We have previously demonstrated that long-term heparin treatment causes cancellous bone loss in rats due in part to an increase in the number of osteoclasts lining the trabecular bone surface. In the present study, we investigated this phenomenon by examining the ability of heparin to synergistically enhance interleukin-11 (IL-11)–induced osteoclast formation. Treatment of murine calvaria and bone marrow cells with IL-11 was found to induce the formation of tartrate-resistant acid phosphatase-positive (TRAP+) multinucleated cells (MNCs) in a dose-dependent fashion. No effect was seen when cocultures were treated with heparin alone. However, when cocultures were treated with both IL-11 and heparin, IL-11's ability to induce TRAP+ MNC formation was enhanced 6-fold. In an attempt to resolve the mechanism responsible for this effect, we examined the ability of heparin to influence IL-11 signaling using murine calvaria cells. Heparin was found to enhance both IL-11–induced STAT3-DNA complex formation and transactivation without altering either STAT3 (signal transducer and activator of transcription-3) tyrosine or serine phosphorylation. Heparin was also found to enhance IL-11's ability to induce the expression of both receptor activator of nuclear factor–κB ligand (RANKL) and glycoprotein (gp) 130. When taken together, these findings suggest a plausible mechanism by which heparin may cause increased osteoclastogenesis and therefore bone loss when administered long-term.
Introduction
Osteoporosis is a well-recognized complication of long-term heparin use, with up to one third of all patients receiving long-term heparin experiencing subclinical reductions in bone density.1-12 Heparin is currently given long-term to prevent and treat venous thromboembolism during pregnancy, to prevent systemic embolism in pregnant women with mechanical heart valves, and to prevent pregnancy loss in women with antiphospholipid antibodies. Long-term heparin therapy is also indicated in nonpregnant patients with venous thromboembolism who have developed recurrences despite “adequate” oral anticoagulant therapy and in some patients who are immobilized for prolonged periods and who require ongoing thromboprophylaxis.11
In an attempt to resolve some of the issues surrounding long-term heparin therapy, we developed an animal model of heparin-induced osteoporosis. Using this model, we demonstrated that heparin induces both a time- and dose-dependent decrease in cancellous bone volume and that this results from a decrease in bone formation and an increase in bone resorption.13 14 We have also shown through histomorphometric analysis that these effects result from decreased osteoblast numbers as well as an increase in osteoclast number and activity. However, using this model we were unable to determine the exact mechanism by which heparin causes such profound effects on either the osteoblast or the osteoclast.
Interleukin-11 (IL-11) is a member of the gp130 receptor family of cytokines, a group that includes IL-6, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT-1). We as well as others have demonstrated that these cytokines are produced by osteoblasts15-17and that members of this cytokine family, including IL-11, are all capable of stimulating osteoclastogenesis in vitro.16-18Receptors for these cytokines are known to be expressed on the osteoblast cell surface, where they can initiate signal transduction via the recruitment and subsequent homodimerization or heterodimerization of membrane-associated glycoprotein (gp) 130.19 This results in the subsequent activation of the Janus family of tyrosine kinases (Jak1, Jak2, and Tyk2), which, once activated, phosphorylate specific tyrosine residues within the cytoplasmic domain of gp130.20 This allows members of the STAT (signal transducer and activator of transcription) family of transcription factors (STAT3 and STAT1) to bind gp130, where they also undergo tyrosine phosphorylation by Jak. Once phosphorylated, STATs form either homodimers or heterodimers and then translocate to the nucleus, where they bind specific promoter elements within their various response genes (reviewed by Heinrich et al21).
Although the sequence of events occurring following STAT activation has been well elucidated, factors modulating this activation are less understood. Here we report that heparin acts synergistically with IL-11 to induce STAT3 activation by a mechanism that is independent of either STAT3 tyrosine or serine phosphorylation. In addition, we report that this results in enhanced osteoclast formation and activity, a finding that may account for the increase in bone resorption that we have observed in our animal models of heparin-induced osteoporosis.
Materials and methods
Reagents
Specific pathogen-free Swiss Webster and 15- to 17-day pregnant female C57 Bl/6 mice were obtained from Charles River Laboratories (St Constant, QC, Canada). Recombinant human IL-11 (rhIL-11) was purchased from R&D Systems (Minneapolis, MN). Mouse monoclonal anti-pTyr antibody was purchased from Transduction Laboratories (Lexington, KY), while phospho-STAT3 (Tyr705) and phospho-STAT3 (Ser727) antibodies were obtained from Cell Signaling Technology (Beverly, MA). All other antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). [32P]orthophosphate was purchased from Perkin Elmer (Boston, MA). Unfractionated heparin was provided as a generous gift from Rhone-Poulenc Rorer (Montreal, QC, Canada).
Calvaria and bone marrow cell isolation
Calvaria cells were isolated from calvaria bones as described previously.16 Briefly, 2 to 4 mm2 pieces of calvaria were isolated from 3- to 5-day-old neonatal mice under sterile conditions. Calvaria cells were then isolated from the calvaria by collagenase II digestion (2.5 mg/mL), washed by centrifugation, and seeded at a concentration of 30 000 cells/cm2 in α-minimal essential medium (α-MEM) containing 10% fetal calf serum (FCS). All calvaria cell populations were expanded for 7 days prior to use.
Bone marrow cells were obtained from the femurs of 30-day-old Swiss Webster mice.16 Briefly, to obtain bone marrow cells, both ends of the disarticulated femoral bone were cut, and the marrow flushed out with phenol red-free α-MEM using a syringe fitted with a 25-gauge needle. The cells were then centrifuged and resuspended to a concentration of 2.0 × 105/mL in α-MEM containing 10% FCS prior to being cocultured with calvaria cells.
In vitro TRAP assay
Bone marrow cells (2 × 105/cm2) and murine calvaria cells (4 × 105/cm2) were cocultured in 24-well plates containing 13-mm Thermanox coverslips (NUNC, Naperville, IL) in the absence or presence of heparin (10 or 25 μg/mL), IL-11 (20 ng/mL), or a combination of the two. Every 3 days, fresh media and drug were added to existing media. Following a 9-day incubation, cells adherent to the 13 mm round Thermanox coverslips were fixed for 30 seconds with a citrate/acetone solution and then stained for tartrate-resistant acid phosphatase (TRAP) (procedure no. 386; Sigma Chemical, St Louis, MO) as described previously.16 The coverslips were then air dried, mounted onto slides, and the number of TRAP-positive (TRAP+) multinucleated cells (MNCs) determined microscopically at × 200 magnification.
Osteoclastic pit formation assay
Osteoclast activation was assessed by measuring the area of resorptive pits formed on the surface of smooth cortical bone slices. Briefly, cocultures of mouse calvaria and bone marrow cells were maintained on top of 1.0 cm2 cortical bone slices in the absence or presence of heparin (10 or 25 μg/mL), IL-11 (20 ng/mL), or a combination of the 2. Twenty-one days later, the cells were solubilized with 0.1% Triton X-100 (Bio-Rad, Hercules, CA) and the bone slices stained with 0.1% hematoxylin to allow for the visualization of resorption pits under light microscopy. Pit area was quantified using the computer imaging software system Northern Eclipse (Empix, Mississauga, ON, Canada).
Cell lysis and nuclear extract preparation
Calvaria cells were cultured to 70% to 80% confluency in α-MEM containing 10% FCS and then stimulated for 20 minutes with either heparin (25 μg/mL), IL-11 (20 ng/mL), or a combination of the 2. The cells were then rinsed once with ice-cold phosphate-buffered saline (PBS), isolated using a cell scraper, pelleted by centrifugation, and then lysed with a hypotonic buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9; 1.5 mM MgCl2; 10 mM KCl; 0.5 mM dithiothreitol [DTT]; and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). The nuclear pellet was then isolated by centrifugation and resuspended in 20 mM HEPES, pH 7.9, containing 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA (ethylenediaminetetraacetic acid), 0.5 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and 25% glycerol. Protein concentrations of the nuclear extracts were measured using a Bio-Rad protein assay (Bio-Rad, Hercules, CA) prior to their use in the electrophoretic mobility shift assay (EMSA).
Electrophoretic mobility shift assays
EMSAs were performed as described previously.22Briefly, a double-strand synthetic consensus oligonucleotide based on the serum-inducible element of the c-fos promoter (SIE) (5′-GTG CAT TTC CCG TAA ATC TTG TCT ACA-3′) was 5′ end-labeled with [γ-32P]adenosine triphosphate ([γ-32P]ATP) using polynucleotide T4 kinase (Boehringer Mannheim, Mannheim, Germany). Nuclear extracts (5 μg) were then incubated with 3 μg of poly(dIdC) (Pharmacia Biotech, Baie d'Urfe, QC, Canada) for 15 minutes on ice before adding 100 000 cpm of the radiolabeled probe in 20 mM HEPES, pH 7.9, containing 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, and 25% glycerol. For supershift assays, nuclear extracts were preincubated with anti-STAT3 antibody for 1 hour on ice. Competitions were performed with either the unlabeled homologous probe or an oligonucleotide mutated in the DNA binding region (5′-GTG CAT CCA CCG TAA ATC TTG TCT ACA-3′). After a 30-minute incubation at room temperature, DNA-protein complexes were run on a 4% native polyacrylamide gel, visualized by autoradiography, and quantified using a PhosphoImager (Molecular Dynamics, Sunnyvale, CA).
Luciferase reporter gene assay
The STAT3 reporter gene construct pTAL-LucSIE was constructed by inserting 3 copies of an annealed oligonucleotide corresponding to the SIE element from the human c-fos gene22 into theKpnI and XmaI sites upstream of the cytomegalovirus (CMV) minimal promoter of the luciferase reporter vector pTAL-Luc (Clontech, Palo Alto, CA). Calvaria cells were grown on 100-mm dishes and transfected with a mixture of plasmid containing the STAT3 reporter contructs (4 μg) and a Renilla luciferase expression plasmid (1 μg; Promega, Madison, WI) using lipofectin reagent as described by the manufacturer (Canadian Life Technologies, Burlington, ON, Canada). Transfected cells were then left untreated or were treated with heparin (25 μg/mL), IL-11 (20 ng/mL), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively) for 8 hours. The cells were then lysed and assayed for luciferase activities using the Dual Luciferase Reporter (DLR) Assay System according to the manufacturer's instructions (Promega). Luciferase activities were normalized to the internal control Renilla luciferase activity and assayed for luciferase activity as directed. Each sample was performed in triplicate in a single experiment and repeated in 4 separate experiments.
Immunoprecipitation and immunoblotting
Murine calvaria cells were grown to subconfluency in α-MEM containing 10% FCS. Cells were then treated with IL-11 (20 ng/mL), heparin (25 μg/mL), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively). After a 10-minute incubation at 37°C, the cells were washed and then lysed with 1% deoxycholic acid and Triton X-100 in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.2) containing the protease and phosphatase inhibitors leupeptin (5 μg/mL), aprotinin (5 μg/mL), pepstatin A (1 μg/mL), PMSF (1 mM), and EDTA (0.25 mM). Supernatants of the cell extracts were then incubated overnight at 4°C with 2 μg of either anti-gp130, anti–Jak-2, or anti-STAT3 antibody and then incubated with protein A sepharose (Pharmacia Biotech) for 1 hour to immunoprecipitate antigen/antibody complexes. Immunoprecipitates were washed 3 to 5 times with lysis buffer, boiled, and then subjected to 7.5% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis before being transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Membranes were blocked with 5% casein for 1 hour at room temperature and then incubated with anti-gp130, anti–Jak-2, or anti-STAT3 antibody for 2 hours. The blots were then incubated with horseradish peroxidase (HRP)–conjugated secondary antibodies (Abs) and protein visualized using an enhanced chemiluminescent system. Identical samples were run on a second gel, transferred to PVDF membranes, and anti-pTyr or anti-pSer727 antibodies were used to probe for phosphorylation.
[32P]orthophosphate labeling
Calvaria cells were starved for 2 hours in phosphate-free media α-MEM (Sigma) containing 10% fetal calf serum that had been dialyzed against a HEPES buffer (1.8 mM CaCl2, 5mM KCl, 0.8 mM MgSO4, 110 mM NaCl, 44 mM NaHCO3, and 25 mM HEPES [pH 7.3]). [32P]orthophosphate was then added to the medium to a final concentration of 0.1 mCi/mL (3.7 MBq; 5 mL labeling medium was used per 100-mm diameter dish). Following a 2.5-hour incubation, cell lysates from heparin, IL-11, and IL-11 plus heparin–treated calvaria cells were prepared and immunoprecipitated with anti-STAT3 antibody as described above. Following immunoblotting, membranes were washed overnight in Tris-buffered saline with 0.05% Tween-20 (TBST) at 4°C prior to autoradiography.
RNA extraction and Northern blot analysis
Calvaria cells were plated at 5 × 105/10-cm dish and cultured for 2 days in α-MEM containing 10% FCS and 100 U/mL penicillin/100 μg/mL streptomycin. The cells were then treated with heparin (25 μg/mL), IL-11 (20 ng/mL), or IL-11 (20 ng/mL) plus heparin (25 μg/mL) for 6 days to examine RANKL expression23 or for 24 hours to examine the expression of gp130. Total RNA was isolated using the RNeasy mini kit from Qiagen (Mississauga, ON, Canada). PolyA+ RNA was isolated from total RNA using the Oligotex procedure (Qiagen). PolyA+ RNA (2.0 μg) was fractionated on a 1% agarose/formaldehyde gel and transferred onto a nitrocellulose membrane. The RNA was cross-linked to the membrane using a UV cross-linker (Statalinker 2400; Stratagene Cloning Systems, La Jolla, CA) prior to hybridization. α-deoxycytidine triphosphate (α-dCTP) 32P-labeled cDNA probes were generated by random priming (Roche Molecular Biochemicals, Laval, QC, Canada); 717- and 734-base pair polymerase chain reaction products corresponding to the partial coding region of RANKL and gp130, respectively, were used as probes. Membranes were exposed to Kodak X-Omat AP film (Rochester, NY) at −70°C for 1 to 3 days and analyzed using a PhosphoImager (Molecular Dynamics). Blots were stripped by boiling for 2 × 20 minutes in 0.1 × SSC/0.5%SDS and rehybridized with additional probes. A partial cDNA fragment for GAPDH was used as loading control.
Statistical analysis
Analysis of variance was used to compare the results in the experimental groups with those in the controls. The significance of differences was determined using an unpaired Student t test with a Bonferroni correction for multiple comparisons. All data are expressed as a mean ± SEM.
Results
The effect of heparin on IL-11–induced in vitro osteoclast formation
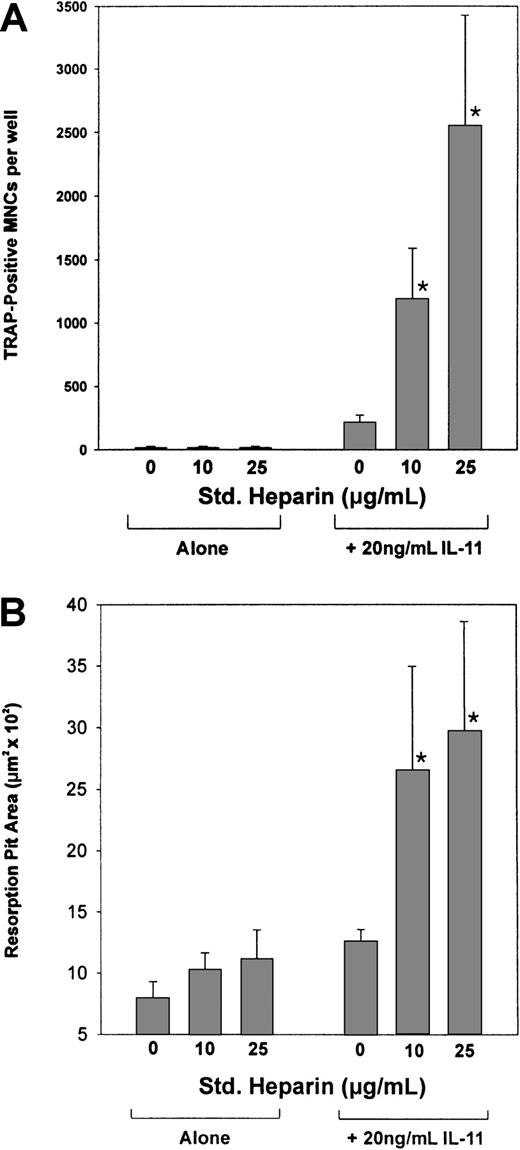
IL-11's ability to induce osteoclast formation was assessed by examining its ability to stimulate TRAP+ MNC formation in cocultures of murine bone marrow and calvaria cells. As shown in Figure1, IL-11 stimulated TRAP+ MNC formation in a dose-dependent manner from concentrations as low as 6.3 ng/mL to concentrations as high as 50.0 ng/mL. In contrast, heparin alone had no effect on the formation of TRAP+ MNCs (Figure2A). However, when cocultures were treated with both heparin and IL-11, IL-11's ability to stimulate TRAP+ MNC formation was enhanced more than 6-fold (Figure 2A).
The effect of IL-11 on the formation of TRAP+ MNCs.
Cocultures of murine calvaria and bone marrow cells were maintained in the absence or presence of increasing doses of IL-11. Nine days later the cells were stained for TRAPase activity and the number of multinucleated TRAP+ cells per well determined as described in “Materials and methods.” Data are expressed as mean ± SEM. *P < .01 when compared with the number of TRAP+ cells counted in the absence of IL-11.
The effect of IL-11 on the formation of TRAP+ MNCs.
Cocultures of murine calvaria and bone marrow cells were maintained in the absence or presence of increasing doses of IL-11. Nine days later the cells were stained for TRAPase activity and the number of multinucleated TRAP+ cells per well determined as described in “Materials and methods.” Data are expressed as mean ± SEM. *P < .01 when compared with the number of TRAP+ cells counted in the absence of IL-11.
The effect of heparin on IL-11–induced osteoclast development and pit formation.
(A) Cocultures of murine calvaria and bone marrow cells were maintained in the absence or presence of 20 ng/mL IL-11 and increasing doses of heparin (10 or 25 μg/mL). Nine days later the cells were stained for TRAPase activity and the number of multinucleated TRAP+ cells per well was determined. (B) Cocultures of murine calvaria and marrow cells were cultured on top of 1.0 cm2 smooth cortical bone slices in the absence or presence of 20 ng/mL IL-11 and increasing doses of heparin (10 or 25 μg/mL). Twenty-one days later the cells were solubilized and the bone slices examined by light microscopy for pit formation. Data are expressed as mean ± SEM. *P < .01 when compared with the number of TRAP+ cells counted in the presence of IL-11 alone.
The effect of heparin on IL-11–induced osteoclast development and pit formation.
(A) Cocultures of murine calvaria and bone marrow cells were maintained in the absence or presence of 20 ng/mL IL-11 and increasing doses of heparin (10 or 25 μg/mL). Nine days later the cells were stained for TRAPase activity and the number of multinucleated TRAP+ cells per well was determined. (B) Cocultures of murine calvaria and marrow cells were cultured on top of 1.0 cm2 smooth cortical bone slices in the absence or presence of 20 ng/mL IL-11 and increasing doses of heparin (10 or 25 μg/mL). Twenty-one days later the cells were solubilized and the bone slices examined by light microscopy for pit formation. Data are expressed as mean ± SEM. *P < .01 when compared with the number of TRAP+ cells counted in the presence of IL-11 alone.
To establish that the TRAP+ MNCs formed were indeed osteoclasts and thus capable of resorbing bone, cocultures of murine calvaria and bone marrow cells were grown over smooth cortical bone slices. Following a 21-day incubation, the cells were lysed and the cortical bone slices stained with hematoxylin to quantify the area of bone resorbed using light microscopy. Figure 2B demonstrates that the TRAP+ MNCs formed in the presence of IL-11 were able to resorb bone and that this activity was increased 2-fold in the presence of heparin. Taken together, these results suggest that heparin acts synergistically with IL-11 to increase not only the formation of osteoclasts but also their activity.
The effects of heparin on IL-11–induced DNA binding and transactivation of STAT3
To determine the mechanism by which heparin enhanced IL-11–induced TRAP+ MNC formation, we treated calvaria cells with IL-11 in the presence or absence of heparin and then quantified STAT-DNA complex formation by EMSA using radiolabeled SIE oligonucleotides. The SIE element is a well-established target of activated STATs, forming STAT-DNA complexes upon incubation in vitro.9 As shown in Figure3, nuclear extracts isolated from IL-11–stimulated cells were able to form protein-DNA complexes containing STAT3 homodimers and, to a lesser extent, STAT1/STAT3 heterodimers. No protein-DNA complexes were detected in the presence of excess cold SIE or in the presence of a DNA probe that differed from native SIE by 3 nucleotides (mSIE). Nuclear extracts isolated from cells treated with heparin alone did not form protein-DNA complexes with radiolabeled SIE, suggesting that heparin alone was unable to induce STAT activation. However, increased protein-DNA complex formation was detected when the nuclear extracts isolated from cells stimulated with IL-11 and heparin were compared with those stimulated with IL-11 alone. When quantified using phosphoimaging analysis, heparin was found to increase IL-11–induced STAT binding to SIE by 2-fold. These results demonstrate that heparin synergizes with IL-11, increasing the amount of activated STAT homodimer and heterodimer binding to DNA.
EMSA using the nuclear extracts of IL-11– and heparin-treated calvaria cells.
Calvaria cells were either left untreated (lane 2) or stimulated with 25 μg/mL heparin (lane 3), 20 ng/mL IL-11 (lanes 4, 6, 7, 8), or a combination of heparin and IL-11 (25 μg/mL and 20 ng/mL, respectively; lane 5) for 10 minutes at 37°C. Nuclear extracts were then prepared and EMSA was performed as described in “Materials and methods.” The EMSA was performed with probe alone (no nuclear extracts; lane 1), and competition was performed with excess (100 ×) unlabeled homologous oligonucleotide (lane 6) or mutated oligonucleotide differing from the SIE by 3 nucleic acids (lane 7), whereas supershifts were performed with anti-STAT3 Ab (lane 8). The arrow indicates STAT3 homodimerization and its association with radiolabeled SIE.
EMSA using the nuclear extracts of IL-11– and heparin-treated calvaria cells.
Calvaria cells were either left untreated (lane 2) or stimulated with 25 μg/mL heparin (lane 3), 20 ng/mL IL-11 (lanes 4, 6, 7, 8), or a combination of heparin and IL-11 (25 μg/mL and 20 ng/mL, respectively; lane 5) for 10 minutes at 37°C. Nuclear extracts were then prepared and EMSA was performed as described in “Materials and methods.” The EMSA was performed with probe alone (no nuclear extracts; lane 1), and competition was performed with excess (100 ×) unlabeled homologous oligonucleotide (lane 6) or mutated oligonucleotide differing from the SIE by 3 nucleic acids (lane 7), whereas supershifts were performed with anti-STAT3 Ab (lane 8). The arrow indicates STAT3 homodimerization and its association with radiolabeled SIE.
To determine if the increase in STAT3-DNA complex formation resulted in increased transcriptional activation of STAT3-responsive genes, murine calvaria cells were transfected with a luciferase reporter plasmid that contained an SIE element upstream of a CMV promotor. The cells were then treated with IL-11 in the presence or absence of heparin for 24 hours. As shown in Figure 4, luciferase activity was significantly increased over control values when transfected cells were treated with IL-11. In contrast, luciferase activity was not significantly increased when transfected cells were treated with heparin alone. However, when transfected cells were treated with both heparin and IL-11, heparin was found to enhance IL-11's ability to induce luciferase activity by more than 2-fold.
Transcriptional activation of SIE-luciferase by STAT3 in heparin- and IL-11–treated calvaria cells.
Calvaria cells were transiently cotransfected with a 3 × SIE-luciferase reporter plasmid and a Renilla luciferase expression plasmid. The cells were then either left untreated or treated with 25 μg/mL heparin, 20 ng/mL IL-11, or a combination of heparin and IL-11 (25 μg/mL and 20 ng/mL, respectively). Twenty-four hours later the cells were lysed and luciferase activities were determined using the Dual Luciferase Reporter (DLR) Assay System as described in “Materials and methods.” Data are expressed as a mean ± SEM of 3 separate experiments. *P < .01 when luciferase activity was compared with that obtained from untreated calvaria cells. **P < .05 when luciferase activity was compared with that obtained from calvaria cells treated with IL-11 alone.
Transcriptional activation of SIE-luciferase by STAT3 in heparin- and IL-11–treated calvaria cells.
Calvaria cells were transiently cotransfected with a 3 × SIE-luciferase reporter plasmid and a Renilla luciferase expression plasmid. The cells were then either left untreated or treated with 25 μg/mL heparin, 20 ng/mL IL-11, or a combination of heparin and IL-11 (25 μg/mL and 20 ng/mL, respectively). Twenty-four hours later the cells were lysed and luciferase activities were determined using the Dual Luciferase Reporter (DLR) Assay System as described in “Materials and methods.” Data are expressed as a mean ± SEM of 3 separate experiments. *P < .01 when luciferase activity was compared with that obtained from untreated calvaria cells. **P < .05 when luciferase activity was compared with that obtained from calvaria cells treated with IL-11 alone.
The effect of heparin on IL-11–induced tyrosine phosphorylation of gp130, Jaks, and STATs in murine calvaria cells
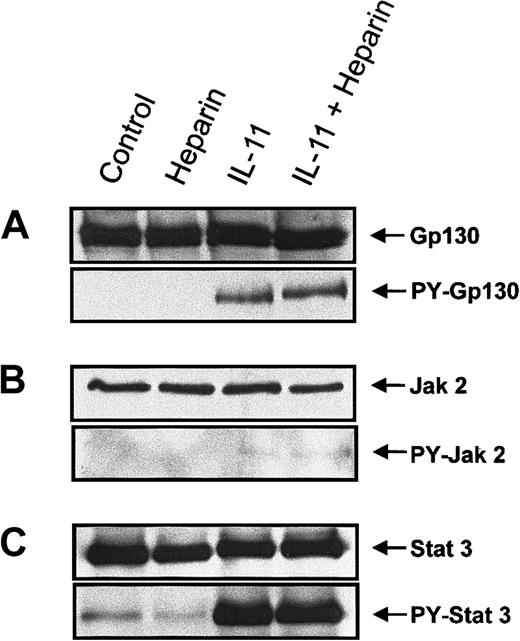
To elucidate the mechanism by which heparin stimulated IL-11–induced STAT activation, proteins involved in the IL-11 signaling pathway were examined for increased tyrosine phosphorylation following their immunoprecipitation from murine calvaria cell lysates. Because tyrosine phosphorylation of the Jak/STAT family proteins has been shown to be essential for gp130-mediated signal transduction,20-22,24 25 we performed immunoprecipitation on whole cell lysates isolated from cells treated with heparin, IL-11, or a combination of the 2 for 10 minutes. The tyrosine phosphorylation status of gp130, Jak1, Jak2, Tyk2, STAT1, and STAT3 was then determined by immunoblotting. Figure 5A demonstrates that IL-11, but not heparin, induced tyrosine phosphorylation of gp130 and that tyrosine phosphorylation of gp 130 by IL-11 was not increased further in the presence of heparin (Figure 5A). IL-11 also induced tyrosine phosphorylation of Jak2 (Figure 5B). However, as shown in Figure 5, tyrosine phosphorylation of Jak 2 by IL-11 was not further amplified in the presence of heparin; nor did it occur in the presence of heparin alone. Neither Jak1 nor Tyk2 tyrosine phosphorylation could be detected in calvaria cells treated with the above conditions (data not shown). STAT3 (Figure 5C) and, to a lesser extent, STAT1 (data not shown) were also found to be tyrosine phosphorylated in response to IL-11 treatment. However, similar to gp130 and Jak2, heparin alone had no effect on the tyrosine phosphorylation status of either STAT3 or STAT1, and no further tyrosine phosphorylation was observed when the cells were treated with IL-11 and heparin. Collectively, these results demonstrate that heparin does not alter the tyrosine phosphorylation status of either gp130, JAK2, or STAT3, suggesting that alternative mechanisms of heparin-induced synergy with IL-11 must be involved.
Tyrosine phosphorylation of gp130, Jak 2, and STAT3 in murine calvaria cells treated with IL-11 and/or heparin.
Confluent calvaria cells were stimulated for 10 minutes with either heparin alone (25 μg/mL), IL-11 alone (20 ng/mL), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively). (A) The cells were lysed and the lysate was immunoprecipitated with a polyclonal rabbit antimouse gp130 antibody. Precipitates were immunoblotted with an anti-gp130 antibody (upper panel) or run on a second gel before immunoblotting with a monoclonal anti-pTyr antibody (lower panel). (B) Cell lysates were immunoprecipitated with a polyclonal rabbit antimouse Jak2 antibody prior to immunoblotting the precipitates with an anti-Jak2 antibody (upper panel) or loaded on a second gel and immunoblotted with a monoclonal anti-pTyr antibody (lower panel). (C) Cell lysates were immunoprecipitated with a polyclonal rabbit antimouse STAT3 antibody prior to immunoblotting the precipitates with an polyclonal anti-STAT3 antibody (upper panel) or running them on a second gel and immunoblotting with a polyclonal pTyr-STAT3 antibody (lower panel).
Tyrosine phosphorylation of gp130, Jak 2, and STAT3 in murine calvaria cells treated with IL-11 and/or heparin.
Confluent calvaria cells were stimulated for 10 minutes with either heparin alone (25 μg/mL), IL-11 alone (20 ng/mL), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively). (A) The cells were lysed and the lysate was immunoprecipitated with a polyclonal rabbit antimouse gp130 antibody. Precipitates were immunoblotted with an anti-gp130 antibody (upper panel) or run on a second gel before immunoblotting with a monoclonal anti-pTyr antibody (lower panel). (B) Cell lysates were immunoprecipitated with a polyclonal rabbit antimouse Jak2 antibody prior to immunoblotting the precipitates with an anti-Jak2 antibody (upper panel) or loaded on a second gel and immunoblotted with a monoclonal anti-pTyr antibody (lower panel). (C) Cell lysates were immunoprecipitated with a polyclonal rabbit antimouse STAT3 antibody prior to immunoblotting the precipitates with an polyclonal anti-STAT3 antibody (upper panel) or running them on a second gel and immunoblotting with a polyclonal pTyr-STAT3 antibody (lower panel).
The effect of heparin on IL-11–induced STAT-3 serine phosphorylation
Phosphorylation of Ser727 has previously been shown to increase STAT3 activation.26 27 To investigate whether serine phosphorylation of STAT3 was important in the synergy between heparin and IL-11, we labeled calvaria cells with [32P]orthophosphate for 2.5 hours and then treated the cells with heparin, IL-11, or IL-11 and heparin for 10 minutes. STAT3 immunoprecipitates were then analyzed by both immunoblotting for Ser727 phosphorylation and by autoradiography (Figure6). Figure 6B demonstrates that incubation of murine calvaria cells with IL-11 resulted in increased Ser727 phosphorylation. In contrast, heparin alone had no effect on Ser727 phosphorylation, nor was it found to enhance IL-11–induced phosphorylation of Ser727. These results demonstrate that heparin's ability to enhance IL-11–induced STAT3 activation is independent of STAT3 Ser727 phosphorylation.
Serine phosphorylation of STAT3 in murine calvaria cells treated with IL-11 and/or heparin.
Phosphate-starved calvaria cells were labeled with 32P for 2 hours and then either left untreated (lane 1) or treated for 10 minutes with heparin alone (25 μg/mL; lane 2), IL-11 alone (20 ng/mL; lane 3), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively; lane 4). The cells were lysed and the lysate was immunoprecipitated with a polyclonal rabbit antimouse STAT3 antibody prior to immunoblotting the precipitates with a polyclonal anti-STAT3 antibody (A) or running them on a second gel and immunoblotting with a STAT-phosphoserine727 Ab (B). Alternatively, total32P labeling of STAT3 was visualized by autoradiography (C).
Serine phosphorylation of STAT3 in murine calvaria cells treated with IL-11 and/or heparin.
Phosphate-starved calvaria cells were labeled with 32P for 2 hours and then either left untreated (lane 1) or treated for 10 minutes with heparin alone (25 μg/mL; lane 2), IL-11 alone (20 ng/mL; lane 3), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively; lane 4). The cells were lysed and the lysate was immunoprecipitated with a polyclonal rabbit antimouse STAT3 antibody prior to immunoblotting the precipitates with a polyclonal anti-STAT3 antibody (A) or running them on a second gel and immunoblotting with a STAT-phosphoserine727 Ab (B). Alternatively, total32P labeling of STAT3 was visualized by autoradiography (C).
Because STAT3 is known to contain phosphoserine residues other than Ser727,28-30 we also explored the possibility that heparin may be increasing serine phosphorylation of STAT3 at these sites. As shown in Figure 6C, basal phosphorylation of STAT3 was not altered in the presence of heparin. Although stimulation with IL-11 did result in enhanced STAT3 phosphorylation, the simultaneous addition of heparin did not further increase total phosphorylation. Because total phosphorylation of STAT3 was not affected by heparin either alone or in the presence of IL-11, these results strongly suggest that heparin is not inducing serine phosphorylation of STAT3 at sites other than Ser727.
Effect of heparin on IL-11–induced gp130 and RANKL expression
Because heparin enhances IL-11–induced STAT3 transactivation, we next examined its ability to enhance the transcription of genes that contain STAT3 binding elements within their promoters. Of those that are capable of modulating osteoclast formation, gp130 and RANKL have recently been demonstrated to play a role in STAT3-mediated osteoclast formation.31-33 We therefore performed Northern blot analysis to examine the expression of both RANKL and gp130 in calvaria cells treated with heparin, IL-11, or a combination of the 2. Figure7A demonstrates that RANKL expression was induced by IL-11, whereas heparin alone had no effect. However, when heparin was combined with IL-11, the expression of RANKL was increased 3.8-fold over that seen with IL-11 alone. Similar results were observed when the expression of gp130 was examined (Figure 7B). When taken together, these results provide a mechanism by which heparin, by increasing IL-11–induced STAT3-DNA binding, can enhance IL-11–induced osteoclast formation.
The effect of heparin and/or IL-11 treatment on RANKL and GP130 expression in murine calvaria cells.
Calvaria cells were stimulated with either heparin alone (25 μg/mL), IL-11 alone (20 ng/mL), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively) as described in “Materials and methods.” Total RNA was then prepared and analyzed by Northern blot analysis for either RANKL (A) or gp130 (B).
The effect of heparin and/or IL-11 treatment on RANKL and GP130 expression in murine calvaria cells.
Calvaria cells were stimulated with either heparin alone (25 μg/mL), IL-11 alone (20 ng/mL), or heparin plus IL-11 (25 μg/mL and 20 ng/mL, respectively) as described in “Materials and methods.” Total RNA was then prepared and analyzed by Northern blot analysis for either RANKL (A) or gp130 (B).
Discussion
We previously developed an animal model of heparin-induced osteoporosis in an attempt to examine heparin's effects on bone. Using this model, we showed that heparin induces both a time- and dose-dependent decrease in cancellous bone volume and that this, in part, results from an increase in osteoclast number and activity.13 14 In the current study, we demonstrate that heparin acts synergistically with IL-11 to induce both STAT3 activation and in vitro osteoclast formation. Furthermore, we demonstrate that the effect of heparin on IL-11–induced STAT3 activation is independent of either STAT3 tyrosine or serine phosphorylation but leads to increased expression of both gp130 and RANKL. To our knowledge, this is the first study demonstrating a synergistic effect between a glycosaminoglycan and a cytokine signaling cascade and suggests a possible mechanism by which heparin may mediate bone loss when given long-term.
Our results are novel in that they clearly demonstrate heparin's ability to synergistically enhance IL-11–induced osteoclast formation and activity in vitro. IL-11 has been shown to act synergistically with other cytokines such as IL-3, stem cell factor, macrophage colony-stimulating factor (M-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) to stimulate megakaryocytopoiesis 34-36 and erythropoiesis37-39 in vivo as well as to enhance both platelet and neutrophil recovery following sublethal irradiation.40However, to date no other factors have been shown to enhance IL-11's ability to induce osteoclast formation. Here we report that heparin enhances the formation of osteoclasts by synergizing with IL-11 to induce RANKL expression (Figure 7A). Osteoblast expression of RANKL, in the presence of M-CSF, is known to be required for the formation of mature osteoclasts.41-45 In addition, IL-11 as well as other gp130 cytokines are known to induce RANKL in a STAT3-dependent manner.23 31 In the current study, we confirm IL-11s' ability to induce RANKL and demonstrate that heparin has the ability to enhance RANKL expression in the presence of IL-11. Although it is possible that heparin in combination with IL-11 may have concurrent effects on osteoclast precursors, the effects of heparin on IL-11–induced RANKL expression suggest a likely mechanism by which heparin enhances osteoclast formation both in vitro and in vivo.
IL-11–type cytokines such as IL-6 have also been shown to up-regulate the expression of gp130 on the surface of both human vascular smooth muscle and HepG2 cells.46,47 Because gp130, like RANKL, contains a STAT3 binding element within its promoter,32 we also examined the ability of heparin to enhance IL-11–induced gp130 expression. We found that, similar to its effect on RANKL, heparin was able to increase gp130 expression in the presence of IL-11. Previous studies have shown that increased gp130 expression can result in increased osteoclast formation in vitro.33 This suggests that up-regulation of gp130 may amplify osteoclast formation by further enhancing RANKL expression and would explain why, in the current study, heparin was able to enhance IL-11–induced osteoclast formation 6-fold even though IL-11–induced STAT3 activation was enhanced only 2-fold.
Heparin is known to bind a number of cytokines and/or growth factors and to thus alter the ability of such factors to bind to their respective receptors. For example, heparin has been shown to bind basic fibroblast growth factor (bFGF), suppressing in vitro collagen synthesis by 21-day-old fetal rat calvariae to a much greater extent than either heparin or bFGF alone.48 In the current study, we show that both IL-11–induced STAT3-DNA complex formation and transactivation are enhanced by heparin (Figures 3 and 4). To explain these findings, we initially hypothesized that heparin might be enhancing IL-11–induced STAT3 activation by binding IL-11 and thereby increasing IL-11–IL-11 receptor (IL-11R) interactions. However, binding studies with radiolabeled IL-11 failed to show increased binding of IL-11 on the calvaria cell surface in the presence of heparin (data not shown). In addition, neither gp130, JAK2, nor STAT3 tyrosine phosphorylation was altered when heparin was added in the presence of IL-11. When taken together, these results suggest that the mechanism by which heparin increases STAT3 activation is independent of IL-11–IL-11R interactions.
Although tyrosine phosphorylation is the primary regulator of STAT activity, maximal activation of STAT requires both serine and tyrosine phosphorylation. For example, STAT3 has been shown to be phosphorylated not only on Tyr705 but also on Ser727, which is located within the putative mitogen-activated protein (MAP) kinase recognition sequence PMSP at the -COOH terminus. Although phosphorylation of Tyr705 is a prerequisite of STAT3 dimerization and DNA binding, phosphorylation of Ser727 has been demonstrated to be required for maximal STAT3 transactivation. In addition, there is evidence to suggest that STAT3 may be phosphorylated on serine residues other than Ser727 and that these may also enhance not only STAT3 transactivation but also STAT3-DNA binding.29,30 In this study, Ser727 was not phosphorylated by heparin alone; nor did heparin alter the phosphorylation status of Ser727 in the presence of IL-11. The lack of involvement of Ser727 in heparin's ability to enhance IL-11–induced STAT3-DNA binding agrees with previous studies that have shown that complex formation is unaffected by a STAT3 Ser727 → Ala mutation.26 In addition, the possibility of other serine residues being phosphorylated in response to heparin was discounted because total STAT3 phosphorylation was not enhanced by heparin either in the presence or absence of IL-11 (Figure 6C). Thus, the results of this study suggest that heparin is not directly affecting STAT3 because neither IL-11–induced tyrosine nor serine phosphorylation of STAT3 is altered in the presence of heparin. To date, a number of factors have been shown to affect the activity of STAT3. Some, such as protein inhibitor of activated STAT3 (PIAS3), inhibit the ability of STAT3 to bind DNA by directly associating with activated STAT3 dimers.49 Other proteins, such as p300, modulate STAT3 transcriptional activity by binding STAT3 (reviewed by Shuai50). Our data demonstrate that heparin alters both DNA binding and transactivation and suggests that heparin acts to modulate the activity of a yet-to-be-identified protein that is capable of mediating STAT3-DNA binding.
In summary, we have demonstrated that heparin enhances IL-11–induced STAT3 activation by a mechanism that does not involve either tyrosine or serine phosphorylation of STAT3 but which leads to the increased expression of both gp130 and RANKL. This provides a plausible mechanism by which heparin may synergize with IL-11 in the formation of osteoclasts and helps to explain why patients treated with long-term heparin have measurable decreases in bone density that can translate to symptomatic fractures.
Supported by a “Premier's Research Excellence Award” to S.G.S. Also supported by the Heart and Stroke Foundation of Ontario (grant no. T 4153) and the Canadian Institutes of Health Research (grant no. MOP-53333). S.G.S. is a Research Scholar of the Heart and Stroke Foundation of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen G. Shaughnessy, Hamilton Civic Hospitals Research Center, 711 Concession St, Hamilton, ON, Canada, L8V 1C3; e-mail: sshaughnessy@thrombosis.hhscr.org.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal