Treatment of the human HL-60 cell line with tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) resulted in rapid (6-24 hours) cytotoxicity associated with progressive maturation of the surviving cells along the monocytic lineage. The occurrence of monocytic maturation was demonstrated by a significant increase of both CD14 and CD11b surface expression, the acquisition of morphologic features typical of mature monocytes, and phagocytic capacity in TRAIL-treated cultures. By using selective pharmacologic inhibitors, it was possible to demonstrate that activation of the caspase cascade played a crucial role in mediating TRAIL cytotoxicity and monocytic maturation of HL-60 cells. Moreover, experiments performed using agonistic polyclonal antibodies, which mimic the interactions between TRAIL and each TRAIL receptor, indicated that TRAIL-R1 was responsible for mediating the TRAIL-induced maturation. Importantly, the maturational effects of TRAIL were observed also in primary normal CD34+ cells, seeded in serum-free liquid cultures for 4 to 8 days in the presence of SCF + GM−CSF. After treatment with TRAIL for 3 additional days, a significant increase in CD14 and CD11b expression, coupled with an increased number of mature monocytes and macrophages, was noticed in the absence of cytotoxicity. These data disclose a novel role for TRAIL as a positive regulator of myeloid differentiation. Moreover, the dichotomous effect of TRAIL on malignant cells (early induction of apoptosis and monocytic maturation of the surviving cells) might have important therapeutic implications for the treatment of acute myeloid leukemia.
Introduction
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL),1 also known as Apo-2 ligand,2 is a member of the structurally related TNF family of cytokines. A subset of these cytokines, including TNF, CD95L, DR3 ligand (TWEAK), and TRAIL, is known to activate the cell death program (reviewed in Gruss and Dower3 and Baker and Reddy4). These cytokines, also called death ligands, induce apoptosis by activating their cognate surface receptors. These death receptors are members of the TNF receptor superfamily and include TNFR1, CD95 (Fas/Apo-1), death receptor (DR)–3, DR-4, DR-5, and DR-6. In particular, TRAIL, which exists as either a type 2 membrane protein or as a soluble protein,5 specifically binds and induces apoptosis by way of DR-4 (also known as TRAIL-R1) and DR-5 (also known as TRAIL-R2).1,2,4 The unique feature of TRAIL, with respect to CD95L and TNF-α, is considered its ability to induce apoptosis in a variety of malignant cells, including several of hemopoietic origin,6-11 displaying minimal toxicity on normal cells and tissues (reviewed in Ashkenazi and Dixit12). The study by Jo et al13 raised a cautionary note for the use of TRAIL in human cancer therapy in consideration of the potential toxicity of TRAIL on in vitro–cultured human normal hepatocytes. A recent study, however, has clarified that recombinant TRAIL prepared for clinical purposes is not toxic either in vivo or in vitro or when used at very high concentrations (500 μg/mL).14
TRAIL induces cell death through recruitment of the adapter molecules FADD15,16 and a hierarchical cascade of cysteine preoteases, termed caspases, that are synthesized as proenzymes and activated by cleavage through upstream caspases or by intermolecular auto-proteolysis.17 They can be grouped into 3 major families: (1) the ICE subgroup (caspases 1, 4, and 5) corresponds to proteases involved in apoptosis and cytokine maturation; (2) the CPP32 subgroup (caspases 3, 6, and 7) includes caspases that mediate downstream steps of proteolysis with preferential cleavage of the DEVD sequence; (3) caspases 2, 8, 9, and 10, which are characterized by the presence of a long prodomain and are related to events of receptor-dependent initiation of apoptosis.17 Initial studies15,16 have outlined the central role of caspase 8 in mediating the apoptotic signal of TRAIL, whereas more recent work has demonstrated that caspases 8 and 10 are recruited by the interaction of TRAIL with either DR-4/TRAIL-R1 or DR-5/TRAIL-R2.18 By inducing proximity between pro–caspase-8 and -10 molecules, which have weak protease activity, death receptor clustering facilitatestrans-proteolysis and consequent activation of the cell death machinery.17 18
It should be emphasized that, although TRAIL has potential as an anticancer therapeutic, its physiologic role is presumably more complex than merely activating caspase-dependent cell death of cancer cells. In this respect, it has been shown by us and other groups of investigators that TRAIL induces a stage of development-specific inhibitory effects on normal immature erythroblasts.19,20 Moreover, it has been shown that caspase activation plays an essential role in the terminal differentiation of human normal erythroblasts,21disclosing a novel role for caspases in the control of hemopoiesis in addition to that of mediating apoptotic cell death. Therefore, because little is still known about possible nonapoptotic functions induced by TRAIL, these data suggest that TRAIL might play different roles in the regulation of normal human hemopoiesis.
The experiments illustrated in this study were designed to investigate the biologic activity of TRAIL on myeloid cells in terms of cytotoxicity or modulation of maturation along the monocytic lineage. As an experimental system, we have chosen the HL-60 myeloblastic leukemia cell line, which can be induced to undergo terminal differentiation along the monocytic and granulocytic lineages by a variety of chemical and biological agents.22-25 Parallel experiments were carried out using primary normal myeloid cells, derived from CD34+ hemopoietic progenitors, to ascertain whether TRAIL also exhibited a maturation-inducing activity on normal myeloid cells.
Materials and methods
Reagents
Recombinant Histidine6-tagged (His) TRAIL and His control peptide were produced in bacteria and purified by chromatography on Ni++ affinity resin. Each batch of recombinant TRAIL was tested for cytotoxicity on the TRAIL-sensitive Jurkat cell line, as previously described.20 26 The absence of endotoxin contamination in the recombinant TRAIL preparation (less than 0.1 endotoxin U/mL) was assessed by Limulus Amoebocyte Lysate assay (BioWhittaker, Walkersville, MD).
Lipopolysaccharide (LPS; Sigma Chemical, St Louis, MO) and polymyxin B (Calbiochem, La Jolla, CA) were used at the final concentration of 10 to 100 ng/mL and 10 μg/mL, respectively. LY294002, a pharmacologic inhibitor of the PI 3-kinase/Akt signal transduction pathway, was purchased from Calbiochem and was used at the final concentration of 10 μM.
For neutralization experiments, either recombinant TRAIL or the supernatant derived from apoptotic TRAIL-treated HL-60 cultures was preincubated with TRAIL-R1-Fc or TRAIL-R2-Fc chimeras, according to the supplier's instructions (R&D, Minneapolis, MN).
The broad caspase inhibitor Cbz-Val-Ala-Asp (Ome)-fluoromethyl ketone (z-VAD-fmk), the peptide control Cbz-Phe-Ala-fluoromethyl ketone (z-FA-fmk), the selective caspase 8 Cbz-Ile-Glu(Ome)-Thr-Asp(Ome)-CH2F(z-IETD-fmk), and caspase 9 Cbz-Leu-Glu(Ome)-His-Asp(Ome)-CH2F (z-LEHD-fmk) inhibitors were from Calbiochem. The activity of most effector caspases3,4 7 and of caspase 1 is blocked by z-VAD-fmk, whereas z-IETD-fmk and z-LEHD-fmk specifically block the activity of caspase 8 and 9, respectively. All caspase inhibitors were dissolved in dimethyl sulfoxide, stocked in aliquots at −20°C, and used at the final concentration of 10 to 100 μM. Inhibitor of inducible nitric oxide (NO) synthase (iNOS) N-nitro-l-arginine methyl ester (L-NAME; Calbiochem) was added to the culture medium at a concentration of 0.5 mM.
Cells
HL-60 cell line, an M2/M3-type acute myeloid leukemia (AML), was grown in RPMI (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Gibco) at an optimal cell density of 3 × 105 to 1.2 × 106 cells/mL. Cell stocks were used between passages 30 to 65 and were re-established from cryopreserved stock cultures. Cell stocks were routinely monitored forMycoplasma contamination by the gene probe method (Gentile Probe, San Diego, CA).
In some experiments, 24 hours after TRAIL treatment, dead HL-60 cells were removed from the cultures by using the Dead Cell Removal kit (Miltenyi Biotech, Auburn, CA). In other experiments, TRAIL-treated HL-60 cultures were enriched in CD14+ cells by positive selection using CD14 MicroBeads (Miltenyi Biotech).
Cord blood (CB) specimens, collected according to institutional guidelines, were obtained during normal full-term deliveries. CB mononuclear cells were isolated by density-gradient centrifugation (Ficoll/Histopaque 1077 g/mL). CB CD34+ cells were then isolated by using a magnetic cell sorting program, Mini-MACS, and the CD34 isolation kit (Miltenyi Biotech) in accordance with the manufacturer's instructions. The purity of CD34+ ranged between 90% and 98%. Myeloid cultures were obtained by seeding CD34+ cells in X-vivo (BioWhittaker) medium, supplemented with nucleosides (10 μg/mL each), 0.5% BSA (fraction V of Chon), 10−4 M BSA-adsorbed cholesterol, 10 μg/mL insulin, 200 μg/mL iron-saturated transferrin, 5 × 10−5 M 2-β-mercaptoethanol (all purchased from Sigma), stem cell factor (SCF, 50 ng/mL), and granulocyte-macrophage colony- stimulating factor (GM-CSF, 10 ng/mL).20 All cytokines were purchased from Genzyme (Cambridge, MA). Every 3 to 4 days, cultures were demi-populated by removing half volume of the medium, which was substituted with fresh medium supplemented with cytokines, and were analyzed for phenotypic expression of myeloid maturative markers. Cells were treated with either control His peptide or TRAIL that was typically added between days 4 and 8 of culture.
Assessment of apoptosis and cell differentiation
After TRAIL treatments, cytotoxicity and maturation-inducing activity were assessed. In particular, at different times (1-4 days) after treatment with TRAIL, samples were analyzed by (1) counting the total number of viable cells by trypan blue dye exclusion; (2) evaluating the degree of apoptosis by propidium iodide (PI) staining and flow cytometry analysis; (3) monitoring cell surface antigen expression by using flow cytometry (for this purpose, surface expression of CD33, CD14, CD15, and CD11b antigens was analyzed); (4) examining the morphology of the cells by staining with May-Grünwald-Giemsa solution or with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) followed by either light or fluorescence microscopy examination, respectively, and by transmission electron microscopy; (5) examining the presence of nonspecific monocyte-associated esterase (NSE) by cytochemical procedure with the NSE kit (Sigma); (6) measuring solid-particle phagocytosis activity by flow cytometry after the addition of 5 × 105/mL FITC-latex microbeads (diameter, 2.0 μm; Polysciences, Warrington, PA) to the cells for 2 hours at 37°C, as previously described.27
Flow cytometry analyses
Apoptosis and surface cellular antigens were analyzed by flow cytometry. For apoptosis detection and quantification, samples containing 2-5 × 105 cells were harvested by centrifugation at 200g for 10 minutes at 4°C, fixed with cold 70% ethanol for at least 1 hour at 4°C, and treated as previously detailed.28 After PI staining, analysis of PI fluorescence was performed by FACScan flow cytometer with the FL2 detector in a linear mode using Lysis II software (Becton Dickinson, San Jose, CA). For quantitative evaluation of apoptosis, the subdiploid (less than 2n) DNA content was calculated as described28and expressed as a percentage of apoptotic versus nonapoptotic cells, regardless of the specific cell cycle phase.
To examine the presence of surface antigens, aliquots of 0.5 × 106 cells per experimental point were subjected to single- or multiple-label staining, as described previously.29 In particular, CD33, CD15, CD11b, and CD14 expression was analyzed by direct staining with either phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody (Becton Dickinson). Nonspecific fluorescence was assessed by using isotype-matched controls. The expression of TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 was analyzed by indirect staining using goat anti-human TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 polyclonal antibodies (all from R&D System, Minneapolis, MN) followed by PE-conjugated rabbit anti-goat IgG (Sigma). Nonspecific fluorescence was assessed by using normal goat IgG followed by a second layer, as described above.
In TRAIL-treated cultures, surface antigens and endocytic activity were analyzed in the gated area detecting viable cells, as established according to the PI staining–negative area, and the forward and side scatter intensities established by multicolor analysis. Data collected from 10 000 cells are reported as either percentage of positive cells or mean fluorescence intensity (MFI) values. All analyses were performed by using a FACScan flow cytometer (Becton Dickinson) and Lysis II software.
Assays for caspase activity
Caspase activity was measured in HL-60 cell extracts by using the fluorometric CaspACE assay system (Promega, Madison, WI). To determine ICE (Caspase-1) and CPP32 (Caspase-3) protease activities, assays were performed in triplicate in 96-well, flat-bottom plates by incubating 10 μg cell proteins/sample with the specific fluorigenic substrates according to the manufacturer's instructions. After 1 hour of incubation, the products of reaction were measured by using a plate reader (Victor 1420; Wallac, Freiburg, Germany). Increases in fluorescence were linear over time and extract concentration.
Caspase activation was also monitored by using the CaspACE FITC-VAD-FMK in situ marker (Promega) according to the manufacturer's instructions. This compound is a FITC-conjugate of the cell-permeable broad caspase inhibitor VAD-fmk, which can be delivered to the cells where it binds to activated caspase. The bound marker was localized by fluorescence detection and quantified by flow cytometry.
Analysis of TRAIL receptor transcripts
The presence of TRAIL receptor mRNA transcripts was examined by reverse transcription–polymerase chain reaction (RT-PCR). RNA purification from HL60 and primary myeloid cultures was performed using the SV total RNA isolation system (Promega) following the manufacturer's protocol. Synthesis of first-strand cDNA and amplification were performed using the Access RT-PCR system (Promega) and specific primer sets according to the manufacturer's protocol. As a control for DNA contamination, equal amounts of RNA were used for PCR without template retro-transcription. RT-PCR reactions were performed using the following primer sets: TRAIL-R1 (forward: 5′-CTG AGC AAC GCA GAC TCG CTG TCC AC-3′; reverse: 5′-TCC AAG GAC ACG GCA GAG CCT GTG CCA T-3′); TRAIL-R2 (forward: 5′-GCC TCA TGG ACA ATG AGA TAA AGG TGG CT-3′; reverse: 5′-CCA AAT CTC AAA GTA CGC ACA AAC GG-3′); TRAIL-R3 (forward: 5′-GAA GAA TTT GGT GCC AAT GCC ACT G-3′; reverse: 5′-CTC TTG GAC TTG GCT GGG AGA TGT G-3′); TRAIL-R4 (forward: 5′-CTT TTC CGG CGG CGT TCA TGT CCT TC-3′; reverse: 5′-GTT TCT TCC AGG CTG CTT CCC TTT GTA G-3′); β-actin (forward: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′; reverse: 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′). Resultant PCR products from RT-PCR were resolved on 2% agarose gels and visualized with ethidium bromide.
Statistical analysis
Data were analyzed using the 2-tailed, 2-sample ttest (Minitab statistical analysis software, State College, PA). Values of P < .05 were considered significant.
Results
TRAIL induces a rapid cytotoxicity on HL-60 cells coupled to maturation of the surviving cells along the monocytic lineage
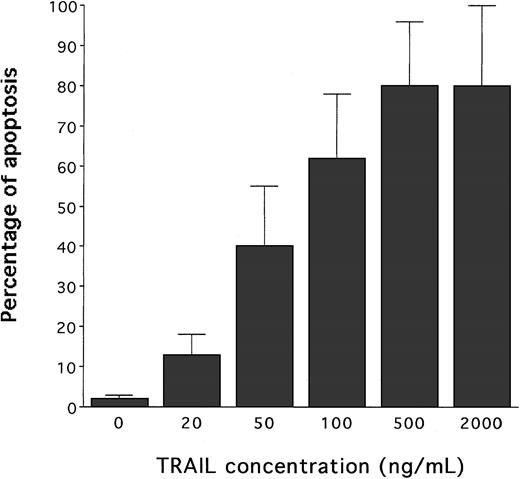
Following treatment with recombinant Histidine6-tagged TRAIL, containing the TRAIL protein fused to a tag of 6 histidine residues, or with a control Histidine6-peptide (His-Pept),26 the extent of apoptosis was determined by PI staining followed by flow cytometry analysis, and cell viability was determined by trypan blue dye exclusion. Although cultures treated with the control His-Pept were indistinguishable from the untreated ones, addition of TRAIL induced a dose-dependent increase of apoptosis (Figure1), accompanied by a drastic decline in the total number of trypan blue dye-negative viable cells (data not shown).
Evaluation of the cytotoxic activity of human recombinant TRAIL on HL60 cells.
Dose-dependent effect of TRAIL on apoptosis quantitatively evaluated by flow cytometry after PI staining. Cells were cultured for 24 hours with the indicated concentrations of TRAIL. Data represent the means ± SD of 5 independent experiments performed in duplicate.
Evaluation of the cytotoxic activity of human recombinant TRAIL on HL60 cells.
Dose-dependent effect of TRAIL on apoptosis quantitatively evaluated by flow cytometry after PI staining. Cells were cultured for 24 hours with the indicated concentrations of TRAIL. Data represent the means ± SD of 5 independent experiments performed in duplicate.
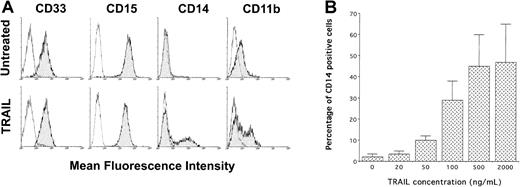
Phenotypic analysis of untreated and His-Pept–treated HL60 cells showed a high expression of CD33 and CD15 early myeloid markers, low expression of CD11b late myeloid marker, and virtually absent expression (less than 7%) of CD14 (Figure2A). Although CD33 and CD15 antigens were unaffected by TRAIL treatment, a significant (P < .01) subset (39% ± 10%; n = 27 independent experiments) of HL-60 cells surviving to the cytotoxic activity of TRAIL unexpectedly showed a selective induction of the surface 55-kD glycoprotein CD14 (Figure2A). It should be noted that CD14 represents an excellent marker of monocytic maturation because it is undetectable on the surface of monocyte precursors and increases dramatically during their differentiation to monocytes.30-32 Surface CD11b, the β2 integrin CR3, is another classical marker of myeloid differentiation that is involved in cell adhesion and also functions as a complement receptor.33 As in the case of CD14, CD11b expression, present at low MFI in a subset of control HL-60 cells (25% ± 9%; n = 12 independent experiments), was significantly (P < .01) increased (58% ± 10%, n = 12 independent experiments) on treatment with TRAIL (Figure 2A). It is also remarkable that the induction of CD14 surface antigen by TRAIL was dose dependent (Figure 2B). By dual-staining analysis performed with anti-CD14 + anti-CD11b mAb, it was also possible to demonstrate that the 2 antigens were coexpressed in the same cell populations (data not shown), further suggesting that TRAIL induced the maturation along the monocytic lineage in at least a subpopulation of HL-60 cells. In agreement with previous studies showing that the up-regulation of CD14 and CD11b antigens constitutes a relatively early maturation event that anticipates morphologic differentiation,25 34 we found that CD14 and CD11b markers were already up-regulated 24 hours after the addition of TRAIL.
Phenotypic characterization of untreated and TRAIL-treated HL60 cells by immunofluorescence staining revealed by flow cytometry.
(A) Representative analysis of surface CD33, CD15, CD14, and CD11b performed at 24 hours of TRAIL treatment (0.2 μg/mL). Analyses were performed by gating viable cells. Control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control mAbs. In some panels, the unshadowed histograms are not visible because they are completely overlapped by the shadowed histograms. The shift of the shadowed histogram along the x-axis illustrates the induction of CD14 and the increased level of CD11b in a subpopulation of the TRAIL-treated culture. Data shown are from a single experiment representative of 8 independent experiments with similar results. (B) Dose-dependent effect of TRAIL on surface CD14 expression, evaluated by flow cytometry and reported as a percentage of CD14+ cells. Data represent the means ± SD of 5 independent experiments performed in duplicate.
Phenotypic characterization of untreated and TRAIL-treated HL60 cells by immunofluorescence staining revealed by flow cytometry.
(A) Representative analysis of surface CD33, CD15, CD14, and CD11b performed at 24 hours of TRAIL treatment (0.2 μg/mL). Analyses were performed by gating viable cells. Control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control mAbs. In some panels, the unshadowed histograms are not visible because they are completely overlapped by the shadowed histograms. The shift of the shadowed histogram along the x-axis illustrates the induction of CD14 and the increased level of CD11b in a subpopulation of the TRAIL-treated culture. Data shown are from a single experiment representative of 8 independent experiments with similar results. (B) Dose-dependent effect of TRAIL on surface CD14 expression, evaluated by flow cytometry and reported as a percentage of CD14+ cells. Data represent the means ± SD of 5 independent experiments performed in duplicate.
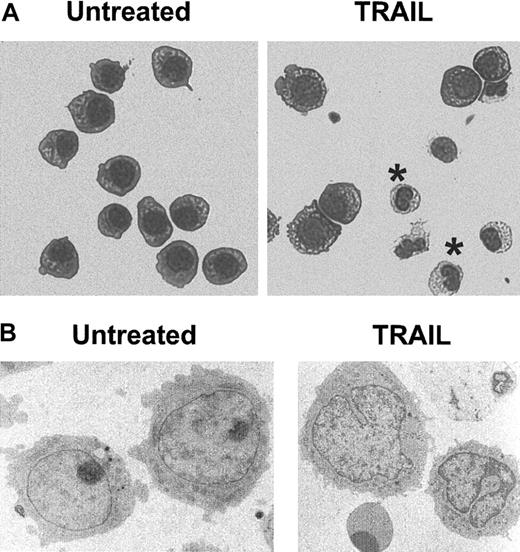
We next investigated whether these phenotypic changes were accompanied by the appearance in culture of cells with monocytic features. Therefore, HL-60 cultures were depleted of the necrotic cells and debris 24 hours after TRAIL treatment and were cultured for an additional 2 to 5 days before morphologic (Figure3) and cytochemical (NSE) analyses (data not shown). During light (Figure 3A) and electron (Figure 3B) microscopy, cells with morphologic features of the monocytic lineage, such as condensation and cleaving of the nucleus, were hardly detectable in untreated and His-Pept–treated cultures. On the contrary, by day 3 of TRAIL treatment, cells with monocytic features represented a significant percentage of the surviving cell population, ranging from 20% to 50%.
Morphologic features of untreated and TRAIL-treated HL60 cells.
HL60 cultures were left untreated or were treated for 72 hours with TRAIL (0.2 μg/mL). After removing dead cells from the culture, cells were cytocentrifuged and stained with May-Grünwald-Giemsa solution (A) or were processed for transmission electron microscopy (B). In panel A, cells with monocytic morphology are indicated by asterisks. Representative fields of 5 separate experiments are shown. Original magnifications: × 400 (A), × 7000 (B).
Morphologic features of untreated and TRAIL-treated HL60 cells.
HL60 cultures were left untreated or were treated for 72 hours with TRAIL (0.2 μg/mL). After removing dead cells from the culture, cells were cytocentrifuged and stained with May-Grünwald-Giemsa solution (A) or were processed for transmission electron microscopy (B). In panel A, cells with monocytic morphology are indicated by asterisks. Representative fields of 5 separate experiments are shown. Original magnifications: × 400 (A), × 7000 (B).
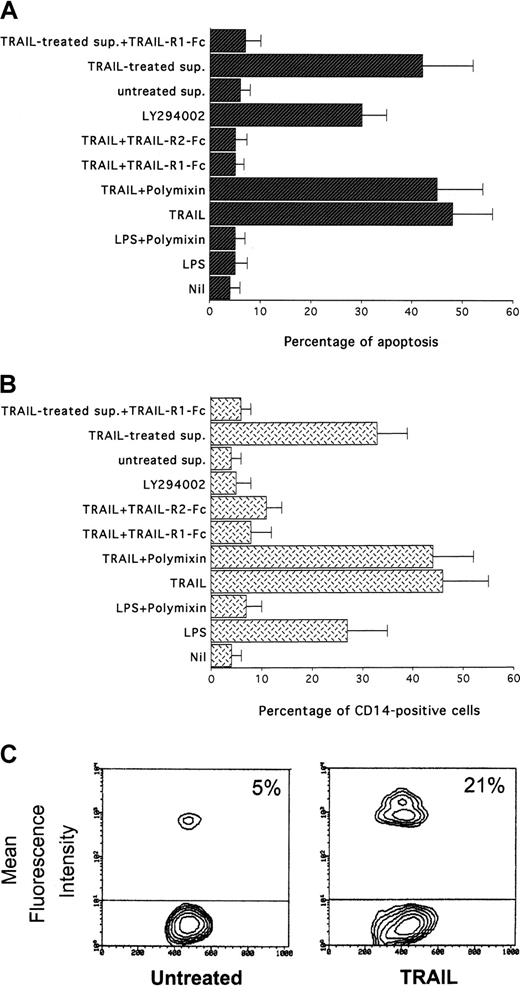
Specificity of the proapoptotic and maturational effects of TRAIL was next demonstrated combining different approaches. To completely rule out the effect of potential contaminating LPS in the TRAIL preparations, cells were treated in the presence of polymyxin, an inhibitor of LPS. TRAIL-mediated cytotoxicity (Figure4A) and up-regulation of surface CD14 (Figure 4B) were unaffected by co-treatment of HL-60 cells with polymyxin. On the other hand, polymyxin completely abrogated the ability of LPS (100 ng/mL) to induce a small increase of surface CD14. The specificity of the effect was confirmed by preincubation of TRAIL with TRAIL-R1-Fc or TRAIL-R2-Fc chimeric proteins, which abrogated the TRAIL-mediated maturational effects (Figure 4A) without exhibiting by themselves any effect on the expression of surface markers in HL-60 cells (Figure 4B). In addition, we considered the possibility that apoptosis of a large proportion of cells, as a consequence of TRAIL treatment, could leave a subpopulation undergoing differentiation in response to signals derived from apoptotic cells, related to the stress of the elimination of large proportions of cells. Therefore, to evaluate whether the differentiation of the surviving cells was truly dependent on a direct TRAIL signal, in some experiments the supernatants of His-Pept (control)– and TRAIL-treated cells were harvested and filtered (0.4 μm) after 24 hours of culture. Incubation of HL60 cells with control supernatant did not result in any modulation of maturative markers. On the other hand, a significant increase of CD14 and CD11b surface expression and the induction of apoptosis was observed on 24-hour incubation of HL60 cells with the supernatant derived from TRAIL-treated cultures (Figure 4A-B). However, such effects were abrogated by preincubating the TRAIL-treated supernatant with the TRAIL-R1-Fc chimeric protein, supporting a direct and specific role of residual TRAIL in the differentiation process (Figure 4A-B). This conclusion was further strengthened by the observation that treatment of HL-60 cells with LY294002, a pharmacologic inhibitor of the PI 3-kinase/Akt survival pathway, induced a significant increase in the percentage of apoptosis without inducing any maturative effect in the surviving HL-60 cells (Figure 4A-B).
Specificity of TRAIL-induced CD14 expression in HL60 cells.
After 24 hours of the indicated treatments, apoptosis (A) was determined by PI staining, and percentages of cells expressing CD14 (B) were evaluated by flow cytometry. Sup. indicates supernatant derived from either untreated or TRAIL-treated HL60 cultures. Data represent the means ± SD of 3 to 5 separate experiments performed in duplicate. (C) Evaluation of the phagocytosis ability of untreated and TRAIL-treated HL60 cells determined by flow cytometry measure of fluorescent latex microbead incorporation. Percentage of fluorescent cells is indicated. Data representative of 4 separate experiments are shown.
Specificity of TRAIL-induced CD14 expression in HL60 cells.
After 24 hours of the indicated treatments, apoptosis (A) was determined by PI staining, and percentages of cells expressing CD14 (B) were evaluated by flow cytometry. Sup. indicates supernatant derived from either untreated or TRAIL-treated HL60 cultures. Data represent the means ± SD of 3 to 5 separate experiments performed in duplicate. (C) Evaluation of the phagocytosis ability of untreated and TRAIL-treated HL60 cells determined by flow cytometry measure of fluorescent latex microbead incorporation. Percentage of fluorescent cells is indicated. Data representative of 4 separate experiments are shown.
TRAIL-treated HL-60 cells were next analyzed by a functional assay, consisting of the evaluation of the phagocytic activity of solid particles. The phagocytic activity in TRAIL-treated cells was significantly (P < .05) increased, as demonstrated by the higher incorporation of microbeads-FITC with respect to untreated cells or cells treated with His-Pept, as evaluated by flow cytometry (Figure 4C).
TRAIL-induced monocytic maturation of HL-60 cells requires the activation of the caspase cascade
We have recently demonstrated that TRAIL activates effector caspases and NOS pathways in the K562 erythroleukemic cell line and that both pathways contribute to the cytotoxicity of TRAIL on these leukemic cells.29 Moreover, it has been shown that certain types of AML can be induced to differentiate along the monocytic lineage by NO.35 Therefore, in the next group of experiments, we sought to investigate whether these pathways were involved in the maturational effects induced by TRAIL in the myeloblastic HL-60 cell line.
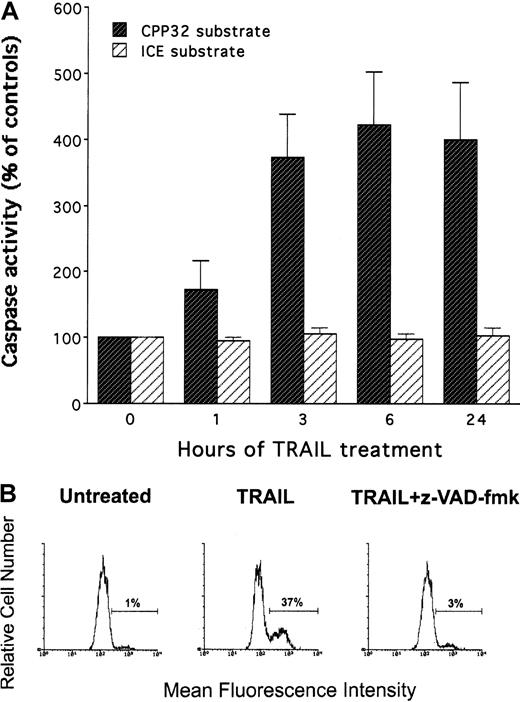
The activity of the effector caspase subgroup (including caspase 3) and the ICE subgroup (including caspase 1) was assessed at different time points after treatment with either control His-Pept or with TRAIL. Although no activation of the ICE subgroup was observed on treatment with TRAIL, the activity of the caspase 3 subgroup showed a significant (P < .01) and persistent increase (Figure5A) with respect to untreated or His-Pept–treated cells. Activation of the caspase 3 subgroup was independently demonstrated by using a fluorometric marker revealed by flow cytometry (Figure 5B).
Evaluation of TRAIL-induced caspase activity in HL60.
Cells were treated with TRAIL (0.2 μg/mL) for the indicated times. (A) Caspase activity was evaluated on cell lysates by using either CPP32 or ICE substrates. Data are expressed as a percentage of control (His peptide–treated) cells. Data represent the means ± SD of 4 independent experiments performed in duplicate. (B) Caspase activation induced by TRAIL, in the presence or absence of z-VAD-fmk inhibitor of effector caspases, has been evaluated by flow cytometry following incubation with CaspACE FITC-VAD-FMK in situ marker. Data are expressed as percentage of positive (fluorescent) cells. Analysis was performed after 6 hours of TRAIL treatment by gating viable cells. Data represent the means ± SD of 4 independent experiments performed in duplicate.
Evaluation of TRAIL-induced caspase activity in HL60.
Cells were treated with TRAIL (0.2 μg/mL) for the indicated times. (A) Caspase activity was evaluated on cell lysates by using either CPP32 or ICE substrates. Data are expressed as a percentage of control (His peptide–treated) cells. Data represent the means ± SD of 4 independent experiments performed in duplicate. (B) Caspase activation induced by TRAIL, in the presence or absence of z-VAD-fmk inhibitor of effector caspases, has been evaluated by flow cytometry following incubation with CaspACE FITC-VAD-FMK in situ marker. Data are expressed as percentage of positive (fluorescent) cells. Analysis was performed after 6 hours of TRAIL treatment by gating viable cells. Data represent the means ± SD of 4 independent experiments performed in duplicate.
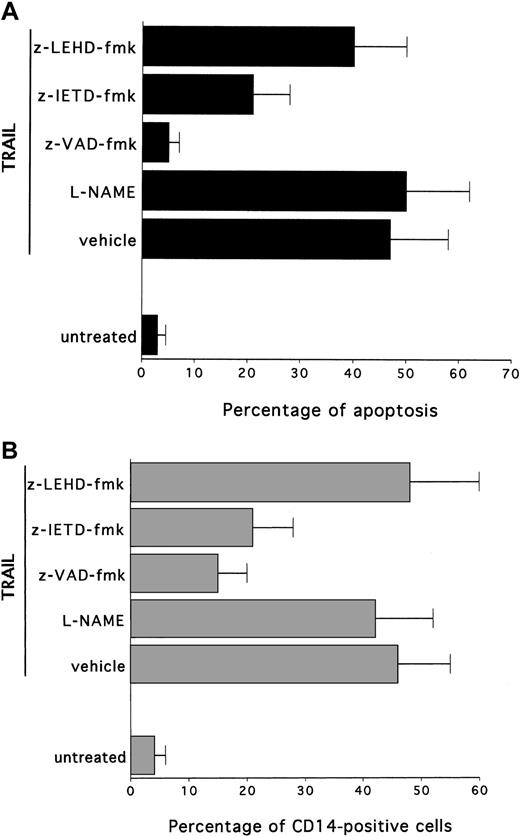
The role of the caspase pathway in TRAIL-mediated maturation of HL-60 cells was next investigated by using selective pharmacologic inhibitors. In this set of experiments, cells were preincubated with each inhibitor for 45 minutes, followed by the co-incubation of TRAIL for an additional 24 to 72 hours. After treatment with TRAIL, cytotoxicity and degree of maturation were examined. Cell viability and expression of surface antigens were not affected by any of the inhibitors, used at indicated concentrations, in control HL-60 cells (data not shown). Incubation of HL-60 cells with the broad inhibitor of effector caspases, z-VAD-fmk, completely abrogated the TRAIL-induced apoptosis (P < .01) (Figure6A) and almost completely prevented (P < .01) the TRAIL maturational effect (Figure 6B). On the other hand, the NOS inhibitor, L-NAME, did not affect (P > .1) the degree of maturation along the monocytic lineage, thus suggesting that effector caspases play a crucial role in TRAIL-mediated maturation, whereas the NO pathway is not involved in this process.
Effect of pharmacologic inhibitors on TRAIL-mediated cytotoxicity and CD14 induction in HL60 cells.
Cultures were left untreated or were treated for 24 hours with TRAIL in the absence (vehicle) or presence of the following inhibitors: L-NAME, inhibitor of NOS; z-VAD-fmk, inhibitor of effector caspases; z-IETD-fmk, specific inhibitor of caspase 8; z-LEHD-fmk, specific inhibitor of caspase 9. (A) Percentage of apoptosis quantitatively evaluated by flow cytometry after PI staining. (B) Percentage of cells expressing surface CD14, evaluated by flow cytometry. Data represent the means ± SD of 3 separate experiments performed in duplicate.
Effect of pharmacologic inhibitors on TRAIL-mediated cytotoxicity and CD14 induction in HL60 cells.
Cultures were left untreated or were treated for 24 hours with TRAIL in the absence (vehicle) or presence of the following inhibitors: L-NAME, inhibitor of NOS; z-VAD-fmk, inhibitor of effector caspases; z-IETD-fmk, specific inhibitor of caspase 8; z-LEHD-fmk, specific inhibitor of caspase 9. (A) Percentage of apoptosis quantitatively evaluated by flow cytometry after PI staining. (B) Percentage of cells expressing surface CD14, evaluated by flow cytometry. Data represent the means ± SD of 3 separate experiments performed in duplicate.
It has been previously shown that members of the TNF superfamily activate 2 main signal transduction pathways. The type 1 pathway is mediated by high levels of caspase 8, which directly activates effector caspases. On the other hand, the type 2 pathway is mediated by mitochondrial dysfunction, which precedes the activation of caspase 9.36 Therefore, the maturation activity of TRAIL was also evaluated in the presence of selective inhibitors for caspase 8 and caspase 9. As shown in Figure 6, z-IETD-fmk, a selective caspase 8 inhibitor, significantly (P < .01) reduced the cytotoxic activity of TRAIL (Figure 6A) and was as efficient as z-VAD-fmk in blocking (P < .01) the TRAIL-induced maturation along the monocytic pathway (Figure 6B). On the other hand, z-LEHD-fmk, a selective caspase 9 inhibitor, was unable to significantly prevent TRAIL-mediated cytotoxicity and maturative effect (Figure 6A-B).
Maturational effect of TRAIL is mediated by TRAIL-R1
It should be emphasized that an extreme complexity characterizes the expression and function of TRAIL receptors (reviewed in Baker and Reddy4). In fact, at least 5 TRAIL receptors have been described so far. TRAIL-R1 (DR4) and TRAIL-R2 (DR5) transduce apoptotic signals on the binding of TRAIL, whereas TRAIL-R3 (DcR1), TRAIL-R4 (DcR2), and osteoprotegerin are homologous to DR4 and DR5 in their cysteine-rich extracellular domain but lack intracellular death domain and apoptosis-inducing capability. Although HL-60 cells expressed the mRNA of all transmembrane TRAIL receptors (TRAIL-R1, -R2, -R3, and -R4) (Table 1), flow cytometry analysis showed detectable surface levels of TRAIL-R1 and TRAIL-R2 expression coupled to low or undetectable surface expression of TRAIL-R3 and TRAIL-R4 (Table 1). Therefore, to elucidate whether the maturational effects of TRAIL on HL-60 cells were specifically mediated by one or more TRAIL receptors, HL-60 cells were challenged with agonistic polyclonal anti–TRAIL-R1, -R2, -R3, and -R4 antibodies, which mimic the interaction between TRAIL and each TRAIL-R without cross-reacting among each other.37
Analysis of TRAIL-receptor expression
| Analyses . | Cells . | |
|---|---|---|
| HL-60 | Primary myeloid cells | |
| RT-PCR | ||
| TRAIL-R1 | + | + |
| TRAIL-R2 | + | + |
| TRAIL-R3 | + | + |
| TRAIL-R4 | + | + |
| Flow-cytometry* | ||
| TRAIL-R1 | 7 | 7 |
| TRAIL-R2 | 9 | 8 |
| TRAIL-R3 | Undetectable | 14 |
| TRAIL-R4 | Undetectable | Undetectable |
| Analyses . | Cells . | |
|---|---|---|
| HL-60 | Primary myeloid cells | |
| RT-PCR | ||
| TRAIL-R1 | + | + |
| TRAIL-R2 | + | + |
| TRAIL-R3 | + | + |
| TRAIL-R4 | + | + |
| Flow-cytometry* | ||
| TRAIL-R1 | 7 | 7 |
| TRAIL-R2 | 9 | 8 |
| TRAIL-R3 | Undetectable | 14 |
| TRAIL-R4 | Undetectable | Undetectable |
Data are representative of 3 separate experiments performed in duplicate.
Data are expressed as MFI. SD were within 15% of the means and are omitted for clarity.
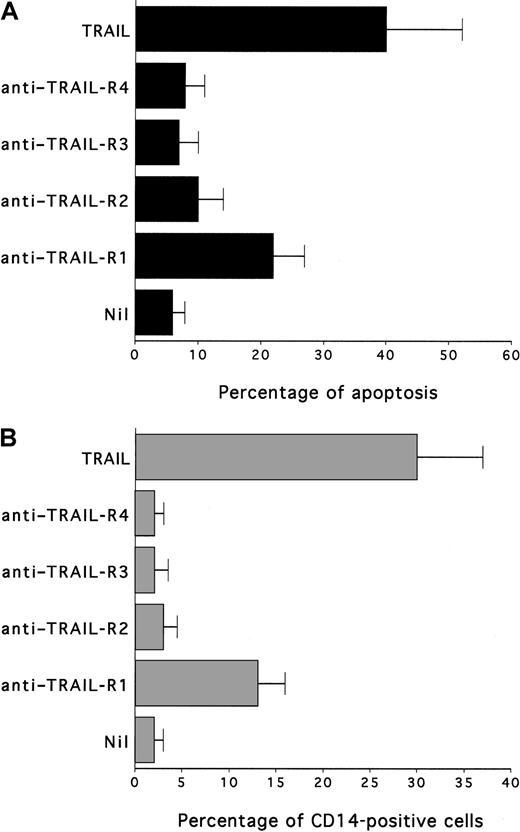
Remarkably, treatment of HL-60 with anti–TRAIL-R1, but not with anti–TRAIL-R2, anti–TRAIL-R3 or anti–TRAIL-R4, induced a significant (P < .05) increase in apoptosis (Figure7A) and CD14 (Figure 7B) surface expression. The biologic activity of anti–TRAIL-R1 antibody was significantly lower (P < .05) than that of recombinant TRAIL, likely because of their differential ability to induce TRAIL-R1 oligomerization.
Effect of agonist antibodies anti-TRAIL receptors on HL60 viability and CD14 induction.
Cultures were left untreated or were treated with TRAIL (0.2 μg/mL) or antibody anti-TRAIL receptors (TRAIL-R1, TRAIL-R2, TRAIL-R3, TRAIL-R4) for 24 hours. (A) Apoptosis quantitatively evaluated by flow cytometry after PI staining. (B) Percentage of cells expressing surface CD14, determined by flow cytometry. Data represent the means ± SD of 3 separate experiments performed in duplicate.
Effect of agonist antibodies anti-TRAIL receptors on HL60 viability and CD14 induction.
Cultures were left untreated or were treated with TRAIL (0.2 μg/mL) or antibody anti-TRAIL receptors (TRAIL-R1, TRAIL-R2, TRAIL-R3, TRAIL-R4) for 24 hours. (A) Apoptosis quantitatively evaluated by flow cytometry after PI staining. (B) Percentage of cells expressing surface CD14, determined by flow cytometry. Data represent the means ± SD of 3 separate experiments performed in duplicate.
TRAIL induces maturation along the monocytic lineage in primary normal CD34-derived myeloid cells
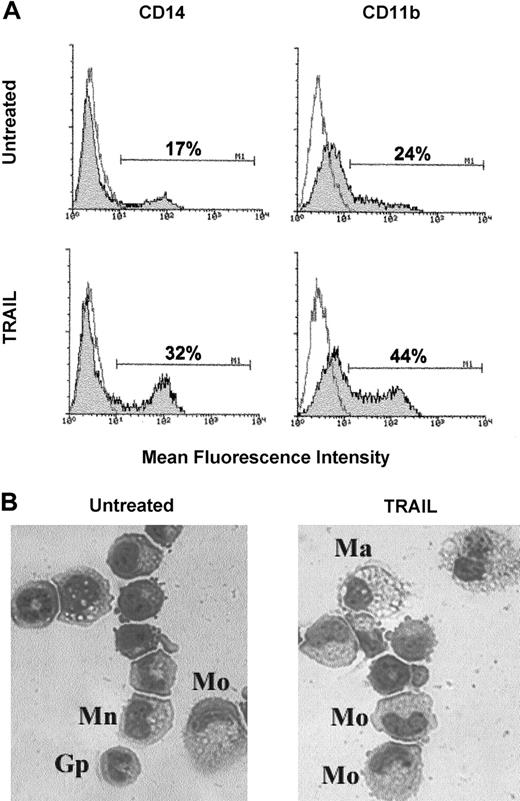
In the last group of experiments, we investigated whether TRAIL affected the maturation of primary normal myeloid cells. For this purpose, we set up serum-free liquid cultures of CB CD34+hemopoietic progenitors supplemented with SCF + GM-CSF. Cells were monitored for phenotypic expression of myeloid maturative surface antigens every 2 days, and TRAIL was typically added in culture between day 4 and day 8, when the cells still showed low expression of CD14. At this time point, primary normal myeloid cells expressed the mRNA for all TRAIL receptors, and, at flow cytometry, dim expression of TRAIL-R, TRAIL-R2, and TRAIL-R3 was clearly observed, whereas TRAIL-R4 was undetectable (Table 1). Thus, with the exception of surface TRAIL-R3, the phenotypic pattern of primary myeloid cells at this culture time (days 4-8) resembled that of HL-60 myeloblastic cell line (Table 1). In agreement with the data obtained on the HL-60 cell line, the addition of TRAIL for 3 days significantly (P < .01) increased the surface expression of CD14 (from 15% ± 7% to 33% ± 6%; n = 6 independent experiments) and CD11b (from 26% ± 8% to 47% ± 9%, n = 6 independent experiments) antigens with respect to untreated or control cells treated with His-Pept (Figure8A). It is particularly remarkable, however, that TRAIL did not induce any significant cytotoxicity on primary normal myeloid cells, as evaluated in terms of apoptosis (from 15 ± 4 to 20 ± 6; n = 6 independent experiments) and viable cell counts (from 126 × 104 to 132 × 104; n = 6 independent experiments). At light microscopy examination, untreated or His-Pept–treated control cultures were characterized by a heterogenous population of cells comprising monoblasts, granulocyte precursors, monocytes, and rare macrophages (Figure 8B). The frequency of monocytes and macrophages in control cultures varied from 19% to 40% in the different experiments performed. In TRAIL-treated cultures, the cell population was still heterogeneous, but cells with morphologic features of mature monocytes and macrophages were more easily detectable and their frequency ranged from 35% to 58%.
Effect of TRAIL on committed myelocytic–monocytic precursors.
At days 4 to 8, serum-free liquid cultures of CB CD34+hemopoietic progenitors supplemented with SCF + GM-CSF were left untreated or were incubated with TRAIL for 3 additional days. (A) Phenotypical characterization of untreated and TRAIL-treated cultures by staining with anti-CD14 and anti-CD11b mAbs. Shadowed histograms represent cells stained with mAbs specific for the indicated antigens, whereas unshadowed histograms represent control cells, stained with control mAbs. Percentages of positive cells are indicated in each panel. Data shown are from a single experiment representative of 6 independent experiments with similar results. (B) Morphologic features of untreated and TRAIL-treated cultures revealed by staining with May-Grünwald-Giemsa solution. Different cell types are indicated. Mo indicates monocyte; Ma, macrophage; Gp, granulocyte precursor; Mn, monoblast. Representative fields of 6 separate experiments are shown. Original magnification, × 400.
Effect of TRAIL on committed myelocytic–monocytic precursors.
At days 4 to 8, serum-free liquid cultures of CB CD34+hemopoietic progenitors supplemented with SCF + GM-CSF were left untreated or were incubated with TRAIL for 3 additional days. (A) Phenotypical characterization of untreated and TRAIL-treated cultures by staining with anti-CD14 and anti-CD11b mAbs. Shadowed histograms represent cells stained with mAbs specific for the indicated antigens, whereas unshadowed histograms represent control cells, stained with control mAbs. Percentages of positive cells are indicated in each panel. Data shown are from a single experiment representative of 6 independent experiments with similar results. (B) Morphologic features of untreated and TRAIL-treated cultures revealed by staining with May-Grünwald-Giemsa solution. Different cell types are indicated. Mo indicates monocyte; Ma, macrophage; Gp, granulocyte precursor; Mn, monoblast. Representative fields of 6 separate experiments are shown. Original magnification, × 400.
Discussion
We have here shown for the first time that, besides inducing a marked cytotoxicity on HL-60, TRAIL promotes maturation of the surviving cells along the monocytic lineage, as shown by combining phenotypic, morphologic, and functional analyses. The possibility that monocytic maturation was the consequence of stress factors released by apoptotic HL-60 cells cannot be completely excluded, but it is unlikely on the basis of experiments performed by using the culture supernatant of apoptotic HL-60 cells that was unable to induce monocytic maturation when added to viable cells in the presence of TRAIL-R1-Fc. Moreover, the induction of apoptosis by LY294002, a pharmacologic compound interfering with the PI 3-kinase/Akt survival pathway, did not induce any monocytic maturation in HL-60 cells. It is particularly remarkable that similar maturational effects were observed in TRAIL-treated primary normal myeloid cells in the absence of cytotoxicity. Therefore, the ability of TRAIL to promote maturation along the monocytic pathway is not confined to the HL-60 experimental cell model, but it involves primary normal myeloid cells. Moreover, the fact that the maturational activity of TRAIL in primary myeloid cells took place in the absence of cytotoxicity strongly suggests that a major physiological role of the TRAIL/TRAIL receptor system in hemopoiesis is to promote normal monocytic development. It should be emphasized that this represents the first demonstration of a positive regulative effect of TRAIL on primary normal cells, whereas previous studies of our and other groups have demonstrated the inhibitory effects of TRAIL on primary normal immature erythroblasts19,20 in the absence of cytotoxicity on normal myeloid cells.11
The ability of TRAIL to promote monocytic differentiation of the HL-60 leukemic cell line, besides inducing cytotoxicity, envisions an additional role of TRAIL as an antineoplastic agent, at least for certain types of acute myeloid leukemia. In fact, inducing death by terminal differentiation represents an alternative approach to cytodestruction of cancer cells by conventional antineoplastic agents with important biologic implications. In fact, it indicates that the malignant state is not an irreversible one and suggests that certain cancers may eventually be treated with agents that initiate terminal maturation, presumably with less morbidity than that produced by cytodestructive agents.38 In this respect, retinoid acids are well-known inducers of granulocytic differentiation of primary acute promyelocytic leukemia (APL) blasts and leukemic cell lines, apparently through the transcriptional regulation of genes critical to this process.39 In fact, retinoids and vitamin D3 interact with nuclear receptors, members of the steroid–thyroid hormone receptor superfamily of transcription factors.40 These are ligand-inducible transregulators that modulate the transcription of genes, which play a critical role in the control of cell growth and differentiation by interacting with retinoic acid or vitamin D3cis-acting DNA responsive elements.39,40However, though some retinoids are used in the treatment of APL, the M3-type of AML, agonists able to induce monocytic differentiation in vitro, such as vitamin D3, have not demonstrated efficacy in clinical trials performed in leukemic patients with different types of AML, mainly because of secondary hypercalcemia that limited the dose of this agent that could be administered.41 Interestingly, it has been proposed that one of the mechanisms of action of retinoic acid in APL is the induction of TRAIL expression in cells differentiating along the granulocytic lineage.42 It has been shown that HL-60 cells become progressively more resistant to TRAIL cytotoxicity as all-trans retinoic acid–induced granulocytic differentiation proceeds.43
Our present study suggests that a therapeutic regimen including TRAIL would optimize the anti-leukemic activity against neoplastic cells by a 2-fold mechanism: cytotoxicity and induction of differentiation along the monocytic lineage. In this respect, TRAIL has garnered the most interest therapeutically, as several studies have demonstrated in vivo tumoricidal activity in animal models without inducing significant toxicity in mice44,45 or nonhuman primates.46Moreover, recombinant TRAIL synergizes with conventional chemotherapy and radiotherapy in inducing cytotoxicity in acute leukemias,37 47-49 which lack functional p53 wild-type genes or overexpress Bcl-2 or MDR genes.
We also started to explore the mechanisms underlying the maturational activity of TRAIL by demonstrating that the activation of effector caspases plays an essential role in this process. These data are in agreement with previous studies showing that the caspase activation in intact cells does not necessarily lead to cell death and that argue for a checkpoint in the apoptotic pathway downstream of caspases. In particular, it has been demonstrated that the terminal differentiation of erythroblasts requires a transient activation of caspases,21 thus indicating that the caspase family of proteases mediates a broader range of biologic activities than originally thought, including the activation and proliferation of quiescent T lymphocytes.50,51 Interestingly, 2 distinct caspase-dependent pathways have been described in response to CD95 ligand,36,52 the member of the TNF superfamily showing the closest amino acid structure to TRAIL. The type 1 pathway triggers the activation of large amounts of caspase 8 followed by the rapid cleavage of caspase-3 before the loss of mitochondria transmembrane potential (Δψm). In the type 2 pathway, low concentrations of caspase 8, insufficient to allow an effective downstream activation of caspase 3, induce the loss of Δψm. This, in turn, induces the release of cytochrome C and procaspase 9 from mitochondria, its activation in the cytosol, and the activation of caspase 3.36 52
We could demonstrate that in HL-60 cells, z-IETD-fmk, a selective pharmacologic inhibitor of caspase 8, almost completely reproduced the ability of z-VAD-fmk, a broad inhibitor of effector caspases, to block TRAIL-mediated cytotoxicity and maturational effects. On the other hand, z-LEHD-fmk, a selective pharmacologic inhibitor of caspase 9, was unable to interfere with TRAIL biologic activities. These findings clearly indicate that the type 2 intrinsic pathway, which has been involved mainly in the induction of apoptosis,36 does not play a role in the maturational effects of TRAIL. Theoretically, because TRAIL preferentially triggers the type 1 pathway in leukemic HL-60 cells, TRAIL with or without chemotherapeutic drugs might substantially enhance the antileukemic activity of conventional chemotherapy in hematologic malignancies.36 52
The experiments performed with agonistic antibodies have indicated that, although TRAIL-R1 and -R2 were expressed on the surfaces of HL-60 cells, only TRAIL-R1 induced maturation along the monocytic lineage. We have not used these agonistic antibodies in primary normal myeloid cultures because of the limited number of cells. However, we have observed that TRAIL-mediated maturation took place only in 4- to 8-day cultures, when primary myeloid cells expressed surface TRAIL-R1, -R2, and -R3. On the other hand, no changes in the surface expression of CD14 and CD15 were observed at later culture times (days 18-22), when TRAIL-R3 was the only TRAIL-R expressed, as also shown by other authors (Zhang et al11 and data not shown). Thus, TRAIL-R1 likely plays an important role in mediating monocytic maturation, possibly by activating effector caspases in primary cells. Moreover, the coexistence of TRAIL-R3 with -R1 and -R2 on the surfaces of primary normal myeloid cells likely protects these cells from apoptosis.
In conclusion, the findings derived from this study might have important implications for understanding the biology of the TRAIL/TRAIL receptor system in normal and leukemic hematopoiesis. In fact, our data disclose a novel physiological role for TRAIL as a positive regulator of monocytic maturation. Moreover, the dichotomous effect of TRAIL on malignant cells (early induction of apoptosis and maturation of the surviving cells) might have therapeutic implications for the treatment of acute myeloid leukemia and possibly of other hematologic malignancies.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-01-0047.
Supported by Consiglio Nazionale delle Ricerche (CNR) and Associazione Italiana per le Ricerca sul Cancro (AIRC) funds. A.G. is supported by a Fondazione Italiana per le Ricerca sul Cancro (FIRC) fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paola Secchiero, Department of Morphology and Embryology, Human Anatomy Section, University of Ferrara, Via Fossato di Mortara 66, 44100 Ferrara, Italy; e-mail:secchier@mail.umbi.umd.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal