A 47-year-old woman with severe macrocytic anemia markedly improved during the second and third trimesters of 3 pregnancies and when breast-feeding her 2 children. Because the serum prolactin level is elevated at these times, we later treated her with metoclopramide (10 mg orally 3 times daily), a medication known to induce prolactin release. Her serum prolactin levels increased from 7 to 133 ng/mL (normal < 20 ng/mL) and hematocrit from 17% to 22% to 35%. With continued therapy (now 10 mg orally daily), her hematocrit has ranged from 30% to 40% for 6 years, although the macrocytosis persists (mean corpuscular volume, 100-112 fL). On the basis of this observation, a pilot study was undertaken of metoclopramide therapy in patients with Diamond-Blackfan anemia who were refractory to low doses of corticosteroids. Fifteen patients were enrolled and 9 completed the planned 16 weeks of therapy. Three individuals responded, suggesting that this therapeutic approach may benefit others. As with the index case, the anemia did not improve until 12 to 15 weeks of therapy had been completed.
Introduction
Diamond-Blackfan anemia is a congenital macrocytic anemia, characterized by a low reticulocyte count, the absence or severe reduction of hemoglobin (Hgb)–containing cells in the marrow, and normal megakaryocytic and granulocytic differentiation.1-3Approximately 30% of patients have physical anomalies, including short stature and craniofacial, neck, and thumb malformations.2-5 Ten percent have a family history of anemia, generally with a dominant inheritance pattern. The genetic basis of Diamond-Blackfan anemia is likely heterogeneous. Abnormalities of the gene encoding ribosomal protein S19 (localized to chromosome 19q13.2) have been reported in 25% of families (as well as 25% of sporadic cases),6-8 whereas linkage to different chromosomes has been implicated in other families.5,7,9,10Although the anemia may initially respond to corticosteroid therapy, many patients require life-long red blood cell (RBC) transfusions, leading to infectious complications and iron overload.2-5Other therapies include androgens, cyclosporine, and interleukin 3 (IL-3), which appear to be efficacious in 10% to 15% of patients,2-5,11-13 and marrow transplantation.4,5,14 Spontaneous remissions, especially during adolescence, have been reported 3.
Our observations in a unique patient suggest that metoclopramide may also be an effective therapy. The patient's severe macrocytic anemia remitted during each of 3 pregnancies and when breast-feeding her 2 children, times when the serum prolactin level is elevated.15 Thus, when she finished childbearing and was again symptomatically anemic (hematocrit [Hct], 17%-22%), we treated her with metoclopramide, which is known to induce the release of prolactin from the pituitary.16-21 The Hct slowly increased and she has remained asymptomatic and transfusion independent (Hct, 30%-40%, mean corpuscular volume [MCV], 100-112 fL) for 6 years. The mechanism by which prolactin might affect erythropoiesis was investigated, and a small pilot study was undertaken to determine if metoclopramide could be effective in patients with Diamond-Blackfan anemia.
Patient, materials, and methods
Index case history
At age 14 years, the index patient received iron for a “very low blood count.” At age 21 years, the patient was a competitive athlete (cross-country skier), yet Hct values of 17% and 19% were obtained when she volunteered as a healthy control for a research study. At age 25 years, Hct values were 27% to 33%, MCV was 107 to 123 fL, and reticulocytes were 0.6% to 1.1%.
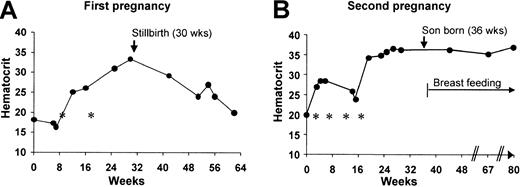
At age 33, during the initial 8 weeks of her first pregnancy, Hct values were 16% to 18% (MCV, 119-122; reticulocytes, 0.3%; Figure1 A). The marrow had diminished erythropoiesis (marrow/erythroid [M/E] > 25:1), normal granulocytic and megakaryocytic differentiation, and a normal karyotype. Although the pregnancy ended with a stillbirth at week 30, by week 25, the patient's Hct spontaneously increased to 31% to 33%. A computed tomography scan of the chest excluded thymoma.
Hematologic data from the index patient.
(A) First pregnancy. At 6 weeks of pregnancy, white blood cell count is 5.5 × 103/μL with 3.4 granulocytes, 0.2 bands, 1.9 lymphocytes, and 0.1 eosinophils; platelets, 409 × 103/μL; and reticulocytes, 0.3%. The Hct increased late in the second trimester of this patient's pregnancy, and then decreased following a stillbirth at 30 weeks of gestation. Asterisks represent transfusions of packed RBCs. (B) Second pregnancy. The Hct increased in the middle of the second trimester and remained elevated during the 103 weeks (23 months) that the patient was breast-feeding her son. Data from the start of pregnancy through the first 44 weeks after birth are shown. Three additional Hct levels were obtained between week 80 (44 weeks after birth) and week 139 (103 weeks after birth) and were 36% to 38%. From week 86 to 103, the patient breastfed her son only once each day. The asterisks represent transfusions of packed RBCs. A third pregnancy followed a comparable course. The Hct was maintained at more than 27% with prednisone 20 to 40 mg daily, and a healthy daughter was born at 40 weeks. With blood loss at the time of the cesarean delivery, the Hct decreased to 18%, but increased to 27% to 30% over the next 3 weeks without transfusion. The Hct also transiently decreased when prednisone was discontinued at 7 months after birth, but spontaneously recovered and remained 30% to 35% for the 16 additional months that she breastfed her daughter one or more times each day.
Hematologic data from the index patient.
(A) First pregnancy. At 6 weeks of pregnancy, white blood cell count is 5.5 × 103/μL with 3.4 granulocytes, 0.2 bands, 1.9 lymphocytes, and 0.1 eosinophils; platelets, 409 × 103/μL; and reticulocytes, 0.3%. The Hct increased late in the second trimester of this patient's pregnancy, and then decreased following a stillbirth at 30 weeks of gestation. Asterisks represent transfusions of packed RBCs. (B) Second pregnancy. The Hct increased in the middle of the second trimester and remained elevated during the 103 weeks (23 months) that the patient was breast-feeding her son. Data from the start of pregnancy through the first 44 weeks after birth are shown. Three additional Hct levels were obtained between week 80 (44 weeks after birth) and week 139 (103 weeks after birth) and were 36% to 38%. From week 86 to 103, the patient breastfed her son only once each day. The asterisks represent transfusions of packed RBCs. A third pregnancy followed a comparable course. The Hct was maintained at more than 27% with prednisone 20 to 40 mg daily, and a healthy daughter was born at 40 weeks. With blood loss at the time of the cesarean delivery, the Hct decreased to 18%, but increased to 27% to 30% over the next 3 weeks without transfusion. The Hct also transiently decreased when prednisone was discontinued at 7 months after birth, but spontaneously recovered and remained 30% to 35% for the 16 additional months that she breastfed her daughter one or more times each day.
When next pregnant, the patient was received transfusions to maintain her Hct over 25% (Figure 1B). At 15 weeks (3 weeks after her last RBC transfusion) she was evaluated at the University of Washington Medical Center. The Hct was 26%, reticulocytes were 3.2%, and the remainder of the complete blood count was normal. The marrow aspirate (M/E = 4:1) had left-shifted erythropoiesis and a normal karyotype. The B12, folate, leukocyte alkaline phosphatase score, creatinine, and liver function studies were normal. Twenty percent of circulating RBCs contained fetal hemoglobin (HgbF). Flow cytometry of blood and marrow did not demonstrate an abnormal population of T, B, or natural killer (NK) cells. Physical examination showed no facial, digital, or other congenital anomalies. There was no family history of anemia.
By 19 weeks of pregnancy, the Hct increased to 35% (as suggested by the marrow morphology and reticulocyte count), and a healthy boy was born at 36 weeks. Remarkably, the Hct then remained at 35% to 38% during the 23 months that she breast-fed her son, including that time (the last 4 months) when he fed only once each day. Both the MCV (107-118 fL) and the percentage of RBCs containing HgbF (13%) remained elevated.
The Hct pattern during and following her third pregnancy was similar to that of earlier pregnancies (Figure 1). When the patient stopped breast-feeding at 26 months after delivery and 4 weeks later, Hct values were 31% and 33%, respectively. The Hct then declined to 20% to 25% at a rate equivalent to that diagrammed in Figure 1A. The anemia responded to prednisone (Hct, 26%-29%), but the patient disliked the side effects, and opted for transfusions (3 U RBCs each 4-6 weeks). A trial of metoclopramide (Reglan) was initiated. At the start of therapy, the Hct was 18%, reticulocytes 0.8%, and serum erythropoietin 2980 mU/mL (normal, 6-20 mU/mL). The white blood cell (7.0 × 103/μL) and platelet (372 × 103/μL) counts were normal. Examination of the marrow confirmed red cell aplasia (M/E = 20:1); 64 ± 6 erythroid burst-forming units (BFU-Es)/105 marrow mononuclear cells (MMNCs), but no erythroid colony-forming units (CFU-Es) were detected.
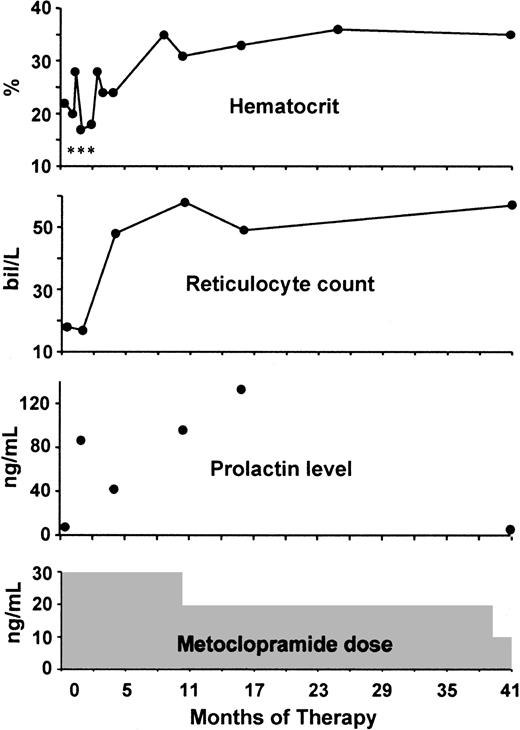
The patient's response to metoclopramide (10 mg orally 3 times daily) is shown in Figure2. The Hct slowly increased to 30% to 37% and remained at this level despite decreases in the metoclopramide dose to 10 mg twice a day (after 10 months) and 10 mg daily (after 39 months). MCVs ranged from 100 to 112 fL.
Hematologic response of the index patient to metoclopramide.
At 2 months, the patient received her last transfusion. After 8.5 months, her Hct was 30% or higher. Serum prolactin levels were elevated with thrice and twice daily dosing, but normal on once-daily metoclopramide therapy. The asterisks represent transfusions of packed RBCs. bil indicates billion.
Hematologic response of the index patient to metoclopramide.
At 2 months, the patient received her last transfusion. After 8.5 months, her Hct was 30% or higher. Serum prolactin levels were elevated with thrice and twice daily dosing, but normal on once-daily metoclopramide therapy. The asterisks represent transfusions of packed RBCs. bil indicates billion.
Four years after beginning metoclopramide, the patient stopped taking her medication, and within 2 months, she developed fatigue with exercise. When these symptoms worsened, metoclopramide was restarted (10 mg 3 times daily), but no blood studies were obtained. However, 4 weeks and 3 months later, Hct values were 28% and 36%, respectively. The metoclopramide dose was then decreased to 10 mg daily. Her most recent Hct, obtained 6 years after the start of the metoclopramide trial, is 40%.
Serum prolactin levels in the index patient
A serum prolactin level was measured prior to the administration of metoclopramide and was 7 ng/mL (normal < 20 ng/mL). With metoclopramide 10 mg thrice and twice daily, serum levels ranged from 42 to 133 ng/mL (Figure 2). When the patient's metoclopramide dose was 10 mg daily, a serum prolactin level (obtained 8 hours after the ingestion of medication) was 5 ng/mL. Five years after beginning metoclopramide, the kinetics of the prolactin response was studied quantitatively and was similar to that reported in pharmacologic studies.17-20 The baseline serum prolactin level was 3 ng/mL. Two hours after ingestion of 10 mg metoclopramide, prolactin was measured at 140 ng/mL.
Marrow culture studies
Marrow cells were obtained from the posterior iliac crest of the index patient and healthy volunteers (after informed consent, as approved by the University of Washington Institutional Review Board [IRB]). MMNCs were separated by centrifugation with a density gradient (1.077 g/mL; LSM, Cappel, Aurora, OH) and were cultured in 1.4% methylcellulose (Dow Chemicals, Costa Mesa, PA) (105cells/mL) in the presence of 30% heat-inactivated fetal bovine serum (Summit Biotechnology, Fort Collins, CO), 1 U/mL recombinant human (rh) erythropoietin (Epo), 3 U/mL rhIL-3 (Genzyme Diagnostics, Cambridge, MA), and 100 ng/mL rh stem cell factor (rhSCF; a gift from Amgen, Thousand Oaks, CA). In some experiments, rh prolactin (R & D Systems, Minneapolis, MN) was added or lower cytokine concentrations (ie, 0.2 U/mL Epo). Erythroid bursts (> 200 erythroid cells) and granulocyte-macrophage colonies (> 50 granulocytic or monocytic cells) were quantitated after 14 days of incubation at 37°C, 5% CO2/95% air. Erythroid colonies (8-50 erythroid cells) were enumerated at day 7.
To determine if prolactin receptors were present on hematopoietic progenitor cells, MMNCs were incubated with monoclonal antibody to the prolactin receptor (clone U5; Affinity Bioreagents, Golden, CO; concentration 2.8-6 μg/106 cells or 19-120 μg/mL, 30 minutes, 4°C), and then with R-phycoerythrin (R-PE)–conjugated F(ab′)2 fragment goat antimouse IgG, F(ab′)2 fragment specific (Jackson Immunoresearch Laboratories, West Grove, PA), final dilution of 1:40, 20-30 minutes, 4°C). Negative and positive subpopulations were isolated using a FACStar Plus cell sorter (Mountain View, CA). Gates were set so that 1% to 2% of marrow cells labeled with a mouse IgG1 isotype control then the R-PE–tagged antimouse IgG were considered positive. Cells from the negative and positive fractions were cultured to enumerate progenitor cells.
Pilot study of metoclopramide in patients with Diamond-Blackfan anemia
Patients with a clinical diagnosis of Diamond-Blackfan anemia1-3 received metoclopramide for 16 weeks at a standard dose for gastrointestinal reflux (eg, 10 mg orally 2-3 times daily for adults). Patients who required transfusions or who were dependent on high-dose corticosteroid therapy were eligible for this trial. Hgb, Hct, and serum prolactin levels were obtained prior to metoclopramide therapy and at weeks 8, 12, and 16. Transfusion intervals were noted. Although the intent was to maintain other medications (ie, prednisone) at baseline doses, these drugs could be adjusted at the discretion of the patient's physician. Each physician obtained IRB or equivalent institutional approval and served as the principal investigator for the local study. Response at 16 weeks (the duration of study), was defined as: (1) a 30% increase in Hct (or Hgb) or (2) a stable or improved Hct (or Hgb) and a 50% or greater increase in the transfusion interval or 50% or greater reduction in the prednisone dose. Because the index patient did not meet the criteria for response until 15 weeks (Figure 2), only patients completing the entire 16-week course of study were considered evaluable.
Results
Results of pilot study of metoclopramide in patients with Diamond-Blackfan anemia
Fifteen transfusion- or steroid-dependent patients were treated with metoclopramide in a multi-institutional study. All had severe macrocytic anemia, low or absent reticulocytes, and marrow erythroid hypoplasia (M/E > 10:1). Six patients were removed from the study at weeks 3 to 12 because of noncompliance (n = 3) or fatigue (n = 3). Patient characteristics and outcomes are listed in Table1. Of the 9 evaluable patients, 3 had clinically significant responses. These patients met response criteria at 12 to 15 weeks of therapy (details in Table 1). Responding patient 1 (a 32-year-old man) remains transfusion independent (Hgb > 12 g/dL) although he still requires prednisone therapy. Responding patient 2 (a 20-year-old woman) has maintained her Hgb more than 9 g/dL without transfusion or other therapies for 2 years. Responding patient 3 (a 3-year-old boy) has received metoclopramide for 10 months, resulting in an increase in Hgb from 6.8 to 9.6 g/dL and the continued slow tapering of prednisone treatment. Maximum recorded prolactin levels in the responding patients averaged 125 ng/mL and in the nonresponding patients, 35 ng/mL (P = .03, student t test).
Patients with Diamond-Blackfan anemia treated for 16 weeks with metoclopramide
| Patient no. . | Sex . | Age at study, y . | Age at diagnosis . | Anomalies . | Prior therapy . | Treatment at initiation of study . | Response . | Highest prolactin level (ng/mL) on metoclopramide . |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 32 | 4 y | Turner-like, piebaldism, short stature | — | P, Tx | Y | 30 |
| 2 | F | 20 | 1 y | Turner-like, short stature | P, C, IL-3 | Tx | Y | 142 |
| 3 | M | 3 | 16 mo | None | Tx | P | Y | 204 |
| 4 | M | 23 | 3 mo | Hypospadia, short stature | A, P, IL-3 | Tx | N | 33 |
| 5 | M | 9 | 11 mo | None | P | Tx | N | 92 |
| 6 | M | 4 | 2 y | None | P | Tx | N | 21 |
| 7 | M | 10 | 4 y | Short stature | — | P | N | 15 |
| 8 | M | 30 | 7 y | None | A, P | Tx | N | 20 |
| 9 | M | 17 | 1 mo | Widespread eyes, short stature | A, P, C, IL-3 | Tx | N | 31 |
| Patient no. . | Sex . | Age at study, y . | Age at diagnosis . | Anomalies . | Prior therapy . | Treatment at initiation of study . | Response . | Highest prolactin level (ng/mL) on metoclopramide . |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 32 | 4 y | Turner-like, piebaldism, short stature | — | P, Tx | Y | 30 |
| 2 | F | 20 | 1 y | Turner-like, short stature | P, C, IL-3 | Tx | Y | 142 |
| 3 | M | 3 | 16 mo | None | Tx | P | Y | 204 |
| 4 | M | 23 | 3 mo | Hypospadia, short stature | A, P, IL-3 | Tx | N | 33 |
| 5 | M | 9 | 11 mo | None | P | Tx | N | 92 |
| 6 | M | 4 | 2 y | None | P | Tx | N | 21 |
| 7 | M | 10 | 4 y | Short stature | — | P | N | 15 |
| 8 | M | 30 | 7 y | None | A, P | Tx | N | 20 |
| 9 | M | 17 | 1 mo | Widespread eyes, short stature | A, P, C, IL-3 | Tx | N | 31 |
Prolactin levels were 2-16 ng/mL (normal, < 20 ng/mL) in patients prior to metoclopramide administration. Responding patients are listed as patients 1, 2, and 3. Although anemia was noted during the first year of his life, Diamond-Blackfan anemia was diagnosed in patient 1 at age 4 years (Hgb, 7.2 g/dL; MCV, 101 fL; marrow demonstrated 4% of RBC precursors). Subsequent testing showed no mutation in the coding sequence of RPS19. At the time of metoclopramide administration, the patient was 32 years old and received prednisone 20 mg daily and RBC transfusions each 5 to 6 weeks. At 15 weeks, 9 weeks past his last transfusion, he met criteria for response. At 16 weeks (the duration of this study), his Hct was 29% (Hgb, 9.9 g/dL). At 6 months with continued therapy, his Hct was 36% (Hgb, 12.2 g/dL). With a prednisone taper, the Hgb decreased to 7.3 g/dL. Prednisone (20 mg daily) was restarted and the Hgb remains more than 12 g/dL for 16 months. He currently takes 10 mg metoclopramide twice a day. Diamond-Blackfan anemia was diagnosed in patient 2 when she was 1 year old (Hct, 21%; MCV, 94 fL; 9% of nucleated marrow cells were RBC precursors). Subsequent studies showed a normal HgbF and no abnormality in the coding sequence of RPS19. She initially responded to steroids (1971-1978), required no therapies between 1980 and 1990 (adolescence), but relapsed in 1990 with steroid-resistant disease. She then failed trials of IL-3 and cyclosporine and required RBC transfusions every 5 weeks. Her Hct (obtained 7 days after transfusion) was 22% and hemoglobin was 7.5 g/dL. After 14 weeks of metoclopramide therapy, her transfusion interval increased, meeting response criteria. At 7 months of therapy, she became transfusion independent (Hgb, 10 g/dL). The Hgb has remained more than 9 g/dL for 2 years (on metoclopramide 20 mg daily). Patient 3 (3-year-old boy) has been steroid dependent since his diagnosis at age 16 months. At that time, his Hct was 15.3%, MCV 103 fL, marrow had 7% erythroid precursors, ADA level was elevated at 1.57, and HgbF was 19.9%. RPS19 status is uncertain. At the time of study, he required daily prednisone at a dose of 5 mg alternating with 10 mg to maintain the Hgb at 6.8 g/dL. After 12 weeks of metoclopramide (0.2 mg/kg 3 times a day), his Hgb was 9.6 g/dL, meeting response criteria. At 16 weeks, the Hgb was 9.9 g/dL and the prednisone dose was tapered to 5 mg daily, then 5 mg alternating with 2 mg daily (Hgb values, 8.4-8.9 g/dL). When the metoclopramide dose was decreased to once a day (at 9 months), the Hgb fell to 6.7 g/dL, but recovered after restarting the 3-times-a-day dose. Because serum prolactin levels were measured at unknown times relative to 3-times-a-day metoclopramide ingestion, or just prior to metoclopramide ingestion, it is unlikely that values reflect peak responses. Three patients (ages 12, 21, and 30) discontinued therapy at weeks 12, 8, and 12, respectively because of toxicity (fatigue). Three patients (ages 4, 12, and 16) were noncompliant and removed from the study at weeks 9, 8, and 3. Their presentations and clinical characteristics were similar to evaluable patients.
A indicates androgens; P, prednisone or methylprednisolone; C, cyclosporine; Tx, RBC transfusions.
Role of prolactin in erythroid differentiation
The prolactin receptor, a type 1 cytokine receptor, is structurally similar to receptors for IL-1 through IL-7, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), Epo, and growth hormone.22 Prolactin and growth hormone (which is able to bind to the prolactin receptor23) have been implicated in erythropoiesis.24-29 Whether this role is direct (mediated by the binding of hormone to a receptor on erythroid progenitor cells) or indirect (mediated through effects on nonerythroid cells within the marrow microenvironment) is uncertain.24 28-30 Thus, in vitro studies were undertaken. The addition of rh prolactin (10-500 ng/mL) to cultures of marrow cells from the index patient or control individuals (n = 3) had no effect on the numbers of detectable BFU-Es (data not shown). The addition of exogenous prolactin also failed to improve erythroid differentiation when cytokine supplementation was suboptimal. When marrow cells (n = 5 independent experiments) were sorted by fluorescence-activated cell sorting (FACS) for the presence or absence of prolactin receptors, 94% ± 3% of BFU-Es, 100% of CFU-Es, and 96 ± 2% of granulocyte-macrophage colony-forming units (CFU-GMs) were in the prolactin receptor–negative fraction. These data suggest that the effects of prolactin on erythropoiesis are indirect.
Discussion
Diamond-Blackfan anemia is a clinically, as well as genetically, heterogeneous disorder. Family members with deficits in the coding sequence of ribosomal protein S19 may have normal Hct values,31,32 suggesting that cofactors must modulate disease phenotype.31 Also, although 95% of patients with Diamond-Blackfan anemia are diagnosed by age 7 years, the disease has been diagnosed in adults, including elderly persons.33 The index patient for this study had a persistent macrocytic anemia, low reticulocyte count, normal granulocyte and platelet production, a paucity of erythroid cells in the marrow aspirate, normal marrow cytogenetics, an elevated number of RBCs containing HgbF, normal erythrocyte adenosine deaminase (ADA), no congenital anomalies, and no evidence of progression to aplastic anemia or leukemia. Her clinical course was particularly instructive, because her anemia remitted in the mid to late second trimester of 3 pregnancies and when she breast-fed her 2 children. Prolactin uniquely increases in pregnancy, and remains elevated, at least intermittently, with breast-feeding, because suckling induces prolactin release.15 Prolactin has been implicated in the support of erythropoiesis in vitro28,29 and in vivo.25,26,34 Case reports from the 1970s suggest that bovine prolactin, administered intramuscularly (600-900 μg/kg/d) may improve anemia in patients.35 In these individuals both iron clearance from serum and iron utilization in RBC production were increased.35
For these reasons, the patient received metoclopramide, a dopamine antagonist that induces prolactin release. Metoclopramide has been both used in diagnostic studies of pituitary function,16-19 and administered chronically.20 21 Our patient received metoclopramide at a time that her Hct was 17% to 22% and had a dramatic response (Figure 2). Because a normal Hct was maintained with 10 mg metoclopramide daily, which caused intermittent (but not sustained) elevations in the serum prolactin level, and also when breast-feeding as infrequently as once each day, it appears that repetitive increases in the serum prolactin level are sufficient to support erythropoiesis.
Fifteen patients with Diamond-Blackfan anemia then received metoclopramide as a therapeutic trial. Nine individuals completed the 16-week course (Table 1), with 3 having clinically significant responses. With continued therapy the Hgb further increased to 12.2 and 10 g/dL at 6 to 7 months in patients 1 and 2, respectively. The Hgb in patient 3 reached 9.9 g/dL at 16 weeks, and remained sufficiently high (8.4-8.9 g/dL) to allow the subsequent taper of his prednisone dose. Thus, the anemia improved late, not until 12 to 15 weeks after the initiation of metoclopramide therapy, and continued to improve after the study formally ended, a pattern of response similar to that observed in the index patient.
Several factors may have contributed to the lack of metoclopramide response in the 6 other patients. Patients 7 and 8, as examples, had serum ferritin concentrations of 4400 and 4521 ng/mL, respectively, and clinical evidence of pituitary dysfunction. Also, prolactin release in response to metoclopramide is greater in women than men17and in older than younger individuals.36 Men and younger individuals were disproportionately represented among study patients (Table 1). Recombinant human prolactin (when available) might be an appropriate therapeutic agent for individuals in whom the serum prolactin remains low, as well as those who experience fatigue or other complications while taking metoclopramide.
In the in vitro experiments, exogenous prolactin did not improve BFU-E differentiation, consistent with some,24 but in contrast to other,28 earlier studies. Prolactin receptors were not present on erythroid progenitor cells, suggesting that the action of prolactin on erythroid differentiation is indirect, potentially mediated by microenvironmental cells,29 such as T cells and monocytes, cells that express prolactin receptors29 30and that produce cytokines known to affect erythropoiesis, such as IL-3, insulinlike growth factor 1, and kit ligand (SCF). That the transient elevations in serum prolactin induced by metoclopramide can lead to improved erythropoiesis is also consistent with a paracrine mechanism.
When the index patient's anemia improved during her pregnancies, with breast-feeding, and when taking metoclopramide, the MCV remained elevated. This suggests that metoclopramide improved erythropoiesis without correcting the underlying erythropoietic defect. Thus, it is possible that metoclopramide could benefit patients with other refractory macrocytic anemias, such as myelodysplasia.
The authors would like to thank Drs Joseph Sack, Nancy Klein, Carl Kjobech, Nancy Kernan, and Richard Minogue for their help in the clinical evaluation of the index and other patients, Dr Bert Glader for determining the erythrocyte ADA level, Dr Thalia Papayannopoulou for determining the percentage of red cells containing fetal hemoglobin, Dr Sandra Catlin for help with the statistical analysis, and Allan Dimaunahan and Zenaida Sisk for their help in the preparation of the manuscript.
Supported by grant R01 HL31823 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Janis L. Abkowitz, Professor of Medicine, Division of Hematology, University of Washington, Box 357710, Seattle, WA 98195; e-mail: janabk@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal