To investigate the molecular mechanisms of the quantitative factor V (FV) deficiency associated with the FV R2 haplotype, 4 missense mutations, Met385Thr, His1299Arg, Met1736Val, and Asp2194Gly, identified in the R2 haplotype allele, were analyzed by in vitro expression studies. The FV variant carrying all 4 mutations showed a markedly lower steady-state expression level than wild-type FV because of low synthesis rate and impaired secretion of the mutant protein. The Asp2194Gly mutation was found to play a key role in the impaired secretion of the mutant FV by interfering with its transport from the endoplasmic reticulum to the Golgi complex. The deleterious effect of the Asp2194Gly mutation was shown to be dominant among the 4 mutations. The Met385Thr mutation and His1299Arg mutation had no effect on steady-state expression levels, but the secretion rates of the mutant proteins were moderately decreased by these mutations. The His1299Arg mutation partially impaired glycosylation in the C-terminal part of the B-domain of the mutant FV, which was supposed to affect the secretion rate, but not the steady-state expression level. It was also suggested that the Met385Thr mutation partially impairs posttranslational modification of the mutant FV without affecting the steady-state expression level. No deleterious effect of the Met1736Val mutation was observed in terms of expression and intracellular processing. Our in vitro data strongly suggest that the naturally existing R2 haplotype mutant FV, which carries all 4 mutations, has the potential to result in quantitative FV deficiency in vivo owing to impaired expression of the mutant protein when the Asp2194Gly mutation is present.
Introduction
Factor V (FV) is a plasma glycoprotein that plays key roles in blood coagulation.1 FV is a single-peptide protein consisting of multiple domains, A1-A2-B-A3-C1-C2, whose domain structure is similar to that of factor VIII (FVIII).1 In the blood coagulation cascade, FV is proteolytically activated by thrombin or activated factor X (FXa).1 The B-domain is released upon activation, whereas the heavy chain (A1-A2) and light chain (A3-C1-C2) form a noncovalently bound heterodimeric molecule, activated FV (FVa).1 FVa binds to FXa and serves as its cofactor in the prothrombinase complex that converts prothrombin to the active enzyme thrombin.1FV also functions as an anticoagulant cofactor in the protein C pathway.2 Activated protein C (APC) down-regulates blood coagulation by inactivating both FVa and activated FVIII (FVIIIa).2 FV and protein S serve as synergistic cofactors in the APC-mediated inactivation of FVIIIa.2
In human plasma, FV exists in 2 isoforms, designated FV1 and FV2, that have slightly different molecular weights because of partial N-linked glycosylation in the C2-domain.3 FV1 and FV2 have different characteristics in terms of procoagulant activity, inactivation by APC, and anticoagulant function in the protein C pathway.4 Consequently, FV1 has the overall potential to generate more thrombin than FV2.
Molecular abnormalities of FV are associated with either bleeding tendency or thrombotic disorders. FV deficiency, which is a rare bleeding disorder, is classified into 2 subclasses. Type I deficiency is a quantitative defect due to decreased plasma FV antigen levels and subsequent reduction of FV activity, whereas type II deficiency is defined as a functional defect caused by a dysfunctional FV molecule in plasma. APC resistance is one of the most common hereditary thrombotic disorders in western populations.2 It is caused by a single missense mutation, Arg506Gln in FV, which results in the loss of one of the APC-cleavage sites.2 The mutant FV, known as FV Leiden, is not only resistant to inactivation by APC, but is also a poor cofactor in the APC-mediated FVIIIa inactivation,2resulting in an increased risk of venous thrombosis in its carriers through this dual mechanism.
The FV R2 haplotype, which consists of multiple nucleotide substitutions on one allele, is a common genetic variation among several distinct populations.5-11 It has been suggested that the R2 haplotype is associated with decreased plasma FV antigen levels,5,7,9 a slightly decreased APC response in the absence of the Arg506Gln mutation,6,7,9,10 and an increased FV1-to-FV2 ratio.12 However, other studies have yielded opposing observations concerning the decreased plasma FV antigen levels6,8,13 and the decreased APC response.8,13 Several population studies have addressed the relationship between the risk of venous thrombosis and the R2 haplotype and have demonstrated that compound heterozygous individuals for FV Leiden and the R2 haplotype have a higher risk of venous thrombosis than heterozygous FV Leiden carriers.7,9,14 However, it remains an unsolved question whether heterozygous carriers for the R2 haplotype without other defects have an increased risk of venous thrombosis.7-9,13 14
Until now, only a few studies have characterized the plasma phenotypes associated with the R2 haplotype in detail. Castoldi et al12 reported that the R2 haplotype affects the plasma FV1-to-FV2 ratio. Hoekema et al15 reported the mutant FV molecule derived from the R2 haplotype allele to be moderately defective as a cofactor in the APC-mediated FVIIIa degradation. However, the decreased plasma FV antigen levels in the R2 haplotype carriers have not yet been characterized by detailed molecular biological approaches. This study focuses on the quantitative FV deficiency associated with the R2 haplotype to determine whether the R2 haplotype indeed results in quantitative reduction of plasma FV and, if so, to investigate the underlying molecular mechanisms by in vitro expression studies.
Materials and methods
FV R2 haplotype mutations analyzed in this study
Until now, more than 10 single nucleotide substitutions have been identified in the R2 haplotype.5,6,9,10 In this study, 4 missense mutations, Met385Thr, His1299Arg, Met1736Val, and Asp2194Gly, were specifically analyzed in terms of their effects on expression and intracellular processing of mutant FV molecules because these 4 mutations have been found to be in linkage disequilibrium in most of the R2 haplotype alleles, except for rare variant alleles that are probably caused by gene recombinations.9,11,12 In addition, some missense mutations other than the above 4 have also been identified as being associated with the R2 haplotype. Although several studies have analyzed linkage of the mutations involved in the R2 haplotype,5,6,9 10 there are some discrepancies among the reports, and, to our knowledge, no definitive consensus has yet been reached concerning the linkage of the mutations, except for the Met385Thr, His1299Arg, Met1736Val, and Asp2194Gly mutations. Thus, we decided to focus on these 4 missense mutations and to exclude other missense mutations in this study. Furthermore, several silent mutations in the R2 haplotype were also neglected in this study.
Construction of expression vectors
The full-length FV cDNA in expression vector pMT2 was kindly provided by Dr R. J. Kaufman.16,17 Compared with the recently published entire FV gene sequence (GenBank Z99572), the cDNA carried several nucleotide substitutions that were previously described as polymorphisms,18,19 and 4 of these caused changes in coding amino acids. Lys830, His837, Lys897, and Met1736 in the published FV gene sequence were replaced by Arg830, Arg837, Glu897, and Val1736, respectively, in the cDNA. This cDNA was designated as the mutant cDNA for Met1736Val in this study because it contained Val1736, which is one of the mutations in the R2 haplotype. Recombinant polymerase chain reaction (PCR) technique20 was used to introduce Met1736 instead of Val1736, and the cDNA obtained was designated wild-type FV (wt-FV) cDNA in this study. The Met385Thr and Asp2194Gly mutations were introduced into the wt-FV cDNA by the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). However, we could not introduce the His1299Arg mutation directly into the wt-FV cDNA because it is located in one of the highly homologous sequence repeats,16 which hampered the site-directed mutagenesis procedure. Thus, genomic DNA from a healthy volunteer who is heterozygous for the R2 haplotype was used as a template of the His1299Arg mutation, after informed consent had been obtained according to the Declaration of Helsinki. Approval was obtained from the review board of University Hospital, Mälmo, Sweden. The DNA fragment containing the His1299Arg mutation was PCR-amplified from the genomic DNA, and the region coding amino acids 874 to 1571 was introduced into the wt-FV cDNA by recombinant PCR technique. The PCR-amplified DNA fragment from the R2 haplotype allele of the volunteer carried Lys897 as well as Arg1299, and it was technically difficult to exclude Lys897. Therefore, in this study, the mutant cDNA for His1299Arg differs from the wt-FV cDNA at both residue 897 and residue 1299. For clarity, this mutant molecule is simply designated His1299Arg, and the combination of these 2 amino acid substitutions is described as a single mutation throughout this study, unless otherwise mentioned specifically. The introduced mutations were confirmed, and unexpected mutations were ruled out by DNA sequencing. By exchanging restriction fragments of the FV cDNA containing each single mutation, we constructed all possible combinations of the mutations in the FV cDNA. The mutant FV carrying quadruple mutations, Met385Thr, His1299Arg, Met1736Val, and Asp2194Gly, was designated the mutant Q. The detailed mutagenesis procedures are available upon request.
Transient expression of FV
COS-1 cells were used for transient expression of recombinant FV in this study because combinations of COS-1 cells and pMT2 or its derivative vectors have been used and well established in previous studies by different groups.3,17 21First, 1 or 8 μg each expression vector was incubated with 2 or 16 μL LipofectAMINE2000 (Gibco BRL, Life Technologies, Grand Island, NY) in 2 or 4 mL OPTI-MEM (Gibco BRL, Life Technologies) for 15 minutes at room temperature; then the mixture was overlaid onto subconfluent COS-1 cells in 35-mm wells of 6-well plates (for enzyme-linked immunosorbent assay [ELISA] and for analysis of the synthesis rate of primary translated peptide) or 90-mm dishes (for pulse-chase analysis). The cells were incubated at 37°C under 5% CO2; then 5 hours after transfection the OPTI-MEM containing the expression vector was replaced by complete Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 44 IU penicillin, and 44 μM streptomycin.
Measurement of steady-state expression levels of FV by ELISA
Culture medium was changed to OPTI-MEM supplemented with 0.5% bovine serum albumin, 2 mM CaCl2, 44 IU penicillin, and 44 μM streptomycin 24 hours after transfection, then harvested 48 hours after transfection. The concentration of FV in the culture media was measured by the FV ELISA kit (Kordia, Leiden, The Netherlands).
Pulse-chase analysis of FV
Pulse-chase analysis of FV by radiolabeling, immunoprecipitation, and electrophoresis was performed essentially as previously described,22 except for some minor modifications. PPACK (2 μM; Calbiochem, San Diego, CA), benzamidine (10 mM), and trypsin inhibitor (50 μg/mL) were added to cell lysis buffer. To immunoprecipitate FV in cell lysates or culture media, protein A-sepharose and a rabbit antihuman FV polyclonal antibody (no. 8806) established in our laboratory were used. This polyclonal antibody recognizes the heavy chain, B-domain, and light chain of FV. Excess amounts of the antibody sufficient to immunoprecipitate all FV in each sample were used. The immunoprecipitate was washed 3 times with washing buffer and subjected to either 7.5% or 4% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The gel was scanned and the radioactivity of FV-specific bands was quantitated by an image scanner.
To compare synthesis rates of primary translated peptides, a control protein was coexpressed to correct for differences in transfection and radiolabeling efficiency among wells. We used wild-type protein S as a control because we had extensive experience with protein S in terms of expression and quantitative immunoprecipitation.22 We cotransfected 1 μg wild-type protein S expression vector22 together with FV expression vectors. Cell lysates were harvested just after pulse-labeling, and radioactivity of primary translated peptides of FV and protein S was quantitated.
Thrombin treatment and deglycosylation of FV
Immunoprecipitated FV was incubated with thrombin as previously described.23 Thrombin-treated or nontreated FV was incubated with either sialidase, O-glycosidase, endoglycosidase H, N-glycosidase F (Roche, Mannheim, Germany), or combinations of these according to protocols described elsewhere,24 and then subjected to SDS-PAGE.
Results
Steady-state expression levels of FV
The concentration of recombinant FV in the medium, reflecting steady-state expression level, was measured by ELISA (Figure1). The mutant Q, and all FV mutants harboring the Asp2194Gly mutation, showed significantly lower expression levels than wt-FV. All other FV mutants showed expression levels comparable to that of wt-FV. This indicated the Asp2194Gly mutation to be the major determinant of the low expression levels and its deleterious effect to be dominant among the 4 mutations.
Steady-state expression levels of FV in culture media.
FV levels in the culture media were measured by ELISA. The histograms and bars represent the mean ± SD (n = 6). The mean value of wt-FV was 562.6 ng/35-mm well per 24 hours and is assigned to be 100% in this figure. 385 indicates Met385Thr; 1299, His1299Arg; 1736, Met1736Val; 2194, Asp2194Gly.
Steady-state expression levels of FV in culture media.
FV levels in the culture media were measured by ELISA. The histograms and bars represent the mean ± SD (n = 6). The mean value of wt-FV was 562.6 ng/35-mm well per 24 hours and is assigned to be 100% in this figure. 385 indicates Met385Thr; 1299, His1299Arg; 1736, Met1736Val; 2194, Asp2194Gly.
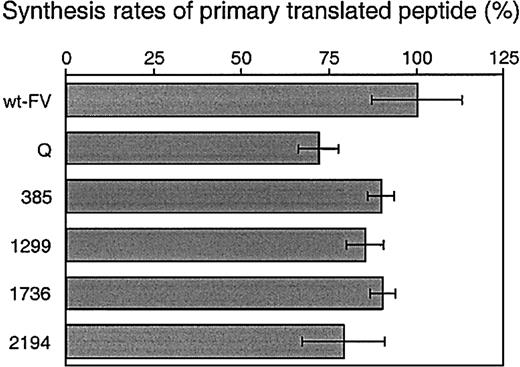
Synthesis rates of primary translated peptide of FV
The primary translated FV polypeptides in the cell lysates at 0 hour of chase were quantified to investigate whether there were any differences in synthesis rates among the FV mutants. Wild-type protein S was coexpressed as a control to correct for transfection and radiolabeling efficiency, and ratios of FV to protein S were calculated (Figure 2). The primary synthesis rates of all FV mutants were lower than that of wt-FV. The mutant Q showed the lowest mean value among the mutants. Thus, the negative effects of the single mutations appeared to be combined and accumulated in the mutant Q.
Synthesis rates of primary translated peptide of FV.
COS-1 cells coexpressing wt-FV or the FV mutants together with wild-type protein S as a control were radiolabeled for 30 minutes. Radiolabeled FV and protein S in cell lysates were quantitatively immunoprecipitated and electrophoresed on polyacrylamide gels. To correct for transfection and radiolabeling efficiency among wells, the FV–to–protein S ratio was calculated by measuring radioactivity of FV- and protein S–specific bands on the gels. The histograms and bars represent the mean ± SD (n = 3). The mean value of wt-FV is assigned to be 100% in this figure. 385 indicates Met385Thr; 1299, His1299Arg; 1736, Met1736Val; 2194, Asp2194Gly.
Synthesis rates of primary translated peptide of FV.
COS-1 cells coexpressing wt-FV or the FV mutants together with wild-type protein S as a control were radiolabeled for 30 minutes. Radiolabeled FV and protein S in cell lysates were quantitatively immunoprecipitated and electrophoresed on polyacrylamide gels. To correct for transfection and radiolabeling efficiency among wells, the FV–to–protein S ratio was calculated by measuring radioactivity of FV- and protein S–specific bands on the gels. The histograms and bars represent the mean ± SD (n = 3). The mean value of wt-FV is assigned to be 100% in this figure. 385 indicates Met385Thr; 1299, His1299Arg; 1736, Met1736Val; 2194, Asp2194Gly.
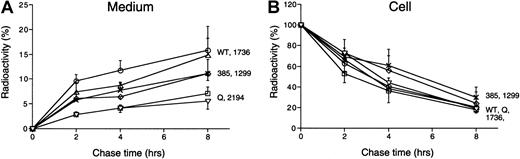
Secretion profiles of FV mutants
Pulse-chase analysis was performed to investigate secretion profiles of FV. Quantitative data are shown in Figure3. In the medium, secretion rates of the mutant Q and mutant Asp2194Gly were significantly lower than that of wt-FV, whereas the mutant Met1736Val showed a secretion rate comparable to that of wt-FV. Secretion rates of the mutant Met385Thr and mutant His1299Arg were moderately decreased compared with that of wt-FV. In the cell, particularly at 4 and 8 hours' chase, higher amounts of the mutant Met385Thr and mutant His1299Arg were detected compared with wt-FV and the other FV mutants. Taken together with the ELISA results, these data suggest that the secretion rates of the mutant Met385Thr and mutant His1299Arg are decreased, but the final amounts of secreted FV are similar to that of wt-FV.
Secretion profiles of FV mutants.
COS-1 cells expressing wt-FV or the FV mutants were radiolabeled for 30 minutes (pulse); then, cell lysates and culture media were harvested at the indicated time points (chase). Radiolabeled FV was quantitatively immunoprecipitated and electrophoresed on 7.5% polyacrylamide gels under reducing conditions. Then, the radioactivity of FV-specific bands on the gels was measured. Quantitative results (mean ± SD, n = 3) of wt-FV (○), mutant Q (■), mutant Met385Thr (⋄), mutant His1299Arg (×), mutant Met1736Val (▵), and mutant Asp2194Gly (▿) are shown. The amount of radioactive FV in the cell lysate at 0 hours is assigned to be 100% for each construct.
Secretion profiles of FV mutants.
COS-1 cells expressing wt-FV or the FV mutants were radiolabeled for 30 minutes (pulse); then, cell lysates and culture media were harvested at the indicated time points (chase). Radiolabeled FV was quantitatively immunoprecipitated and electrophoresed on 7.5% polyacrylamide gels under reducing conditions. Then, the radioactivity of FV-specific bands on the gels was measured. Quantitative results (mean ± SD, n = 3) of wt-FV (○), mutant Q (■), mutant Met385Thr (⋄), mutant His1299Arg (×), mutant Met1736Val (▵), and mutant Asp2194Gly (▿) are shown. The amount of radioactive FV in the cell lysate at 0 hours is assigned to be 100% for each construct.
Posttranslational intermediate forms of wt-FV
Impairment of posttranslational modifications in the Golgi complex could account for the decreased secretion rates of the FV mutants. Therefore, the posttranslational modifications of FV were investigated by analyzing the glycosylation patterns.
The wt-FV was detected as a single band when cell lysates were electrophoresed on 7.5% polyacrylamide gels (Figure4A). However, on 4% gels, wt-FV appeared as multiple bands (Figure 4B). To analyze which bands were FV-specific, mock-transfected samples and wt-FV samples at 2 hours' chase were compared (Figure 4C). The cell lysate of wt-FV (lane 2) contained 4 FV-specific bands (1 to 4) and 2 that were nonspecific (ns). Band 1 was already present at 0 hour of chase (Figure 4B), that is, just after pulse-labeling. Thus, it supposedly represented the newly synthesized primary translated peptide of wt-FV in the endoplasmic reticulum (ER). The faint band 4 in the cell (lane 2) had the same molecular weight as wt-FV in the medium (lane 3), indicating that it represented the mature form of wt-FV. Two bands seen between the primary translated peptide and the mature form (lane 2, bands 2 and 3) were supposed to be intermediate forms of wt-FV appearing during the posttranslational modification. To confirm that bands 2 and 3 indeed represented the posttranslational intermediates, the cell lysate of wt-FV at 2 hours' chase was subjected to deglycosylation analysis. Bands 2 and 3 increased in mobility after sialidase treatment (Figure 4D, lane 2), indicating that these bands had sialic acids on their carbohydrates. Because enzymes involved in the sialylation process exist only in the Golgi complex,24we concluded that bands 2 and 3 had already been transported to the Golgi complex and partially modified posttranslationally. The wt-FV in the cell lysate was also subjected to endoglycosidase H digestion to determine the intracellular localization of these bands more precisely. All 3 wt-FV bands were sensitive to endoglycosidase H and decreased in mobility (lane 5). In contrast, the mature wt-FV in the medium was endoglycosidase H resistant and did not change in molecular mass on the gel (data not shown). These results indicated that all 3 wt-FV bands still had high-mannose–type or hybrid-type N-linked carbohydrates and existed in the ER or the early regions of the Golgi complex.24 Thus, it was clearly shown by these deglycosylation analyses that bands 2 and 3 were the posttranslational intermediates localized in the early regions of the Golgi complex.
Posttranslational intermediate forms of wt-FV.
The wt-FV in cell lysates was immunoprecipitated and electrophoresed on 7.5% (panel A) and 4% (panel B) polyacrylamide gels under reducing conditions. (C) Cell lysate and medium samples of wt-FV and mock-transfected control harvested at 2 hours' chase were subjected to 4% SDS-PAGE under reducing conditions. The gel was run longer than the gel in Figure 4B to obtain better separation of the bands. Lane 1, mock cell lysate; lane 2, wt-FV cell lysate; lane 3, wt-FV medium; lane 4, mock medium. Arrow 1, primary translated peptide; arrows 2 and 3, posttranslational intermediates; arrow 4, mature form, nonspecific (ns) bands. (D) The wt-FV in cell lysates at 2 hours' chase was immunoprecipitated and incubated with indicated deglycanases, then electrophoresed on 4% polyacrylamide gels under reducing conditions. Lanes 1, 4, and 6 had no deglycanase. S indicates sialidase; O, O-glycosidase; H, endoglycosidase H; N, N-glycosidase F. Arrow 1, primary translated peptide; arrows 2 and 3, posttranslational intermediates. (E) The wt-FV in cell lysates at 2 hours' chase was immunoprecipitated and incubated with thrombin and indicated deglycanases, then subjected to 7.5% SDS-PAGE under reducing conditions. FV is cleaved into the following 4 fragments by thrombin digestion: the heavy chain (amino acids 1 to 709), the N-terminal part of the B-domain (amino acids 710 to 1018), the C-terminal part of the B-domain (amino acids 1019 to 1545), and the light chain (amino acids 1546 to 2196). The N-terminal part of the B-domain is not visible on the gel because there are only 3 methionine and no cysteine residues that can be radioactive in this region. Thus, the radioactivity of this fragment is too weak compared with the other fragments. Lane 1 had no deglycanase. S indicates sialidase; O, O-glycosidase; B, fragments representing the C-terminal part of the B-domain; HC, heavy chain, LC, light chain. Arrows a to e indicate distinct forms of the C-terminal part of the B-domain.
Posttranslational intermediate forms of wt-FV.
The wt-FV in cell lysates was immunoprecipitated and electrophoresed on 7.5% (panel A) and 4% (panel B) polyacrylamide gels under reducing conditions. (C) Cell lysate and medium samples of wt-FV and mock-transfected control harvested at 2 hours' chase were subjected to 4% SDS-PAGE under reducing conditions. The gel was run longer than the gel in Figure 4B to obtain better separation of the bands. Lane 1, mock cell lysate; lane 2, wt-FV cell lysate; lane 3, wt-FV medium; lane 4, mock medium. Arrow 1, primary translated peptide; arrows 2 and 3, posttranslational intermediates; arrow 4, mature form, nonspecific (ns) bands. (D) The wt-FV in cell lysates at 2 hours' chase was immunoprecipitated and incubated with indicated deglycanases, then electrophoresed on 4% polyacrylamide gels under reducing conditions. Lanes 1, 4, and 6 had no deglycanase. S indicates sialidase; O, O-glycosidase; H, endoglycosidase H; N, N-glycosidase F. Arrow 1, primary translated peptide; arrows 2 and 3, posttranslational intermediates. (E) The wt-FV in cell lysates at 2 hours' chase was immunoprecipitated and incubated with thrombin and indicated deglycanases, then subjected to 7.5% SDS-PAGE under reducing conditions. FV is cleaved into the following 4 fragments by thrombin digestion: the heavy chain (amino acids 1 to 709), the N-terminal part of the B-domain (amino acids 710 to 1018), the C-terminal part of the B-domain (amino acids 1019 to 1545), and the light chain (amino acids 1546 to 2196). The N-terminal part of the B-domain is not visible on the gel because there are only 3 methionine and no cysteine residues that can be radioactive in this region. Thus, the radioactivity of this fragment is too weak compared with the other fragments. Lane 1 had no deglycanase. S indicates sialidase; O, O-glycosidase; B, fragments representing the C-terminal part of the B-domain; HC, heavy chain, LC, light chain. Arrows a to e indicate distinct forms of the C-terminal part of the B-domain.
N-glycosidase F treatment of wt-FV in the cell yielded increased mobility of all 3 FV bands on the gel; however, bands 2 and 3 were still clearly distinguishable from band 1 (Figure 4D, lane 7). This demonstrated that the observed differences in molecular weight between the primary translated peptide (band 1) and the posttranslational intermediates (bands 2 and 3) were not caused by differences in N-linked carbohydrates. Thus, it was concluded that the differences in sialic acid in O-linked carbohydrates were responsible for the gel-mobility differences of bands 1 to 3. The cell lysate was also incubated with O-glycosidase after sialidase treatment, but further molecular weight changes were not apparent on the 4% gel (Figure 4D, lane 3).
To determine which part of the FV molecule contained the O-linked carbohydrates that were responsible for the observed gel-mobility differences of bands 1 to 3, wt-FV from the cell lysate at 2 hours' chase was cleaved by thrombin before being incubated with sialidase and O-glycosidase. After thrombin digestion, the heavy chain and light chain of wt-FVa appeared on the gel as well as 3 bands (a toc) located above the heavy chain (Figure 4E, lane 1). The 3 bands represented different forms of the C-terminal part of the B-domain (amino acids 1019 to 1545) because only this FVa fragment is larger than the heavy chain. Sialidase treatment had no effect on banda, whereas bands b and c increased in gel mobility (lane 2), revealing that bands b andc had sialic acids although band a did not. These results suggested that the gel-mobility differences between the primary translated peptide and the posttranslational intermediates of wt-FV in Figure 4C-D were related to sialic acid content in the C-terminal part of the B-domain. Although bands b andc increased in mobility and were detected as bandd after sialidase treatment, still there was a small difference in molecular weights between bands a andd (lane 2). After O-glycosidase treatment, band dfurther increased in mobility and was detected as band e,which comigrated with band a (lane 3). These results demonstrated that O-linked carbohydrates were present in the C-terminal part of the B-domain from the posttranslational intermediates (bands b and c), but not in that from the primary translated peptide (band a). This also indicated transport of bands 2 and 3, which produced bands band c by thrombin digestion, into the Golgi complex where O-linked glycosylation takes place.24
Impaired intracellular processing of FV mutants
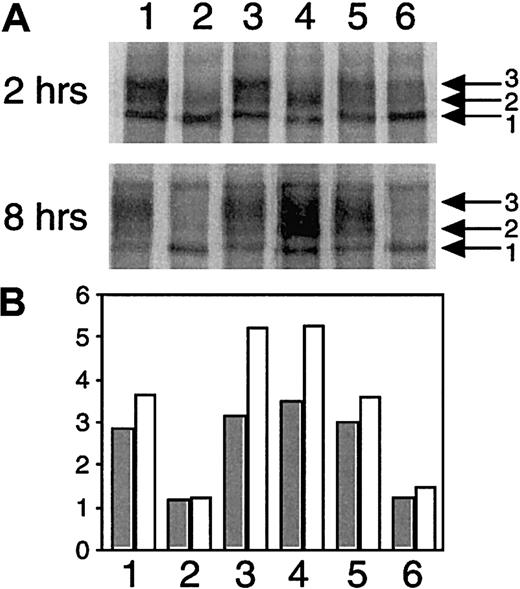
To investigate whether there were any differences in intracellular processing between wt-FV and the FV mutants, the cell lysates at 2 hours' and 8 hours' chase were compared (Figure5).
Impaired intracellular processing of FV mutants.
The wt-FV and the FV mutants in cell lysates at 2 hours' chase (upper panel) and 8 hours' chase (middle panel) were subjected to 4% SDS-PAGE under reducing conditions. It should be noted that the gel of 8 hours' chase was overexposed to make faint bands visible. Lane 1, wt-FV; lane 2, mutant Q; lane 3, mutant Met385Thr; lane 4, mutant His1299Arg; lane 5, mutant Met1736Val; lane 6, mutant Asp2194Gly. The radioactivity of the primary translated peptide (band 1) and the posttranslational intermediates (bands 2 and 3) in each lane was quantitated, and the ratios of the posttranslational intermediates over the primary translated peptide (bands 2 and 3/band 1) were calculated (lower panel). Closed and open histograms indicate the ratios at 2 hours' chase and 8 hours' chase, respectively.
Impaired intracellular processing of FV mutants.
The wt-FV and the FV mutants in cell lysates at 2 hours' chase (upper panel) and 8 hours' chase (middle panel) were subjected to 4% SDS-PAGE under reducing conditions. It should be noted that the gel of 8 hours' chase was overexposed to make faint bands visible. Lane 1, wt-FV; lane 2, mutant Q; lane 3, mutant Met385Thr; lane 4, mutant His1299Arg; lane 5, mutant Met1736Val; lane 6, mutant Asp2194Gly. The radioactivity of the primary translated peptide (band 1) and the posttranslational intermediates (bands 2 and 3) in each lane was quantitated, and the ratios of the posttranslational intermediates over the primary translated peptide (bands 2 and 3/band 1) were calculated (lower panel). Closed and open histograms indicate the ratios at 2 hours' chase and 8 hours' chase, respectively.
The cell lysates of the mutant Asp2194Gly (lane 6) contained decreased amounts of the posttranslational intermediates (bands 2 and 3) relative to the primary translated peptide (band 1) (upper and middle panels). Especially at 8 hours' chase, the posttranslational intermediates were hardly seen in the mutant Asp2194Gly, whereas wt-FV showed a strong signal on the gel (middle panel). This was more obvious when ratios of the posttranslational intermediates over the primary translated peptide (bands 2 and 3/band 1) were calculated (bottom panel). Because the primary translated peptide and the posttranslational intermediates were localized in the ER and the Golgi complex, respectively, these data indicated that the transport of the mutant Asp2194Gly from the ER to the Golgi complex was impaired, which supported the results by the ELISA and pulse-chase described above.
In analysis of the mutant His1299Arg, 2 effects on the posttranslational modification were identified (Figure 5). First, whereas wt-FV (lane 1) showed 2 major bands of posttranslational intermediates (bands 2 and 3), band 3 was hardly visible in the mutant His1299Arg (lane 4) on the gel (upper panel). As the gel-mobility differences of bands 1 to 3 were related to sialic acid content in the C-terminal part of the B-domain, these data indicated the posttranslational modification, especially the sialylation process in the C-terminal part of the B-domain of the mutant His1299Arg, to be somewhat different from that of wt-FV. The second observation was an increased ratio of the posttranslational intermediates (bands 2 and 3) over the primary translated peptide (band 1), especially at 8 hours' chase (middle and bottom panels). This indicated that the mutant His1299Arg accumulated in the Golgi complex, probably as a result of the impaired posttranslational modification in the Golgi complex. After thrombin digestion of the mutant His1299Arg in the cell, only 2 B-domain bands (a and b) were observed, in contrast to the 3 bands (a to c) observed for wt-FV (data not shown). In addition, the mature wt-FV and mutant His1299Arg in the culture media were also subjected to the thrombin digestion and deglycosylation analysis. In contrast to the cell lysate samples, no clear difference between them was observed (data not shown), suggesting that the effect of the His1299Arg mutation on sialylation was temporary during the posttranslational modification, rather than an absolute defect.
The mutant Met385Thr (lane 3) also showed a higher ratio of the posttranslational intermediates over the primary translated peptide (bands 2 and 3/band 1) (Figure 5, lower panel), indicating its accumulation in the Golgi complex due to possible impairment of posttranslational modification. However, we found no evidence of the impaired posttranslational modification of the heavy chain containing the Met385Thr mutation on the gel analysis.
The mutant Met1736Val (lane 5) yielded results similar to those of wt-FV (Figure 5), which suggested that this mutation did not affect the posttranslational modification.
The mutant Q (lane 2), like the mutant His1299Arg (lane 4), did not have band 3 on the gel (Figure 5, upper panel), indicating that the mutant Q had the impaired posttranslational modification because of the effect of the His1299Arg mutation. In addition, like the mutant Asp2194Gly (lane 6), the mutant Q (lane 2) contained decreased amounts of the posttranslational intermediates, demonstrating that the mutant Q had the impaired transport from the ER to the Golgi complex owing to the presence of the Asp2194Gly mutation. Thus, in the mutant Q, the impaired transport by the Asp2194Gly mutation masked the accumulation in the Golgi complex by the Met385Thr mutation and His1299Arg mutation.
FV variants carrying double or triple mutations were also analyzed. Consistently, in all FV mutants having the His1299Arg mutation, band 3 was hardly visible (data not shown). Moreover, all FV mutants carrying the Asp2194Gly mutation showed decreased amounts of the posttranslational intermediates (data not shown).
Discussion
The first study concerning the FV R2 haplotype was reported by Lunghi et al.5 They described an association of the His1299Arg mutation with decreased plasma FV antigen levels. Several population studies have subsequently analyzed the relationship between the R2 haplotype and the quantitative FV deficiency on the basis of plasma assays.5-9,13,14 However, there are some discrepancies among the results, and it has not yet been clarified whether the R2 haplotype indeed leads to the quantitative FV deficiency in vivo. Until now, only 2 studies have analyzed the mechanism of the quantitative FV deficiency associated with the R2 haplotype. De Visser et al9 sequenced the promoter region of the R2 haplotype allele without finding plausible candidate mutations. Castaman et al25 described that in vivo accumulation of the mutant mRNA derived from the R2 haplotype allele was decreased, suggesting that the R2 haplotype affects accumulation of the mutant mRNA in vivo. However, further detailed mechanisms of the quantitative FV deficiency have not yet been characterized. In this study, we have now focused on the quantitative FV deficiency associated with the R2 haplotype, and investigated its molecular basis by in vitro expression studies.
Measurement of expressed protein levels in culture medium by ELISA is a useful method by which to study the overall efficiency of protein expression because the results of ELISA depend on the efficiency of every step involved in the protein-expression pathway. Our ELISA results showed that the steady-state expression level was significantly lower for the mutant Q than for wt-FV; this was in agreement with the previous in vivo observations that the R2 haplotype carriers have decreased plasma FV antigen levels.5,7,9 The ELISA results also indicated that the Asp2194Gly mutation played a key role in the impaired expression of the mutant FV and that its negative effect was dominant among the 4 missense mutations. A variant R2 haplotype lacking the Asp2194Gly mutation has been identified in 2% to 11% of the R2 haplotype alleles.9,11,12 Furthermore, several individuals have been recently found who have only the Asp2194Gly mutation without any other mutations involved in the R2 haplotype.11 The measurements of their plasma FV antigen levels are of interest; however, detailed plasma data have not been described.
From the ELISA results, it was impossible to identify which steps in the protein-expression pathway were impaired. In contrast, a defect in the step of primary protein synthesis or earlier steps was clearly demonstrated by analyzing the synthesis rate of the primary translated peptide of the mutant Q. However, it should be noted that our in vitro expression system did not exactly reproduce the in vivo situation concerning promoter activity, transcription, and RNA processing because, in our expression studies, the FV cDNA was used for transfection owing to a technical difficulty in transfecting the entire huge FV gene including introns.
To study the next steps from primary protein synthesis to secretion in the protein-expression pathway, pulse-chase analysis was performed. We found that secretion of wt-FV was much less efficient than that of other coagulation factors22,26-31 and that the majority of wt-FV was degraded in the cell before its secretion (data not shown). A similar poor secretion efficiency has been observed in the homologous protein FVIII.17 32 Our pulse-chase analysis demonstrated the clear differences in secretion rates between wt-FV and the FV mutants. It was revealed that the secretion of the mutant Q and mutant Asp2194Gly were significantly impaired. This was in good agreement with the ELISA results and again suggested that the Asp2194Gly mutation was the major determinant of the impaired expression of the mutant FV. The secretion rates of the mutant Met385Thr and mutant His1299Arg were also decreased, but the final amounts recovered in the medium were similar to the amount of wt-FV. In the cell, the amounts of the mutant Met385Thr and mutant His1299Arg were higher than the amount of wt-FV. This suggested that these mutants were more stable and resistant to degradation and/or were processed more slowly in the cell than wt-FV. Thus, it seemed possible that, in spite of the lower secretion rates, the steady-state expression levels of the mutant Met385Thr and mutant His1299Arg assessed by ELISA were compensated for by the presence of the mutant proteins that were more stable and resistant to degradation in the cell.
We speculated that the molecular mechanisms causing low secretion rates in the mutant Asp2194Gly and in the mutant Met385Thr or mutant His1299Arg might be different. To investigate this, we compared intracellular processing of these mutants with that of wt-FV and determined the intracellular localization of the distinct forms of FV by deglycosylation analysis. It was revealed that the mutant Asp2194Gly had an impaired transport from the ER to the Golgi complex and was retained in the ER. In contrast, the mutant Met385Thr and mutant His1299Arg were demonstrated to accumulate in the Golgi complex. It is known that misfolded proteins are retained in the ER and trapped by a quality control system, which leads to intracellular degradation.33,34 However, the Golgi complex in mammalian cells does not play a role in the quality control of secretory proteins.33 Thus, it appeared that the low steady-state expression level and the low secretion rate of the mutant Asp2194Gly resulted from the retention in the ER and the subsequent intracellular degradation by the quality control machinery.33 34 In contrast, the mutant Met385Thr and mutant His1299Arg that had accumulated in the Golgi complex were supposed to be secreted into the medium without further degradation. This was consistent with the pulse-chase results, suggesting that the mutant Met385Thr and mutant His1299Arg were more stable and resistant to degradation in the cell than wt-FV.
The mutant His1299Arg was found to have impaired sialylation of O-linked carbohydrates located in the C-terminal part of the B-domain. As mentioned in “Materials and methods,” the mutant His1299Arg in fact carried 2 amino acid substitutions, Lys897Glu and His1299Arg, unlike wt-FV. The Lys897Glu mutation is localized in the N-terminal part of the B-domain (amino acids 710 to 1018), whereas the His1299Arg mutation exists in the C-terminal part of the B-domain (amino acids 1019 to 1545). Thus, it was reasonable to suppose that the impaired sialylation in the C-terminal part of the B-domain was due to the His1299Arg mutation and not to the Lys897Glu mutation. The His1299Arg mutation is located in 1 of the 31 highly conserved 9–amino acid repeats in the B-domain, and each repeat contains one potential target site of O-linked glycosylation.35Therefore, it was likely that the His1299Arg mutation affected the O-linked glycosylation process in this region. Despite clear differences in the posttranslational intermediates in the cell, the mature forms of wt-FV and the mutant His1299Arg in the medium were indistinguishable on the gel. Thus, the effect of the His1299Arg mutation was not so deleterious as to fully block the glycosylation process of the mutant FV even though the sialylation process of O-linked carbohydrates in the B-domain was partially affected by the mutation. We supposed that this partial defect in the glycosylation process in the mutant His1299Arg was responsible for its accumulation in the Golgi complex and its moderately decreased secretion rate. A similar explanation could account for the secretion defect of the mutant Met385Thr, although we found no difference in posttranslational modification of the heavy chain containing the Met385Thr mutation. De Visser et al9 studied several individuals having only the Met385Thr mutation without any other mutations involved in the R2 haplotype and found these individuals to have normal FV antigen levels. This in vivo finding supports our observation that the steady-state expression level of the mutant Met385Thr, as assessed by ELISA, was comparable to that of wt-FV.
The last factor affecting steady-state expression levels of proteins is the stability of the mature proteins secreted in the medium. However, this did not affect the FV mutants analyzed in this study because no significant differences in stability were observed among wt-FV and all FV mutants (G.A.F.N., unpublished observation, April 2001).
In the present study, we have investigated the effects of the 4 missense mutations, Met385Thr, His1299Arg, Met1736Val, and Asp2194Gly, on intracellular processing and expression of the recombinant FV mutants. As described in “Materials and methods,” the naturally existing FV variant derived from the R2 haplotype allele carries not only the 4 missense mutations studied here, but also several other missense mutations.5,6,9 10 Thus, there is a possibility that the other missense mutations could affect the expression of the naturally existing FV R2 haplotype mutant. Furthermore, several silent mutations in the R2 haplotype, which were excluded in this study, might affect transcription, RNA processing, and/or mRNA stability in vivo, although they do not result in a change in amino acid. Thus, one should be careful in comparing our in vitro observations directly with in vivo data in the R2 haplotype carriers because the mutant Q in this study does not exactly mimic the naturally existing FV R2 haplotype mutant. However, our data clearly demonstrated that the mutant Q had the impairment in both primary synthesis and secretion, leading to the markedly decreased steady-state expression level compared with wt-FV. Furthermore, our results revealed that the Asp2194Gly mutation was the major determinant of the impaired expression of the mutant proteins, and its deleterious effect was dominant among the 4 missense mutations. Thus, it is strongly suggested that the R2 haplotype has the potential to result in quantitative FV deficiency in vivo when the Asp2194Gly mutation is present.
We thank Dr F. Tokunaga (Department of Life Science, Himeji Institute of Technology, Hyogo, Japan); Dr K. Uchimura (Department of Biochemistry, Nagoya University School of Medicine, Japan); Dr M. Souri (Department of Molecular Pathological Biochemistry, Yamagata University School of Medicine, Japan); and Dr A. Katsumi (Department of Vascular Biology, The Scripps Research Institute, La Jolla, CA) for their helpful discussions. We are also grateful to M. Steen, E. A. Norstrøm, E. Ajzner, and I. Balogh for their critical reading of the manuscript.
Supported by grants from the Swedish Research Council (07143); a Senior Investigator Award from the Swedish Foundation for Strategic Research; Påhlsson's trust; grant no. 902-26-227 from the Dutch Organization for Scientific Research (G.A.F.N.); and research funds from the University Hospital, Malmö, Sweden.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomio Yamazaki, Department of Laboratory Medicine, Division of Clinical Chemistry, Lund University, Wallenberg Laboratory, University Hospital, S-20502 Malmö, Sweden; e-mail:tomio.yamazaki@klkemi.mas.lu.se.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal