85 (15-100) | 80 (5-100) | .37 |
| Role physical | 50 (0-100) | 75 (0-100) | .61 |
| Pain | 100 (41-100) | 74 (10-100) | .03 |
| General health | 62 (15-100) | 70 (15-100) | .64 |
| Vitality | 55 (0-100) | 50 (5-90) | .26 |
| Social functioning | 88 (13-100) | 75 (25-100) | .23 |
| Role emotional | 100 (0-100) | 100 (0-100) | .37 |
| Mental health | 76 (16-100) | 78 (36-100) | .74 |
| Quality of life index (QLI) | 0.92 (0.49-1.00) | 0.91 (0.61-1.00) | .50 |
| Rating scale | 86 (50-100) | 80 (40-100) | .12 |
Presented as median (range).
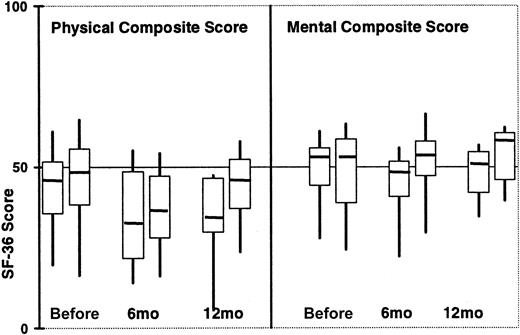
There were 12 TCD and 33 IST surviving patients who responded to the 6-month survey (response rate 80% vs 97%). Similar scores were seen on the physical composite SF-36 scale (32 vs 36,P=.60), mental composite SF-36 score (47 vs 54,P=.02), calculated utilities from the QLI (0.85 vs 0.85,P=.61) and the rating scales (70 vs 75, P=.10). Reported rates of rehospitalization were similar (33% vs 38%,P=.99) and 42% of TCD patients compared with 28% of IST patients reported very good or excellent health (P=.48). There were 9 TCD and 24 IST survivors who responded to the 12-month survey (response rate 100% vs 90%). The raw SF-36 data are plotted in Figure 2.
Composite SF-36 scores according to type of GVHD prophylaxis over time.
For each time point, TCD patients are represented by the first box, followed by IST patients. Medians are depicted by the horizontal bars, the 25th and 75th percentiles by the upper and lower bounds of the boxes, and the full range of values by the whiskers. At the pretransplantation, 6-month, and 12-month time points, there are 25, 12, and 9 TCD patients and 53, 33, and 24 IST patients, respectively.
Composite SF-36 scores according to type of GVHD prophylaxis over time.
For each time point, TCD patients are represented by the first box, followed by IST patients. Medians are depicted by the horizontal bars, the 25th and 75th percentiles by the upper and lower bounds of the boxes, and the full range of values by the whiskers. At the pretransplantation, 6-month, and 12-month time points, there are 25, 12, and 9 TCD patients and 53, 33, and 24 IST patients, respectively.
Days of hospitalization and costs
Days of hospitalization and costs were available for 47 TCD and 98 IST patients. One patient was excluded from the cost analysis due to research administrative error. Hospital days and costs were correlated (r2 = 0.39) as expected. Univariate comparisons are shown in Table 2. TCD patients had fewer days of hospitalization (34 vs 46, P = .0006) and lower costs ($113 000 vs $155 000, P < .0001) during the first year after transplantation. This was primarily due to the initial hospitalization, as there was no difference in length of stay and costs in subsequent hospitalizations between the 2 groups.
Results from the separate ANOVA analyses modeling the natural logarithm of costs and length of stay are presented in Table5. The model provides estimates of the differences in means on the log scale; transformation back to the original scales results in ratios with the standard interpretation. For example, a ratio of 1.22 for costs corresponds to a 22% increase in costs when patients with high-risk disease are compared with those with low-risk disease, controlling for all other characteristics. Our data show that after controlling for patient age, patient-donor sex matching, patient CMV serologic status, degree of HLA matching, and disease stage, costs were significantly lower for TCD patients ($125 000 vs $173 000, ratio 0.73, 95% CI 0.63-0.84,P < .0001) and significantly higher for high-risk patients (ratio 1.2, 95% CI 1.1-1.4, P = .01). When time to engraftment was included in the model, an effect of TCD on overall costs was still detectable (P = .02). Length of stay was also longer for IST patients (49 vs 40 days, ratio 1.2, 95% CI 1.1-1.4, P = .004) adjusting for all other patient characteristics. Thus, TCD patients have approximately 9 fewer days of hospitalization and cost $48 000 less than IST patients after adjusting for age, disease stage, patient-donor sex matching, degree of HLA matching, and patient CMV status.
Analysis of variance for costs and length of stay
| Variables5-150 . | Cost . | Length of stay . | ||||
|---|---|---|---|---|---|---|
| Ratio . | 95% CI . | P . | Ratio . | 95% CI . | P . | |
| T-cell depletion vs immunosuppressive medications | 0.73 | 0.63 -0.84 | < .001 | 0.81 | 0.71 -0.93 | .004 |
| Age, older than 50 y vs 50 y or younger | 1.04 | 0.90 -1.20 | .60 | 1.05 | 0.91 -1.20 | .47 |
| High-risk disease vs low-risk disease | 1.22 | 1.05 -1.40 | .01 | 1.21 | 1.05 -1.39 | .01 |
| Male patient/female donor vs other | 1.20 | 1.02 -1.40 | .03 | 1.19 | 1.02 -1.38 | .03 |
| HLA-mismatched vs HLA-matched | 1.08 | 0.88 -1.32 | .46 | 0.97 | 0.80 -1.19 | .80 |
| Patient CMV serologic status negative vs positive | 0.86 | 0.75 -0.99 | .03 | 0.88 | 0.77 -1.00 | .05 |
| Variables5-150 . | Cost . | Length of stay . | ||||
|---|---|---|---|---|---|---|
| Ratio . | 95% CI . | P . | Ratio . | 95% CI . | P . | |
| T-cell depletion vs immunosuppressive medications | 0.73 | 0.63 -0.84 | < .001 | 0.81 | 0.71 -0.93 | .004 |
| Age, older than 50 y vs 50 y or younger | 1.04 | 0.90 -1.20 | .60 | 1.05 | 0.91 -1.20 | .47 |
| High-risk disease vs low-risk disease | 1.22 | 1.05 -1.40 | .01 | 1.21 | 1.05 -1.39 | .01 |
| Male patient/female donor vs other | 1.20 | 1.02 -1.40 | .03 | 1.19 | 1.02 -1.38 | .03 |
| HLA-mismatched vs HLA-matched | 1.08 | 0.88 -1.32 | .46 | 0.97 | 0.80 -1.19 | .80 |
| Patient CMV serologic status negative vs positive | 0.86 | 0.75 -0.99 | .03 | 0.88 | 0.77 -1.00 | .05 |
Excludes one patient missing cost and days of hospitalization data due to research administrative error.
CI indicates confidence interval; HLA, human leukocyte antigen; and CMV, cytomegalovirus.
Reference groups: immunosuppressive medications, age ≤ 50 years, low-risk disease, other than male patient/female donor, HLA-matched, patient CMV serologic status positive.
Discussion
We report the costs, quality of life, and clinical outcomes of 48 TCD and 98 IST recipients of URD bone marrow transplants for hematologic diseases. Although the IST group had a higher percentage of mismatched and high-risk patients, their clinical outcomes were remarkably similar to the TCD group. Specifically, rates of engraftment, acute GVHD, extensive chronic GVHD, relapse, and survival were similar. This is consistent with other observational reports in the literature for URD transplantation,2,5 and contrasts with findings in related donor transplantation in which TCD is associated with less acute and chronic GVHD but more relapse.20
Approximately half (53%) of patients participated in a longitudinal quality of life study. Comparison of participants and nonparticipants suggests that the studied population is clinically representative of the entire cohort. TCD and IST patients reported similar pretransplantation quality of life, but contrary to our hypothesis, changes in quality of life were similar over time between the 2 groups and may be due to the comparable GVHD and complication rates. However, total days of hospitalization within the first year and costs were lower for patients undergoing TCD procedures. This finding is largely attributable to the initial period of hospitalization. Subsequent admissions for TCD and IST patients were of similar length and cost. There was a high degree of correlation between time to engraftment and length of initial hospitalization, which in turn drove some of the cost difference. Some supportive care measures were protocol specific, such as filgrastim in the TCD procedures or methotrexate in the IST procedures, and may have contributed to differences in time to engraftment and thus costs. However, an independent effect of TCD on costs was detected despite adjustment for time to engraftment (P = .02).
We note a few caveats to our conclusions. First, this is a single-institution, observational study with a median follow-up of 1.5 years for survivors. Patients undergoing TCD procedures were more likely to be HLA matched while patients undergoing IST procedures had more advanced disease. Although conclusions were similar even after adjustment for these differences, residual selection bias may be obscuring significant differences. In addition, late relapses and transplant complications could still result in clinical, quality of life, or cost differences between TCD and IST patients. Second, due to the participation of only half the patients in the quality of life study, the power to detect statistically meaningful differences may be limited and selection bias might be important. However, we believe that factors associated with nonresponse are likely to apply equally to TCD and IST patients. Finally, different methods of TCD and different stem cell products have their own profile of complications and clinical outcomes. Our results may only apply to TCD of marrow accomplished through anti-CD6 (a narrow specificity antibody) and complement.21
In summary, we observed similar clinical outcomes and quality of life following TCD and IST URD bone marrow transplantation. However, days of hospitalization and costs were significantly lower with TCD procedures even after adjustment for clinical characteristics in the population. Longer follow-up of this cohort and additional randomized clinical trials may help address the relative merits of different GVHD prophylaxis regimens. A large, prospective, multicenter, randomized study comparing TCD against IST in URD marrow transplantation is being supported by the National Heart, Lung and Blood Institute (NHLBI) with quality of life and costs as secondary endpoints. Enrollment is complete and follow-up is ongoing. However, multiple strategies for TCD are represented in the NHLBI trial, and results will not be available for some time. We strongly support attention to quality of life and costs in evaluating GVHD prophylaxis regimens. Allogeneic hematopoietic stem cell transplantation is a costly procedure with long-term health consequences, and these data are important to help society and individual patients choose which approach offers the best outcomes.
We wish to thank our colleagues at the Dana-Farber Cancer Institute and Brigham and Women's Hospital for the excellent care of the patients who participated in the study. We especially thank our patients for participating in this research and sharing their experiences with us.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-03-0984.
Supported in part by National Institutes of Health grant nos. CA75267-01 and AI 29530, the Amy Strelzer-Manasevit Scholars Program, and the Leukemia and Lymphoma Society of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephanie Lee, Center for Outcomes and Policy Research, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: stephanie_lee@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal