In children with acute lymphoblastic leukemia (ALL), response to treatment is assessed by bone marrow aspiration. We investigated whether minimal residual disease (MRD) can be effectively monitored in peripheral blood. We used flow cytometric techniques capable of detecting 1 leukemic cell among 10 000 or more normal cells to compare MRD measurements in 718 pairs of bone marrow and peripheral blood samples collected from 226 children during treatment for newly diagnosed ALL. MRD was detected in marrow and blood in 72 pairs and in marrow but not in blood in 67 pairs; it was undetectable in the remaining 579 pairs. Remarkably, findings in marrow and blood were completely concordant in the 150 paired samples from patients with T-lineage ALL: for each of the 35 positive marrow samples, the corresponding blood sample was positive. In B-lineage ALL, however, only 37 of 104 positive marrow samples had a corresponding positive blood sample. Notably, peripheral blood MRD in these patients was associated with a very high risk for disease recurrence. The 4-year cumulative incidence of relapse in patients with B-lineage ALL was 80.0% ± 24.9% for those who had peripheral blood MRD at the end of remission induction therapy but only 13.3% ± 9.1% for those with MRD confined to the marrow (P = .007). These results indicate that peripheral blood may be used to monitor MRD in patients with T-lineage ALL and that peripheral blood MRD may provide strong prognostic information in patients with B-lineage ALL.
Introduction
Response to therapy for acute lymphoblastic leukemia (ALL) is assessed by identifying residual cells that express morphologic, immunophenotypic, or genotypic characteristics of leukemia.1 Residual disease investigations at the completion of remission induction therapy are universally performed on bone marrow samples. However, the frequency with which residual leukemia can be monitored is limited, especially in children, by the discomfort and practical difficulties posed by bone marrow aspiration.
After sensitive methods for minimal residual disease (MRD) detection became available,2-4 a few investigators recognized the potential advantage of performing MRD studies on peripheral blood instead of bone marrow and assessed their value.5-8 It was found that the detection of MRD in peripheral blood paralleled that in bone marrow in most patients, but levels of MRD in peripheral blood were, at most, one tenth of those measured in paired bone marrow samples. These results suggested the potential usefulness of MRD studies in peripheral blood, but their interpretation was limited by the small number of patients in each series and by the lack of correlative comparisons with presenting features and outcomes. Moreover, the reported studies included only patients with B-lineage ALL.
Monitoring of MRD in bone marrow samples is known to provide strong prognostic information about childhood ALL,9-15 and such assays are increasingly used to select the intensity of treatment.16 We hypothesized that MRD studies in peripheral blood could also be helpful. ALL of T-cell origin may well derive from progenitor cells that naturally reside in the thymus rather than in the bone marrow.17,18 If leukemic T-cell lymphoblasts migrate to the bone marrow through the circulating blood, studies of peripheral blood might be as informative as studies of bone marrow in that subset of patients. In patients with B-lineage ALL (which originates from bone marrow progenitor cells),18 19the presence of circulating lymphoblasts at the time of clinical remission might indicate a propensity of the malignant cells to exit the bone marrow prematurely. This feature may be associated with invasion of extramedullary sites, residence in pharmacologic sanctuaries, and unfavorable outcomes. Here, we present results suggesting that monitoring MRD in the peripheral blood of children with ALL may be clinically useful.
Patients, materials, and methods
Patients
We studied 718 pairs of bone marrow and peripheral blood samples (total, 1436 samples) obtained from 226 children with newly diagnosed ALL (173 with B-lineage and 53 with T-lineage ALL) enrolled in the St Jude Children's Research Hospital Total Therapy studies between May 1997 and February 2002. The only criterion for inclusion in this study was that leukemic cells express an immunophenotype suitable for flow cytometric studies of MRD at the time of diagnosis. Thirty-four additional patients were potentially eligible for the study but were not analyzed because their leukemic cells did not have a suitable immunophenotype. Thus, MRD studies by flow cytometry could be performed in 226 of 260 eligible patients (86.9%); at least 90% of patients are amenable to these studies with currently available markers.20 21 Paired samples were obtained at days 19 (n = 106) and 26 (n = 13) of remission induction therapy, at the end of remission induction therapy (day 46; n = 222), and during weeks 14 (n = 135), 32 (n = 122), 56 (n = 81), and 120 (n = 39) of continuation therapy. These studies were approved by the St Jude Institutional Review Board, and informed consent was obtained from the parent(s) or guardian(s) of each child.
Treatment protocol
Patients initially received methotrexate followed by a 6-week remission induction regimen of prednisone, vincristine, daunorubicin, asparaginase, and etoposide plus cytarabine.22 After entering complete clinical remission, each patient received consolidation therapy with methotrexate and mercaptopurine followed by risk-directed continuation therapy. Continuation chemotherapy consisted of multiple drug pairs administered in weekly rotation for patients with high-risk leukemia and daily mercaptopurine, weekly methotrexate with prednisone, and a vincristine pulse every 4 weeks for patients at lower risk. Reinduction therapy (similar to induction therapy) was administered from week 16 to week 21 after remission induction. All patients received intrathecal therapy with methotrexate, hydrocortisone, and cytarabine for 1 year. Those treated in an earlier protocol (Total Therapy Study XIII) also received cranial irradiation after 1 year of continuation therapy if they were at very high risk for relapse (18 Gy) or had central nervous system status 3 at diagnosis (24 Gy).23
Flow cytometric assessment of MRD
Aspirated bone marrow and peripheral blood were collected in preservative-free heparin. Leukemia-associated immunophenotypes (combinations of cell markers found on leukemic cells but not on normal bone marrow or peripheral blood cells) were detected by multiparameter flow cytometry with various combinations of monoclonal antibodies and heterologous antisera conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein, and allophycocyanin.15,21,24 Matched, nonreactive, fluorochrome-conjugated antibodies served as controls. The staining procedure has been described.24,25 For each patient, one or more marker combinations that allowed the identification of 1 leukemic cell in 104 or more normal nucleated bone marrow or peripheral blood cells—as determined by testing samples from healthy donors, patients undergoing treatment, and artificial mixtures of leukemic and normal cells24,26—were selected at the time of diagnosis and applied during treatment. In the early part of the study, we used 3-color analysis with a single-laser FACScan flow cytometer; after August 1998, all samples were assayed by 4-color analysis and processed with a dual laser FACSCalibur flow cytometer (both cytometers were from Becton Dickinson, San Jose, CA). The flow cytometry protocol used for MRD detection has been described in detail previously.24 25 In each test of each sample, we acquired data from more than 105 mononuclear cells. Detectable MRD was defined as 0.01% or more cells expressing a leukemia-associated immunophenotype among mononuclear cells in the sample.
Statistical analysis
Exact χ2 analysis and Fisher exact test were used to compare differences in the distribution of clinicobiologic presenting features at the end of remission induction therapy for B-lineage ALL according to the presence of MRD in peripheral blood. The cumulative incidence of relapse of ALL in patients treated on earlier protocols with adequate follow-up was calculated by the Gray method, adjusting for other competing risks (ie, second malignancy and death during remission). The cut-off date for follow-up observations was February 2002. Patients who underwent hematopoietic stem cell transplantation were censored at the time of relapse, a competing event, or last follow-up date.
Results
In 139 (19.4%) of the 718 bone marrow samples studied, 0.01% or more of the mononuclear cells expressed the leukemia-associated immunophenotype determined at diagnosis. Levels of residual disease ranged from 0.01% to 53.21% (median, 0.20%). Of the corresponding 139 peripheral blood samples, 72 (51.8%) contained 0.01% or more cells with the leukemic phenotype, whereas 67 (48.2%) contained no leukemic cells detectable by flow cytometry (less than 0.01%). In the peripheral blood samples that were positive for MRD, findings ranged from 0.01% to 50.04% (median, 0.05%). The remaining 579 bone marrow samples were MRD-negative; all of their paired peripheral blood samples were MRD-negative.
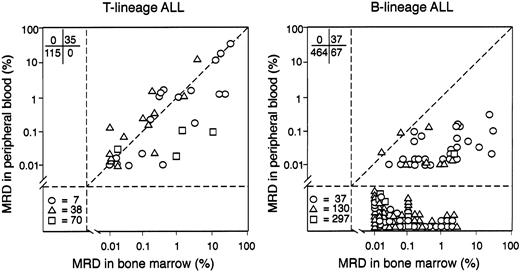
The distribution of residual disease differed markedly between patients with T-lineage ALL and patients with B-lineage ALL. In patients with T-lineage ALL, detectable MRD in bone marrow was consistently accompanied by detectable MRD in peripheral blood, at all time points. Of the 150 pairs of samples from patients with T-lineage ALL, 35 (23.3%) showed detectable MRD in bone marrow and blood, and the paired samples generally had similar proportions of leukemic cells (Figure1). The Pearson correlation coefficient was 0.8183, and, by regression analysis, the slope of the regression line was not significantly different from that of the line of unity (P = .703). In only 5 of the 35 (14.3%) sample pairs was the level of MRD in the bone marrow sample more than 10 times that in the blood sample, and, in one pair, MRD in the blood sample was more than 10 times that in the marrow sample. Therefore, in T-lineage ALL, the proportion of MRD in peripheral blood generally reflected that in the bone marrow.
Distribution of MRD in bone marrow and peripheral blood of patients with T-lineage ALL and B-lineage ALL.
Values represent the percentage of residual disease determined by flow cytometry in paired bone marrow and peripheral blood samples. Symbols indicate samples collected on days 19 and 26 of remission induction therapy (○), at the end of remission induction (day 46; ▵), and during weeks 14, 32, 56, or 120 of continuation chemotherapy (■). In the lower left quadrant, numbers of MRD-negative paired samples collected at the various treatment intervals are shown.
Distribution of MRD in bone marrow and peripheral blood of patients with T-lineage ALL and B-lineage ALL.
Values represent the percentage of residual disease determined by flow cytometry in paired bone marrow and peripheral blood samples. Symbols indicate samples collected on days 19 and 26 of remission induction therapy (○), at the end of remission induction (day 46; ▵), and during weeks 14, 32, 56, or 120 of continuation chemotherapy (■). In the lower left quadrant, numbers of MRD-negative paired samples collected at the various treatment intervals are shown.
In patients with B-lineage ALL, MRD was more prevalent in bone marrow than in peripheral blood. Of the 568 pairs of B-lineage ALL samples, 104 (18.3%) had detectable MRD in the bone marrow, but only 37 of these had detectable disease in the corresponding peripheral blood sample, a result significantly different from that obtained in T-lineage ALL, where all 35 bone marrow samples with detectable MRD had detectable disease in the corresponding blood sample (P < .001, Fisher exact test). Moreover, the level of MRD in blood was generally lower than that in the paired bone marrow sample (Figure 1). The Pearson correlation coefficient was 0.4162, and, by regression analysis, the slope of the regression line was significantly different from that of the line of unity (P = .012). In 24 of the 37 sample pairs in which the peripheral blood was positive (64.9%), the bone marrow sample had a level of MRD more than 10 times as high; the reverse did not occur. Of the 47 MRD-positive marrow samples collected during clinical remission, 9 had a paired blood sample that was MRD-positive. The presence of MRD in the blood was generally associated with higher levels of MRD in marrow. The 9 patients who had peripheral blood MRD had a median of 0.7% MRD in the marrow (range, 0.02%-3.3%), whereas the 38 patients who had MRD only in the marrow had a median of 0.05% MRD (0.01%-3.8%). However, levels of MRD in the marrow in the 2 groups largely overlapped, and the presence of MRD in blood was not limited to patients with high MRD in the marrow. This finding suggested that MRD in the peripheral blood is dependent on specific biologic features of the leukemic cells rather than on physical disruption of the blood–bone marrow barrier.
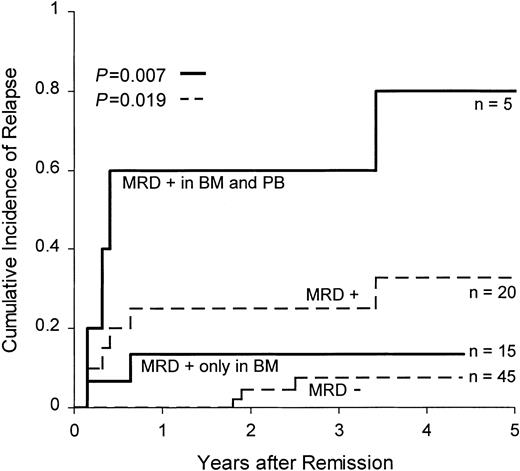
We assessed the relationship between the presenting cellular and biologic features of patients with B-lineage ALL and the presence of MRD in peripheral blood at the end of remission induction therapy (day 46). Of the 171 patients studied at this time point (all in clinical remission), 41 had detectable MRD in the bone marrow and 8 of the 41 also had detectable MRD in the blood. We detected no obvious association among the presence of MRD in the peripheral blood and leukemic cell ploidy, rearrangement of the TEL orMLL genes, presence of the BCR-ABL fusion gene, or age, sex, race, or leukocyte count at diagnosis, though the low number of patients studied limited the power of the statistical analysis. We also found no apparent relationship between MRD in peripheral blood and previous treatment with G-CSF. We then investigated whether the presence of MRD in the peripheral blood could identify patients at higher risk for relapse. We restricted the analysis to 65 patients with B-lineage ALL who were treated on earlier protocols, had been followed for at least 2.5 years, and had not undergone risk-adapted therapy based on MRD findings. All 65 patients were in clinical remission at the end of remission induction therapy (day 46), but 20 had detectable MRD (0.01% or more) in the bone marrow at that time. Five of these 20 patients also had detectable MRD in the peripheral blood. We found no apparent differences in the morphologic, immunophenotypic, or karyotypic features of the malignant cells or in clinical presenting features between patients who had MRD only in the bone marrow and those who had MRD in bone marrow and peripheral blood. The 4-year cumulative incidence of relapse was 7.4% ± 4.2% for the 45 patients with no detectable MRD and 32.9% ± 12.0% for the 20 with detectable MRD (P = .019). Remarkably, 4 of the 5 patients with detectable MRD in the peripheral blood had relapses compared with only 2 of the 15 patients with MRD only in the bone marrow (4-year estimated cumulative incidence of relapse, 80.0% ± 24.9% vs 13.3% ± 9.1%; P = .007) (Figure2).
Cumulative incidence of relapse in patients with B-lineage ALL according to MRD distribution at the end of remission induction therapy.
Dashed lines indicate cumulative incidence of relapse of patients with 0.01% or more leukemic cells in bone marrow (MRD+) and of patients with less than 0.01% leukemic cells (MRD−). Solid lines indicate the cumulative incidence of relapse of MRD+ patients with MRD confined to the bone marrow (BM) or detectable MRD in the peripheral blood (PB).
Cumulative incidence of relapse in patients with B-lineage ALL according to MRD distribution at the end of remission induction therapy.
Dashed lines indicate cumulative incidence of relapse of patients with 0.01% or more leukemic cells in bone marrow (MRD+) and of patients with less than 0.01% leukemic cells (MRD−). Solid lines indicate the cumulative incidence of relapse of MRD+ patients with MRD confined to the bone marrow (BM) or detectable MRD in the peripheral blood (PB).
Discussion
We found that the distribution of MRD differs radically in patients with T-lineage and B-lineage ALL. In patients with T-lineage ALL, cells expressing a leukemia-associated immunophenotype were consistently present in the peripheral blood when they were detected in the bone marrow. Further, their proportions in peripheral blood were remarkably similar to those obtained in the bone marrow, irrespective of the time point at which they were measured. This finding suggests that peripheral blood could be used for MRD studies in patients with T-lineage ALL and warrants further study in patients enrolled in different treatment protocols, monitored at different intervals, or both. Use of peripheral blood would allow closer monitoring of leukemia while sparing patients the discomfort of bone marrow aspirations.
In sharp contrast to findings in patients with T-lineage ALL, those with B-lineage ALL often had no detectable cells with leukemia-associated immunophenotypes in peripheral blood, despite their presence in bone marrow. This result is consistent with those of previous studies of MRD in peripheral blood, which have focused on patients with B-lineage ALL. Brisco et al5 used polymerase chain reaction (PCR) amplification of immunoglobulin genes to quantify MRD in 35 pairs of bone marrow and blood samples from 15 children receiving remission induction therapy. They found that the levels of MRD in blood were lower than those in bone marrow by a factor of 10. Van Rhee et al6 used reverse transcription–PCR (RT-PCR) amplification to study p190 BCR-ABL transcripts in 29 pairs of samples from 18 patients receiving treatment for Philadelphia chromosome–positive B-lineage ALL. As a whole, the numbers ofBCR-ABL transcripts detected in peripheral blood were not significantly different from those detected in bone marrow. However, MRD was detectable in the bone marrow but not in the blood in 4 sample pairs; in 3 additional pairs, MRD was detected in both samples but was more than 10 times as high in marrow than in blood. Using the same approach, Martin et al7 studied 9 pairs of samples from 6 patients and found that MRD levels in marrow exceeded those in blood by a factor of 10 or more in every case. These findings, taken together with our results, clearly indicate that in B-lineage ALL, levels of MRD in bone marrow are usually higher than those in peripheral blood. However, in our series, the presence of MRD in peripheral blood denoted more aggressive leukemia with an extremely high risk for recurrence—4 of our 5 patients with B-lineage ALL who had MRD in the peripheral blood have experienced relapse, whereas only 2 of the 15 patients with MRD confined to the bone marrow have experienced relapse. These results indicate that peripheral blood MRD assays in patients with B-lineage ALL could have clinical usefulness. Larger trials are necessary to conclusively establish this and to determine the relation between peripheral blood MRD and other clinical and biologic features of B-lineage ALL.
We thank Peixin Liu and Mo Mehrpooya for technical assistance, Yinmei Zhou for assistance with the statistical analysis, Geoffrey Neale for helpful discussions, and Sharon Naron for editorial suggestions.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-04-1130.
Supported by grants CA60419, CA21765, and CA20180 from the National Cancer Institute, by the Rizzo Memorial Grant from the Leukemia Research Foundation, and by the American Lebanese Syrian Associated Charities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dario Campana, Department of Hematology-Oncology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis TN 38105-2794; e-mail: dario.campana@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal