Many of the mechanisms that govern T-cell homeostasis remain obscure. Here we report that repeated administration of synthetic oligodeoxynucleotides containing unmethylated cytosine-guanine motifs (CpG-ODN) into mice induces a systemic antigen-independent expansion of naive and memory T cells in a full T-cell compartment. Expansion of T cells was observed on both CD4+ and CD8+ T-cell subsets and was produced not by inducing the proliferation of the cells but by preventing their death. The antiapoptotic effects of CpG-ODN on T cells were observed against activation-induced death and growth factor withdrawal–mediated death. The ability of CpG-ODN to protect T cells from these forms of death was associated with the up-regulation of antiapoptotic gene products including c-FLIP, bcl-xL, and, to some extent, bcl-2. The effect of CpG-ODN on naive and memory T cells required the expression of CD28 and was not dependent on the presence of B lymphocytes, suggesting that other antigen-presenting cells that respond to CpG-ODN, such as dendritic cells, may provide antiapoptotic signals to T cells in an antigen-independent but CD28/B7-dependent fashion. The present findings suggest that CpG-ODN can disrupt normal T-cell homeostasis not by acting as a mitogen but by preventing T-cell death that normally takes place as a mechanism to maintain steady-state levels of T cells. These findings support a potential means to expeditiously replenish and maintain the peripheral lymphocyte population after severe immunodepletion such as that which occurs in HIV-infected individuals and individuals undergoing cytoablative therapies.
Introduction
Under normal conditions tight homeostatic control ensures that the number of peripheral naive and antigen-primed T lymphocytes remains steady.1-4 In mice the peripheral pools of T cells are continuously replenished by 1 × 106to 2 × 106 thymic T-cell emigrants each day regardless of the existing peripheral T-cell population.5-7 The thymic emigrants that survive in the periphery may continue to endure throughout life, but their persistence is at the expense of other resident T-cell inhabitants. The survival and expansion of T cells in the periphery can also depend upon the state of activation that the T cells have acquired. Naive T cells (CD44low, CD62Lhigh, CD45RAhigh) require constant T-cell receptor (TCR) “tickling”8 through the interaction with major histocompatibility complex (MHC) molecules presenting self-peptides.3,4,9 Moreover, cytokines such as interleukin-7 (IL-7) and perhaps IL-4 play a pivotal role in conveying survival signals to naive T cells.10-13Adoptively transferred T cells are unable to expand significantly in an already full T-cell compartment unless “space” is created by reducing the size of the existing peripheral T-cell pool via irradiation or antibody depletion. Under these conditions, adoptively transferred naive T cells will survive and divide, sometimes transiently, acquiring memory T-cell–like characteristics.14 15
Antigen-experienced T cells (CD44high, CD62Llow, CD45RO+) rapidly divide in response to antigen-MHC complex stimulation, resulting in the generation of effector T cells that disappear soon after the responsible pathogen is eliminated and memory T cells, which survive for long periods of time without further need of TCR tickling.1,8,16 In the absence of antigen, memory T cells divide slowly in response to IL-15, which is produced throughout various tissues.17Yet IL-2 can play a dual role, serving as a survival factor for resting T cells or as a proapoptotic factor during TCR stimulation.18
In spite of the tight controls that seek to maintain constant T-cell numbers throughout life, homeostasis is frequently broken. For example, at the onset of an immune response during an acute infection, the numbers of lymphocytes rapidly rise in both the circulation and the lymphoid organs. The marked increase in T cells results not only from the expansion of pathogen-specific lymphocytes, but also from the bystander accumulation of nonspecific cells responding to high levels of proinflammatory lymphokines or to pathogen-associated molecular patterns that activate the innate immune system. For example, the accumulation of both antigen-specific and bystander T cells occurs after exposure to nonspecific stimuli such as type-I interferons or bacterial lipopolysaccharide resulting from the prevention of T-cell death and/or the enhancement of T-cell proliferation.19 20
Synthetic oligodeoxynucleotides (ODN) containing unmethylated cytosine-guanine (CpG) motifs that mimic bacterial DNA correspond to a class of pathogen-associated molecular patterns that skew the immune response to a Th1 phenotype function.21-23 As a consequence, CpG-ODNs have been used as adjuvants to stimulate cytotoxic T lymphocyte (CTL) responses to various vaccines.24-27 We reported that CTL responses to peptide immunization were significantly increased in a vaccination protocol that included 9 daily injections of CpG-ODN.27 During the course of these experiments we observed a dramatic increase in the size of the spleen and the draining lymph nodes. The increase in the size of the lymphoid organs induced by the repeated administration of CpG-ODN did not require antigen and resulted in the rise of T-cell numbers by approximately 4- to 5-fold. Furthermore, a 40-fold increase in the number of dendritic cells (DCs) in the spleen of mice receiving the 9-day CpG therapy was observed.28
Here we describe how the repeated administration (9 daily 100 μg injections) of CpG-ODN promotes the antigen-independent peripheral expansion of both memory and naive subsets of CD4+ and CD8+ T cells in an already full T-cell compartment. The effect of CpG-ODN does not appear to be mediated through the induction of T-cell proliferation, as it occurs with B cells and other antigen-presenting cells (APCs), but through the decrease in the rate of T-cell death. Most interestingly, T-lymphocytes isolated from CpG-ODN–treated mice were highly resistant to growth factor withdrawal–induced apoptosis and activation-induced cell death (AICD). The effects of CpG therapy in T cells appear to be mediated through an increased expression of the antiapoptotic gene products c-FLIP and bcl-xL. Experiments using gene knockout mice suggest that a CD28/B7-dependent interaction of naive and memory T cells with CpG-activated APCs other than B lymphocytes results in the expansion of these T-cell pools. The present findings not only bear relevance for the induction of potent and long-lasting immune responses but also may be of use in other areas of immune therapy such as the repopulation of T cells in deficient individuals or in other circumstances where T-cell apoptosis plays a critical role in pathogenesis.
Materials and methods
Mice and immunizations
Female 6- to 8-week-old C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Wilmington, MA). CD28−/− and μMT−/−(B-cell deficient) mice were produced at the Mayo Animal Facility from breeding stocks obtained from The Jackson Laboratory. All experiments were routinely performed in groups of 3 to 5 mice each. Mice received 9 daily injections of 100 μg of CpG-ODN (5′-TCCATGACGTTCCTGACGTT-3′) and 100 μg/mL Neg-ODN (5′-TCCATGAGCTTCCTGAGCTT-3′), which were dissolved in sterile endotoxin-free phosphate-buffered saline (PBS), or PBS alone. All injections were administered subcutaneusly at the nape of the neck. Lymphoid tissues were obtained 1 day after final immunization. ODNs were synthesized at the Mayo Molecular Core facility using a phosphorothioate backbone.
Apoptotic assays
T lymphocytes were purified from spleens and lymph nodes of CpG-ODN–treated or control mice by negative selection using T-cell separation columns (R&D Systems, Minneapolis, MN). Purified T cells were first made susceptible to AICD by stimulating the cells with anti-CD3 and anti-CD28 monoclonal antibodies for 48 hours in IL-2 (50 IU/mL) prior to the apoptosis assays. For induction of AICD, 1 × 106 previously activated T cells were incubated in 24-well plates coated with 1 ug/mL of anti-CD3 antibody (Ab) for 14 hours. Cells were centrifuged and washed once with PBS and resuspended in a PBS buffer containing 0.01% (wt/vol) sodium Azide (Sigma) and 1% fetal calf serum. Cells were then incubated with fluorescein isothiocyanate (FITC)–labeled annexin-V (BD Pharmingen, San Diego, CA) at room temperature in the dark for at least 20 minutes. Flow cytometry was performed in a FACScan machine (Becton Dickinson, Mountain View, CA). Specific apoptosis was calculated as follows: (percentage of experimental apoptosis − percentage of spontaneous apoptosis)/(100 − percentage of spontaneous apoptosis) × 100.
For induction of growth factor deprivation–induced death, naive CD62L+, CD4+, or CD8+ T cells were purified using T-Cell Separation Columns (R&D Systems) and were resuspended at a density of 2 × 105 cells/mL for the induction of apoptosis. Apoptosis was induced by serum and cytokine withdrawal for the indicated time periods and quantitated by flow cytometry annexin-V as described above. In one experiment (Figure 4C), the purified naive T cells were labeled with the fluorescent probe 5,6-carboxyfluorescein diacetate, succimidyl ester (CFSE; Molecular Probes, Eugene, OR). Briefly, freshly purified T cells from Neg-ODN–treated mice were washed once and resuspended at 2 × 107 cells/mL in serum-free RPMI 1640 medium. An equal volume of 2 μM CFSE was added to the cells, and they were mixed gently and incubated at 37°C for 10 minutes. After the incubation period, an equal volume of fetal bovine serum (FBS) was added to quench the reaction, and the cells were washed 2 times in medium containing 10% FBS. CFSE-labeled T cells were then mixed with equal numbers of unlabeled naive T cells from CpG-ODN–treated mice in order to determine the numbers of apoptotic (annexin-V+) cells from each subpopulation at various time points.
Western blots
For Western blot analyses, about 30 μg whole cell extract protein was separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto a Hybond C nitrocellulose membrane, and blocked with 2% bovine serum albumin in Tris-buffered saline (TBS)/Tween 20 (0.05% Tween 20). After washing with TBS/Tween 20, blots were incubated with protein A–horseradish peroxidase (1/10 000) for 1 hour at room temperature, washed again, and developed with the ECL Western Blotting Detection kit (Amersham Pharmacia Biotech, Piscataway, NJ). All antibodies used for Western blot analysis were purchased from Santa Cruz Biotechnology, Santa Cruz, CA.
RNase protection assays
Total RNA was extracted from lymph node (LN) or splenic cells using the RNeasy minikit (Qiagen, Valencia, CA). The RNA was redissolved in RNase-free water, and yield was estimated by spectrophotometry. RNase protection assays (RPAs) were performed using RiboQuant from BD PharMingen according to the manufacturer's protocol. The multiprobe template sets were the mouse APO-3 and a custom design consisting of the mouse APO-2; however, bf1 was replaced for a FLICE inhibitory protein (FLIP) probe. The templates were used to synthesize the 32[p]-UTP–labeled probes (3000 Ci/mmol [111 000 GBq/mmol], 10 mCi/mL [370 MBq/mL]; NEN Life Science Products, Boston, MA) in the presence of a deoxynucleotide pool using a T7 RNA polymerase (BD PharMingen). Hybridization with 10 μg RNA was performed for 12 to 14 hours at 56°C, and the products were digested with RNase A and T1 mixture. The samples were treated by proteinase K in proteinase K buffer with yeast tRNA and then extracted with phenol and chloroform-isoamyl alcohol (50:1) and precipitated in the presence of ammonium acetate. The samples were loaded on acrylamide-urea gel and run at 40 W with 0.5 × Tris-borate-EDTA (ethylenediaminetetraacetic acid) electrophoresis buffer for 2 hours. The gel was adsorbed to filter paper, vacuum dried, and then exposed on film (X-AR; Kodak, Rochester, NY) with intensifying screens at −70°C.
Cytofluorometric analyses
Numbers of cells from lymphoid organs were determined by cytofluorography using fluoresceinated antibodies specific for these cell populations. For the analysis of cell surface expression, 1 × 106 cells were stained with monoclonal antibody (mAb) for 45 minutes at 4°C in 50 μL of PBS containing 0.1% sodium azide and 2% fetal calf serum (FCS). Cells were then washed and analyzed for CD8, CD3, CD4, CD11c, CD25, CD44, CD45RA, and CD69 expression on a Becton Dickinson FACScan (San Jose, CA). The mAbs used were purchased from PharMingen. For studies involving CFSE staining, naive T cells were stained with CFSE (Molecular Probes). In brief, cells were resuspended in PBS at 1 × 107/mL. CFSE was added to the cell suspension at a final concentration of 10 μM and incubated for 10 minutes at 37°C. Cells were washed 3 times in ice-cold RPMI 1640 with 20% FCS, recounted, and resuspended at 1.5 × 107/mL.
Evaluation of in vivo cell expansion
Normal C57BL/6, CD28−/−, or μMT−/−mice received 9 daily injections of 100 μg CpG-ODN or Neg-ODN. One day after the last injection, the draining axillary lymph nodes and spleens were harvested and pooled for each group. The total numbers of live cells were determined via trypan blue exclusion of viable cells by counting the cells in a hemocytometer. The numbers of naive (CD44low) and memory (CD44high) CD4+/CD3+ and CD8+/CD3+T cells, activated (CD80high) CD19+ B cells, and activated (CD80high) CD19-CD11c+ DCs for each treatment group was determined by 3-color flow cytometry. The total fold increase for each cell type/subset was calculated by multiplying the percentage of the corresponding group by the total number of live cells obtained per organ. The error bars in Figures 8 and 9 represent the standard error of the means obtained from 3 separate experiments.
Results
CpG-ODN induces a systemic but transient expansion of T lymphocytes in the absence of antigen
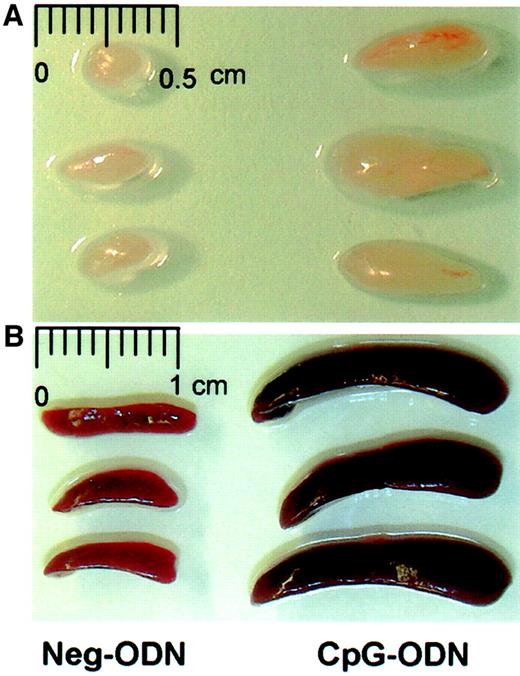
We first observed that the repeated daily subcutaneous injections of 100 μg of CpG-ODN into the nape of the neck resulted in a significant increase not only in the size of the corresponding draining axillary lymph nodes, but also in the size of the spleens (Figure1). Control synthetic ODN not containing a CpG motif (Neg-ODN) failed to induce splenomegaly, lymphoadenopathy, or to increase the number of cells in these organs. In CpG-ODN–treated mice the numbers of both CD4+ and CD8+ T lymphocytes in spleens were significantly increased as compared to the control (Neg-ODN) mice (Table 1). Interestingly, both the naive (CD44low) and the memory/activated (CD44high) T-cell subsets were increased approximately 3- to 4-fold. Similar effects were observed in the cell numbers obtained from the draining lymph nodes (data not shown). In addition to the effects of CpG-ODN on T lymphocytes, there was an average 12-fold increase in the number of B cells, 13-fold increase in NK cells, and a 15- to 40-fold increase in the number of CD11c+ DCs and CD11b+ macrophages obtained from CpG-treated mice as compared to PBS-treated mice (data not shown). The effects of CpG-ODN were transient since the total numbers of T cells were restored to normal numbers approximately 20 days after the last CpG-ODN injection (data not presented). The decrease in T-cell numbers during this phase paralleled with a decrease in B cells, DCs, and macrophages. These results indicate that the repeated administration of CpG-ODN results in a dramatic antigen-independent increase in the overall number of lymphocytes as well as accessory cells, with a systemic yet transient lymphoadenopathy and splenomegaly ensuing.
Repeated administration of CpG-ODN induces lymphoadenopathy and splenomegaly.
C57BL/6 mice underwent 9 daily injections of CpG-ODN or Neg-ODN (lacking CpG motifs). One day after the last ODN injection the draining axillary lymph nodes (A) and spleens (B) were harvested and photographed.
Repeated administration of CpG-ODN induces lymphoadenopathy and splenomegaly.
C57BL/6 mice underwent 9 daily injections of CpG-ODN or Neg-ODN (lacking CpG motifs). One day after the last ODN injection the draining axillary lymph nodes (A) and spleens (B) were harvested and photographed.
CpG-ODN therapy increases the number of naive and activated T cells
| . | Cell number per spleen, millions . | Fold increase . | |
|---|---|---|---|
| CpG-ODN . | Neg-ODN . | ||
| Total CD4+ | 35.4 ± 2.2 | 9.44 ± 0.95 | 3.75 |
| CD4+CD44low | 18.4 ± 2.4 | 5.74 ± 0.85 | 3.21 |
| CD4+CD44high | 17.0 ± 2.6 | 3.70 ± 0.74 | 4.59 |
| Total CD8+ | 20.9 ± 3.1 | 5.50 ± 0.85 | 3.81 |
| CD8+CD44low | 12.4 ± 3.0 | 3.30 ± 0.70 | 3.76 |
| CD8+CD44high | 8.6 ± 0.5 | 2.20 ± 0.09 | 3.89 |
| . | Cell number per spleen, millions . | Fold increase . | |
|---|---|---|---|
| CpG-ODN . | Neg-ODN . | ||
| Total CD4+ | 35.4 ± 2.2 | 9.44 ± 0.95 | 3.75 |
| CD4+CD44low | 18.4 ± 2.4 | 5.74 ± 0.85 | 3.21 |
| CD4+CD44high | 17.0 ± 2.6 | 3.70 ± 0.74 | 4.59 |
| Total CD8+ | 20.9 ± 3.1 | 5.50 ± 0.85 | 3.81 |
| CD8+CD44low | 12.4 ± 3.0 | 3.30 ± 0.70 | 3.76 |
| CD8+CD44high | 8.6 ± 0.5 | 2.20 ± 0.09 | 3.89 |
Repeated CpG-ODN administration induces an increase in CD4+ and CD8+ T-cell numbers. The number of CD44low (naive) and CD44high (antigen experienced) CD4+/CD3+ and CD8+/CD3+ T cells in spleens for each treatment group was determined by triple-color flow cytometry. The total cell yield was calculated by multiplying the proportion of each cell subset by the total number of live cells obtained per organ. Values represent the means and standard deviations obtained from 3 animals. Results are representative of 3 separate experiments.
Lack of mitogenic effect of CpG-ODN on T lymphocytes
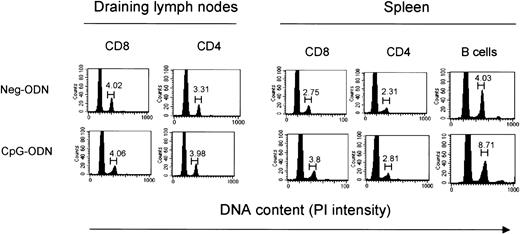
The significant increase in T-cell numbers in CpG-ODN–treated mice suggested that this compound could be functioning as a typical mitogen such as concanavalin-A, inducing the proliferation of these cells in an antigen nonspecific manner. To investigate whether CpG-ODN induced the expansion of T cells via increased proliferation, we quantitated the numbers of dividing T lymphocytes via cell-cycle analysis using propidium iodide (PI) DNA staining. Data in Figure2 indicate that the number of CD4 and CD8 T cells entering cell cycle (high content of DNA) slightly increased (about 16%) in the CpG-ODN–treated animals as compared to the Neg-ODN–treated mice. Yet, consistent with the knowledge that CpG-ODNs function as mitogens for B lymphocytes,29the number of these cells entering the cell cycle more than doubled in the CpG-ODN–treated mice as compared to the controls (Figure 2). Similar results were obtained when the proportion of dividing T cells was determined by bromodeoxyuridine (BrdU) incorporation into DNA, which was measured by flow cytometry by double-staining permeabilized CD3+ T cells with an anti-BrdU antibody. In these experiments we observed a small increase (7%) in the number of dividing T cells in the CpG-ODN–treated mice as compared to the untreated controls after receiving 4 daily injections of CpG-ODN. In addition, after 9 injections of CpG-ODN, there was no further increase in the number of BrdU-positive T cells (data not shown). Moreover, the effect of various concentrations of CpG-ODN in the induction of T-cell proliferation as measured by 3[H]-thymidine incorporation into DNA was evaluated both in the presence and absence of APCs. Under all conditions examined, CpG-ODN did not induce a significant proliferative response in either CD4+ or CD8+ T cells (data not presented). Collectively, these results indicate that CpG ODNs do not function as T-cell mitogens and that other mechanisms must be involved in the expansion of various T-cell subsets in mice receiving repeated doses of CpG-ODN.
CpG-ODN does not induce T-cell proliferation.
C57BL/6 mice underwent 9-day therapy with CpG-ODN or Neg-ODN. One day after the last ODN injection the draining axillary lymph nodes and spleens were harvested, stained, and analyzed via flow cytometry. Cell cycle analysis of various lymphocyte subsets was evaluated by double-staining of LN or spleen cells with specific antibodies and PI and gating on the CD8, CD4, or CD19 populations. Numbers above the gates indicate the percentage of cells with double the content of DNA, which initiated cell division.
CpG-ODN does not induce T-cell proliferation.
C57BL/6 mice underwent 9-day therapy with CpG-ODN or Neg-ODN. One day after the last ODN injection the draining axillary lymph nodes and spleens were harvested, stained, and analyzed via flow cytometry. Cell cycle analysis of various lymphocyte subsets was evaluated by double-staining of LN or spleen cells with specific antibodies and PI and gating on the CD8, CD4, or CD19 populations. Numbers above the gates indicate the percentage of cells with double the content of DNA, which initiated cell division.
CpG-ODNs induce the expansion of T cells in the peripheral lymphoid tissues
The increase in the number of T cells could be the result of either a rise in the number of T lymphocytes emerging from the thymus or, alternatively, via the expansion of these cells occurring in the periphery. Thus, to ascertain the anatomical site of the CpG-ODN–induced increase in the T-cell numbers, we compared the effect of CpG therapy in the expansion of CD4+ and CD8+ T cells subsets in normal and thymectomized mice. The results in Figure 3 show that CpG therapy had similar effects on both normal and thymectomized mice, with respect to the increase in total T-cell numbers in their lymph nodes and spleens. In addition, CpG-ODN therapy affected both naive (CD44low) and memory (CD44high) CD4+ and CD8+ T cells (data not presented). These results suggest that CpG therapy augments T-cell numbers in the periphery even in the absence of thymic output and that both naive and antigen-experienced T cells are affected. However, it is possible that CpG-ODNs may also increase thymic output by altering positive/negative selection functions. Nevertheless, analysis for the various thymocyte subsets using flow cytometry showed that the numbers of the various thymic T-cell progenitor populations including double-negative, double-positive, or single-positive CD4 or CD8 cells were not altered in CpG-ODN–treated mice as compared to the PBS controls (data not shown).
CpG-ODN therapy increases the number of naive (CD44low) and memory (CD44high) T cells in normal and thymectomized mice.
Thymectomized (white bars) and normal (gray bars) C57BL/6 mice (3 animals per group) were treated with 9 injections of the CpG-ODN (hatched bars) or PBS (solid bars) as described in “Materials and methods.” The percentages of the various subsets of T cells on draining lymph nodes or spleens were determined by 2-color flow cytometry. Results represent the total cell yield calculated by multiplying the proportion of cells from each subset by the number of cells from CpG-ODN mice divided by the PBS control for each treatment group. The error bars represent the standard error of the means obtained from 3 separate experiments.
CpG-ODN therapy increases the number of naive (CD44low) and memory (CD44high) T cells in normal and thymectomized mice.
Thymectomized (white bars) and normal (gray bars) C57BL/6 mice (3 animals per group) were treated with 9 injections of the CpG-ODN (hatched bars) or PBS (solid bars) as described in “Materials and methods.” The percentages of the various subsets of T cells on draining lymph nodes or spleens were determined by 2-color flow cytometry. Results represent the total cell yield calculated by multiplying the proportion of cells from each subset by the number of cells from CpG-ODN mice divided by the PBS control for each treatment group. The error bars represent the standard error of the means obtained from 3 separate experiments.
CpG-ODN protects T cells against growth factor deprivation–induced cell death
If the increase in T-lymphocyte numbers observed in CpG-ODN–treated mice is not the result of a stimulation of cell proliferation or an increase in thymic output, it would most likely be due to a decrease in the normal turnover of these cells. Thus, we proceeded to study whether CpG-ODN affects T-cell apoptosis, which is an important homeostatic mechanism that ensures that the total number of T lymphocytes remains relatively constant in the periphery. One mechanism that may help keep T-cell numbers in check is the competition for survival signals (growth factors and cell interactions with APCs) occurring between resident naive T cells and recent thymic emigrants. We tested the hypothesis that CpG-ODN may function in part by preventing growth factor deprivation–induced death in T cells. This possibility was evaluated by measuring the in vitro survival of purified naive T cells from normal and CpG-ODN–treated mice in the absence of growth factors (serum, lymphokines) and without APCs or other feeder cells. The results from these experiments showed an improved survival of both CD4+ and CD8+ T cells that were obtained from CpG-ODN–treated mice, as compared with T cells from control Neg-ODN–treated or untreated mice (Figures4A,B). We considered the possibility that low numbers of contaminating non–T cells such as APCs in these cultures could be responsible for providing survival signals to the naive T cells from CpG-ODN–treated mice. Equal numbers of naive T cells from CpG-ODN–treated mice were mixed with CFSE-labeled naive T cells from Neg-ODN control mice, and cell numbers for both populations were evaluated at various time points. The results presented in Figure4C demonstrate that in these mixed cultures, only the naive T cells from the CpG-ODN–treated mice survived in the absence of exogenous growth factors, indicating that the survival signals are not likely to be derived from contaminating APCs in these cultures. The overall data indicate that CpG therapy can confer protection against death resulting from growth factor withdrawal in both CD4+ and CD8+ T cells in the absence of APCs.
CpG-ODN therapy increases resistance to growth factor withdrawal–induced death in naive T lymphocytes.
Purified T cells were obtained from untreated (■), CpG-ODN–treated (●), or Neg-ODN–treated (⋄) mice one day after the last ODN injection. T cells were purified from splenocytes and lymph nodes, and the naive T cells were further selected using anti-CD62L antibody coated magnetic beads. Purified naive CD4+ (A) or CD8+ (B) T cells were cultured separately in the absence of serum and exogenous cytokines for up to 12 hours. As a positive control, naive T cells from untreated mice were cultured in the presence of 5% FCS and 25 IU IL-2/mL (▵). The percent apoptotic cells were evaluated 6 and 12 hours after the initiation of the experiment by flow cytometry using annexin-V staining. (C) Purified naive T cells from Neg-ODN–treated mice were first labeled with CFSE and then mixed with an equal number of naive T cells (unlabeled) from CpG-ODN–treated mice. The cell mixture was placed in culture in medium lacking growth factors, and the numbers of viable CFSE-positive and CFSE-negative cells were estimated at various time points as described above. Error bars represent the standard errors of the means obtained from 3 different mice per group.
CpG-ODN therapy increases resistance to growth factor withdrawal–induced death in naive T lymphocytes.
Purified T cells were obtained from untreated (■), CpG-ODN–treated (●), or Neg-ODN–treated (⋄) mice one day after the last ODN injection. T cells were purified from splenocytes and lymph nodes, and the naive T cells were further selected using anti-CD62L antibody coated magnetic beads. Purified naive CD4+ (A) or CD8+ (B) T cells were cultured separately in the absence of serum and exogenous cytokines for up to 12 hours. As a positive control, naive T cells from untreated mice were cultured in the presence of 5% FCS and 25 IU IL-2/mL (▵). The percent apoptotic cells were evaluated 6 and 12 hours after the initiation of the experiment by flow cytometry using annexin-V staining. (C) Purified naive T cells from Neg-ODN–treated mice were first labeled with CFSE and then mixed with an equal number of naive T cells (unlabeled) from CpG-ODN–treated mice. The cell mixture was placed in culture in medium lacking growth factors, and the numbers of viable CFSE-positive and CFSE-negative cells were estimated at various time points as described above. Error bars represent the standard errors of the means obtained from 3 different mice per group.
CpG-ODN prevents T cells from TCR-induced death
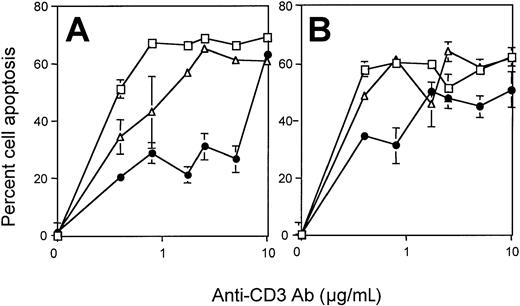
The homeostatic control of naive and activated/memory T-cell numbers appears to be regulated differently. AICD is one of the mechanisms by which effector T-cell responses are down-regulated as a means to control the size of the peripheral T-cell pool.30 31 We thus proceeded to examine whether CpG therapy was affecting the size of the antigen-experienced CD44high T-cell population (Table 1) by rendering these T cells more resistant to AICD. To test this hypothesis, purified CD4+ and CD8+ T cells from untreated, CpG-ODN–treated, or Neg-ODN–treated mice were compared for their susceptibility to TCR-mediated AICD. For these experiments, the resting T cells were first rendered susceptible to AICD by TCR activation with plate-bound anti-CD3 and anti-CD28 antibodies (see “Materials and methods” for details). The recently activated T cells were then assayed for AICD mediated by TCR stimulation with various concentrations of plate-bound anti-CD3 antibodies. The results show that both CD4+ and CD8+ T cells obtained from CpG-treated mice were more resistant to AICD than the T cells obtained from untreated mice or Neg-ODN (Figure5). However, the resistance to AICD was observed only at low to intermediate concentrations of anti-CD3 antibody, and the effects were more apparent in CD4+ T cells (Figure 5A) than in the CD8+ (Figure 5B) population. Resistance to AICD in T cells from CpG-ODN–treated mice remained evident even when the cells were obtained 5 days after the mice received the last CpG-ODN injection (data not presented). However, no significant protection against AICD was observed if the T cells were harvested 10 days after the final CpG-ODN injection (data not shown), indicating that although this effect persists for some time, the cells ultimately revert to the AICD-susceptible phenotype. The increased resistance to AICD in T cells from CpG-treated mice was not the result of an inhibition in the capacity of the T cells to respond to TCR activation, since these cells secreted similar levels of IL-2 upon anti-TCR stimulation as compared with the T cells from the untreated controls (data not shown). These results demonstrate that T cells are rendered more resistant against AICD and remain functionally active to TCR stimulation in response to CpG-ODN therapy.
CPG therapy increases T-cell resistance to AICD.
CD4+ (A) or CD8+ (B) T cells were purified splenocytes derived from untreated (■), CpG-ODN–treated (●) or Neg-ODN–treated (▵) mice one day after the final ODN injection. Purified T cells were cultured in anti-CD3 (1 μg/mL) antibody–coated culture plates together with an anti-CD28 antibody (1 μg/mL) for 48 hours in the presence of 50 U IL-2/mL. T cells were then induced to undergo AICD by plating these cells with varying concentrations of immobilized anti-CD3 Ab for about 14 hours. The percent of dead cells was evaluated via flow cytometric analysis using annexin-V staining. Error bars represent the standard error obtained from 3 different mice. Asterisks denote points where statistical significance was observed between the CpG-ODN–treated group and the control groups.
CPG therapy increases T-cell resistance to AICD.
CD4+ (A) or CD8+ (B) T cells were purified splenocytes derived from untreated (■), CpG-ODN–treated (●) or Neg-ODN–treated (▵) mice one day after the final ODN injection. Purified T cells were cultured in anti-CD3 (1 μg/mL) antibody–coated culture plates together with an anti-CD28 antibody (1 μg/mL) for 48 hours in the presence of 50 U IL-2/mL. T cells were then induced to undergo AICD by plating these cells with varying concentrations of immobilized anti-CD3 Ab for about 14 hours. The percent of dead cells was evaluated via flow cytometric analysis using annexin-V staining. Error bars represent the standard error obtained from 3 different mice. Asterisks denote points where statistical significance was observed between the CpG-ODN–treated group and the control groups.
CpG-ODN alters the expression of apoptotic-related genes in T cells
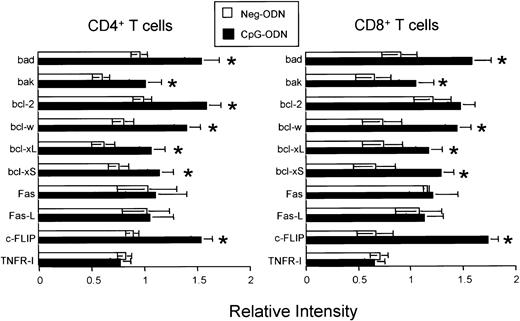
Based on the data presented so far indicating that CpG-ODN is not mitogenic for T cells but prolongs T-cell survival under various conditions (growth factor withdrawal and AICD), we reasoned that CpG therapy may provoke the accumulation of peripheral T cells in vivo by inhibiting the apoptotic pathways responsible for maintaining T-cell homeostasis. We proceeded to study the effects of CpG therapy on the expression of apoptosis-related genes by analysis of RNA prepared from purified CD4+ and CD8+ T cells obtained from CpG-ODN–treated and Neg-ODN–treated mice. Upon treatment with CpG-ODN but not with Neg-ODN, the expression of the antiapoptotic genes bcl-w, bcl-xL, and c-FLIP (for cellular FLICE-inhibitory protein) was significantly up-regulated in both CD4+ and CD8+ T cells (Figure 6). In addition, the expression of the antiapoptotic gene bcl-2 also was found markedly up-regulated in CD4+ T cells derived from CpG-ODN–treated mice (Figure 6). However, the T-cell expression of various membrane-associated death-inducing molecules including Fas-L, Fas, and TNF-RI did not appear to differ between the CpG-ODN and Neg-ODN–treated mice. Paradoxically, CpG therapy appeared to produce a slight up-regulation of the proapoptotic genes bcl-xS and bak.
Effect of CpG-ODN therapy in the T-cell RNA levels for various apoptotic-related genes.
CD4+ (A) or CD8+ (B) T cells were purified from untreated, CpG-ODN– (black bars), or Neg-ODN–treated (white bars) mice one day after the final ODN injection. Total RNA was isolated, and the amounts of specific transcripts were determined using a custom-designed RNA protection assay containing the indicated genes. After normalization against a housekeeping gene (GAPDH), the expression of each gene from the ODN-treated groups was compared with the expression observed in the untreated mice. Results represent the expression levels for each transcript found in ODN-treated mice over the untreated controls. The asterisks represent a greater than 33% increase in gene expression. The error bars represent the standard errors of means obtained from 3 different experiments.
Effect of CpG-ODN therapy in the T-cell RNA levels for various apoptotic-related genes.
CD4+ (A) or CD8+ (B) T cells were purified from untreated, CpG-ODN– (black bars), or Neg-ODN–treated (white bars) mice one day after the final ODN injection. Total RNA was isolated, and the amounts of specific transcripts were determined using a custom-designed RNA protection assay containing the indicated genes. After normalization against a housekeeping gene (GAPDH), the expression of each gene from the ODN-treated groups was compared with the expression observed in the untreated mice. Results represent the expression levels for each transcript found in ODN-treated mice over the untreated controls. The asterisks represent a greater than 33% increase in gene expression. The error bars represent the standard errors of means obtained from 3 different experiments.
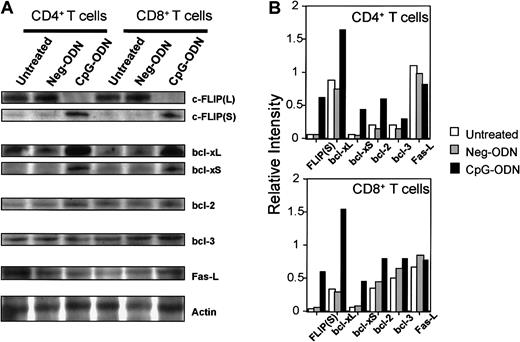
The presence of apoptotic-related proteins on purified CD4+and CD8+ T cells derived from CpG-ODN–treated, Neg-ODN–treated, and untreated mice was also examined by Western blot analysis using antibodies specific for c-FLIP, bcl-x, bcl-2, bcl-3, and Fas-L. The results presented in Figure 7clearly show that CD4+ and CD8+ T cells from CpG-ODN–treated mice expressed higher levels of the short form of c-FLIP protein, c-FLIP(S), and at the same time the levels of the long form of c-FLIP, c-FLIP(L), were diminished, as compared with the T cells from control mice. The protein levels of bcl-xL, which like c-FLIP has an antiapoptotic function for AICD, were found substantially increased in both CD4+ and CD8+ T cells from CpG-ODN–treated mice as compared with the controls. A small and less dramatic increase of bcl-xS, which is correlated with proapoptotic activity, was also observed in the T cells from CpG-ODN–treated mice. Additionally, we detected only a small increase in the levels of bcl-2 and a moderate and most likely insignificant increase in the levels of bcl-3 in T cells derived from CpG-ODN–treated mice as compared to the controls. Taken together these data suggest that CpG-ODN can alter T-cell homeostasis by rendering naive and antigen-experienced T cells resistant to various apoptotic stimuli, which normally serve to eliminate excess numbers of T cells.
CpG-ODN therapy alters the protein expression of apoptotic-related genes in T lymphocytes.
(A) CD4+ and CD8+ T cells were purified from untreated, CpG-ODN–treated, and Neg-ODN–treated mice one day after the final ODN injection. Total detergent cell lysates were used for Western blot analysis as described in “Materials and methods.” (B) Relative intensities were normalized to the corresponding actin intensity bands.
CpG-ODN therapy alters the protein expression of apoptotic-related genes in T lymphocytes.
(A) CD4+ and CD8+ T cells were purified from untreated, CpG-ODN–treated, and Neg-ODN–treated mice one day after the final ODN injection. Total detergent cell lysates were used for Western blot analysis as described in “Materials and methods.” (B) Relative intensities were normalized to the corresponding actin intensity bands.
Role of the CD28 costimulatory receptor in CpG-mediated T-cell expansion
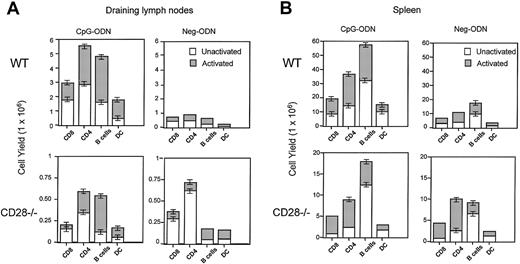
Up-regulation of antiapoptotic molecules such as bcl-xL and c-FLIP on T cells can occur as the result of CD28 costimulation.32,33 Since CpG-ODN are known to increase the expression of the CD28 ligands B7.1 and B7.2 on various APCs,34 35 we reasoned that the interaction of T cells with CpG-ODN–activated APCs involving CD28/B7 costimulation could be somehow involved in the expansion of T-lymphocyte pools resulting from CpG therapy. To assess the role of CD28 on the CpG-ODN–induced expansion of T cells, we compared the effects of CpG therapy in normal wild-type (WT) and CD28-deficient (CD28−/−) mice. As previously noted, CpG therapy in normal, WT mice significantly increased the numbers of naive and antigen-experienced CD4+and CD8+ T cells in the draining lymph nodes and spleens (Figure 8). In addition, the numbers of APCs (B lymphocytes and DCs) also were substantially increased as the result of CpG therapy. In contrast, CpG therapy in CD28−/− mice did not result in the expansion of any of the T-cell subsets, and the effects on APCs were less marked than those observed in the WT mice (Figure 8). These results are strongly indicative that the CpG-mediated expansion of T cells requires direct cell-to-cell interaction with B7-expressing APCs, and this interaction may involve CD28 stimulation.
CD28 is required for the CpG-ODN–induced expansion of CD4+and CD8+ T cells.
C57BL/6 mice and CD28−/− mice underwent 9 daily injections of CpG-ODN or Neg-ODN. One day after the last ODN injection, the draining axillary lymph nodes (A) and spleens (B) were harvested and analyzed by flow cytometry. The total numbers of naive/unactivated (CD44low) and memory/activated (CD44high) CD4+/CD3+ and CD8+/CD3+T cells, unactivated (CD80low) or activated (CD80high) CD19+ B cells, and unactivated (CD80low) or activated (CD80high) CD11c+ dendritic cells for each group were estimated. The total cell numbers in each organ were evaluated via trypan blue exclusion of viable cells on a hemocytometer. The total cell yield for each subset was calculated by multiplying the proportion of the corresponding group by the total number of live cells obtained per organ. The error bars represent the standard error of the means obtained from 3 separate experiments.
CD28 is required for the CpG-ODN–induced expansion of CD4+and CD8+ T cells.
C57BL/6 mice and CD28−/− mice underwent 9 daily injections of CpG-ODN or Neg-ODN. One day after the last ODN injection, the draining axillary lymph nodes (A) and spleens (B) were harvested and analyzed by flow cytometry. The total numbers of naive/unactivated (CD44low) and memory/activated (CD44high) CD4+/CD3+ and CD8+/CD3+T cells, unactivated (CD80low) or activated (CD80high) CD19+ B cells, and unactivated (CD80low) or activated (CD80high) CD11c+ dendritic cells for each group were estimated. The total cell numbers in each organ were evaluated via trypan blue exclusion of viable cells on a hemocytometer. The total cell yield for each subset was calculated by multiplying the proportion of the corresponding group by the total number of live cells obtained per organ. The error bars represent the standard error of the means obtained from 3 separate experiments.
B lymphocytes are not required for the CpG-ODN–induced expansion of T cells
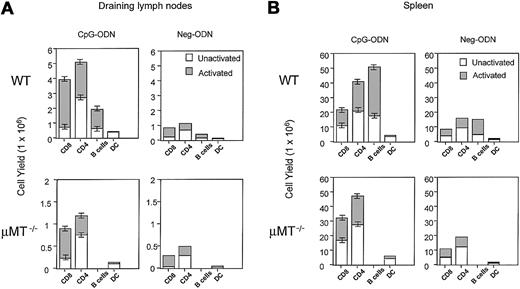
Based on the findings that CpG-ODN increases the expression of several costimulatory molecules including B7.1 and B7.2 on B cells and that this compound dramatically increases the number of B cells (6- to 11-fold increase, Figure 8 and Krieg et al29), we investigated the role of B cells in the CpG-mediated T-cell expansion. For this purpose we used the μMT−/− mice, which have a genetic alteration in the IgM transmembrane domain resulting in the lack mature B lymphocytes. Again, the effects of CpG therapy in WT mice showed a clear expansion of various subsets of T cells and APCs on both lymph nodes and spleens (Figure 9). Interestingly, CpG therapy in the μMT−/− mice also resulted in a significant expansion of all subsets of T cells (Figure9). These effects were almost identical in the spleen, but the total cell numbers on both CpG-ODN– and Neg-ODN–treated μMT−/− mice were substantially lower on the lymph nodes as compared to the WT mice. These results indicate that the CpG-ODN–mediated expansion of CD8+ and CD4+ T cells is not solely dependent on B lymphocytes, but it does not rule out the possibility that these APCs may participate in the effects of CpG-ODN in WT mice.
The CpG-ODN–induced expansion of CD4 and CD8 T cells is not dependent on the presence of B cells.
C57BL/6 mice and μMT−/− (B-cell deficient) mice underwent 9 daily injections of CpG-ODN or Neg-ODN. One day after the last ODN injection, the draining axillary lymph nodes (A) and spleens (B) were harvested. Cell numbers for each subset were estimated by flow cytometry as described in the legend of Figure 8.
The CpG-ODN–induced expansion of CD4 and CD8 T cells is not dependent on the presence of B cells.
C57BL/6 mice and μMT−/− (B-cell deficient) mice underwent 9 daily injections of CpG-ODN or Neg-ODN. One day after the last ODN injection, the draining axillary lymph nodes (A) and spleens (B) were harvested. Cell numbers for each subset were estimated by flow cytometry as described in the legend of Figure 8.
Discussion
In the present study, we have shown that repeated administration of CpG-ODN leads to a disruption in T-cell homeostasis by significantly increasing the numbers of naive and antigen-experienced T lymphocytes in various lymphoid compartments. Although the effect was mostly apparent in the draining lymph nodes close to the CpG-ODN injection site, systemic effects were also observed in the spleen. Interestingly, we did not observe any alterations in T-cell homeostasis when mice were repetitively injected with purified bacterial DNA (9 daily doses of 100 μg/mice), which is rich in CpG motifs (data not presented). Thus, it is possible that the phosphorothioate groups in the CpG-ODN, which protect the ODN against degradation by nucleases, are critical for this activity. Similar requirements for the presence of phosphorothioate groups have been reported for the extramedullary hematopoiesis activity of CpG-ODN.36 37
The increase in T-cell numbers produced by CpG therapy does not appear to result from an induction of cell proliferation, but from the generation of an apoptotic resistance state that protects both CD4+ and CD8+ T cells against growth factor–deprivation cell death and AICD. The resistance to these different modes of cell death correlated with an increase in the expression of antiapoptotic molecules including c-FLIP, bcl-xL, and bcl-2. We believe that CpG therapy does not directly induce these changes on the T cells, but most likely the effect is mediated via accessory cells such as APCs. It has been established that CpG-ODN stimulate cell functions through Toll-like receptor-9 (TLR-9), which has not been detected on T cells but is present on numerous types of APCs such as DCs and B lymphocytes.38,39 In effect, CpG-ODN activates both DCs and B cells to proliferate and become more potent APCs by increasing the expression of adhesion molecules and costimulatory ligands and inducing the secretion of numerous cytokines.23,35,40-44 Moreover, our results with CD28−/− mice reinforce the idea that B7-expressing APCs may play a role in the expansion of T cells by CpG-ODN. Thus, we propose that CpG therapy first increases the number of activated APCs, which results in the generation of additional “space” for T lymphocytes. Therefore, “space” for T cells is not necessarily a measure of volume but could be viewed more appropriately as a reflection of the access of T cells to APCs and their costimulatory factors, which are required to maintain the viability of the T cells. Our laboratory and other groups have shown that CpG-ODNs function as a potent adjuvant for the induction of T-cell responses against various types of vaccines.21,24-27,45Typically, adjuvants are thought to function by creating an inflammatory response at the injection site, resulting in the activation of APCs, which take up the antigen and transport it to the lymphoid organs where it is presented to the T lymphocytes. However, Marrack recently demonstrated that some adjuvants, such as bacterial lipopolysaccharide and vaccinia virus, increase the survival of activated T cells via the induction of the antiapoptotic molecule bcl-3.46 Our results indicate that CpG-ODN has a similar effect in blocking AICD but possibly not through bcl-3, which did not appear to be highly up-regulated in T cells derived from CpG-ODN–treated mice. Instead, the antiapoptotic molecules that may be enhancing the adjuvant effect of CpG-ODN appear to be bcl-xL and c-FLIP, which are potent inhibitors of AICD.47-51Interestingly, CpG therapy increased the levels of c-FLIP(S), but at the same time decreased c-FLIP(L) (Figure 7). Although both of these splice form variants of c-FLIP can prevent apoptosis and are recruited to the death-inducing signaling complex,52 resistance to AICD correlates with up-regulation of c-FLIP(S).33 53 Our results suggest that CpG therapy may up-regulate c-FLIP(S) in T cells at the expense of the c-FLIP(L) form.
At present we do not know all the exact mechanism(s) of how CpG-ODNs are able to enhance the expression of antiapoptotic genes in T cells, especially in the case of naive T cells and in the absence of antigen. It was recently reported that CpG-ODNs are able to inhibit apoptosis in DCs by up-regulating cellular inhibitors of apoptosis proteins, bcl-2 and bcl-xL.54 However, DCs, as well as other APCs, are capable of responding directly to CpG-ODN through the action of the TLR-9 receptor.38,39 Since TLR-9 does not appear to be expressed on T cells, the effect of CpG-ODN in increasing gene expression of antiapoptotic molecules must be through an accessory APC via direct cell-to-cell interactions and/or through soluble factors (cytokines). One possibility is that CpG induces the production of vast quantities of type I interferon (IFN) by various types of cells,7,55 but mostly in plasmocytoid DCs.56-58 It has been reported that type I IFN can induce survival signals through the activation of PI-3K and Akt and the sequential activation of nuclear factor (NF)-kB.59Moreover, the induction in the production of IL-15 by these interferons will enhance the survival and expansion of memory CD8+ T cells,17 and the antiproliferative effect of type I IFN on lymphocytes60 61 could also have an effect in blocking cell death in those circumstances where cells need to progress through cell cycle to undergo apoptosis.
Besides type I IFN, other cytokines that are induced by CpG-ODN such as IL-1, TNF-alpha, IL-6, IFN-gamma, and IL-1241-43 will likely influence the survival of T lymphocytes. Many of these proinflammatory cytokines are strong activators of NF-kB, resulting in the up-regulation of strong antiapoptotic factors such as c-FLIP and bcl-xL,62-64 both of which in our hands were induced in T cells by CpG therapy (Figure7). As indicated by the present findings, the generation of survival signals for T cells by CpG therapy probably also derives from direct cell interactions with APCs. For instance, CpG up-regulates the expression of CD80 and CD86 in APCs,34,40 potentiating CD28 costimulation, which enhances T-cell survival by inducing expression of bcl-xL and c-FLIP.32 33
Some observations suggest that alterations of T-cell homeostasis by CpG-containing DNA may contribute to autoimmune diseases. Serum DNA from systemic lupus erythematosus (SLE) patients contains an enriched fraction of the CpG motifs, capable of activating monocytes.65 Immune complexes of anti-DNA antibodies with CpG-DNA may be effectively delivered to APCs through Fc receptors, potentiating their activation and cytokine production.66Thus, it is possible that the release of CpG-DNA from necrotic cells could render autoreactive T cells more resistant to apoptosis, aggravating the chronic inflammatory response in autoimmune disorders. However, there is no experimental data to substantiate this possibility.
Our findings suggest that besides their use as vaccine adjuvants, CpG-ODNs could have several additional clinical applications. For instance, CpG-ODN therapy could benefit HIV-infected individuals or patients receiving adoptive T-cell therapy by rendering these T cells more resistant to apoptosis, increasing their survival and effectiveness. If CpG-ODNs were to prevent T-cell death induced by other proapoptotic agents, we could also envision the use of CpG therapy in combination with conventional cytoablative treatments (radiation, chemotherapy) to diminish the induction of immunosuppression, which is often accompanied by these treatments. Providing support for this possibility are the observations that the expression of bcl-xL confers resistance to cell death induced by chemotherapeutic agents and irradiation.67 68
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-02-0401.
Supported by grants R01CA80782, R01CA82677, and RR-00585 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Esteban Celis, Department of Immunology, GU421A, Mayo Clinic, Rochester, MN 55905; e-mail: celis.esteban@mayo.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal