α-Synuclein has been implicated in the pathogenesis of Parkinson disease (PD) and related neurodegenerative disorders. More recently, it has been suggested to be an important regulatory component of vesicle transport in neuronal cells. α-Synuclein is also highly expressed in platelets and is loosely associated with the membrane of the secretory α-granules. However, the functional significance of these observations is unknown. In this study, the possible function of α-synuclein in vesicle transport, with particular regard to α-granule release from the platelets, was investigated. The results showed that ionomycin- or thrombin-induced α-granule secretion was inhibited by exogenous α-synuclein addition in a dose-dependent manner. However, [3H]5-HT release from the dense granules and hexosaminidase release from the lysosomal granules were not affected. Two point mutants (A30P and A53T) found in some familial types of PD, in addition to β-synuclein and α-synuclein112, effectively inhibited PF4 release from the α-granules. However, the deletion mutants, which completely lacked either the N-terminal region or the C-terminal tail, did not affect α-granule release. Interestingly, exogenously added α-synuclein appeared to enter the platelets but did not change the Ca++ level in the platelets at the resting state and the increase in the Ca++level on stimulation. Electron microscopy also supported that α-synuclein inhibits α-granule release. These results suggest that α-synuclein may function as a specific negative regulator of α-granule release in platelets.
Introduction
α-Synuclein is an acidic neuronal protein containing 140 amino acids.1,2 It is highly expressed in brain tissues and is primarily localized at the presynaptic terminals of neurons.3 In addition to its expression in neuronal cells, α-synuclein is expressed in other tissues, such as the heart, skeletal muscle, pancreas, and placenta, but it is less abundant than in the brain.1,2 α-Synuclein consists of 3 distinct regions.4 The N-terminal region contains KTKEGV repeats, which form amphipathic α-helices that are similar to the lipid-binding domain of apolipoproteins. The central region is a hydrophobic NAC (non-Aβ component of Alzheimer disease) peptide, and the C-terminal region is primarily composed of acidic amino acids. In addition to α-synuclein, β- and γ-synuclein and synoretin, which belongs to the synuclein family, have been identified in humans.1 4-6
α-Synuclein has also been identified as a major component of intracellular fibrillar protein deposits (Lewy bodies) in several neurodegenerative diseases, including Parkinson disease,7,8 diffuse Lewy body disease,9 and multiple systemic atrophy.10 Interest in the pathologic role of α-synuclein was enhanced when 2 different mutations, A30P and A53T, were found in some patients with early-onset familial Parkinson disease.11,12 Although significant progress has been made in understanding the pathologic role of α-synuclein in neurodegenerative diseases,13-17 the biologic function of α-synuclein is unclear. Several hypotheses for the normal function of α-synuclein have been proposed. First, α-synuclein may play a role in the neuronal plasticity responses because its avian homolog, synelfin, is regulated during a critical period of song learning.18 Second, α-synuclein is a presynaptic protein that can interact with particular types of lipid bilayers, such as with the synthetic, small, unilamellar phospholipid vesicles containing acidic phospholipids.19 Third, α- and β-synucleins may play an important regulatory role in the vesicular transport process because these proteins appear to selectively inhibit phospholipase D-2 (PLD2).20 Recently, α-synuclein knockout mice were produced.21 These mice appear to be viable and fertile, exhibit an intact brain architecture, and have a normal complement of dopaminergic cell bodies, fibers, and synapses, which suggests that α-synuclein is not essential for neuronal development and differentiation. However, they exhibit an accelerated recovery of dopamine release when presented with multiple stimuli. This suggests that α-synuclein might negatively regulate the activity-dependent dopamine release. Overall, it is highly likely that the normal function of α-synuclein is associated with the trafficking of vesicles and that functional disorders in this process might be associated with neurodegenerative diseases.
In a previous paper, α-synuclein was shown to be highly expressed in various hematopoietic cells including T cells, B cells, NK cells, and monocytes in addition to neuronal cells.22 This result suggests that the α-synuclein function might not be restricted to just the neurons. Hashimoto et al23 also showed that α-synuclein is abundant in platelets and that α-synuclein immunoreactivity is loosely associated with the membrane of α-granules and plasma membrane. These results led us to suggest that α-synuclein may play an important role in vesicle release from hematopoietic cells, particularly in α-granule release from the platelets.
In this study, the effect of exogenous α-synuclein addition on granule release in platelets was investigated. α-Synuclein is shown to specifically inhibit α-granule secretion from platelets when they are stimulated with either ionomycin or thrombin.
Materials and methods
Construction of expression vectors for α-synuclein point mutants
Three mutant form constructs of α-synuclein were made using polymerase chain reaction (PCR)–based, site-directed mutagenesis from pRK172 encoding the wild-type α-synuclein (a kind gift from Dr R. Jakes, Medical Research Council, Cambridge, United Kingdom). The 2XcmI sites in the α-synuclein gene were used for subcloning. Briefly, A30P and A53T point mutations were introduced by PCR with pRK172 as the template, the 5′-oligonucleotide primer agaaaaccaaacagggtgtggcagaagaccaggaagacaaaagaggt and the 3′-oligonucleotide primer cgagctctcaagcttggatggaacatctgtgtcagcag (the mutated codon is underlined) for A30P, and the 5′-oligonucleotide primer ccaaaaccaaggagggagtggtgcatggtgtgacaacagtggctgagaag and the 3′-oligonucleotide primer cgagctctcaagcttggatggaacatctgtgtcagcag (the mutated codon is underlined) for A53T, respectively. An A30P–A53T double-mutant construct was generated by ligating the XcmI-digested DNA fragment from the A30P construct into the pRKA53TΔXcmI. All constructs were confirmed by DNA sequencing.
Purification of α-synuclein, β-synuclein, and α-synuclein point mutants and of α-synuclein deletion mutants
The α- and β-synucleins and mutant forms of α-synuclein were overexpressed in Escherichia coli (Bl21), and the recombinant proteins were purified as described previously.24 α-Synuclein112 (also called NACP112) was purified using a method described previously.25α-Synuclein deletion mutants (Syn61-140 and Syn96-140) were prepared as described previously.26 The α-synuclein1-97 protein (Syn1-97) was prepared by ASPN digestion as described previously.27
Platelet preparation and stimulation assay
Fresh platelets were obtained from the Yonsei University Medical Center blood bank and were prepared from the peripheral blood of healthy donors after they gave informed consent, as described previously.28 Fifty microliters (more than 5 × 107) platelets in HEPES-buffered saline (140 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, 11 mM glucose, 15 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.4) containing 0.1% bovine serum albumin (BSA) was mixed with 50 μL HEPES-buffered saline containing an appropriate amount of the protein sample. Solutions were then incubated for 30 minutes on ice to minimize spontaneous release. Samples were warmed to room temperature for 5 minutes, then 0.5 to 1 μM ionomycin (Calbiochem, Nottingham, United Kingdom) or 0.1 to 1 U/mL thrombin (Sigma, St Louis, MO) was added and the reactions were incubated for a further 5 minutes at room temperature. Reactions were stopped by placing the samples on ice for 4 minutes followed by centrifugation at 13 000 rpm for 1 minute. Supernatants were collected and assayed as below. All the experiments (Figures1-5) were performed at least 3 times using the platelets from 3 different donors. The error bar indicates the results from triplicate experiments.
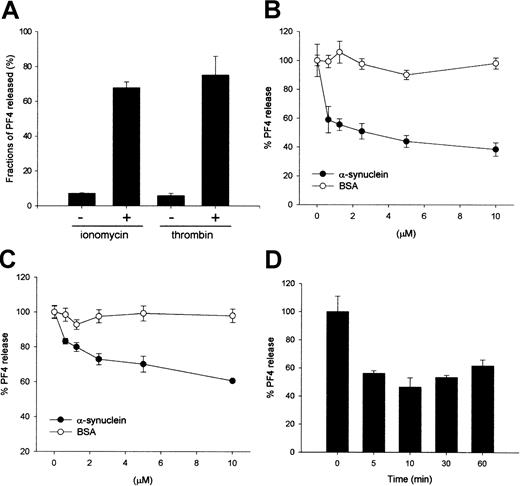
Exogenously added α-synuclein inhibits ionomycin- or thrombin-induced PF4 release from purified platelets.
(A) Ionomycin- and thrombin-induced PF4 release from the α-granules in platelets. Purified platelets (100 μL, more than 106cells/μL) in HEPES-buffered saline containing 0.1% BSA were incubated for 30 minutes on ice to minimize a spontaneous release. Samples were warmed to room temperature for 5 minutes, then 0.5 μM ionomycin or 0.1 U/mL thrombin was added and the reactions were further incubated for 5 minutes at room temperature. Reactions were quenched by placing the samples in ice followed by centrifugation. Supernatants were collected and assayed by quantitative ELISA to determine the amount of PF4. (B) α-Synuclein inhibits ionomycin-induced PF4 release from the α-granules in a dose-dependent manner. (C) α-Synuclein inhibits thrombin-induced PF4 release from the α-granules in a dose-dependent manner. (D) Effect of the incubation time with α-synuclein. Platelet solutions were incubated with 10 μM α-synuclein for the indicated times on ice before stimulation with ionomycin. The PF4 released was quantified using the same method shown in panel A.
Exogenously added α-synuclein inhibits ionomycin- or thrombin-induced PF4 release from purified platelets.
(A) Ionomycin- and thrombin-induced PF4 release from the α-granules in platelets. Purified platelets (100 μL, more than 106cells/μL) in HEPES-buffered saline containing 0.1% BSA were incubated for 30 minutes on ice to minimize a spontaneous release. Samples were warmed to room temperature for 5 minutes, then 0.5 μM ionomycin or 0.1 U/mL thrombin was added and the reactions were further incubated for 5 minutes at room temperature. Reactions were quenched by placing the samples in ice followed by centrifugation. Supernatants were collected and assayed by quantitative ELISA to determine the amount of PF4. (B) α-Synuclein inhibits ionomycin-induced PF4 release from the α-granules in a dose-dependent manner. (C) α-Synuclein inhibits thrombin-induced PF4 release from the α-granules in a dose-dependent manner. (D) Effect of the incubation time with α-synuclein. Platelet solutions were incubated with 10 μM α-synuclein for the indicated times on ice before stimulation with ionomycin. The PF4 released was quantified using the same method shown in panel A.
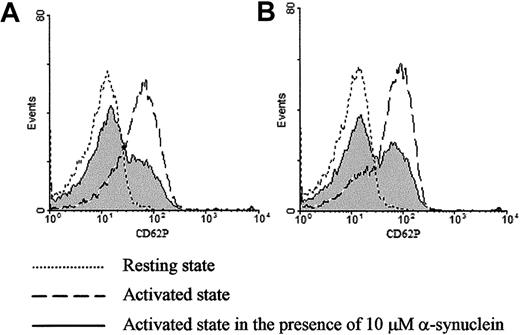
Exogenously added α-synuclein inhibits ionomycin- or thrombin-induced CD62P expression on purified platelets.
Surface expression of CD62P was analyzed by flow cytometry before and after stimulation with 1 μM ionomycin (A) or 0.1 U/mL thrombin (B) in the presence and absence of α-synuclein.
Exogenously added α-synuclein inhibits ionomycin- or thrombin-induced CD62P expression on purified platelets.
Surface expression of CD62P was analyzed by flow cytometry before and after stimulation with 1 μM ionomycin (A) or 0.1 U/mL thrombin (B) in the presence and absence of α-synuclein.
Exogenously added α-synuclein has no effect on ionomycin- or thrombin-induced [3H]5-HT release and hexosaminidase release from platelets.
(A) Platelet-rich plasma was incubated with 0.037 MBq (1 μCi) per milliliter [3H]5-HT for 60 minutes at room temperature to enable sufficient uptake. Platelets were resuspended in HEPES-buffered saline containing 0.1% BSA and were stimulated with either 1 μM ionomycin or 1 U/mL thrombin in the presence or absence of 2 μM imipramine, as described in “Materials and methods.” After the platelet stimulation assay, 50 μL supernatant was added to a 5 mL cocktail solution, and the radioactivity was measured using a liquid scintillation counter. (B) α-Synuclein has no effect on ionomycin-induced [3H]5-HT release from the dense granules. (C) α-Synuclein has no effect on thrombin-induced [3H]5-HT release from the dense granules in the presence of 2 μM imipramine. (D) Ionomycin-induced lysosomal granule release was measured by a quantitative β-hexosaminidase assay, as described in “Materials and methods.” (E) Ionomycin-induced hexosaminidase release from lysosomal granules is not affected by exogenously added α-synuclein, GST-α-synuclein, or α-synuclein112.
Exogenously added α-synuclein has no effect on ionomycin- or thrombin-induced [3H]5-HT release and hexosaminidase release from platelets.
(A) Platelet-rich plasma was incubated with 0.037 MBq (1 μCi) per milliliter [3H]5-HT for 60 minutes at room temperature to enable sufficient uptake. Platelets were resuspended in HEPES-buffered saline containing 0.1% BSA and were stimulated with either 1 μM ionomycin or 1 U/mL thrombin in the presence or absence of 2 μM imipramine, as described in “Materials and methods.” After the platelet stimulation assay, 50 μL supernatant was added to a 5 mL cocktail solution, and the radioactivity was measured using a liquid scintillation counter. (B) α-Synuclein has no effect on ionomycin-induced [3H]5-HT release from the dense granules. (C) α-Synuclein has no effect on thrombin-induced [3H]5-HT release from the dense granules in the presence of 2 μM imipramine. (D) Ionomycin-induced lysosomal granule release was measured by a quantitative β-hexosaminidase assay, as described in “Materials and methods.” (E) Ionomycin-induced hexosaminidase release from lysosomal granules is not affected by exogenously added α-synuclein, GST-α-synuclein, or α-synuclein112.
Mutant forms of α-synuclein (A30P, A53T, A30P/A53T), β-synuclein, and α-synuclein112 function like the wild-type α-synuclein.
(A) α-Synuclein point mutants (A30P, A53T), found in the early-onset familial PD patients, and a double mutant (A30P/A53T) inhibits the ionomycin-induced PF4 release in a similar way to that found in wild-type. Below the figure, each protein for the quantitative comparison was loaded in SDS-PAGE and stained by Coomassie brilliant blue R-250. (B) β-Synuclein and (C) α-synuclein112 also inhibit the PF4 release. The PF4 released was measured as in Figure 1.
Mutant forms of α-synuclein (A30P, A53T, A30P/A53T), β-synuclein, and α-synuclein112 function like the wild-type α-synuclein.
(A) α-Synuclein point mutants (A30P, A53T), found in the early-onset familial PD patients, and a double mutant (A30P/A53T) inhibits the ionomycin-induced PF4 release in a similar way to that found in wild-type. Below the figure, each protein for the quantitative comparison was loaded in SDS-PAGE and stained by Coomassie brilliant blue R-250. (B) β-Synuclein and (C) α-synuclein112 also inhibit the PF4 release. The PF4 released was measured as in Figure 1.
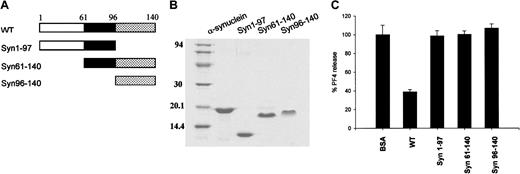
Fragments of α-synuclein have no effect on ionomycin-induced PF4 release from platelets.
(A) Diagram of the wild-type α-synuclein and its deletion mutants. (B) Purified proteins of α-synuclein, α-synuclein fragments (Syn1-97, Syn61-140, Syn96-140) were separated on 12% SDS polyacrylamide gel and stained with Coomassie brilliant blue R-250. (C) Both N-terminally and C-terminally truncated α-synuclein proteins did not appear to inhibit the ionomycin-induced PF4 release from platelets. For these experiments 10 μM synuclein proteins was used, and the PF4 released was measured as in Figure 1.
Fragments of α-synuclein have no effect on ionomycin-induced PF4 release from platelets.
(A) Diagram of the wild-type α-synuclein and its deletion mutants. (B) Purified proteins of α-synuclein, α-synuclein fragments (Syn1-97, Syn61-140, Syn96-140) were separated on 12% SDS polyacrylamide gel and stained with Coomassie brilliant blue R-250. (C) Both N-terminally and C-terminally truncated α-synuclein proteins did not appear to inhibit the ionomycin-induced PF4 release from platelets. For these experiments 10 μM synuclein proteins was used, and the PF4 released was measured as in Figure 1.
Measurement of α-granule secretion
α-Granule release was measured by quantitative enzyme-linked immunosorbent assay (ELISA) for the α-granule protein, platelet factor 4 (PF4), as described previously.29 Supernatants obtained from the platelet stimulation assay were added to the wells of a high-binding ELISA plate (Costar, Cambridge, MA) containing 150 μL binding buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6). Samples were incubated at 37°C for 2 hours. Wells were washed 4 times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T). Blocking was carried out at 37°C for 2 hours using a blocking solution (5% nonfat dried milk in PBS-T). Sheep anti-PF4 primary antibodies (Accurate Chemical and Scientific, Westbury, NY) were diluted to 10 μg/mL in the blocking solution and were added to the wells. The resultant mixture was then incubated for 2 hours at 37°C. The wells were washed 4 times, and anti-sheep secondary antibodies conjugated to horseradish peroxidase (Sigma) diluted in the blocking solution were added. Samples were then allowed to incubate for 1 hour at 37°C. Wells were washed 4 times, and 100 μL of 0.4 mg/mL ς-phenylenediamine (Sigma) in a citrate-phosphate buffer (pH 5.5) was then added. After 15 minutes, the reaction was quenched by the addition of 150 μL of 2.5 N H2SO4. Samples were quantified using a Spectra MAX 340 ELISA Reader (Molecular Devices, Sunnyvale, CA) at a 490-nm wavelength. The percentage release was calculated using the following equation: [OD490 (release from sample) − OD490 (spontaneous release)]/[OD490 (release from no addition of protein) − OD490 (spontaneous release)] × 100. Cells were lysed by 2% Triton X-100 (Sigma) to measure the total amount of granule (Figures 1-5).
Flow cytometric analysis
CD62P (P-selectin) expression was measured by flow cytometric analysis using a slight modification of a previously described method.30 31 Five microliters platelet-rich plasma was diluted to 95 μL HEPES-buffered saline containing 0.1% BSA and anti-CD62P antibodies (AC1.2; BD PharMingen, San Diego, CA). Samples were incubated for 30 minutes at room temperature in the presence or the absence of 10 μM α-synuclein. After the samples were stimulated with either 1 μM ionomycin or 0.1 U/mL thrombin for 5 minutes at room temperature, 100 μL of 2% paraformaldehyde was added for fixation, and the mixture was further incubated for 10 minutes. When thrombin was used as the stimulus, 5 mM Gly-Pro-Arg-Pro (GPRP; Sigma) was added to prevent platelet aggregation. Samples were subsequently incubated with fluorescein isothiocyanate (FITC)–conjugated secondary antibodies for 30 minutes. Then samples were diluted 10- fold and were analyzed in a Becton Dickinson FACScalibur (Franklin Lakes, NJ).
Measurement of dense granule secretion
Dense granule secretion was measured by a [3H]5-HT release assay. Platelet-rich plasma was incubated with 0.037 MBq (1 μCi) per milliliter [3H]5-HT (New England Nuclear, Boston, MA) for 60 minutes at room temperature to allow for sufficient uptake. After incubation, the platelet-rich plasma was washed twice with platelet-poor plasma and once with Tyrode solution. Platelets were resuspended in HEPES-buffered saline containing 0.1% BSA. A platelet stimulation assay was then performed as described above. Before stimulation, 2 μM imipramine (Calbiochem) was added to prevent reuptake in some cases. After the platelet stimulation assay, radioactivity was measured using a liquid scintillation counter.32 Percentage release was calculated similarly to the method used for the α-granule release assay.
Measurement of lysosomal granule release
Lysosomal granule release was measured by a quantitative β-hexosaminidase assay, as described previously.33 After the platelet stimulation assay, 20 μL each supernatant was added to a 100 μL citrate-phosphate buffer (pH 4.5), and 50 μL of 20 mMp-nitrophenyl-β-d-glucosaminides (Sigma) was added to the reaction mixture. Reaction mixtures were incubated at 37°C for 30 minutes. Reactions were quenched by the addition of 250 μL of 0.08 N NaOH. Samples were quantified using a Spectra MAX 340 ELISA Reader at a 410-nm wavelength. Percentage release was calculated similarly to the method used for α-granule release assay.
Confocal microscopy and Western blot analysis
Platelet-rich plasma was incubated for 30 minutes at room temperature in either the presence or absence of α-synuclein and then was washed twice with platelet-poor plasma. For confocal microscopy, the platelets were attached to slide glasses and were fixed for 5 minutes with 4% paraformaldehyde. Fixed platelets were washed several times with a washing buffer (PBS containing 0.1% saponin and 0.1% BSA or PBS containing 0.1% BSA only) and were incubated for 30 minutes at room temperature with anti–α-synuclein antibodies (Synuclein-1; Transduction Laboratories). After washing 4 times, the platelets were incubated for 30 minutes at room temperature with FITC-conjugated secondary antibodies and then were washed several times again with the washing buffer. Immunostained platelets were observed using confocal microscopy (Reica, TCSNT system; Chatsworth, CA). α-Synuclein was also detected by Western blotting. Briefly, the platelets were lysed with 1× sodium dodecyl sulfate (SDS) sample buffer, and the lysates were loaded onto a 12% SDS polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Western blot analysis was then performed with anti–α-synuclein antibodies (LB509; Zymed, South San Francisco, CA).
Measurement of Ca++ concentrations
Platelet-rich plasma was prepared as described above and incubated for 1 hour at room temperature with 1 μM Fura-2/am (Molecular Probes, Eugene, OR). The platelets were then washed twice by platelet-poor plasma, and the pellets were resuspended in HEPES-buffered saline containing 0.1% BSA to a final concentration of more than 107 cells/mL. The fura-2/am fluorescence was measured using 2-mL aliquots of the platelets on a spectrofluorometer (Photon Technology International, Brunswick, NJ). Excitation wavelengths were alternated between 340 nm and 380 nm every second, and the emission wavelength was 510 nm. Data were analyzed to give a fluorescence intensity ratio at the excitation wavelengths of 340/380nm.
Preparation of platelets for electron microscopy
After the platelets were stimulated by ionomycin in the presence or absence of α-synuclein, the samples were fixed with 0.1 M cacodylate buffer (pH 7.4) containing 2% glutaraldehyde, 2% paraformaldehyde, and 0.5% CaCl2 for 6 hours and were then washed with a 0.1 M cacodylate buffer (pH 7.4). Samples were postfixed with 1.33% OsO4 in a cacodylate buffer for 2 hours. Fixed samples were dehydrated with alcohol and then were incubated with propylene oxide for 10 minutes. Embedding EPON mixtures (EPON812, nadic methyl anhydride [MNA], dodecenyl succinic anhydride [DDSA], and tridimethyl phenol [DMP30]) were prepared, and the samples were sectioned using an ultramicrotome followed by double staining with uranyl acetate and lead citrate. Samples were observed using a transmission electron microscope (TEM; Philips CM-10).
Results
Exogenous α-synuclein addition specifically inhibits the α-granule secretion
An in vitro granule release assay system was established using purified platelets as a model system, and either ionomycin or thrombin was used as a stimulus to investigate the molecular mechanism and the regulation of the granule release from cells. First, the amount of secreted platelet factor 4 (PF4) was measured to monitor α-granule release after ionomycin or thrombin stimulation. When the platelets were stimulated with 0.5 μM ionomycin for 5 minutes at room temperature, approximately 70% of the total PF4 in the α-granules was released (Figure 1A). Thrombin, a natural platelet stimulator, was as effective as ionomycin (Figure 1A). The effect of α-synuclein on the α-granule secretion from platelets was next investigated using the established granule release assay system. When the platelets were incubated with α-synuclein, Ca++-induced PF4 release was inhibited in a dose-dependent manner, but Ca++-induced PF4 release was not affected by BSA (Figure 1B). PF4 release was inhibited up to 60% of its normal maximal release when the platelets were incubated with 10 μM α-synuclein. α-Synuclein also appeared to inhibit PF4 release when the platelets were stimulated with 0.1 U/mL thrombin (Figure 1C).
Subsequently, the time course of inhibition caused by α-synuclein treatment was then investigated. A platelet solution was incubated with 10 μM α-synuclein for 5, 10, 30, and 60 minutes before stimulation, respectively, and the amount of PF4 release was measured. As shown in Figure 1D, there was no significant difference in the inhibition level at all the tested times, which suggests that the effect of α-synuclein occurs early. The incubation temperature (4°C, 25°C, or 37°C) did not affect the inhibitory role of α-synuclein on α-granule release (data not shown).
To confirm the observation that α-synuclein inhibits α-granule release, CD62P expression was analyzed by flow cytometry before and after stimulation in the presence or absence of α-synuclein. CD62P is a component of the α-granule membrane that is only expressed on the platelet surface after α-granule secretion.31 As shown in Figure 2A, the platelets stimulated with ionomycin demonstrate a marked increase in CD62P surface expression (dashed line). Interestingly, the surface CD62P surface expression level was greatly reduced in the presence of α-synuclein (solid line). Similar results were obtained when the platelets were stimulated with thrombin (Figure2B). α-Synuclein did not affect CD62P surface expression in resting platelets (data not shown). Flow cytometry data demonstrate that the platelets exposed to α-synuclein and then stimulated by an agonist, particularly thrombin, fall into 2 discrete populations: those that are still stimulated and those that are inhibited essentially to the resting levels of CD62P expression. The reason for this result is unclear, but it may be related to heterogeneous incorporation of α-synuclein.
Abeliovich et al21 suggested that α-synuclein negatively regulates dopamine release in neuronal cells. In addition, Forloni et al34 reported that an externally applied NAC had an effect on dopamine level at concentrations 5 to 50 times lower than those affecting the cell viability in dopaminergic neuronal cells. Because dense granules in the platelets have properties similar to those of small dense core granules containing catecholamine in neuron cells,35 α-synuclein was investigated to determine whether it could also affect the dense granule release from platelets. To investigate the effect of α-synuclein on the dense granule release, platelets were incubated with [3H]5-HT and then stimulated with 1 μM ionomycin or 1 U/mL thrombin in the presence or absence of α-synuclein. As shown in Figure 3A, the released [3H]5-HT was approximately 34% and 60% of total [3H]5-HT uptake in the absence or presence of 2 μM imipramine, respectively. When the platelets were incubated with α-synuclein, [3H]5-HT release was not affected by α-synuclein in either the presence or the absence of imipramine (Figure 3B,C, respectively). This indicates that α-synuclein does not play a role in the regulation of dense granule release and reuptake. We also investigated the effect of α-synuclein on lysosomal granule release to observe whether it specifically inhibited α-granule release. Lysosomal granule release was measured by assaying the released hexosaminidase activity (Figure 3D). As shown in Figure 3E, lysosomal granule release was unaffected by either α-synuclein or α-synuclein112. Overall, these results suggest that α-synuclein specifically regulates α-granule release in platelets.
Effects of α-synuclein point mutants, α-synuclein112, and β-synuclein
The 2 point mutants of α-synuclein (A30P and A53T), which are associated with a few cases of familial Parkinson disease,11,12 have been thoroughly examined to elucidate the role of α-synuclein in the pathogenesis of Parkinson disease. These mutant forms of α-synuclein appear to have different properties than the wild-type with respect to the aggregation patterns36,37 and cytotoxicity to cells.38However, the pathologic role of the α-synuclein mutation in Parkinson disease is still controversial because most cases of Parkinson disease are sporadic, and the point mutants are found only in the rare cases of inherited Parkinson disease. In addition, these mutations did not cause a change in the secondary structure of the protein, its interaction with itself,39 or the intracellular distribution of the protein in the cell cultures.40 The α-synuclein point mutants were also investigated to determine whether they have a different effect in regulating Ca++-induced exocytosis of α-granules. When the platelets were incubated with these mutant proteins, Ca++-induced PF4 release was also inhibited similarly to wild-type α-synuclein (Figure 4A), suggesting that the point mutations (A30P, A53T, and A30P/A53T) do not affect the regulatory function of α-synuclein in α-granule release.
The effects of β-synuclein on α-granule release from platelets were also investigated. β-Synuclein is a homolog of α-synuclein. The N-terminal amphipathic region of β-synuclein is similar to that of α-synuclein, showing a 90% amino acid identity. However, the NAC region lacks 11 central amino acids (amino acids 73-83), and the C-terminal acidic tail has only 33% identity.41Interestingly, our data demonstrate that β-synuclein was also able to negatively regulate α-granule release from the platelets (Figure 4B). In addition to β-synuclein, α-synuclein112, which is a deletion mutant of α-synuclein and lacks 28 amino acids at the C-terminal acidic region (amino acids 103-130), was also able to negatively regulate the α-granule release from platelets (Figure 4C). α-Synuclein112 occurs naturally and is predominantly expressed in the heart, skeletal muscles, and pancreas.42 The observations that the PF4 release was inhibited by β-synuclein and α-synuclein112, almost as effectively as α-synuclein, suggest that the conserved N-terminal region of the synuclein proteins may play an important role in regulating α-granule exocytosis.
Effects of α-synuclein deletion mutants
Three α-synuclein deletion mutants, Syn1-97, Syn61-140, and Syn96-140, were synthesized to determine which part of α-synuclein is responsible for its negative regulatory function in α-granule release from platelets (Figure 5A,B). As shown in Figure 5C, none of these deletion mutant proteins appeared to inhibit PF4 release. This suggests that the deletion mutants, which completely lack either the N-terminal amphipathic region or the C-terminal acidic tail, do not function as a negative regulator of α-granule release in platelets, although a partial deletion of either the NAC peptide (as in β-synuclein) or the C-terminal acidic tail (as in α-synuclein112) does not eliminate the α-synuclein function.
To better understand how exogenously added α-synuclein inhibits α-granule release from the platelets, α-synuclein was further investigated to determine whether it enters platelets and whether it is confined to granules or actually moves into platelet cytosol. After treating the platelets with α-synuclein, the localization of α-synuclein was analyzed using confocal microscopy, and any α-synuclein that entered the cytosol was detected by Western blot analysis. As shown in Figure 6, more α-synuclein was observed in the platelets treated with exogenous α-synuclein (Figure 6Aii) than in the normal platelets (Figure 6Ai). Interestingly, exogenous α-synuclein addition appeared to be localized near the plasma membrane more abundantly than in the cytosol. However, α-synuclein was barely detected when the platelets were not permeabilized with saponin in either the presence or absence of α-synuclein (Figure 6Aiii,Aiv, respectively). This suggests that α-synuclein penetrates the platelets and localizes in the cytosol and near the plasma membrane. Previous studies have shown that endogenous α-synuclein loosely associates with the plasma membrane and the α-granules in platelets.23 Western blot analysis showed that exogenously added α-synuclein penetrated the platelets (Figure6B), as had been previously observed in neuronal cells.17Interestingly, Syn61-140 also appeared to penetrate the platelets, but Syn96-140 did not (Figure 6B). This indicates that the NAC region (residues 61-95) plays a critical role in the membrane translocation of α-synuclein.
Exogenously added α-synuclein and Syn61-140 penetrate platelets.
(A) Confocal microscopic analysis of the platelets treated or not treated with 10 μM α-synuclein in the presence (i,ii) or absence (iii,iv) of 0.1% saponin. (B) Western blot analysis of the platelets either treated or not treated with 10 μM synuclein proteins. Lane 1, platelets not treated with α-synuclein; lane 2, platelets treated with α-synucleinl; lane 3, platelets treated with Syn61-140; lane 4, platelets treated with Syn96-140.
Exogenously added α-synuclein and Syn61-140 penetrate platelets.
(A) Confocal microscopic analysis of the platelets treated or not treated with 10 μM α-synuclein in the presence (i,ii) or absence (iii,iv) of 0.1% saponin. (B) Western blot analysis of the platelets either treated or not treated with 10 μM synuclein proteins. Lane 1, platelets not treated with α-synuclein; lane 2, platelets treated with α-synucleinl; lane 3, platelets treated with Syn61-140; lane 4, platelets treated with Syn96-140.
α-Synuclein does not affect the intracellular Ca++level and the increase of intracellular Ca++ level on stimulation
The intracellular Ca++ level was measured by using Fura-2/am to determine whether α-synuclein changes the intracellular Ca++ level on activation. When α-synuclein was added to the platelets, there was no difference in the Ca++ level for up to 5 minutes (Figure7A). The Ca++ level was also unchanged when platelets were incubated with α-synuclein for a long time (up to 1 hour; data not shown). The intracellular Ca++level was then measured using ionomycin stimulation in the presence or absence of α-synuclein. Figure 7B shows that α-synuclein did not affect the increase in the intracellular Ca++ level on activation in the platelets. The intracellular Ca++ level was also unaffected by α-synuclein treatment when the platelets were stimulated with 0.1 U/mL thrombin (data not shown). Therefore, these results suggest that exogenous α-synuclein addition has no effect on Ca++ homeostasis in platelets.
Intracellular Ca++ level and increase of intracellular Ca++ level on stimulation in the presence and absence of α-synuclein.
Purified platelets were incubated with Fura-2/am for 1 hour at room temperature and were washed with Tyrode solution. Fura-2/am fluorescence was then measured using a fluorescence spectrophotometer. (A) At t = 60, after the start of the run, 10 μM α-synuclein was added. (B) At t = 60, after the start of the run, 0.5 μM ionomycin was added in the presence or absence of 10 μM α-synuclein.
Intracellular Ca++ level and increase of intracellular Ca++ level on stimulation in the presence and absence of α-synuclein.
Purified platelets were incubated with Fura-2/am for 1 hour at room temperature and were washed with Tyrode solution. Fura-2/am fluorescence was then measured using a fluorescence spectrophotometer. (A) At t = 60, after the start of the run, 10 μM α-synuclein was added. (B) At t = 60, after the start of the run, 0.5 μM ionomycin was added in the presence or absence of 10 μM α-synuclein.
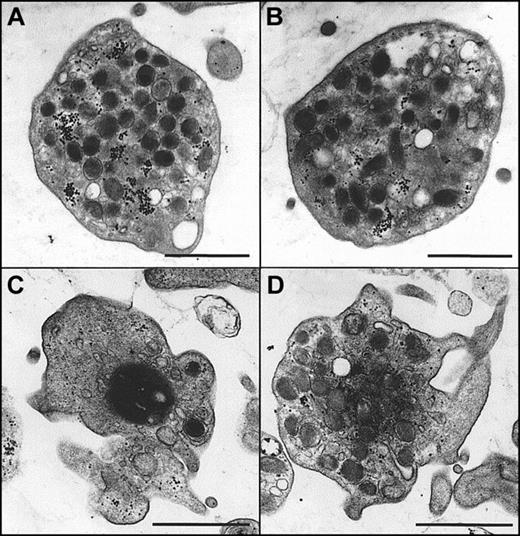
Morphologic changes in the presence and absence of α-synuclein
Morphologic changes in platelets at the resting and ionomycin-stimulated states in the presence or absence of α-synuclein were next examined using TEM to observe the effects of the inhibition of granule release. As shown in Figure8A-B, platelets at the resting state maintained a normal morphology and evenly dispersed granules regardless of the presence of α-synuclein. When the platelets were stimulated with ionomycin, they underwent morphologic changes typical of platelet stimulation (Figure 8C). In particular, there was cytoskeletal contraction, which crowds granules toward the center of the cell. The central, darker area represents the residual cytoskeletal cage surrounding the granules and forcing them to the center of the cell on activation. This is a unique feature of granule exocytosis that is commonly observed in platelets.43 44 Interestingly, the morphology of ionomycin-stimulated platelets in the presence of α-synuclein (Figure 8D) was clearly different from that of ionomycin-stimulated platelets in the absence of α-synuclein (Figure8C). It was also different from that at the resting state (Figure8A,B). In particular, more intracellular granules were observed than in the ionomycin-stimulated platelets, and the granules were more homogenous than in the platelets at the resting state.
Morphologic changes of platelets on stimulation in the presence and absence of α-synuclein in electron microscopy.
Purified platelets were prepared as described in “Materials and methods” and were fixed for TEM analysis. (A, B) Platelets in the resting state in the absence (A) and presence (B) of recombinant α-synuclein. (C) Platelets that have been stimulated with 0.5 μM ionomycin for 5 minutes before fixation. (D) Platelets that were preincubated with 10 μM recombinant α-synuclein before stimulation with 0.5 μM ionomycin. Scale bars, 1μm.
Morphologic changes of platelets on stimulation in the presence and absence of α-synuclein in electron microscopy.
Purified platelets were prepared as described in “Materials and methods” and were fixed for TEM analysis. (A, B) Platelets in the resting state in the absence (A) and presence (B) of recombinant α-synuclein. (C) Platelets that have been stimulated with 0.5 μM ionomycin for 5 minutes before fixation. (D) Platelets that were preincubated with 10 μM recombinant α-synuclein before stimulation with 0.5 μM ionomycin. Scale bars, 1μm.
Discussion
Previous studies have suggested that α-synuclein may have a regulatory function in dopamine release from neuronal cells.21 In this study, the negative regulatory function of exogenous α-synuclein addition in granule release from platelets was investigated for the following reasons. First, platelets are easy to prepare, and they contain many secretory granules. In addition, platelets have been proposed to be good peripheral models for studying the aminergic neuronal function because they have some similarities to neurons, including their ability to manufacture, store, release, and take up monoamines.45 Second, previous reports have shown that α-synuclein is also expressed in hematopoietic cells, suggesting that the biologic function of α-synuclein is not restricted to neuronal cells.22 In particular, the α-synuclein function has been implicated in the differentiation of megakaryocytes, platelet precursors.23 In addition, many abnormalities of platelets have been found in patients with a wide variety of neurodegenerative diseases, including Alzheimer disease,46Huntington disease,47 and Parkinson disease.32,48-50 Moreover, Sharma et al51reported that the incidence of ischemic stroke in patients with Parkinson disease is lower than in the controls, and platelet aggregation is also significantly decreased,51 suggesting that granule release might be blocked. Factor et al52reported that the platelets of patients with Parkinson disease have more and larger intracytoplasmic vacuoles, containing numerous granular molecules around the open canalicular system, than those of the controls. This suggests that the platelets from patients with Parkinson disease have unknown defects in their secretory pathways.
These experiments were designed and conducted on the basis of recent observations showing that α-synuclein can penetrate cells.34,53 In support to these observations, Forloni et al34 showed that fluorescent NAC peptides can penetrate cells and accumulate in the perinuclear region. In addition, Volles et al54 recently reported that the protofibrillar form of α-synuclein binds the synthetic vesicles tightly and transiently permeabilizes these vesicles. In a previous report, α-synuclein was demonstrated to be able to penetrate a neuronal cell and induce cell death.17 The data in this study also demonstrate that α-synuclein penetrates the platelets (Figure 6) and subsequently affects α-granule release on stimulation (Figures 1, 2). It is believed that exogenously added α-synuclein translocates the plasma membrane and that the increased α-synuclein level in the cytoplasm affects the α-granule release from the platelets. Interestingly, Syn61-140 also appeared to enter platelets but Syn96-140 did not (Figure 6B), suggesting that the NAC region (residues 61-95) plays a critical role in α-synuclein penetration of platelets. This observation is consistent with a previous report showing that NAC peptide can penetrate neuronal cells.34
Like the wild type of α-synuclein, the 2 point mutants (A30P and A53T) found in a familial type of Parkinson disease and the double mutant (A30P/A53T) effectively inhibited PF4 release (Figure 4A). β-Synuclein and α-synuclein112 also inhibited the α-granule release from platelets (Figure 4B,C). Interestingly, however, the deletion mutants, Syn1-97, 61-140, and 96-140—which completely lack the C-terminal acidic tail, the N-terminal amphipathic region, and the N-terminal and NAC regions, respectively—did not inhibit α-granule release (Figure 5). These findings show that the N-terminal and the C-terminal regions of α-synuclein are essential for the negative regulation of α-granule release by exogenously added α-synuclein. Based on previous observations showing that the N- and C-terminal regions have the potential to interact with the vesicle membranes and other proteins, respectively,19,38 39 it is proposed that the N-terminal region (residues 1-95) is important for membrane translocation and the interaction with vesicles and that the C-terminal region is necessary for the effector function, although the detailed molecular mechanism requires further investigation.
Previous studies have demonstrated that some amyloidogenic proteins are able to modulate the intracellular Ca++ level in platelets.55 However, in our experiments, α-synuclein did not appear to modulate the intracellular Ca++ levels (Figure 7). Furthermore, α-synuclein did not appear to interrupt the increase in the intracellular Ca++ level caused by stimulation with either ionomycin or thrombin, suggesting that exogenously added α-synuclein has no effect on the Ca++homeostasis in platelets. These observations can be explained by the suggestion that α-synuclein regulates α-granule release by a specific interaction with the α-granules, as was reported by Hashimoto et al.23
For the exocytosis of granules, 3 steps are required: docking, for moving the vesicles to the target membrane; priming, for preparing the interaction between the vesicular membrane and the target membrane; and fusion, for secreting the vesicles.35 It is well known that the N-ethylmalemide attachment protein receptor (SNARE) complex is mainly involved in the final fusion step.56 On the other hand, some proteins are known to play a role in the movement of granules by regulating the interaction between the vesicular membranes and the cytoskeletal proteins. For example, a neuron-specific protein, synapsin I, is involved in the movement of the synaptic vesicles.57 It has been proposed that the function of α-synuclein found in the presynaptic terminals may be associated with synaptic transmission, including neurotransmitter release and uptake.41 In this study, α-synuclein appeared specifically to inhibit the α-granule release induced by the increase in the intracellular Ca++ level in platelets. If α-synuclein were to inhibit all 3 kinds of granules, the α-granule, the dense granule, and the lysosomal granule, in the platelets, then α-synuclein could act at either the proximal or the distal step in the cascade of events leading to the agonist-induced granule secretion. In contrast, the observation that α-synuclein inhibits only α-granule secretion suggests that α-synuclein may act distally in the mechanism of granule release. Considering that the lipid compositions of the various vesicles differ from each other and that there is also an asymmetric distribution of lipid components in each vesicle,58 it is not surprising that α-synuclein appears to specifically interact with the α-granules. In fact, earlier studies have shown that the interaction between α-synuclein and the synthetic vesicles is affected by the lipid composition of the membranes and by the size of the vesicles themselves.59 In addition, the TEM images of the platelets that were stimulated with ionomycin in the presence of α-synuclein (Figure 8D) suggest that α-synuclein may interrupt the step that occurs before membrane fusion, presumably by disrupting the integrity of the α-granule membrane or by blocking the movement of the α-granules to the plasma membrane. Alternatively, α-synuclein itself may have no effect on the cytoskeleton but may inhibit granule centralization by preventing α-granule–dependent autocrine stimulation because the TEM image suggests that incubation with α-synuclein prevents granule centralization after agonist stimulation (Figure 8D).
In summary, exogenous α-synuclein addition negatively regulates Ca++-dependent α-granule release in platelets, and this effect is unaffected by the Parkinson disease–associated point mutations. α-Synuclein112 and β-synuclein have the same properties as α-synuclein. However, a complete deletion of either the N-terminal or the C-terminal region eliminates the negative regulatory effect of α-synuclein on α-granule release. The mechanism of the negative regulatory effect of α-synuclein appears to be related to the specific binding of this protein to the α-granule membrane. Elaborating the consequence of the specific binding and searching for the binding partner of α-synuclein in the platelets is necessary to clarify the molecular mechanism for the negative regulatory function of α-synuclein in granule release. Additional investigations designed to reveal the association between these findings and the pathogenesis of Parkinson disease would lead to useful tools for diagnosis and pathologic study of the disease.
We thank Dr R. Jakes (Medical Research Council, Cambridge) for the recombinant α- and β-synuclein cDNAs; Drs. W. Y. Lee, M. K. Lee, E. C. Shin, H. M. Kim, and H. I. Kim for their helpful discussions; and Dr J. T. Seo and H. J. Shin for technical assistance. We also thank Dr J. T. Seo for giving us access to the spectrofluorometer used for Ca++ level measurements.
Supported by a research grant from the Korea Research Foundation (KRF-2001-DP0517).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jongsun Kim, Department of Microbiology, Yonsei University College of Medicine, 134 Shinchon-dong, Seodaemoon-gu, Seoul 120-752, Korea; e-mail: jkim63@yumc.yonsei.ac.kr.

![Fig. 3. Exogenously added α-synuclein has no effect on ionomycin- or thrombin-induced [3H]5-HT release and hexosaminidase release from platelets. / (A) Platelet-rich plasma was incubated with 0.037 MBq (1 μCi) per milliliter [3H]5-HT for 60 minutes at room temperature to enable sufficient uptake. Platelets were resuspended in HEPES-buffered saline containing 0.1% BSA and were stimulated with either 1 μM ionomycin or 1 U/mL thrombin in the presence or absence of 2 μM imipramine, as described in “Materials and methods.” After the platelet stimulation assay, 50 μL supernatant was added to a 5 mL cocktail solution, and the radioactivity was measured using a liquid scintillation counter. (B) α-Synuclein has no effect on ionomycin-induced [3H]5-HT release from the dense granules. (C) α-Synuclein has no effect on thrombin-induced [3H]5-HT release from the dense granules in the presence of 2 μM imipramine. (D) Ionomycin-induced lysosomal granule release was measured by a quantitative β-hexosaminidase assay, as described in “Materials and methods.” (E) Ionomycin-induced hexosaminidase release from lysosomal granules is not affected by exogenously added α-synuclein, GST-α-synuclein, or α-synuclein112.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/7/10.1182_blood.v100.7.2506/3/m_h81923182003.jpeg?Expires=1769168592&Signature=lfVTyR5tukYU76dEwa8dv6g4W0Dfr4Xsc~0KwcOzuqk8EyGxkT7DPwmE~KTNOhOaMdEGOR0cqxOJo1pa5R3QxhIgISCKUDqcC94PJQFi5ImTu9gPWhzE0I-9zKPQmPomOaIrhXkfbxjGV49z8VjO29isdP2zXLS9tvBHNgqT1Rh4182eN7hsWSQIqCz4ZFc4sh8uOvlTUP3bNbKwS79P7un5Tj07H8XVbJAAjGFHsHF6AKwIMKAKGWfMaA6Byf8ZIx~-qBUyLOxynwu1zvZBBDaS9vDVUKcGw5DabHWHlSQtp6eKsRb9cqpshaWHopahGDpbSMjFXrNvWkgzXT~6ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal