Major changes have occurred in the transplantation of hematopoietic stem cells (HSCs) during the last decade. This report reveals the changes, reflects current status, and provides medium-term projections of HSC transplantation (HSCT) development in Europe. Data on 132 963 patients, 44 165 with allogeneic HSC transplant (33%) and 88 798 with an autologous HSC transplant (67%), collected prospectively from 619 centers by the European Group for Blood and Marrow Transplantation (EBMT) in 35 European countries between 1990 (4234 HSCTs) and 2000 (19 136 HSCTs) illustrate utilization of HSCT. HSCT increased in all European countries and for all indications. There were major differences depending on disease indication and donor type. Transplantation rates (numbers of HSCTs per 10 million inhabitants) varied from less than 1 for some rare indications to 37.7 ± 4.1 for acute myeloid leukemia in allogeneic HSCT or 95.5 ± 13.5 for non-Hodgkin lymphoma in autologous HSCT. There were indications with a steady, continuing increase and others with initial increase but subsequent decrease. Projections on medium-term development for each disease based on a weighted sensitivity analysis predict an ongoing increase in allogeneic HSCT except for chronic myeloid leukemia. In autologous HSCT they predict an increase for lymphoproliferative disorders, acute myeloid leukemia, myelodysplastic syndromes, and some solid tumors but a decrease for most solid tumors, acute lymphoid leukemia, and chronic myeloid leukemia. Transplantation rates can be predicted with reasonable sensitivity for most disease indications. Despite marked changes in the rapidly developing field of HSCT, this information on current use, trends, and midterm predictions forms a rational basis for patient counseling and health care planning.
Introduction
Transplantation of hematopoietic stem cells (HSCs) is established therapy for many congenital or acquired severe disorders of the hematopoietic system as well as for chemosensitive or radiosensitive malignancies.1-3Hematopoietic stem cells from bone marrow, peripheral blood, or cord blood are used for autologous or allogeneic HSC transplantation (HSCT).4,5 Donors for allogeneic transplants include HLA-identical siblings, other family members, or unrelated volunteers from the vast worldwide donor pools.6
Major changes have occurred in HSCT over the last decade. Stem cell source has changed from bone marrow to peripheral blood for almost all disease indications in autologous HSCT. In allogeneic HSCT, more than 50% of the HLA-identical sibling transplants, most haploidentical and twin transplants, and more than one third of the unrelated donor transplants were peripheral blood derived in the year 2000.7,8 Expansion of unrelated donor pools to a current state of more than 7.5 million registered donors worldwide, the establishment of cord blood banks in North America and Europe, and successful introduction of haploidentical HSCT have made allogeneic HSCT available to patients without an HLA-identical sibling donor.4,6,9 New technologies, such as reduced-intensity conditioning, limit early toxicity and allow allogeneic HSCT for patients above the previous age limit and for those with concomitant organ toxicity.10,11 At the same time, the role of HSCT has been put to question for certain disease indications. Excessive hopes first put forward HSCT for breast cancer, but frustration about the results then halted its use.12 Furthermore, novel therapeutic strategies have emerged, such as the specific bcr/abl tyrosine kinase inhibitor imatinib mesylate for treatment of chronic myeloid leukemia.13,14 It made an immediate impact on HSCT use for this disease. All these changes occurred in a field of high technology and high-cost medicine. Health care providers, hospital administrators, and reimbursement agencies alike are challenged in this constantly changing field to have the resources available when needed. Correct assessment of current status, trends, and predictions for the near future are essential for planning health care strategies.7
Ten years ago the European Group for Blood and Marrow Transplantation (EBMT) initiated an annual activity survey as a new tool. All HSCTs are registered by disease indication, donor type, and stem cell source on an annual basis.15 Introduced as an instrument for quality control, the activity survey gained rapid acceptance and presently more than 95% of all HSCTs in Europe are covered by this survey. It allows a precise description of current status each year, illustrates differences and similarities between European countries, allows calculations of transplantation rates and team densities between the countries, reveals quantification of consensus for indications, and permits detailed observations of changes in technologies.16 17 As presented in this report, observations over a decade now give a precise assessment of current status for the individual disease indications and permit a forecast with substantial sensitivity for the immediate future.
Patients and methods
Data collection and validation
Data collection is based on the EBMT activity surveys introduced in 1990.15 Since then, all European centers, EBMT members as well as nonmembers, are requested annually to report on a survey sheet the numbers of new patients by indication, stem cell source, and donor type. Transplantations are defined as the infusion of HSCs following a conditioning regimen with the intention of replacing the existing hematopoiesis by injected stem cells. The EBMT survey was adopted by the General Assembly as a mandatory self-reporting system and forms an integral part of a prospective quality assurance program (http://www.EBMT.org). The latter includes revalidation of a computer print-out of entered data by reporting teams, cross-checking with national transplant registries, and on-site visits.
Participating teams
Six hundred nineteen teams in 36 European countries were contacted over time. Five hundred eighty (94% return) replied in 2000. This includes all 470 EBMT member teams. No major transplant team in Europe is missing from this list. In 1990, the report began with 142 teams. The contacted teams are listed in the in alphabetical order according to country, city, and EBMT center code. In 2000, according to unofficial information received, no blood or marrow transplantations were performed in these European countries: Albania, Andorra, Armenia, Azerbaijan, Bosnia-Herzegovina, Georgia, Iceland, Latvia, Liechtenstein, Malta, Moldavia, Monaco, San Marino, and the Vatican.
Transplantation rates
Transplantation rates were defined as number of HSCTs per 10 million inhabitants. They were computed as previously defined for each year, disease indication, donor type, and country. For each disease indication transplantation rates were assessed for all HSCTs and separately for autologous, allogeneic, and unrelated HSCTs. Population data have been obtained from the US census office (http://www.census.gov) since 1996 and from the annual Fischer Weltalmanach for the years prior to 1996.
Statistical analysis, prediction of transplantation rates, and sensitivity analysis
Mean, median, and SDs of numerical variables were calculated on an Excel spreadsheet. Groups were compared with χ2tests.
To assess changes in transplantation rates over time for each disease indication and to recognize trends, the following approach was used. All countries with at least 100 transplantations during all the years since 1990 were selected; these include Belgium, France, Germany, The Netherlands, Switzerland, Italy, Spain, Sweden, and the United Kingdom. For each of these countries transplantation rates were calculated for all indications as listed in Table 1 for autologous HSCT and Table 2 for allogeneic HSCT. For calculations of transplantation rates a weighted analysis was used considering the size of each of the 9 individual countries. For each disease indication weighted means and SDs were calculated and in a regression analysis the best fitting curve was computed.
Indications for allogeneic HSCT in Europe 1990-2000
| . | No. of patients . | |||
|---|---|---|---|---|
| Total 1990-2000 . | % . | Total 2000 . | % . | |
| Leukemias | 33 509 | 75.9 | 4653 | 72.7 |
| Acute myeloid leukemia | 10 990 | 24.9 | 1627 | 25.4 |
| Acute lymphocytic leukemia | 8 871 | 20.1 | 1135 | 17.7 |
| Chronic myeloid leukemia | 10 559 | 23.9 | 1315 | 20.5 |
| Myelodysplastic syndromes | 2 680 | 6.1 | 454 | 7.1 |
| Chronic lymphocytic leukemia | 409 | 0.9 | 122 | 1.9 |
| Lymphoproliferative disorders | 3 930 | 8.9 | 820 | 12.8 |
| Multiple myeloma | 1 470 | 3.3 | 295 | 4.6 |
| Hodgkin disease | 320 | 0.7 | 68 | 1.1 |
| Non-Hodgkin lymphoma | 2 140 | 4.8 | 457 | 7.1 |
| Solid tumors | 272 | 0.6 | 104 | 1.6 |
| Neuroblastoma | 30 | 0.1 | 1 | 0.0 |
| Glioma | 2 | 0.0 | 1 | 0.0 |
| Soft tissue sarcoma | 13 | 0.0 | 5 | 0.1 |
| Germinal tumors | 10 | 0.0 | 3 | 0.0 |
| Breast cancer | 57 | 0.1 | 16 | 0.2 |
| Ewing sarcoma | 28 | 0.1 | 4 | 0.1 |
| Lung cancer | 1 | 0.0 | 0 | 0.0 |
| Ovarian cancer | 11 | 0.0 | 7 | 0.1 |
| Other solid tumors | 120 | 0.3 | 67 | 1.0 |
| Nonmalignant disorders | 5 695 | 12.9 | 700 | 10.9 |
| Aplastic anemia + Fanconi | 2 483 | 5.6 | 287 | 4.5 |
| Thalassemia | 1 349 | 3.1 | 147 | 2.3 |
| Combined immune deficiencies | 724 | 1.6 | 103 | 1.6 |
| Inborn errors | 1 112 | 2.5 | 153 | 2.4 |
| Autoimmune diseases | 27 | 0.1 | 10 | 0.2 |
| Others | 759 | 1.7 | 127 | 2.0 |
| Total | 44 165 | 6404 | ||
| . | No. of patients . | |||
|---|---|---|---|---|
| Total 1990-2000 . | % . | Total 2000 . | % . | |
| Leukemias | 33 509 | 75.9 | 4653 | 72.7 |
| Acute myeloid leukemia | 10 990 | 24.9 | 1627 | 25.4 |
| Acute lymphocytic leukemia | 8 871 | 20.1 | 1135 | 17.7 |
| Chronic myeloid leukemia | 10 559 | 23.9 | 1315 | 20.5 |
| Myelodysplastic syndromes | 2 680 | 6.1 | 454 | 7.1 |
| Chronic lymphocytic leukemia | 409 | 0.9 | 122 | 1.9 |
| Lymphoproliferative disorders | 3 930 | 8.9 | 820 | 12.8 |
| Multiple myeloma | 1 470 | 3.3 | 295 | 4.6 |
| Hodgkin disease | 320 | 0.7 | 68 | 1.1 |
| Non-Hodgkin lymphoma | 2 140 | 4.8 | 457 | 7.1 |
| Solid tumors | 272 | 0.6 | 104 | 1.6 |
| Neuroblastoma | 30 | 0.1 | 1 | 0.0 |
| Glioma | 2 | 0.0 | 1 | 0.0 |
| Soft tissue sarcoma | 13 | 0.0 | 5 | 0.1 |
| Germinal tumors | 10 | 0.0 | 3 | 0.0 |
| Breast cancer | 57 | 0.1 | 16 | 0.2 |
| Ewing sarcoma | 28 | 0.1 | 4 | 0.1 |
| Lung cancer | 1 | 0.0 | 0 | 0.0 |
| Ovarian cancer | 11 | 0.0 | 7 | 0.1 |
| Other solid tumors | 120 | 0.3 | 67 | 1.0 |
| Nonmalignant disorders | 5 695 | 12.9 | 700 | 10.9 |
| Aplastic anemia + Fanconi | 2 483 | 5.6 | 287 | 4.5 |
| Thalassemia | 1 349 | 3.1 | 147 | 2.3 |
| Combined immune deficiencies | 724 | 1.6 | 103 | 1.6 |
| Inborn errors | 1 112 | 2.5 | 153 | 2.4 |
| Autoimmune diseases | 27 | 0.1 | 10 | 0.2 |
| Others | 759 | 1.7 | 127 | 2.0 |
| Total | 44 165 | 6404 | ||
Indications for autologous HSCT in Europe 1990-2000
| . | No. of patients . | |||
|---|---|---|---|---|
| Total 1990-2000 . | % . | Total 2000 . | % . | |
| Leukemias | 15 052 | 17.0 | 1 764 | 13.9 |
| Acute myeloid leukemia | 8 129 | 9.2 | 1 047 | 8.2 |
| Acute lymphocytic leukemia | 3 765 | 4.2 | 302 | 2.4 |
| Chronic myeloid leukemia | 1 967 | 2.2 | 148 | 1.2 |
| Myelodysplastic syndromes | 297 | 0.3 | 50 | 0.4 |
| Chronic lymphocytic leukemia | 894 | 1.0 | 217 | 1.7 |
| Lymphoproliferative disorders | 48 917 | 55.1 | 8 498 | 66.7 |
| Multiple myeloma | 16 206 | 18.3 | 3 388 | 26.6 |
| Hodgkin disease | 9 078 | 10.2 | 1 226 | 9.6 |
| Non-Hodgkin lymphoma | 23 633 | 26.6 | 3 884 | 30.5 |
| Solid tumors | 24 016 | 27.0 | 2 286 | 18.0 |
| Neuroblastoma | 1 912 | 2.2 | 262 | 2.1 |
| Glioma | 544 | 0.6 | 73 | 0.6 |
| Soft tissue sarcoma | 1 013 | 1.1 | 144 | 1.1 |
| Germinal tumors | 2 658 | 3.0 | 304 | 2.4 |
| Breast cancer | 12 598 | 14.2 | 781 | 6.1 |
| Ewing sarcoma | 1 401 | 1.6 | 244 | 1.9 |
| Lung cancer | 318 | 0.4 | 62 | 0.5 |
| Ovarian cancer | 628 | 0.7 | 100 | 0.8 |
| Other solid tumors | 2 944 | 3.3 | 316 | 2.5 |
| Nonmalignant disorders | 321 | 0.4 | 100 | 0.8 |
| Aplastic anemia + Fanconi | 7 | 0.0 | 1 | 0.0 |
| Thalassemia | 0 | 0.0 | 0 | 0.0 |
| Combined immune deficiencies | 10 | 0.0 | 2 | 0.0 |
| Inborn errors | 4 | 0.0 | 0 | 0.0 |
| Autoimmune diseases | 300 | 0.3 | 97 | 0.8 |
| Others | 492 | 0.6 | 84 | 0.7 |
| Total | 88 798 | 12 732 | ||
| . | No. of patients . | |||
|---|---|---|---|---|
| Total 1990-2000 . | % . | Total 2000 . | % . | |
| Leukemias | 15 052 | 17.0 | 1 764 | 13.9 |
| Acute myeloid leukemia | 8 129 | 9.2 | 1 047 | 8.2 |
| Acute lymphocytic leukemia | 3 765 | 4.2 | 302 | 2.4 |
| Chronic myeloid leukemia | 1 967 | 2.2 | 148 | 1.2 |
| Myelodysplastic syndromes | 297 | 0.3 | 50 | 0.4 |
| Chronic lymphocytic leukemia | 894 | 1.0 | 217 | 1.7 |
| Lymphoproliferative disorders | 48 917 | 55.1 | 8 498 | 66.7 |
| Multiple myeloma | 16 206 | 18.3 | 3 388 | 26.6 |
| Hodgkin disease | 9 078 | 10.2 | 1 226 | 9.6 |
| Non-Hodgkin lymphoma | 23 633 | 26.6 | 3 884 | 30.5 |
| Solid tumors | 24 016 | 27.0 | 2 286 | 18.0 |
| Neuroblastoma | 1 912 | 2.2 | 262 | 2.1 |
| Glioma | 544 | 0.6 | 73 | 0.6 |
| Soft tissue sarcoma | 1 013 | 1.1 | 144 | 1.1 |
| Germinal tumors | 2 658 | 3.0 | 304 | 2.4 |
| Breast cancer | 12 598 | 14.2 | 781 | 6.1 |
| Ewing sarcoma | 1 401 | 1.6 | 244 | 1.9 |
| Lung cancer | 318 | 0.4 | 62 | 0.5 |
| Ovarian cancer | 628 | 0.7 | 100 | 0.8 |
| Other solid tumors | 2 944 | 3.3 | 316 | 2.5 |
| Nonmalignant disorders | 321 | 0.4 | 100 | 0.8 |
| Aplastic anemia + Fanconi | 7 | 0.0 | 1 | 0.0 |
| Thalassemia | 0 | 0.0 | 0 | 0.0 |
| Combined immune deficiencies | 10 | 0.0 | 2 | 0.0 |
| Inborn errors | 4 | 0.0 | 0 | 0.0 |
| Autoimmune diseases | 300 | 0.3 | 97 | 0.8 |
| Others | 492 | 0.6 | 84 | 0.7 |
| Total | 88 798 | 12 732 | ||
Results
Development of transplantation activity
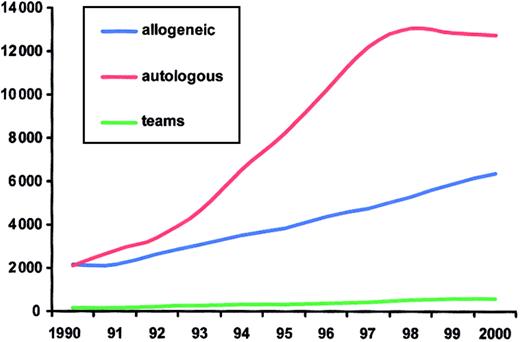
The development of HSCT in Europe during the last decade is illustrated in Figure 1. There has been an increase in HSCT activity for both allogeneic and autologous transplants. In 1991, there were equal numbers for both technologies. Autologous HSCT showed a marked increase after 1993, culminating in 1998 with almost 13 000 patients and a decline thereafter. As a total, there were 19 136 first transplantations, including 6404 (33%) allogeneic and 12 732 (67%) autologous HSC transplantations in 2000.
Evolution of HSCT in Europe from 1990 to 2000.
Annual numbers of allogeneic and autologous HSCT and of participating teams.
Evolution of HSCT in Europe from 1990 to 2000.
Annual numbers of allogeneic and autologous HSCT and of participating teams.
Increase in transplantation activity during the last decade is based both on increase in team numbers and increase in transplantations within participating teams. Teams increased from 143 in 1990 to 619 in 2000. Of those, 579 responded to this survey. Fifty-three percent performed allogeneic and autologous transplantations, 41% restricted their activity to autologous transplantations only, and 2% performed allogeneic transplantations only.
Activity between teams varied widely; 137 (24%) teams performed fewer than 10 transplantations, 128 (22%) teams between 10 and 20 transplantations, 180 (31%) between 20 and 50 transplantations, 107 (18%) between 50 and 100 transplantations, and 27 teams (5%) more than 100 transplantations in 2000.
Indications for HSCT and donor type
Numbers of patients treated with HSCT over the last decade are listed in Tables 1 and 2 according to disease indication and donor type. Table 1 lists the numbers of patients with allogeneic transplants by disease indication as a total for the years 1990-2000 and for the year 2000. Table 2 lists the autologous HSCT. Overall, from 1990 to 2000 there were 132 963 first transplantations in Europe, of which 44 165 (33%) were allogeneic and 88 798 (67%) autologous HSCTs. They are grouped into 4 main disease categories, namely, lymphoproliferative disorders with 52 847 patients (39.7%), leukemias with 48 561 patients (36.5%), solid tumors with 24 288 patients (18.3%), and nonmalignant disorders with 6016 patients (0.5%).
Not all indications increased at the same rate, as given in Tables 1and 2 and as reflected in Figure 2. Some indications appeared only recently, such as allogeneic HSCT for solid tumors or HSCT for autoimmune disorders. Concerning allogeneic HSCT, a marked increase was observed for leukemias and a relatively stable rate for nonmalignant disorders, including aplastic anemia (Figure 2A). For lymphoproliferative disorders, a trend toward more allogeneic HSCT has been observed during the last 2 years. For autologous HSCT, the pattern is different (Figure 2B). Breast cancer showed a marked increase after 1994 with a peak in 1997 and a continuous decline thereafter, whereas lymphoproliferative disorders and multiple myeloma continue to rise. Lymphoproliferative disorders (lymphoma) showed the most pronounced increase. For leukemias and other solid tumors, there are signs of a plateau developing over the last 2 years.
Absolute numbers of HSCT in Europe from 1990 to 2000 according to main indications.
(A) Allogeneic transplantations for leukemias, lymphoproliferative disorders, and nonmalignant disorders. (B) Autologous transplantations for leukemias, lymphomas, multiple myelomas, breast cancer, and other solid tumors.
Absolute numbers of HSCT in Europe from 1990 to 2000 according to main indications.
(A) Allogeneic transplantations for leukemias, lymphoproliferative disorders, and nonmalignant disorders. (B) Autologous transplantations for leukemias, lymphomas, multiple myelomas, breast cancer, and other solid tumors.
There are distinct differences between the disease groups with regard to donor type. Patients with solid tumors were almost exclusively treated with autologous HSCT (98.0%). In contrast, patients with aplastic anemia, hemoglobinopathies, immunodeficiency disorders, or inborn errors, in the group of nonmalignant disorders, almost exclusively underwent allogeneic HSCT (98%-100%). The few patients with congenital disorders and autologous HSCT are those given genetically modified autologous HSC transplants. Patients with lymphoproliferative disorders were treated predominantly with autologous HSCT (92.6%). Similarly, patients with autoimmune disorders were primarily treated with autologous HSCT (92%). Patients with leukemias were mainly treated with allogeneic HSCT (69.0%) even though for some subgroups, such as acute myeloid leukemia, numbers of autologous and allogeneic procedures were equal.
Of the 6404 allogeneic HSCTs in 2000, 3955 (62%) recipients received cells from an HLA-identical sibling donor, 437 (7%) from another family member, 58 (1%) from a syngeneic twin, and 1954 (31%) from an unrelated volunteer donor. Over the decade, the percentage of twin donors has remained stable; the percentage of unrelated donors has increased from less than 10% to more than 30% in 2000.
Stem cell source
Stem cell source varied over time and was dependent on donor type. In 1990, almost all HSC transplants were bone marrow derived. This has changed within the decade. Of the 19 136 HSC transplants in 2000, only 3555 (19%) were still bone marrow derived; 15 581 (81%) were from peripheral blood stem cells or were combined bone marrow and peripheral blood stem cell transplants. There are differences in stem cell source for autologous and allogeneic HSCT. Of the 12 732 autologous HSCTs, only 566 (4%) used bone marrow and 12 166 (96%) used peripheral blood stem cells. Of the 6404 allogeneic HSCTs, 2989 (47%) were bone marrow derived and 3415 (53%) were peripheral blood stem cell transplants. Peripheral blood was used in 57% of HLA-identical sibling donor transplants, in 81% of transplants from other family members, in 76% of twin donors, and in 39% of unrelated donors.
Changes in transplantation rates over time
Transplantation rates differed between the European countries but increased in all European countries over the decade (Figure 3). These rates differed depending on indication, donor type, and time. Changes in transplantation rates for the 9 selected countries described above are given in Tables 3 and4.
Transplantation rates in participating European countries in 2000.
Shades reflect number of total HSCTs (autologous and allogeneic) per 10 million inhabitants. (A) Transplantation rates in 1990. (B) Transplantation rates in 2000.
Transplantation rates in participating European countries in 2000.
Shades reflect number of total HSCTs (autologous and allogeneic) per 10 million inhabitants. (A) Transplantation rates in 1990. (B) Transplantation rates in 2000.
Changes in allogenic HSCT rates from 1990 to 2000 in 9 selected countries with predictions for 2003
| . | Year of transplantation . | Maximum transplantation rate . | Predictions 2003 . | ||||
|---|---|---|---|---|---|---|---|
| 1990 . | 1995 . | 2000 . | Year . | Transplantation rate . | Transplantation rate . | r2 . | |
| Leukemias | |||||||
| Acute myeloid leukemia | 13.2 ± 2.2 | 22.4 ± 3.2 | 37.7 ± 4.1 | 2000 | — | 49 | 0.997 |
| Acute lymphocytic leukemia | 12.7 ± 2.8 | 21.0 ± 2.5 | 26.9 ± 3.3 | 2000 | — | 32 | 0.975 |
| Chronic myeloid leukemia | 14.4 ± 2.1 | 20.3 ± 3.1 | 28.3 ± 4.6 | 1999 | 30.7 ± 3.7 | (36)3-150 | 0.925 |
| Myelodysplastic syndromes | 2.2 ± 1.9 | 6.3 ± 1.2 | 10.5 ± 1.2 | 2000 | — | 14 | 0.986 |
| Chronic lymphocytic leukemia | 0.4 ± 0.2 | 0.7 ± 0.5 | 2.9 ± 0.8 | 2000 | — | 5 | 0.951 |
| Lymphoproliferative disorders | |||||||
| Multiple myeloma | 1.4 ± 1.0 | 2.7 ± 1.2 | 7.4 ± 1.5 | 2000 | — | 11 | 0.971 |
| Hodgkin disease | 0.6 ± 0.3 | 0.4 ± 0.2 | 1.7 ± 0.5 | 2000 | — | 3 | 0.901 |
| Non-Hodgkin lymphoma | 2.2 ± 0.8 | 4.7 ± 1.5 | 10.7 ± 1.8 | 2000 | — | 16 | 0.985 |
| Nonmalignant disorders | |||||||
| Aplastic anemia + Fanconi | 4.0 ± 1.0 | 4.8 ± 1.3 | 5.7 ± 0.5 | 2000 | — | 5.3 | 0.530 |
| Combined immune deficiencies | 0.8 ± 0.5 | 1.3 ± 0.5 | 2.2 ± 0.6 | 2000 | — | 2.5 | 0.892 |
| Inborn errors | 0.9 ± 0.7 | 2.2 ± 0.6 | 3.4 ± 0.6 | 1999 | 3.8 ± 0.6 | 4.5 | 0.868 |
| . | Year of transplantation . | Maximum transplantation rate . | Predictions 2003 . | ||||
|---|---|---|---|---|---|---|---|
| 1990 . | 1995 . | 2000 . | Year . | Transplantation rate . | Transplantation rate . | r2 . | |
| Leukemias | |||||||
| Acute myeloid leukemia | 13.2 ± 2.2 | 22.4 ± 3.2 | 37.7 ± 4.1 | 2000 | — | 49 | 0.997 |
| Acute lymphocytic leukemia | 12.7 ± 2.8 | 21.0 ± 2.5 | 26.9 ± 3.3 | 2000 | — | 32 | 0.975 |
| Chronic myeloid leukemia | 14.4 ± 2.1 | 20.3 ± 3.1 | 28.3 ± 4.6 | 1999 | 30.7 ± 3.7 | (36)3-150 | 0.925 |
| Myelodysplastic syndromes | 2.2 ± 1.9 | 6.3 ± 1.2 | 10.5 ± 1.2 | 2000 | — | 14 | 0.986 |
| Chronic lymphocytic leukemia | 0.4 ± 0.2 | 0.7 ± 0.5 | 2.9 ± 0.8 | 2000 | — | 5 | 0.951 |
| Lymphoproliferative disorders | |||||||
| Multiple myeloma | 1.4 ± 1.0 | 2.7 ± 1.2 | 7.4 ± 1.5 | 2000 | — | 11 | 0.971 |
| Hodgkin disease | 0.6 ± 0.3 | 0.4 ± 0.2 | 1.7 ± 0.5 | 2000 | — | 3 | 0.901 |
| Non-Hodgkin lymphoma | 2.2 ± 0.8 | 4.7 ± 1.5 | 10.7 ± 1.8 | 2000 | — | 16 | 0.985 |
| Nonmalignant disorders | |||||||
| Aplastic anemia + Fanconi | 4.0 ± 1.0 | 4.8 ± 1.3 | 5.7 ± 0.5 | 2000 | — | 5.3 | 0.530 |
| Combined immune deficiencies | 0.8 ± 0.5 | 1.3 ± 0.5 | 2.2 ± 0.6 | 2000 | — | 2.5 | 0.892 |
| Inborn errors | 0.9 ± 0.7 | 2.2 ± 0.6 | 3.4 ± 0.6 | 1999 | 3.8 ± 0.6 | 4.5 | 0.868 |
Numbers reflect weighted transplantation rates per 10 million inhabitants.
indicates limitations of mathematical model; see “Results.”
Changes in autologous HSCT rates from 1990 to 2000 in 9 selected countries with predictions for 2003
| . | Year of transplantation . | Maximum transplantation rate . | Predictions 2003 . | ||||
|---|---|---|---|---|---|---|---|
| 1990 . | 1995 . | 2000 . | Year . | Transplantation rate . | Transplantation rate . | r2 . | |
| Leukemias | |||||||
| Acute myeloid leukemia | 10.8 ± 2.2 | 19.3 ± 4.8 | 23.7 ± 4.4 | 1998 | 23.9 ± 4.0 | 24 | 0.968 |
| Acute lymphocytic leukemia | 8.1 ± 1.8 | 9.9 ± 2.6 | 6.2 ± 2.1 | 1991 | 10.0 ± 2.8 | 3 | 0.67 |
| Chronic myeloid leukemia | 2.4 ± 1.4 | 5.5 ± 1.5 | 3.6 ± 1.3 | 1998 | 8.1 ± 2.7 | (3.6)4-150 | 0.656 |
| Myelodysplastic syndromes | 0.1 ± 0.1 | 1.1 ± 1.3 | 1.4 ± 0.8 | 2000 | 1.4 ± 0.8 | 1.7 | 0.901 |
| Chronic lymphocytic leukemia | — | 1.3 ± 0.7 | 5.6 ± 1.5 | 2000 | 5.6 ± 1.5 | 11.2 | 0.933 |
| Lymphoproliferative disorders | |||||||
| Multiple myeloma | 3.8 ± 2.4 | 34.3 ± 12.1 | 82 ± 15.2 | 2000 | — | 121.6 | 0.99 |
| Hodgkin disease | 9.3 ± 2.7 | 21.9 ± 4.5 | 24.9 ± 3.2 | 2000 | — | 23.1 | 0.979 |
| Non-Hodgkin lymphoma | 13.7 ± 5.0 | 56.6 ± 8.4 | 95.5 ± 13.6 | 2000 | — | 118.9 | 0.986 |
| Solid tumors | |||||||
| Neuroblastoma | |||||||
| Glioma | 1.7 ± 1.6 | 1.6 ± 1.1 | 1.8 ± 0.9 | 2000 | — | 1.4 | 0.45 |
| Soft tissue sarcoma | 1.2 ± 0.6 | 2.0 ± 0.8 | 3.8 ± 1.1 | 1997 | 4.0 ± 1.3 | 3.98 | 0.882 |
| Germinal tumors | 3.6 ± 1.9 | 7.3 ± 2.5 | 7.3 ± 2.5 | 1997 | 8.9 ± 2.4 | 4.1 | 0.951 |
| Breast cancer | 27.0 ± 1.5 | 33.3 ± 14.4 | 20.0 ± 8.8 | 1997 | 66.3 ± 19.3 | 04-150 | 0.734 |
| Ewing sarcoma | 1.1 ± 0.7 | 3.0 ± 0.8 | 5.6 ± 2.0 | 2000 | — | 6.7 | 0.961 |
| Lung cancer | NA | NA | 1.4 ± 0.7 | 1999 | 2.3 ± 1.3 | 04-150 | 0.882 |
| Ovarian cancer | NA | NA | 2.7 ± 1.1 | 1998 | 5.0 ± 1.5 | 04-150 | 0.999 |
| Other solid tumors | NA | NA | 7.6 ± 1.9 | 2000 | — | 3.04 | 0.883 |
| . | Year of transplantation . | Maximum transplantation rate . | Predictions 2003 . | ||||
|---|---|---|---|---|---|---|---|
| 1990 . | 1995 . | 2000 . | Year . | Transplantation rate . | Transplantation rate . | r2 . | |
| Leukemias | |||||||
| Acute myeloid leukemia | 10.8 ± 2.2 | 19.3 ± 4.8 | 23.7 ± 4.4 | 1998 | 23.9 ± 4.0 | 24 | 0.968 |
| Acute lymphocytic leukemia | 8.1 ± 1.8 | 9.9 ± 2.6 | 6.2 ± 2.1 | 1991 | 10.0 ± 2.8 | 3 | 0.67 |
| Chronic myeloid leukemia | 2.4 ± 1.4 | 5.5 ± 1.5 | 3.6 ± 1.3 | 1998 | 8.1 ± 2.7 | (3.6)4-150 | 0.656 |
| Myelodysplastic syndromes | 0.1 ± 0.1 | 1.1 ± 1.3 | 1.4 ± 0.8 | 2000 | 1.4 ± 0.8 | 1.7 | 0.901 |
| Chronic lymphocytic leukemia | — | 1.3 ± 0.7 | 5.6 ± 1.5 | 2000 | 5.6 ± 1.5 | 11.2 | 0.933 |
| Lymphoproliferative disorders | |||||||
| Multiple myeloma | 3.8 ± 2.4 | 34.3 ± 12.1 | 82 ± 15.2 | 2000 | — | 121.6 | 0.99 |
| Hodgkin disease | 9.3 ± 2.7 | 21.9 ± 4.5 | 24.9 ± 3.2 | 2000 | — | 23.1 | 0.979 |
| Non-Hodgkin lymphoma | 13.7 ± 5.0 | 56.6 ± 8.4 | 95.5 ± 13.6 | 2000 | — | 118.9 | 0.986 |
| Solid tumors | |||||||
| Neuroblastoma | |||||||
| Glioma | 1.7 ± 1.6 | 1.6 ± 1.1 | 1.8 ± 0.9 | 2000 | — | 1.4 | 0.45 |
| Soft tissue sarcoma | 1.2 ± 0.6 | 2.0 ± 0.8 | 3.8 ± 1.1 | 1997 | 4.0 ± 1.3 | 3.98 | 0.882 |
| Germinal tumors | 3.6 ± 1.9 | 7.3 ± 2.5 | 7.3 ± 2.5 | 1997 | 8.9 ± 2.4 | 4.1 | 0.951 |
| Breast cancer | 27.0 ± 1.5 | 33.3 ± 14.4 | 20.0 ± 8.8 | 1997 | 66.3 ± 19.3 | 04-150 | 0.734 |
| Ewing sarcoma | 1.1 ± 0.7 | 3.0 ± 0.8 | 5.6 ± 2.0 | 2000 | — | 6.7 | 0.961 |
| Lung cancer | NA | NA | 1.4 ± 0.7 | 1999 | 2.3 ± 1.3 | 04-150 | 0.882 |
| Ovarian cancer | NA | NA | 2.7 ± 1.1 | 1998 | 5.0 ± 1.5 | 04-150 | 0.999 |
| Other solid tumors | NA | NA | 7.6 ± 1.9 | 2000 | — | 3.04 | 0.883 |
NA indicates not applicable. Numbers reflect weighted transplantation rates per 10 million inhabitants.
indicates limitations of mathematical model; see “Results.”
For allogeneic HSCT, transplantation rates increased for all indications continuously with 2 exceptions (Table 3): chronic myeloid leukemia and inborn errors, for which the maximum was reached in 1999. For chronic myeloid leukemia, the likely explanation is the advent of imatinib mesylate. For inborn errors, it could be a chance phenomenon at the beginning of stabilization. Mathematical models cannot separate chance variations from first signs of a new trend, but they predict an increase for both indications for 2003. Patterns of increase were not the same for all indications (Figure4A,B). In aplastic anemia, the increase was relatively small over time with a slow steady increase, and a decrease in SD (trend mean y = 0.0066 ×2 + 0.187x + 4.0116:r2 0.5338); in AML the increase is marked with a doubling of transplantation rate almost every 5 years (trend mean y =0.0961 ×2 + 1.3861x + 11 105; r2 = 0.9973).
Transplantation rates for selected indications in 9 European countries from 1990 to 2000.
Refer to Figure 3. Weighted transplantation rates (per 10 million inhabitants) are given (♦) and best fitting curves (lines). (A) Aplastic anemia, allogeneic HSCT. (B) Acute myeloid leukemia, allogeneic HSCT. (C) Multiple myeloma, autologous HSCT. (D) Breast cancer, autologous HSCT.
Transplantation rates for selected indications in 9 European countries from 1990 to 2000.
Refer to Figure 3. Weighted transplantation rates (per 10 million inhabitants) are given (♦) and best fitting curves (lines). (A) Aplastic anemia, allogeneic HSCT. (B) Acute myeloid leukemia, allogeneic HSCT. (C) Multiple myeloma, autologous HSCT. (D) Breast cancer, autologous HSCT.
For autologous HSCT, the changes in transplantation rate differed from the pattern seen in allogeneic HSCT (Table 4). For some indications, a continuous increase occurred throughout the decade. This was the case for lymphoproliferative disorders, as exemplified by the curves for multiple myeloma (Figure 4C) and a doubling every 3 to 4 years (trend mean y = 0.33 ×2 + 4.3538x − 4.0192; r2 = 0.9899). The same trend was observed for some leukemias (myelodysplastic syndromes, chronic lymphocytic leukemia) and some solid tumors (glioma, Ewing sarcoma). In contrast, for other indications, most marked for breast cancer (Figure4D), the rapid increase in the early 1990s with a peak between 1997 and 1998 was followed by a rapid decline. Mathematical models would even predict a value of 0 at 3 years. Similarly, for chronic myeloid leukemia, predictions cannot yet capture the decline in 2000 and show a continuing rise.
Discussion
These data give an overview of the status of HSCT in Europe during the last decade and today. They illustrate the main changes in technology and the overall increase over time with substantial differences between European countries. They give detailed insight for individual disease indications and allow a precise medium-term forecast.
Based on the analysis of weighted transplantation rates in 9 countries with the highest transplantation numbers, it can be predicted that transplantation rates for allogeneic HSCT will continue at the same or higher level in the immediate future for all indications. Only one exception is chronic myeloid leukemia, which has been the leading indication for allogeneic HSCT up to the year 1999.8,15 It is likely that the decline in 2000 is not a chance phenomenon but due to the introduction of imatinib mesylate, a novel specific tyrosine kinase inhibitor.13,14 It is of interest to note that reduction in transplantation rates for chronic myeloid leukemia occurred before the first publication on the results of the initial phase 1 trial, which were published in the spring of 2001. This sequence of events suggests that anticipation of therapeutic success was a major factor in decision-making. The same phenomenon that changes in transplantation rates occurred before the key publications was observed earlier in the decade with regard to breast cancer. This phenomenon of anticipation needs to be recognized at times of high praise for evidence-based medicine.18 The issue of HSCT for chronic myeloid leukemia is still not settled. Imatinib failures continue to occur and therapists are reverting to HSCT in these cases. More observation time is needed for reevaluation of HSCT in chronic myeloid leukemia.
Predictions in autologous HSCT do not show a general pattern but rather different trends. The situation for autologous HSCT in breast cancer, as mentioned above, has received broad attention beyond the medical literature and was summarized in headlines, such as “transplants decline, research continues.”19 Hopes for cure initially stimulated autologous HSCT for breast cancer as well as solid tumors in general.20 Results did not meet expectations and induced a similar, marked decline.21,22 The pattern varies substantially depending on indications. HSCT rates declined for germ cell tumors and acute lymphocytic leukemia. In contrast, they continued for neuroblastoma and Ewing tumors at similar rates and increased for non-Hodgkin lymphoma, multiple myeloma, or acute myeloid leukemia. For the latter 3 indications, autologous HSCT continued to increase with the highest transplantation rates in the year 2000. Importantly, these indications correspond to those where HSCTs were shown to provide a clinical benefit compared to standard therapy in prospective randomized studies.23-26 It is also noteworthy that indications with the highest transplantation rate in 2000 were compatible and concordant with so-called accepted indications for HSCT.3
Health care today has many aspects of a market. Demand is there, if a given technology is recognized as the treatment of choice. Health care providers should be in a position to offer these therapies if the need arises. For high-cost, complex techniques, such as HSCT, planning is essential. Medium-term horizon scanning (http://wwww.bham.ac.uk/PublicHealth/horizon/glossary.htm) has become vital for health care management. Novel tools are required in this field. The annual activity survey of the EBMT presents one such unique instrument. Thanks to a nearly complete coverage of a medical technology across several countries within a continent and the rapid return of information, trends can be discovered very early and predictions can be made with reasonable sensitivity and accuracy.27 The limitation of the approach is that new events, for example, the introduction of a novel drug, such as imatinib mesylate, which can change treatment strategies within 1 year, cannot be anticipated. Despite the best mathematical models, statistical analyses cannot distinguish in the first year between chance events and the beginning of a new era.
Several factors influence transplantation rates for individual disease indications. Prevalence of the disease, consensus on indication, and the economic situation within a country are the main determinants. In addition, the technology has to be available and needs to be disseminated within a country.28 This can clearly be influenced by health care planning even though optimal team density (number of transplantation teams per 10 million inhabitants) needs to be defined.
In summary, these data based on 10 years of cumulative transplantation activity data in Europe show that transplantation rates for individual disease indications can change. Such shifts are not necessarily based on evidence but rather on anticipation. Nevertheless, transplantation rates are not erratic but highly predictable at medium term.
The cooperation of all participating teams and their staff (listed in the ), the EBMT secretariat (A. Urbano-Ispizua, A. Baur), the European EBMT Data Office in Paris (V. Chesnels, P. Palut, N. C. Gorin), the EBMT Registry Subcommittee (P. Ljungman, C. Ruiz de Elvira), the French Registry SFGM (J. P. Vernant, M-L Tanguy), the Dutch Registry (T. de Witte, A. v. Biezen, N. Tazelaar), the Austrian Registry (D. Niederwieser, B. Gritsch), the Italian Registry (M. Vignetti, A. Bacigalupo, R. Oneto, C. Palazzi), the German Registry (H. Ottinger, C. Müller, B. Kubanek, N. Schmitz, U. W. Schaefer), the Swiss Registry (J. Passweg, H. Baldomero), the British Registry, the Belgium Registry, (Y. Beguin) and the Spanish Transplantation Office (ONT) (M. Naya) is greatly appreciated. The authors also thank A. Maerki for excellent secretarial assistance, A. Wodnar-Filipowicz for reviewing the manuscript, as well as L. John and O. Baldomero for technical assistance with data management.
List of transplant centers in 2000 (numbers show total number of patients with first transplantations in year 2000 (total number of transplantations) followed by the allografts/autografts). The total number teams in 2000 was 600; total number of transplantations, 22 496 (allo, 7146; auto, 15 350); and total number of first transplantations 19 366 (allo, 6456; auto, 12 910). *indicates no report; †late data, included only in Figure 3B.
Albania
No report.
Andorra
No report.
Armenia
No report.
Austria (15 teams; 329 [396], 127/202)†
Graz, University Hospital, CIC 308, W. Linkesch (52 [66], 10/42); Graz, University Hospital, Onco, CIC 278, H. Samonigg, M. Schmid (4 [9], 0/4)†; Graz, Universitäts-Kinderklinik, CIC 593, Ch. Urban (12 [13], 5/7)†; Innsbruck, Universitätsspital (hem, onco), CIC 271, G. Gastl, D. Nachbaur (29 [33], 24/5); Innsbruck, Universitätsspital (Internal Medicine), CIC 516, E. Woell (4 [7], 0/4); Klagenfurt, General Hospital Klagenfurt CIC 716, D. Geissler, M. Heistinger (8 [8], 0/8); Linz, 1 Medizinische Abteilung, AO Krankenhaus, M. A. Fridrik (1 [1], 0/1); Linz, AOK der Elisabethinen, CIC 594, D. Lutz, O. Krieger (32 [43], 7/25)†; Salzburg, LKA Salzburg (Onco), CIC 356, Prof. Hausmaninger (10 [10], 0/10); Vienna-Lainz, Krankenhaus der Stadt Wien-Lainz, 5. Med Onko, CIC 362, G. Baumgartner, E. Ulsperger, Dr. Mayer (1 [1] 0/1) ; Vienna, St Anna Kinderspital, CIC 528, H. Gadner, C. Peters (35 [43], 20/15); Vienna, Donauspital, CIC 767, W. Hinterberger (14 [18], 0/14)†; Vienna, Universitätsklinik für Innere Medizin I-AKH, CIC 227, H.T Greinix, P. Kalhs (89 [97], 61/28)†; Vienna, Wilhelminerspital, CIC 828, H. Ludwig (31 [40], 0/31); Vienna, Hanusch-Krankenhaus, CIC 743, R. Reisner, E. Pittermann, E. Koller (9 [10], 0/9).†
Azerbaijan
No report.
Republic of Belarus (3 teams; 41 [42], 13/28)
Minsk, Belorussian Center, CIC 591, O. Aleinikova (15 [16], 6/9); Minsk, Hospital No. 9, CIC 801, N. Milanovitch (26 [26], 7/19); Minsk, Institute of Haematology, V. Ivanov ().*
Belgium (24 teams; 553 [647], 170/383)
Aalst, OLV Ziehenhuis, E. Wouters ()*; Antwerpen, AZ Middelheim, CIC 783, R. de Bock (7 [7], 0/7); Antwerpen, Stuivenberg ZH, CIC 339, P. Zachée (35 [41], 6/29); Brugge, AZ St Jan, CIC 506, D. Selleslag, A. Van Hoof (40 [53], 12/28); Brussels, Clinique Général Saint Jean, CIC 779, C. Dubois (4 [7], 0/4); Brussels, Hôpital Erasme, CIC 596, W. Feremans (14 [16], 0/14); Brussels, Clinique universitaire St Luc (Adults), CIC 234, A. Ferrant (49 [52], 26/23); Brussels, Institut Jules Bordet +Children's University Hospital, CIC 215, D. Bron C. Devalck, E. Sariban (48 [56], 24/24); Brussels, University Hospital, CIC 630, B. Van Camp, A. Schots (25 [25], 12/13); Brussels, Cliniques Universitaires St Luc, (onco), M. Symann (3 [3], 0/3); Brussels, Institute Edith Cavelle Marie Depage (onco), C. Vanhaelen ()*; Brussels, Clinique Universitaire St Luc (peds), CIC 234, C. Vermylen (13 [13], 6/7); Charleroi, Hopital Notre-Dame, M. André (27 [29], 1/26); Edegem, University Antwerpen, CIC 648, W. Schroyens (26 [28], 3/23); Gent, University Hospital, CIC 744, L. A. Noens (50 [53], 20/30); Haine, St Paul, Hôpital de Jolimont, CIC 234, A. Delannoy, C. Ravoet (13 [16], 0/13); Hasselt, Virgajesse Ziekenhuis CIC 632, D. Vanstraelen, Dr. Janssen (25 [27], 0/25); Jumet, Hôpital Civil de Jumet, A. Duvivier ()*; Leuven, University Hospital Gasthuisberg, CIC 209, M. A. Boogaerts, P. Vandenberghe, J. Maertens (76 [89], 29/47); Liège, University Hospital Sart-Tilman, CIC 726, Y. Béguin (49 [71], 20/29); Liège, CHR-Citadelle, CIC 353, B. De Prijck (6 [7], 0/6); Liège, Centre Hospitalier St Joseph (hem), L. Longree ()*; Roeselare, H. Hartziekenhuis, F. Van Aelst, J. Tytgat, J. Demol (13 [14], 2/11); Yvoir, Clinique universitaire de Mont-Godinne CIC 234, C. Doyen (30 [40], 9/21).
Bosnia-Herzegovina
No report.
Bulgaria (1 team; 15 [15], 3/12)
Sofia, Uni Hospital “Queen Johanna,” CIC 346, (peds hem-onco), D. Bobev (15 [15], 3/12).
Croatia (2 teams; 93 [99], 24/69)
Zagreb, Hospital Merkur, CIC 159, B. Jaksic, H. Minigo (21 [21], 1/20); Zagreb, Clinical Hospital Center, CIC 302, B. Labar, D. Nemet, M. Mrsic (72 [78], 23/49).†
Cyprus (1 team; 14 [14], 0/14)
Nicosia Makarious Hospital lll, N. Papaminas (14 [14], 0/14).
Czech Republic (10 teams; 392 [465], 103/289)
Brno, Masaryk University Hospital, CIC 597, J. Vorlicek (75 [91], 18/57); Hradec Kralové, Charles University, CIC 729, S. Filip, M. Blaha (51 [59], 10/41); Olomouc, University Hospital, CIC 574, K. Indràk (52 [61], 10/42); Pilsen, Faculty Hospital, CIC 718, V. Koza (62 [67], 19/43); Prague, Thomayer Memorial Hospital, CIC 375, J. Abrahamova, J. Nepomucka, L. Boublikova (7 [8], 0/7); Prague, University Hospital Motol (peds onco), P. Kavan (22 [25], 0/22); Prague, Clinical Haematology, Charles University, CIC 318, T. Kozak (22 [29], 0/22); Prague, University Hospital Motol (peds hem), CIC 656, J. Stary (21 [23], 20/1); Prague, Charles University, CIC 745, M. Trneny (47 [66], 0/47); Prague, Institute of Hematology and Blood Transfusion, CIC 656, A. Vitek, P. Kobylka (33 [36], 26/7).
Denmark (3 teams; 161 [179], 47/114)
Aarhus, Amtssygehus, CIC 634, A. Boesen (38 [43], 0/38); Copenhagen, Rigshospitalet, CIC 206, N. Jacobsen (93 [100], 47/46); Copenhagen, Herlev Hospital, University, CIC 568, H. E. Johnson (30 [36], 0/30).
Estonia (1 team; 16 [16], 1/15)
Tartu, University Hospital, CIC 746, H. Everaus (16 [16], 1/15).
Finland (7 teams; 248 [271], 99/149)
Helsinki, University Hospital, Department Oncology, CIC 833, H. Joensuu, T. Wiklund (12 [13], 0/12); Helsinki, University Hospital, Third Department of Medicine, CIC 515, T. Ruutu (88 [91], 63/25); Helsinki, Children's Hospital, CIC 219, U. Pihkala, S. Vettenranta (28 [32], 18/10); Kuopio, Department of Medicine, University Hospital, E. Jantunen, T. Nousiainen (28 [28], 0/28); Oulu, University Central Hospital (haem/onco), CIC 690, P. Koistinen, T. Turpeenniemi-Hujanen (20 [20], 0/20); Tampere, University Hospital, CIC 635, E. Koivunen, R. Silvennoinen (30 [41], 0/30)†; Turku, University Central Hospital, CIC 225, K. Remes (42 [46], 18/24).
France (85 teams; 3103 [3624], 711/2392)†
Amiens, CHU d'Amiens, B. Desablens, ()*; Angers, Paul Papin, Dr Gamelin ()* Angers, Centre Hospitalier, CIC 650, N. Ifrah (53 [64], 12/41); Argenteuil, Centre hospitalier, M. Urbajtel (28 [28], 7/21); Besançon, Hôpital Jean Minjoz and Hôpital St Jacques (adults and peds), CIC 233, P. Hervé, J.-Y. Cahn, M. N. Cailleux, Dr Surowka (86 [105], 26/60); Bobigny, Hôpital Avicenne (hem), P. Casassus ()*; Bordeaux, CHU Hôpital de Bordeaux Enfants, Y. Perel ()*; Brest, Centre Hospitalier, C. Berthou (40 [51], 0/40); Caen, Hôpital Cote de Nacre (peds hem onco), P. Boutard (3 [3], 0/3); Caen, Centre Hospitalier Régional, CIC 251, O. Reman (22 [25], 0/22); Caen, Centre Régional François Baclesse, A. M. Peny (19 [20], 0/19); Clermont Ferrand, Hotel Dieu (peds), F. Démeocq ()*; Clermont Ferrand, Centre Jean Perrin, CIC 273, J.-O. Bay, (76 [76], 14/62); Clichy, Hôpital Beaujon, J. Brière ()*; Colmar, Hôpital civil, B. Audhuy (8 [8], 0/8); Corbeil Essonne, Hôpital Gilles de Corbeil, A. Devidas (11 [11], 0/11); Créteil, Hôpital H. Mondor, CIC 252, C. Cordonnier, M. Kuentz (60 [62], 22/38); Dijon, Hôpital d'Enfants, D. Caillot (70 [91], 0/70); Dunkerque, Centre Hospitalier (hem), M. Wetterwald (9 [9], 0/9); Grenoble, Centre Hospitalier (ads, allo peds), CIC 270, J. J. Sotto, F. Garban, P. Drillat (50 [55], 13/37); Grenoble, Centre Hospitalier (auto peds), D. Plantaz, M. Bost (8 [8], 0/8); Lille, Hôpital Claude Huriez, CIC 277, F. Bauters, J. P. Jouet (112 [126], 43/69); Lille, Hôpital Jeanne de Flandre, Dr. Nelken (2 [2], 0/2); Lille, Centre Oscar Lambret (onco), Dr Depadt, Dr Defachelles (20 [20], 0/20); Lille, Centre Hospitalier Saint Vincent, N. Cambier (15 [15], 0/15); Limoges, Centre Hospitalier Dupuytren (ads), CIC 977, D. Bordessoule, P. Turlure (37 [43], 0/37); Limoges, Centre Hospitalier Dupuytren (peds), Prof De Lumley (2 [2], 0/2); Lyon Sud (Pierre Benite), Centre Hospitalier, B. Coiffier (124 [135], 0/124); Lyon, Hôpital Edouard Herriot, CIC 671, D. Fiere, E. Archimbaud, A. Belhabri, M. Michallet (61 [68], 30/31); Lyon, Centre Léon Bérard, CIC 241, P. Biron, T. Philip (66 [78], 0/66); Lyon, Hôpital Debrousse, N. Philippe, G. Souillet, Y. Bertrand (24 [27], 24/0); Marseille, Inst. Paoli-Calmettes, CIC 230, D. Blaise (196 [283], 19/177); Marseille, Hôpital d'Enfants de la Timone (onco), CIC 301, C. Coze, J. L. Bernard (9 [10], 0/9); Marseille, Hôpital d'Enfants de la Timone, G. Michel (16 [18], 16/0); Meaux, Centre Hospitalier de Meaux, C. Soussain (6 [8], 0/6); Metz, Thionville Hôpital Notre-Dame de Bon-Secours (hem), V. Dorvaux (18 [24], 0/18); Montpellier, CHU de Montpellier Hôpital Arnaud de Villeneuve, F. Bernard (11 [11], 2/9); Montpellier, Centre Rég De Lutte contre de Cancer, M. Fabbro, J- B. Dubois (13 [13], 0/13); Montpellier, CHR Lapeyronie, J. F. Rossi (95 [100], 18/77); Mulhouse, Hôpital du Hasenrain, Ph. Hénon, Dr Becker (15 [16], 0/15); Nantes, Hotel Dieu, CIC 253, J. L. Harousseau, N. Milpied (146 [203], 30/116); Nice, Hôpital de Cimiez, CIC 523, J. G. Fuzibet, J. P. Cassuto, N. Gratecos (52 [55], 15/37); Nice, Fondation Lenval (peds), Dr Soler, Dr. De Ricaud (1 patient received transplant in Marseille (1 [1], 0/1); Nice, Centre Antoine Lacassagne, A. Thyss (20 [21], 0/20); Paris, Hôpital Européen GP, J. M. Andrieu, C. Le Maignan (7 [10], 0/7); Paris, Hôpital d'Instruction des Armées Percy, Clamart, T. de Revel, G. Nedellec (28 [31], 3/25); Paris, Hôpital Cochin, J - P. Levy, F. Dreyfus (36 [36], 0/36); Paris, Hôpital Necker des enfants malades, CIC 210, A. Fischer (39 [43], 36/3); Paris, Hôpital St Antoine, CIC 213, C. Gorin, L. Fouillard (48 [59], 8/40); Paris, Hôpital St Louis (auto), CIC 805, G. Gisselbrecht (58 [61], 0/58); Paris, Hôpital St Louis (allo), CIC 207, E. Gluckman (87 [92], 86/1); Paris, Hôpital St Louis (peds), CIC 748, A. Baruchel, M-F. Auclerc (3 [3], 0/3); Paris, Hôpital St Louis (auto immuno-Haem), CIC 969, J-C. Brouet, B. Royer, J- P. Fermand (57 [58], 0/57); Paris, Hôpital St Louis (auto-leuk), CIC 960, H. Dombret, L. Degos, P. Rousselot (14 [16], 0/14); Paris, Hôpital Pitié Salpétière (hem), CIC 262, J-P. Vernant, V. Leblond (91 [100], 42/49); Paris, Hôpital D'enfants Armand-Trousseau, G. Leverger, A. Auvrignon (10 [10], 0/10); Paris, Hôpital Tenon, J. P. Lotz (32 [57], 0/32); Paris, Hôpital Robert Debré, P. Rohrlich, E. Vilmer (22 [22], 21/1); Paris, Hôpital Necker (ads), CIC 160, B. Varet, C. Bélanger, A. Veil (63 [66], 28/35); Paris, Hôtel Dieu (hem), CIC 222, J-P. Marie, B. Rio (50 [57], 13/37); Paris, Hotel Dieu (onco), Prof Bernadou, L. Chauvenet (2 [2], 0/2); Paris, Institut Curie (ads/onco/peds), CIC 702, P. Pouillart, J. Michon, J. M. Zucker ()*; Pessac, Hôpital Haut-Lévèque, CIC 267, J. Reiffers, Dr Fabères (98 [133], 31/67)†; Poitiers, Hôpital Jean Bernard, CIC 264, A. Sadoun ()*; Pontoise, Hospital Renè Dubois, CIC 961, Dr. Morvan, Y. Kernéis (17 [19], 0/17); Reims, Hopital Robert Debré, J. C. Etienne (29 [32], 0/29); Reims, Institute Jean Godinot (onco), Dr. Eymard ()*; Rennes, Hôpital Pontchaillou, C. Dauriac ()*; Rennes, CHRU, Clinique Médical Infantil, E. Le Gall, V. Gandemer (17 [17], 7/10); Rouen, Centre Henri Becquerel, H. Tilly, P. Lenain (66 [71], 17/49); Rouen, Hôpital Charles Nicolle, P. Tron (16 [17], 10/6); St Cloud, Centre René Huguenin, M. Janvier (6 [7], 0/6); St Etienne, Hôpital Etienne, D. Guyotat, J. L. Stephan ()*; Strasburg, Hôpital de Hautepierre, B. Lioure (72 [88], 15/57); Strasburg, Hospices Civils, Service de Pédiatrie 5, P. Lutz (13 [13], 10/3); Toulouse, Hôpital de Purpan (hem), CIC 624, M. Attal (75 [82], 28/47); Toulouse, Hôpital de Purpan (peds), A. Robert, H. Rubie (8 [8], 1/8); Toulouse, Centre Claudius Régaud, H. Roche, C. Chevreau (9 [9], 0/9); Tours, Hôpital Bretonneau, CIC 272, P. Colombat (89 [99], 0/89); Valenciennes, Hosp De Valenciennes, M. Simon (19 [19], 0/19); Vandeuvre-les-Nancy, Hôpital d'Enfants, P. Bordigoni (33 [38], 28/5); Vandeuvre-les-Nancy, CHU Nancy-Brabois (hem auto), P. Lederlin, F. Witz (63 [73], 0/63); Villejuif, Institut G. Roussy (peds), CIC 503, O. Hartmann (53 [86], 0/53); Villejuif, Institut G. Roussy (ads), CIC 666, J-H. Bourhis, C. Boccaccio, J-M. Vantelon (106 [118], 26/80); Villejuif, Hôpital Paul Brousse, B. Delmas-Marsalet (8 [8], 0/8).
Georgia
No report.
Germany (106 teams; 3541 [4395], 1432/2109)
Aachen, Universitätsklinikum RWTH, Med Klinik lV, R. Osieka, U. Fabry (12 [18] 0/12); Augsburg, Zentralklinikum, Med Klinik ll, G. Schlimok, P. Müller (37 [40], 8/29); Bad Saarow, Humaine Klinikum, G. Schultze, H. Fuss (49 [49], 0/49); Berlin, Universitätsklinikum Charité Campus Mitte, ll Med Klinik, CIC 807, K. Possinger, R. Arnold (53 [58], 40/13); Berlin, Univ Charité der Humboldt Universität Campus, Robert- Rössle Klinik (onco), CIC 518, B. Dörken, G. Maschmeyer (10 [15], 0/10); Berlin, KH Neukölln, A. C. Mayr, C. Kerschgens (1 [1], 0/1); Berlin, Charité Virchow Klinikum (peds), CIC 336, W. Ebell, G. Gaedicke (30 [36], 30/0); Berlin, Charité Virchow Klinikum (ads), CIC 293, W. Siegert, D. Huhn (60 [89], 29/31); Berlin, Universitäts-Klinik der FU Benjamin Franklin, CIC 590, W. Knauf, E. Thiel (47 [52], 14/33); Bielefeld, Bethel KKS Gilead, R. Kolloch, F. K. Lindemann (0 [0], 0/0); Bielefeld, Franziska Hospital, H. J. Weh, A. Zumsprekel (7 [14], 0/7); Bochum, Knappschaftskrankenhaus, U. Graeven, W. Schmiegel (12 [17], 0/12); Bonn, Medical University Klinik Bonn, T. Sauerbruch, I. Schmidt-Wolf, R. Kleinschmidt (27 [45], 0/27); Bonn, Universitäts Kinderklinik, U. Bode, C. Hasan (7 [8], 0/7); Bremen, CIC 602, Zentralkran kenhause mitte, C. Meier, H. Rasche (11 [15], 0/11); Bremen, DIAKO, DRST 28001, T. Wolff, K. H. Pflüger (9 [10], 0/9); Chemnitz, KH Küchwald, F. Fliedler, R. Nowak (20 [21], 0/20); Cottbus, Carl-Thiem Klinikum, Med Klinik ll, Ch. Rudolf, H. Steinhauer (21 [35], 0/21); Dortmund, St Johannes Hospital, H. Plelken, M. Nahler (3 [3], 0/3); Dresden, Universitätsklinikum Carl Gustav Carus, CIC 808, G. Ehninger, M. Bornhäuser (153 [188], 94/59); Duisburg, St Johannes Hospital, CIC 519, C. Aul, J. Anhuf (21 [29], 0/21); Duisburg, Klinikum Kalkweg (onco), H. Gerhartz (0 [0], 0/0); Düsseldorf, Heinrich-Heine Universität; Zentrum für Kinderheilkunde, CIC 651, K. Göbel, W. Nürnberger, D. Dilloo (23 [26], 16/7); Düsseldorf, Heinrich-Heine Universität; Medizinische Klinik (haem, onco), CIC 390, R. Haas, P. Schneider (76 [90], 22/54); Erlangen, Universitäts-Klinik für Kinder und Jugendliche, CIC 809, W. Rascher, W. Holter, J. D. Beck, J. Greil (8 [11], 3/5); Erlangen, Universität Erlangen-Nuremberg, Med Klinikum lll, CIC 809, M. Gramatzki, J-R. Kalden (37 [40], 16/21); Eschweiler, St Antonius Hospital, R. Fuchs (2 [3], 0/2); Essen, Evangelisches Krankenhaus Essen-Werden GmbH, CIC 784, W. Heit, M. Wattad (66 [73], 0/66); Essen, Universitäts-Klinik (hem), G. Brittinger, U. Dührsen, R. Noppeney (12 [18], 0/12); Essen, West German Cancer Center, S. Seeber, P. Bojko (44 [93], 0/44); Essen, Universitäts-Klinik (ads), CIC 259, U. W. Schaefer, D. W. Beelen, V. Runde, W. Havers, O. Basu (118 [125], 112/6); Essen, Universitäts-Klinik (peds), CIC 259, W. Havers, B. Kremens (20 [21], 17/3); Frankfurt a M, JW Goethe-Universität (ads, peds), CIC 297+CIC 138, D. Hoelzer, H. Martin B. Kornhuber, D. Schwabe (65 [72], 34/31); Frankfurt, KH Nordwest, A. Knuth, E. Jäger (1 [1], 0/1); Freiburg i Br, Universitätsklinik (ads), Med Klinik l, CIC 810, J. Finke, W. Lange (126 [136], 82/44); Freiburg i Br, UniversitätsKinderklinik, CIC 810, C. Niemeyer, M. Brandis, U. Duffiser, B. Bächle (22 [23], 20/2); Göttingen, Georg-August Universität, G. Brittinger, T. Hagemann, B. Wörmann (25 [28], 0/25); Greifswald, Ernst-Moritz-Arndt Universität (ads + peds), CIC530, G. Dölken, T. Kiefer (37 [44], 6/31); Gütersloh, Städtkrankenhaus, C. Gropp (2 [3], 0/2); Hagen, Kath Krankenkaus, H. Eimermacher, W. Kalitschke (8 [12], 0/8); Halle, Martin Luther Universität, CIC 338 + CIC 654, H-J. Schmoll, H. Wolf, S. Burdach (20 [38], 0/20); Hamburg, Allgemeines Krankenhaus, D. Braumann, U. Hahn, (19 [23], 0/19); Hamburg, Eppendorf-Krankenhaus (hem, onco), CIC 673, D. Hossfeld, K. Kösters (35 [46], 0/35); Hamburg, Eppendorf-Krankenhaus (KMT) CIC 614, A. R. Zander (83 [97], 74/9); Hamburg, KH St George, B. Lamersdorf, R. Kuse (0 [0], 0/0); Hameln, Kreiskrankenhaus Hameln, H. Schmidt, K. Buhrmann (9 [16], 0/9); Hamm, St Marien Hospital, H. Dürk, B. Schmid (7 [7], 0/7); Hannover, Medizinische Hochschule, CIC 295, A. Ganser, B. Hertenstein (64 [74], 33/31); Hannover, Medizinische Hochschule, Abt Kinderheilkunde, CIC 295, K. Welte, K. Sykora (24 [24], 19/5); Hannover, KH Siloah, H. Kirchner, M. Sosada (13 [13], 0/13); Heidelberg, Ruprecht-Karls Universitäts-Poliklinik, CIC 524, A. D. Ho (181 [217], 41/140); Homburg/Saar, Universität des Saarlandes, CIC 785, L. Trümper, M. Pfreundschuh (38 [52], 16/22); Idar-Oberstein, Klinik für Hämato-/Onkologie, CIC 592, A. A. Fauser, M. Kiehl (63 [69], 58/5); Jena, Klinik fur Innere Medizin ll, CIC 533, H. G. Sayer, K. Hoeffken (45 [62], 23/22); Jena, Universitäts-Kinderklinik, CIC 750, F. Zintl, D. Fuchs (24 [28], 17/7); Kaiserslautern, Westpfalzklinikum, F.-G. Hagmann, H. Link, C. Wollermann (8 [11], 0/8); Karlsruhe, Städtisches Klinikum, J. Fischer, T. Kubin (14 [22], 0/14); Kassel, Städtische Kliniken, W. D. Hirschmann, K. Schultes, E. Steinhauer (5 [5], 0/5); Kiel, Christian-Albrechts-Universität (ads, peds), CIC 256, N. Schmitz, M. Kneba, J. Schaub, M. Suttorp (99 [115], 25/74); Köln, Kinderonkologie der Universitäts-Klinik, F. Berthold, T. Simon (2 [2], 0/2); Köln, Universitäts-Klinik, CIC 534, V. Diehl, D. Söhngen, Ch. Scheid (64 [94], 15/49); Krefeld, Klinikum Krefeld, Med Klinik lll, M. Planker, R. Peceny (2 [2], 0/2); Leipzig, Universitäts-Klinik, D. Niederwieser, W. Helbig, R. Krahl, W. Pönisch (92 [105], 59/33); Lemgo, Klinikum Lippe, H. P. Lohrmann (6 [6], 0/6); Lübeck, Städtisches KH Sud, M. Thalheimer, H. Bartels (11 [12], 0/11); Lübeck, Med. Universität, J. Fehm, T. Wagner (13 [21], 1/12); Lübeck, Klinik für Kinder und Jugendmedizin, P. Bucsky, Ch. Schultz, K. Kruse (1 [1], 0/1); Magdeburg, Otto-von-Guericke Universität, A. Frank, M. Koenigsmann (3 [3], 0/3); Magdeburg, Städt Klinikum Magdeburg, E. Kettner, H. Kröning (10 [10], 0/10); Mainz, Medizinische Klinik der Universität, CIC 786, C. Huber, K. Kolbe, H-G. Derigs (103 [113], 46/57); Mannheim, IlI Med Klinik, R. Hehlmann, J. Hastka (15 [18], 0/15); Marburg, Med Universitätsklinik der Philipps Universität, CIC 645, A. Neubauer, R. Weide, U. Kaiser (43 [51], 18/25); Minden/Westfallen, Med Klinik, H. Bodenstein, H. J. Tischler (13 [18], 0/13); Mönchengladbach, KH Maria Hilf ll, Dr Berkovic, D. Kohl, H-E. Reis (7 [9], 0/7); Munich, Städt Krankenhaus Schwabing (peds), P. Emmrich, L. Stengel-Rutkowski (6 [6], 6/0); Munich, Klinikum Innenstadt, B. Emmerich, C. Straka (34 [55], 0/34); Munich, Klinikum Grosshadern (ads) CIC 513, H.-J. Kolb, W. Hiddemann (135 [174], 93/42); Munich, Klinikum Grosshadern (peds), CIC 513, C. Bender-Götze (7 [7], 7/0); Munich, Dr v Haunersches Kinderspital (hem and onco) R. J. Haas, D. Stachel, S. Schulz (9 [9], 8/1); Munich, SKH München-Harlaching, R. Hartenstein, R. Munker (21 [28], 0/21); München, SKH München-Schwabing, Ch. Nerl, N. Fischer, C. Waterhaus (16 [23], 1/15); München, Klinikum rechts der Isar, M. Sandherr C. Peschel, C. v Schilling (56 [69], 0/56); Münster, Westfälische Wilhelms-Universitäts Klinik, Innere Med CIC 680, W. Berdel, J. Kienast, Th. Büchner, H. Ostermann (86 [115], 27/59); Münster, Westfälische Wilhelms-Universitäts kinderkKlinik (hem and onco), CIC 505, H. Jürgens, M. Paulussen, J. Vormoor (21 [21], 13/8); Neuss, Lukaskrankenhaus, T. Wieberding, P. Czygan, T. Nieberding (3 [3], 0/3); Nürnberg, Klinikum, CIC 625, W. Gallmeier, H. Wandt, K. Schäfer-Eckart (52 [56], 20/32); Offenburg, Klinikum Offenburg, Med Klinik lll, B. Weber, F. Hirsch (0 [0], 0/0); Oldenburg, Städtische Kliniken, CIC 749, H. Illiger, B. Metzner (46 [63], 0/46); Osnabrück, Paracelsus Klinik, O. M. Koch, G. Innig (0 [0], 0/0); Potsdam, Klinikum Potsdam, A. Haas, R. Pasold (8 [13], 0/8); Regensburg, Universitäts Klinikum, CIC 787, E. Holler, R. Andreesen, A. Reichle (80 [132], 26/54); Rostock, Universitäts Klinikum, CIC 585, M. Mathias, M. Freund, J. Casper (46 [72], 22/25); Siegen, St Marien Krankenhaus, T. Gaska (3 [4], 0/3); Stuttgart, Bürgerhospital, H. Benöhr, W. Grimminger, D. Hahn (17 [23], 0/17); Stuttgart, Olgahospital, Pädiatrisches Zentrum, CIC 701, U. Gross, J. Treuner, E. Koscielniak (7 [8], 1/6); Stuttgart, Diakonissen Krankenhaus, E. Heidemann, J. Kaesberger (8 [8], 0/8); Stuttgart, Robert-Bosch-Krankenhaus, CIC 145, S. Martin, W. Aulitzky (28 [41], 0/28); Stuttgart, Katharinenhospital, J. Schleicher, H-G. Mergenthaler (13 [26], 0/13); Tübingen, Medizinische Universitäts-Klinik, CIC 223, L. Kanz, H. Einsele, W. Brugger, C. Faul (135 [170], 54/81); Tübingen, Medizinische Universitäts-Klinik, Abteilung Pädiatrie, CIC 535, D. Niethammer, T. Klingebiel (39 [49], 24/15); Ulm, Medizinische Universitäts-Klinik, CIC 204, H. Döhner, D. Bunjes (110 [123], 52/58); Ulm, Kinderklinik der Universität, CIC 204, W. Friedrich, K. Debatin (27 [32], 26/1); Wiesbaden, Deutsche Klinik für Diagnostik, CIC 311, R. Schwerdtfeger, M. Prumbaum (73 [77], 64/9); Wiesbaden, Dr Horst-Schmidt Klinikum, CIC 586, N. Frickhofen, B. Jung (10 [16], 0/10); Wuppertal, Klinikum Wuppertal GmbH, A. Raghavachar (4 [4], 0/4); Würzburg, Universitätsklinikum, Würzburg, M. Wilhelm, K. Wilms, M. Braun (22 [22], 0/22).
Greece (11 teams; 216 [226],83/133)
Alexandroupolis, Thrace University Medical School (Haem), G. Bourikas (3 [4], 0/3); Athens, Hellenic Cancer Institute St Savas, CIC 751, A. Efremedis, M. Stamatellou, K. Papanastassiou, M. Pouli (24 [27], 1/23); Athens, “Aghia Sophia” Children's Hospital, CIC 752, S. Graphakos (29 [29], 18/11); Athens, Evanghelismos Hospital, CIC 622, D. Karakasis, A. Skandalis, N. Harhalakis, E. Nikiforakis (49 [53], 31/18); Athens, Diagnosis and Therapy Centre “Hygeia,” Maroussi, CIC 643, G. Karianakis (18 [20], 5/13); Athens, Medical Center, CIC 603, A. Pigadito (3 [3], 0/3); Athens, University of Athens, CIC 604, I. Dervenoulas (6 [6], 1/5); Athens, Laikon General Hospital, CIC 328, Y. Rombos, D. Boutsis, V. Kalotychou (13 [13], 0/13); Crete, University Hospital of Heraklion (peds, hem-onco), CIC 352, M. Kalmanti (1 [1], 0/1); Thessaloniki, The George Papanicolaou General Hospital, CIC 561, A. S. Fassas (58 [58], 25/33); Patras, University Medical School, N. C. Zoumbos, M. Tiniakou (12 [12], 2/10).
Hungary (4 teams; 126 [130], 39/87)
Budapest, National Institute of Hematology, CIC 504, K. Palocz, R. Denes (29 [29], 11/18); Budapest, Szent Laszlo Hospital, CIC 739, T. Masszi, P. Reményl, G. Kriván (69 [72], 23/46); Miskolc, Postgraduate Medical School (peds), CIC 599, N. Kalman, K. Kiss, G. Marton (14 [15], 5/9); Pécs, University of Pécs, CIC 682, H. Losonczy (14 [14], 0/14).
Iceland (1 team; 0 [0], 0/0)
Reykjavik, National University Hospital, CIC 605, S. Reykdal (0 [0], 0/0).
Iran (1 team; 105 [108], 86/19)
Teheran, Shariati Hospital (Hem-Onco), CIC 633, A. Ghavamzadeh (105 [108], 86/19).
Ireland (3 teams; 62 [83], 30/32)
Dublin, St James's Hospital, CIC 257, S. R. McCann (34 [38], 20/14); Dublin, St Vincent's Hospital, CIC 541, J. Crown (16 [32], 0/16)†; Dublin, Our Lady's Hospital of Sick Children, Crumlin, CIC 774, A. O'Meara (12 [13], 10/2).
Israel (7 teams; 359 [383], 181/178)
Haifa, Rambam Medical Center, J. Rowe (123 [134], 40/83); Jerusalem, Hadassah University Hospital, CIC 258, R. Or, S. Slavin (96 [101], 80/16); Petach-Tikva, Children's Medical Center, CIC 755, J. Stein (26 [28], 16/10); Revohot, Kaplan Hospital, CIC 327, A. Berribi (9 [12], 0/9); Tel Aviv, Sourasky Medical Center, CIC 161, E. Naparstek ()*; Tel Hashomer, Chaim Sheba Medical Center (hem) CIC 754, I. Ben-Bassat (89 [90], 33/56); Tel Hashomer, Chaim Sheba Medical Center (peds), CIC 572, A. Toren, H. Golan, B. Bielorai (16 [18], 12/4).
Italy (94 teams; 3132 [3932], 963/2169)†
Alessandria, SS Antonio e Biagio e C Arrigo, CIC 825, A. Levis, A. Allione, M. Pini, F. Salvi (19 [23], 0/19); Ancona, Nuovo Ospedale Torrette, CIC 788, P. Leoni, A. Olivieri (46 [52], 6/40); Aviano, CRO Aviano, CIC 162, M. Michieli, M. Rupolo, M. Mazzucato, F. Lollo (5 [5], 0/5); Avellino, AOS Giuseppe Moscati, CIC 789, E. Volpe, N. Cantore (18 [18], 2/16); Avezzano, Ospedale Civile di Avezzano, CIC 921, F. Recchia (0 [0], 0/0)†; Bari, Policlinico, V. Pavone, V. Liso (0 [0], 0/0)†; Bergamo, Ospedale Riuniti, CIC 658, T. Barbui, A. Rambaldi (64 [90], 20/44); Bologna, St Orsola-Malpighi (haem), CIC 240, G. Bandini, S. Tura, M. Baccarani (88 [108], 36/52); Bologna, St Orsola-Malpighi, Oncologia Medica, CIC 657, A. Martoni, C. Zamagni (12 [16], 0/12); Bologna, Poli. S. Orsola, Clinica pediatrica III, CIC 790, A. Pession, G. Paolucci (27 [35], 17/10); Bolzano, Ospedale S. Maurizio, CIC 299, M. Casini, P. Fabris, P. Coser (36 [40], 6/30); Brescia, Ospedali Civili, CIC 288, T. Izzi (34 [37], 4/30); Brescia, Università, CIC 741, F. Porta, A. Ugazio (18 [23], 16/2); Brindisi, Ospedaliera “A. Di Summa,” Perrino Hospital, CIC 920, G. Quarta, S. Pinna (6 [9], 0/6); Cagliari, Ospedale Oncologica “AB,” CIC 791, G. Broccia, P. Dessalvi (26 [31], 6/20); Cagliari, lI Clinica Pediatrica, CIC 820, F. Argiolu, A. Cao (13 [14], 12/1); Cagliari, Cattedra di Genetica, University of Cagliari CIC 811, L. Contu, G. La Nasa (11 [13], 8/3); Catania, Ospedale Ferrarotto, CIC 792, R. Giustolisi, G. Milone (31 [35], 11/20); Cremona, Ospedale Maggiore, Medicina II, CIC 226, S. Morandi, C. Bergonzi (12 [16], 0/12); Cuneo, Hospital S. Croce E Carle (hem), CIC 606, A. Gallamini, M. Grasso (22 [25], 3/19); Ferrara, St Anna Hospital, CIC 330, F. Lanza, G. Castoldi (21 [22], 0/21); Firenze, Policlinico di Careggi, CIC 304, A. Bosi (61 [61], 39/22); Firenze, Azienda Ospedale, “A Meyer,” CIC 600, L. Faulkner, G. Bernini (8 [10], 0/8); Forli, Morgagni-Pierantoni Hospital, CIC 298, G. L. Frassineti, D. Amadori (9 [11], 0/9); Genova, Ospedale S Martino, CIC 217, A. Bacigalupo, A. Carella, G. Santini (165 [175], 93/72); Genova, Istituto Giannina Gaslini, CIC 274, G. Dini (41 [63], 21/20); Genova, Università, CIC 139, F. Patrone, A. Ballestrero (54 [77], 0/54); Genova, 1st Nat per la Ricerca s. Cancro, CIC 340, M. Venturini, R. Rosso (0 [0], 0/0); Latina, Ospedale S Maria Goretti, A. De Blasio (12 [16], 0/12); Lodi, Ospedale Maggiore Lodi, G. Nalli, V. Fregoni, (8 [11], 0/8); Milano, Istituto Scientifico H.S. Raffaele, CIC 813, M. Bregni (62 [96], 13/49)†; Milano, Istituto Nazionale Tumori, CIC 616, A. Gianni (123 [180], 0/123); Milano, Università, CIC 265, G. Lambertenghi Deliliers (47 [54], 29/18); Milano, Ist Nazionale Tumori di Milano (onco, peds), CIC 381, R. Luksch (25 [35], 0/25); Milano, Ospedale di Niguarda, CIC 294, P. Marenco, R. Cairoli (47 [53], 13/34); Milano, Ospedale di Niguarda (hem/oncoST), CIC 294/2, S. Siena, P. Pedrazzoli, R. Schiavo (33 [35], 7/26); Milano, Istituto Europeo di Oncologia, CIC 331, G. Martinelli (121 [290], 0/121); Milano, Ospedale Fatebenefratelli e Oftalmico (onco), CIC 269, A. Scanni, C. Bianchi, D. Pedretti (4 [6], 0/4); Milano, 1st Clinico Humanitas (hem-onco), CIC 354, A. Santoro, L. Castagna (62 [94], 10/52); Milano, S Carlo Borromeo Hospital (onco), L. Tedeschi (2 [2], 0/2); Modena, University of Modena, CIC 543, F. Narni, G. Torelli, R. Sabbatini (29 [46], 2/27); Monza, Ospedale S Gerardo, CIC 279, C. Uderzo (28 [30], 21/7); Monza, Ospedale S Gerardo de' Tintori, CIC 544, P. Pioltelli, E. Pogliani (32 [42], 5/27); Napoli, Division Di Oncologia, CIC 313, C. Battista, G. Pacilio, B. Chiurazzi, G. Iodice (0 [1], 0/0)†; Napoli, Università, CIC 766, B. Rotoli, C. Selleri, G. De Rosa (34 [34], 14/20); Napoli, Hospital “Pausilipon” (hem peds), CIC 341, V. Poggi, M. Ripaldi (3 [3], 3/0); Napoli, Cardarelli Hospital (hem), CIC 607, F. Ferrara (20 [21], 1/19); Noale, Civic Hospital (onco), CIC 563, O. Vinante, G. Azzarello (17 [20], 1/16); Nuoro, Ospedale San Francesco, CIC 793, A. Gabbas, A. Palmas (7 [15], 0/7); Orbassano, Ospedale San Luigi Gonzaga, G. Saglio (34 [41], 0/34); Padova, Centro Leucemie Infantili, CIC 285, C. Messina, S. Cesaro, L. Zanesco (30 [35], 15/15); Padova, Centro Oncologia Regionale, CIC 319, S. Aversa, S. Monfardini (14 [18], 0/14); Palermo, Uni degli studi di Palermo (hem), CIC 814, G. Mariani (10 [11], 0/10); Palermo, Ospedale V. Cervello, CIC 392, R. Scimè, A. Cavallaro (65 [74], 26/39); Palermo, Ospedale “La Maddalena,” M. Musso, F. Porretto, A. Crescinanno (39 [52], 4/35); Parma, Ospedaliera Di Parma (onco), CIC 364, V. Franciosi, S. Cascinu, G. Vasini (1 [1], 0/1); Parma, Università degli studi, CIC 245, V. Rizzoli (19 [26], 4/15); Pavia, Policlinico S. Matteo (hem), CIC 286, C. Bernasconi (69 [74], 27/42); Pavia, Policlinico St Matteo (peds), CIC 557, F. Locatelli (80 [87], 59/21); Pavia, Policlinico St Matteo (onco), CIC 562, E. Ascari, M. Danova (23 [33], 0/23); Pavia, Fondazione Clinica del Lavoro, CIC 771, A. Zambelli, G. Robustelli della Cuna (42 [45], 6/36); Perugia, Silvestrini Hospital, CIC 815, A. Amici (2 [3], 2/0); Perugia, Policlinico Monteluce, Università, CIC 794, M. F. Martelli, F. Aversa, A. Tabilio (94 [95], 54/40); Perugia, Policlinico Monteluce, CIC 573, A. M. Liberati, F.Grignani (14 [18], 0/14)†; Pesaro, Ospedale, CIC 529, G. Lucarelli (64 [64], 48/16); Pescara, Ospedale Civile, CIC 248, P. di Bartolomeo (36 [40], 29/7); Piacenza, Ospedale Civile (hem-onco), CIC 163, L. Cavanna (6 [7], 0/6); Pisa, University of Pisa (ads hem, peds hem and onco), CIC 795, P. Macchia, M. Petrini, (45 [54], 19/26); Pisa, St Chirara Hospital (ads onco) CIC 320, P. F. Conte, C. Bengala (7 [10], 1/6); Ravenna, Ospedale Civile, CIC 306, G. Rosti (46 [79], 0/46); Reggio di Calabria, Azienda Ospedale “Riuniti e Morelli,” CIC 587, P. Iacopino (69 [94], 13/56); Roma, Università S. Eugenio, CIC 756, S. Amadori, L. Cudillo (36 [41], 18/18); Roma, Università“La Sapienza,” CIC 232, W. Arcese, F. Mandelli, G. Meloni (166 [180], 51/115); Roma, Università Cattolica, CIC 307, S. Cuore, S. Sica, G. Leone (42 [43], 17/25); Roma, Ospedale Bambino Gesu, CIC 796, G. Deb (17 [23], 0/17); Roma, Ospedale S. Camillo, CIC 287, I. Majolino, A. Locasciulli (38 [45], 2/36); Roma, Ospedale Bambino Gesu, G. De Rossi (4 [4], 3/1); San Giovanni Rotondo, Hospital Casa Sollievo Sofferenza (onco), CIC 314, G. Lelli (12 [12], 0/12); San Giovanni Rotondo, Hospital Casa Sollievo Sofferenza (hem), CIC 526, M. M. Greco, A. Carella (29 [31], 6/23); San Giovanni Rotondo, Hospital Casa Sollievo Sofferenza (peds), CIC 350, P. Paolucci (8 [13], 0/8); Siena, Ospedale Sclavo, CIC 321, F. Lauria (21 [22], 3/18); Taranto, Ospedale Nord, CIC 332, P. Mazza, G. Palazzo, B. Amurri (48 [58], 8/40); Taranto, Ospedale SS Annunziata, Dr Pezzella (0 [0], 0/0)†; Torino, S. Giovanni Antica Sede Hospital, M. Airoldi (0 [0], 0/0)†; Torino, Ospedale Mauriziano Umberto 1, CIC 377, M. Aglietta, A. Capaldi; G. Garetto (19 [26], 0/19); Torino, University Hospital of Turin, Magg San Giovanni Battista, CIC 231a, M. Falda, F. Locatelli, E. Gallo (95 [101], 52/43); Torino, Department of Pediatrics, University, CIC 305, E. Madon, F. Fagioli (33 [38], 12/21); Torino, Ospedale S Giovanni, CIC 696, M. Boccadoro, M. Massaia, C. Tarella, B. Bruno, D. Caracciolo, A. Pileri (5 [10], 5/10)†; Trieste, Istituto per I'Infanzia, Clinical Pediatrica, M. Andolina, A. de Manzini (19 [21], 10/9); Udine, Policlinico Universitario, CIC 705, A. Sperotto, R. Fanin (65 [74], 19/46); Venezia, Ospedale Civile Riuniti di Venezia, CIC 502, T. Chisesi, M. Vespignani, M. Chinello (17 [28], 0/17); Verbania Pallanza, Ospedale di Verbania, CIC 385, M. Bersi (6 [10], 0/6); Verona, Policlinico di Borgo Roma (hem, onco), CIC 623+CIC 514, G. Perona, F. Benedetti, G. Cetto (40 [52], 13/27); Vicenza, Ospedale S Bortolo (hem), CIC 797, R. Raimondi, F. Rodeghiero (54 [63], 18/36).
Latvia
No report.
Liechtenstein
No report.
Lithuania (1 team; 7 [7], 2/5)
Vilnuis, University Hospital (hem), I. Trociukas (7 [7], 2/5).
Luxemburg (2 teams; 5 [6], 0/5)
Centre Hospitalier, M. Dicato ()*; Esch-Alrette, Hopital de la Ville Esch/Alzette, CIC 545, F. Le Moine (5 [6], 0/5).
Macedonia:(1 team; 5 [5], 1/4)
Skopje, Medical Faculty (haem), B. Georgievski (5 [5], 1/ 4).
Malta
No report.
Moldova
No report.
Monaco
No report.
Netherlands (15 teams; 499 [528], 323/267)†
Amsterdam, Free University Hospital (Haem), CIC 588, G. M. Ossenkoppele (60 [64], 15/45); Amsterdam, Free University Hospital (onco), CIC 380, E. van der Wall (0 [0], 0/0); Amsterdam, Academic Medical Center (ads, peds), CIC 247, J. van der Lelie, H. van den Berg (peds) (23 [25], 8/15); Amsterdam, The Netherlands Cancer Institute, CIC 976, S. Rodenhuis J. Baars (13 [20], 0/13); Enschede, The Medisch Spectrum Twente, CIC 360, Dr. Schaafsma (15 [15], 0/15); Groningen, University Hospital (onco), CIC 395, E. de Vries ()*; Groningen, University Hospital (hem), CIC 546, E. Vellenga (29 [29], 0/29); The Hague, Leyenburg Hospital, CIC 547, P. W. Wijermans (13 [13], 0/13); Leiden, University Medical Centre (ads, peds), CIC 203, J. Vossen, R. Willemze (70 [75], 52/18); Maastricht, University Hospital (haem, onco), CIC 565, H. C. Schouten, J. Wagstaff (36 [37], 21/15); Nieuwegein, St Antonius Hospital, CIC 200, D. Biesma, G. Veth, O. de Weerdt () reported with CIC 239 Nijmegen, University Hospital (ads, peds, onco), CIC 237, A. Schattenberg, L. Beex, P. Hoogerbrugge (81 [85], 50/31)†; Rotterdam, Dr Daniel den Hoed Cancer Center, CIC 246, J. J. Cornelissen (72 [73], 30/42); Utrecht, University Hospital (ads and peds), CIC 239, L. F. Verdonck, N. M. Wulffraat, D. Biesma (81 [86], 56/25); Zwolle, Isala Klinieken/Sophia Ziekenhuis, CIC 548, M. von Marwijk Kooy (6 [6], 0/6).†
Norway (5 teams; 120 [132], 45/75)
Bergen, Haukelands Sjukhus, P. Ernst (9 [9], 0/9); Oslo, Rikshospitalet, CIC 235, D. Albrechtsen, L. Brinch (58 [58], 45/13); Oslo, The Norwegian Radium Hospital, CIC 782, S. Kvaloy (32 [44], 0/32); Oslo, Ullevals Sjukhus (haem), F. Wisslöf, J-M.Tangen (14 [14], 0/14); Trondheim, Regionsjukhuset, J. Hammerstrom, A. Waage (7 [7], 0/7).
Poland (14 teams; 581 [646], 201/380)
Gdansk, Medical University, CIC 799, A. Hellmann (52 [53], 18/34); Katowice, Silesian Medical Academy, CIC 677, J. Holowiecki (139 [149], 39/100); Krakow, CMUJ, CIC 553, A. Skotnicki (41 [47], 10/31); Lublin, Ped Hem Onco, CIC 678, J. Kowalczyk (20 [20], 6/14); Lublin, University Medical School, CIC 695, A. Dmoszynska, M. Wach, A. Walter-Croneck, W. Legiec (28 [31], 0/28); Poznan, Medical Academy, CIC 730, J. Hansz (66 [73], 38/28); Poznan, Institute of Pediatrics, CIC 641, J. Wachowiak (21 [21], 16/5); Warsaw, Central Clinical Hospital, Military Medical Academy, CIC 816, K. Sulek (19 [19], 7/12); Warsaw, Central Military Hospital (onco), CIC 824, C. Szczylik (11 [12], 0/11); Warsaw, Maria Sklodowska-Curie, Centre of Oncology, CIC 800, J. Walewski (41 [43], 3/38); Warsaw, Institute of Haematology and Blood Transfusion, CIC 693, B. Marianska, L. Konopka (13 [15], 0/13); Warsaw, Central Clinical Hospital, CIC 954, W. Wiktor-Jedrzejczak, A. Dzwigala, M. Rokicka-Piotrowicz (28 [40], 8/20); Wroclaw, University of Medicine, Department of Children, CIC 817, J. Boguslawska-Jaworska (45 [55], 23/22); Wroclaw, K. Diuske Hospital, CIC 538, A. Lange (57 [68], 33/24).
Portugal (6 teams; 206 [230], 70/136)
Coimbra, University Hospital, CIC 164, N. Costa (15 [15], 0/15); Lisbon, Instituto Portugues de Oncologia, CIC 300, M. Abecasis, F. Leal Costa (68 [72], 22/46); Lisbon, Hospital de Santa Maria, CIC 636, J. Alves do Carmo, F. de Lacerda (39 [41], 20/19); Lisboa, Hospital dos Capuchos, A. Botelho de Sousa (9 [9], 0/9); Porto, Instituto Portugues de Oncologia, CIC 291, P. Pimentel, F. Campilho (64 [82], 28/36); Porto, Hospital S Joao (hem. onco), CIC 329 + CIC 572, F. Principe, J. E. Guimaraes (11 [11], 0/11).
Romania (1 team; 0 [0], 0/0)
Bucharest, Fundeni University Hospital, CIC 296, A. D. Moicean, D. Colita, C. Arion (0, 0/0)* starting in 2001.
Russia (14 teams; 190 [199], 48/142)
Ekaterinburg, City Hospital No 7, L. B. Filatov (4 [4], 0/4); Ekaterinburg, Regional Hospital No 1, T. S. Konstantinova, V. A. Shalaev (8 [10], 0/8); Moscow, Institute of Biophysics, A. E. Baranov (10 [10], 3/7); Moscow, Cancer Research Center, CIC 757, V. Ptuschkin (16 [17], 0/16); Moscow, Cancer Research Center peds Hem/onco, G. Mentrevich (18 [22], 6/12); Moscow, Russian Children's Hospital, CIC 694, A. Maschan, E. Skorobogato, E. Pachanov (20 [20], 9/11); Moscow, Research Hematology Center of RAS, V. Savtchenko (28 [29], 16/12); Novosibirsk, Institute of Clinical Immunology, CIC 376, I. Lisukov (3 [3], 0/3); Samara, Regional Hospital, V. A. Rossiev (32 [32], 2/30); St Petersburg, Research Institute of Hematology, CIC 724, K. M. Abdulkadirov (10 [10], 4/6); St Petersburg, Military Medical Academy, CIC 520, A. Novik (5 [5], 0/5)†; St Petersburg, Clinical Center for Advanced Medical Tech, CIC 370, E. Podoltseva, V. Soldatenkov, O. Ryasnyanskaya (16 [16], 3/13); St Petersburg, State Pavlov Medical University, CIC 725, B. V. Afanassiev, L. Zubarovskaya (20 [21], 5/15); Yaroslavl, City Hospital No. 8, V.A. Lapin ()*.
San Marino
No report
Slovakia (4 teams; 107, [117] 26/81)
Bansra Bystrica, Roosevelt Hospital, CIC 333, K. Mocikova (21 [28], 0/21); Bratislava, 2nd Children's Clinic, University Hospital, J. Lukac (19 [20], 10/9); Bratislava, University Hospital, CIC 610, M. Mistrik (25 [27], 14/11); Bratislava, National Cancer Institute, CIC 560, J. Lakota (42 [42], 2/40).
Slovenia (1 team; 18 [21], 8/10)
Ljubljana, University Medical Centre, CIC 640, J. Pretnar (18 [21], 8/10).
Spain (77 teams; 1916 [2036], 451/1465)
Alicante, Hospital General, C. Rivas-Gonzales (11 [11], 0/11); Barcelona, Instituto de Oncologia Corachan, D. Alfonso-Modolell (5 [5], 0/5); Barcelona, Santa Creu I Sant Pau (adults), CIC 260, J. Sierra, S. Brunet (78 [85], 26/52); Barcelona, Santa Creu I San Pau (peds), CIC 260, I. Badell Serra, J. Cubells-Riero (9 [9], 5/4); Barcelona, Santa Creu I Sant Pau (onco), CIC 260, Dr J. J. Lopez, C. Solia ()*; Barcelona, Hospital Sant Joan de Deu, CIC 668, J. Estella Aguado (12 [12], 0/12); Barcelona, Hospital Duran i Reynals (Hem), Institut Catala d'Oncologia, CIC 759, A. Granena, C. Ferra, J. Berlanga (54 [58], 10/44); Barcelona, Hospital General “Vall d'Hebron,” CIC 583, A. Julia Font (24 [25], 4/20); Barcelona, Hospital Mutua de Terrasa (hem-onco), CIC 556, J. Marti (7 [7], 0/7); Barcelona, Hospital Universitario Germans Trias i Pujol, CIC 613, J. Rivera (35 [35], 0/35); Barcelona, Sant Cugat del Vallés, Hospital General de Cataluna, M. Sureda-Gonzales (2 [2], 0/2); Barcelona, Hospital M Infantil, CIC 527, J. Ortega (44 [45], 32/12); Barcelona, Hospital Clinic, CIC 214, E. Montserrat, E. Carreras (112 [116], 42/70); Barcelona, Instituto Hematologico Torre Vilana, CIC 777, P. Vivancos (5 [5], 0/5); Barcelona, Instituto Dexeus (hem), CIC 670, A. Granena, J. Sarra, J. Garcia (3 [3], 0/3); Caceres, Hospital San Pedro de Alcantara, J. Bergua Burgues (10 [10], 0/10); Cadiz, Hospital del SAS de Jérez, A. Leon (26 [28], 6/20); Cadiz, Hospital Universitario “Puerta del Mar,” CIC 679, J. Gil (20 [25], 0/20); Canary Isles, Las Palmas, Hospital Insular, CIC 335, F. Fernandez-Fuentes, J. Gonzalez-San Miguel (13 [14], 0/13); Canary Isles, Las Palmas, Hospital Materno-Infantil (haem, onco), J. Lodos Rojas, A. Molinés (3 [3], 0/3); Canary Isles, Las Palmas, Hospital General de Gran Canaria, T. Molero, R. Mataix, C. Campo, T. Negrin (36 [39], 18/18); Canary Isles, Teneriffe, Hospital Universitario de Canarias, L. Hernandez Nieto, M. T. Hernandez Garcia (15 [16], 0/15); Canary Isles, Teneriffe, Hospital Nuestra Senora de la Candelaria, P. Rios Ru (12 [12], 0/12); Castellon de La Plana, Hospital General de Castellon (haem), R. Garcia-Boyero (4 [6], 0/4); Cordoba, Hospital de la Cruz Roja de Cordoba (haem), J-M. Garcia-Castellano (2 [2], 0/2); Cordoba, Hospital Reina Sofia, CIC 238, A. Torres Gomez (46 [50], 28/18); Cruces-Barakaldo, Hospital de Cruces, I. Zuazua-Verde (34 [37], 0/34); Galdakao, Hospital de Galdakao, Hem, J. Ojanguren, K. Atucha (12 [12], 0/12); Granada, Hospital Virgen de la Nieves, J. M. de Pablos Gallego (22 [23], 4/18); Jaen, Hospital Cuidad de Jaen (haem), Dr Alcalo, (10 [10], 0/10); La Coruna, Complexo Hospitalario Juan Canalejo, F. J. Batlle, C. Ramirez, P. Torres, R. Varela (28 [28], 3/25); Lérida, Hospital Arnan de Villanova, J. Macia (10 [10], 0/10); Lugo, Hospital Xeral-Calde, M. Gonzales-Lopez (8 [8], 0/8); Madrid, Clinica La Luz, H. Cortés-Funes, J. Hornedo (0 [0], 0/0); Madrid, Clinica Moncloa (hem), J. M. Fernandez, Q. Escudero (17 [17], 0/17); Madrid, Hospital Universitario de Getafe (hem), F. Oña Compan, N. Somolinos, (12 [12], 0/12); Madrid, Hospital Universitario San Carlos, CIC 733, J. Diaz Mediavilla, L. Llorente (30 [30], 0/30); Madrid, Hospital Universitario San Carlos, Onco, CIC 733, M. Martin, E. Diaz-Rubio, A. Casado, J. A. Lopez-Martin (7 [9], 0/7); Madrid, Hospital Ruber Internacional, J. Diaz Mediavilla ()*; Madrid, Unidad de TMO-ONC 4, Hospital Gregorio Maranon, CIC 819, J.L. Diez Martin (31 [34], 14/17); Madrid, Clinica Ruber, J.M. Fernandez-Ranada, Q. Escudero (19 [19], 0/19); Madrid, Hospital de la Princesa, CIC 236, J. M. Fernández Rañada, A. Figuera, A. Alegre (78 [83], 35/43); Madrid, Clinica Puerta de Hierro, CIC 728, M. N. Fernandez (30 [34], 20/10); Madrid, Hospital General La Paz (ads), F. Hernandez Navarro, M. Canales (55 [55], 5/50); Madrid, Hospital Doce de Octubre, CIC 382, J. J. Lahuerta (hem), H. Cortés Funes (onco), J. Lopez Perez (peds) (60 [61], 7/53); Madrid, Hospital Nino Jesus, L.M. Madero (35 [35], 13/22); Madrid, Hospital La Paz Infantil, CIC 734, A. Martinez-Rubio, A. Sastre, P. Garcia-Miguel (15 [15], 6/9); Madrid, Hospital Ramon y Cajal (peds), CIC 615, A. Munoz Villa, M. S. Maldonado (12 [12], 5/7); Madrid, Hospital Ramon y Cajal (ads), CIC 615, J. Odriozola, J. Pérez de Oteyza, J. Lopez, J. Garcia Larana (38 [42], 7/31); Madrid, Fundacion Jimenez Diaz, J. Tomas, C. Paniagua, F. Lobo (20 [23], 4/16); Madrid, Hospital Militar Gomez Ulla, F. Sancho-Cuesta, S. Enrech-Frances (6 [6], 0/6); Malaga, Hospital Regional, CIC 576, J. Maldonado (33 [34], 16/17); Murcia, Hospital Virgen de la Arrivxaca, CIC 323, R. Candel Parra (12 [13], 0/12); Murcia, Hospital General Uni. Morales Meseguer, CIC 735, J. M. Moraleda, V. Vicente-Garcia, I. Heras (29 [31], 9/20); Orense, Hospital Cristal-Pinor (hem), J-L. Sastre-Moral (9 [10], 0/9)†; Oviedo, Hospital Covadonga, CIC 642, D. Carrera Fernandez, C. Rodriguez Pinto (35 [37], 6/29); Palma de Mallorca, Hospital Son Dureta, CIC 722, J. Besalduch, H. S. Dureta (24 [26], 4/20); Palma de Mallorca, Policlinica Miramar, J. Besalduch, A. Sampol (10 [10], 0/10); Pamplona, Hospital Provincial de Navarra, CIC 577, E. Pérez Equiza, M. J. Uriz Pascual, J.Gastearena (23 [23], 0/23); Pamplona, Clinica Universitario de Navarra, CIC 737, J. Rifon (14 [17], 3/11); Pontevedra, Hospital Montecelo, CIC 549, M. Constela (17 [17], 0/17); Salamanca, Complejo Hospital, CIC 727, D. Caballero (71 [87], 20/51); San Sebastian, Hospital Nostra Senora de Aranzazu, CIC 598, J. Marin, D. Martinez (35 [42], 6/29); Santander, Hospital Universitario M. de Valdecilla, CIC 242, A. Iriondo, E. Conde, E. Bureo, A. Zubizarreta-Pina (55 [56], 21/34); Santiago de Compostela, Hospital Xeral de Galicia, CIC 570, J. L. Bello (17 [18], 3/14); Sevilla, Hospital Universitario Virgen del Rocio, CIC 769, J. M. Rodriguez Fernandez (52 [52], 16/36); Sevilla, Clinica Del Sagrado Corazon, J. M. Rodriguez ()*; Tarragona, Hospital de Tarragona Joan XXlll (hem), A. Llorente Cabrera (11 [11], 0/11); Valencia, Hospital Universitario La Fe (peds), CIC 653, V. Castel, A. Verdeguer (16 [17], 4/12); Valencia, Hospital Clinico Universitario, CIC 282, J. Garcia-Conde, C. Solano (76 [80], 11/65); Valencia, Instituto Valenciano de Oncologia, V. Guillen, J. Palau (15 [17], 0/15); Valencia, Hospital Universitario La Fe, CIC 663, M. A. Sanz, G. F. Sanz (69 [81], 33/36); Valencia, Hospital Doctor Peset (hem), P. Ribas Garcia (11 [11], 0/11); Valladolid, Hospital Rio Hortega, J. Garcia Frade (16 [16], 0/16); Vigo, Hospital Xeral-Cies, A. Martinez-Dalmau (18 [21], 3/15); Zaragoza, Hospital Miguel Servet (hem and onco) M. Giralt, G. Pérez-Lugmus, D. Rubio-Félix, A. Anton (29 [29], 2/27); Zaragoza, Clinico Universitario Lozano Blesa (Haem, onco), A. Tres, L.Palomera, M. Gutierrez, J. Mayordomo, (42 [42], 0/42).
Sweden (10 teams; 450 [513], 186/264)
Goteborg, Medical Clinic, CIC 715, M. Brune (82 [108], 23/59); Goteborg, East Hospital, CIC 289, A. Fasth, S. Rodjer (16 [21], 10/6); Huddinge, Hospital, CIC 212, P. Ljungman (114 [127], 79/35); Linköping, University Hospital, CIC 740, G. Juliusson (43 [43], 19/24); Lund, University Hospital, CIC 283, A. N. Bekassy (54 [61], 15/39); Malmö, University Hospital, I. Turesson (6 [6], 0/6); Örebro, Medical Center Hospital, CIC 738, U. Tidefelt (7 [7], 0/7); Stockholm, Karolinska Hospital, CIC 626, M. Björkholm (26 [30], 0/26); Umea, Norrland University Hospital, CIC 731, A. Wahlin, P. Hörnsten, J. Lindh, L. Eliasson (34 [36], 13/21); Uppsala, University Hospital, CIC 266, B. Simonsson, K. Carlson, G. Oberg (68 [74], 27/41).
Switzerland (10 teams; 293 [376], 95/198)
Aarau, Kantonsspital, CIC 316, M. Wernli (11 [11], 0/11); Basel, Kantonsspital, CIC 202, A. Gratwohl, T. Kühne, R. Herrmann (60 [80], 40/20); Bellinzona, Ospedale San Giovanni, CIC 829, F. Cavalli, M. Ghielmini, L. Leoncini (10 [14], 0/10); Berne, Inselspital, CIC 221, A. Tobler, K. Leibundgut, M. Fey (27 [31], 0/27); Geneva, Hôpital Cantonal Universitaire, CIC 261, B. Chapuis, P. Wacker (25 [27], 22/3); Lausanne, CHUV, CIC 820 + CIC 579, D. Schapira, T. Kovacsovics, S. Leyvraz, N. Ketterer, N. Nenadov-Beck (50 [65], 0/50); St Gallen, Kantonsspital, CIC 324, U. Hess (8 [8], 0/8); Zurich, University Hospital (ads, hem/onco), CIC 208, U. Schanz, J. Halter, R. Stahel, L. Jost (82 [107], 26/56); Zurich, University Hospital (peds), CIC 334, R. Seger (12 [15], 7/5); Zurich, Klinik Im Park, J. Gmür, U. Breitenstein, A. von Rohr (8 [18], 0/8).
Turkey (24 teams; 403 [413], 182/221)
Ankara, Numune Education and Research Hospital, CIC 691, T. Demirer (64 [64], 19/45); Ankara, Ibn-i Sina Hospital, CIC 617, H. Koc (52 [53], 41/11); Ankara, Hacettepe University, Institute of Oncology Hematopoietic Stem Cell Transplantation Unit CIC 292, E. Kansu, C. Akyüz (12 [12], 0/12); Ankara, Children's Hospital Hacettepe University, A.Tuncer, D. Uckan (19 [20], 19/0); Ankara-Sihhiye, Hacettepe University Medical School (hem), CIC 168, O. Ozcebe (0 [0], 0/0), starting in 2001; Ankara-Besevler, Gazi University (hem), CIC 169, R. Haznedar (0 [0], 0/0)†; Ankara, University of Ankara (peds), CIC 620, E. Unal (4 [5], 4/0); Ankara-Etlik, GATA BMT Center, CIC 372, A. Yalcin, F. Arpaci, A.Özet, C. Beyan, A. Ural (44 [45], 15/29); Antalya, Akdeniz University hospital, CIC 618, M.A. Yesilipek, V. Hazar, O. Yegin (12 [12], 11/1); Antalya, Akdeniz University Hospital, CIC 685, L. Undar (14 [14], 10/4); Balcali, Hospital, CIC 821, A. Tanyeli (5 [5], 4/1); Eskisehir, Osmangazi University, CIC 686, Z. Güblas (7 [7], 2/5); Istanbul, Marmara University, Altunizade, CIC 714, S. Ratip, T. Akoglu (8 [9], 4/4); Istanbul, Maltepe Medical Faculty, CIC 210, K. Ozerkan, A. Tamkan (0 [0], 0/0) starting in 2004; Istanbul, Cerrahpasa Medical School, CIC 761, B. Ferhanoglu, T. Soysal, Z. Baslar (12 [12], 6/6); Istanbul, Tip Fakultesi, CIC 762, G. Gedikoglu (23 [23], 13/10); Istanbul, University of Istanbul, CIC 760, S. Kalayoglu-Besisik (30 [34], 17/13); Istanbul, GATA Haydarpasa Egitim Hst, CIC 687, A. Öztürk (3 [3], 1/2)†; Istanbul, Institute of Oncology, CIC 689, H. Onat (12 [12], 0/12); Izmir, Ege University Medical Faculty (ads), CIC 628, S. Cagirgan (48 [48], 5/43); Izmir, Ege University Medical Faculty (peds), CIC 621, S. Kansoy (8 [8], 5/3); Izmir, Dokuz Eylul University, CIC 688, U. Yilmaz (3 [3], 0/3); Kayseri, Erciyes University Hospital, CIC 627, A. Unal, M. Cetin, (17 [17]. 2/15); Trabzon, Karadeniz Technical University, CIC 170, E. Ovali (6 [7], 4/2).
Ukraine (1 team; 12 [13], 1/12)
Kiev, Kiev Regional Oncologic Hospital, S. Donska, O. Ryzhak (12 [13], 1/12).
United Kingdom (54 teams; 2009 [2184], 787/1222)
Aberdeen, The Royal Infirmary, CIC 344, D.J. Culligan (19 [20], 5/14); Bangor, Gwynedd Hospital, CIC 736, H. Parry (8 [8], 0/8); Bath, Royal United Hospital, CIC 619, J. G. Smith (6 [6], 0/6); Belfast, Royal Victoria Hospital, CIC 268, F. Jones, M. F. McMullin, P. Burnside (15 [16], 9/6); Belfast, City Hospital, CIC 753, T. C. M. Morris, L. Ranaghan (13 [15], 0/13); Birmingham, The Birmingham Childrens Hospital, CIC 781, P. J. Darbyshire, M. W. Williams (31 [36], 27/4); Birmingham, Queen Elizabeth Hospital, CIC 387, P. Mahendra, C. Craddock (91 [96], 42/49); Birmingham, Heartlands Hospital, CIC 284, D. W. Milligan (34 [40], 14/20); Bournemouth, Royal Bournemouth Hospital, CIC 765, S. Killick (11 [13], 0/11); Bristol, Royal Hospital for Sick Children, CIC 386, J. M. Cornish and Southmead Hospital, J. Hows, M. G. Rainey (84 [90], 65/19); Cambridge, Addenbrooke's Hospital and Norwich Hospital, CIC 566 + 391, R. E. Marcus, J. Craig, M. Deane (45 [46], 11/34); Cardiff, University Hospital of Wales, CIC 303, K. Wilson, C. H. Poynton, (40 [45], 11/29); Coventry, Walsgrave Hospital, N. Jackson (6 [6], 0/6); Dundee, Ninewells Hospital, CIC 719, D. Bowen (9 [9], 0/9); Edinburgh, Western General Hospital, (hem) CIC 228, J. M. Davies, P. R. E. Johnson, M. J. Mackie, P. H. Roddie (39 [39], 6/33)†; Edinburgh, Western General Hospital (onco) CIC 228, R. Leonard (0 [0], 0/0); Exeter, Royal Devon and Exeter Hospital, CIC 571, M. Joyner (13 [13], 0/13); Glasgow, Royal Infirmary, CIC 244, A. Parker, I. G. McQuaker (60 [62], 31/29); Glasgow, The Western Infirmary, CIC 325, T. Fitzsimons (30 [32], 0/30); Glasgow, Royal Hospital for Sick Children, CIC 707, B. Gibson (12 [13], 7/5); Leeds, St James's University Hospital and The General Infirmary, D. Barnard, S. Kinsey, J. A. Child (102 [106], 26/76); Leicester, Royal Infirmary, CIC 713, A. E. Hunter (51 [55], 13/38); Liverpool, Royal Liverpool University Hospital, CIC 501, R. E. Clark (48 [48], 13/35); Liverpool, Alder Hay, M. Caswell (12 [12], 10/2); London, Hammersmith and Charing Cross Hospital, CIC 205, J. M. Goldman, J. Apperley, D. Samson, C. Giles, E. Kanfer (120 [128], 45/75); London, University College Hospital, CIC 224, A. H. Goldstone (143 [167], 54/89); London Oncology Marrow Transplantation Group, CIC 263, P. J. Gravett (7 [9], 1/6); London, St George's Hospital, CIC 539, J. Marsh, S. Ball, E. C. Gordon-Smith, C. Dearden (12 [12], 7/5); London, King's College, CIC 763, A. Pagliuca, G. J. Mufti (65 [75], 41/24); London, Royal Marsden Hospital, CIC 218, R. Powles, J. Mehta (154 [182], 46/108); London, Royal Free Hospital, CIC 216, H. G. Prentice, M. Potter (61 [67], 44/17); London, St Bartholomew's, CIC 768 and the Royal London Hospital, CIC 269, A. Rohatiner, A. C. Newland (53 [55], 19/34); London, Guy's Hospital, CIC 721, S. Schey (25 [30], 8/17); London, Institute of Child Health, CIC 243, P. Veys, I. M. Hann (52 [55], 38/14); Manchester, Christie Hospital, G. Morgenstern (86 [101], 20/66); Manchester, Royal Children's Hospital, CIC 521, A. M. Will (24 [27], 13/11); Manchester, The Royal Infirmary, J. A. Yin (53 [57], 32/21); Manchester, Trafford General Hospital, P. A. Carrington (1 [1], 0/1); Manchester, Hope Hospital, P. A. Carrington (2 [2], 0/2); Newcastle upon Tyne, Royal Victoria Infirmary, CIC 276, G. H. Jackson, S. J. Proctor, P. Taylor, A. Cant, R. Skinner (84 [87], 41/43); Norwich, Norfolk and Norwich Hospital (hem), CIC 391, M. Deane (transplantations performed in 2000 at Norwich are reported through Addenbrookes CIC 566); Nottingham, City Hospital, CIC 717, N. Russell (85 [91], 43/42); Oxford, John Radcliffe Hospital, Headington, CIC 255, T. J. Littlewood, C. Bunch, C. Mitchell, C.Hatton, G. Hall, J. Wainscoat (40 [41], 14/26); Plymouth, Derriford Hospital, CIC 823, M. D. Hamon (33 [33], 11/22); Poole, Dorset Cancer Centre, CIC 580, A. Bell (20 [25], 0/20); Rotherham, General Hospital, CIC 778:5, H. Barker (1 [1], 0/1); Sheffield, The Royal Hallamshire, Weston Park and the Children's Hospitals (ads, peds, onco) CIC 778:1/2/3, E. Vandenberghe, A. Vora, P. Lorigan (45 [46], 19/26); Somerset, Taunton and Somerset Hospital, S. A. Johnson, S. Bolam (8 [8], 1/7); CRC Wessex, Southhampton, CIC 704, A. Smith, A. Duncombe, J. Sweetenham, J. Kohler (20 [20], 0/20); Stoke-on-Trent, North Staffordshire Royal Infirmary, R. Chasty (12 [12], 0/12); Sunderland, The Sunderland Royal, P. J. Carey (4 [4], 0/4); Swansea, Singleton Hospital, Sketty, S. Al Ismail (7 [8], 0/7); Swindon, Princess Margaret Hospital (hem), CIC 608, N. E. Blesing, A. Gray, S. Green, A. Koster (4 [5], 0/4); Wakefield, Pinderfield's and Pontefract Hospitals NHS Trust, CIC 764, M. C. Galvin, D. Wright (9 [9], 0/9).
Yugoslavia (Serbia and Montenegro) (4 teams; 23 [24], 9/14)
Belgrade, Clinical Centre of Serbia, CIC 373, M. Colovic, A. Bogdanovic (1 [1], 0/1); Belgrade, Mother and Child Health Institute, CIC 358, D. Makic, D. Vujic (2 [2], 0/2); Belgrade, Military Medical Academy, CIC 582, M. Malesevic (20 [21], 9/11); Novi Sad, Institute of Internal Diseases, CIC 655, D. Pejin ().*
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-03-0675.
Supported in part by a grant from the Swiss National Research Foundation, 32-52756.97, the Swiss Cancer League, and the Horton Foundation. EBMT is supported by grants from the corporate members: Hoffmann-La Roche Ltd, Amgen Europe, Chugai Rhone-Poulenc Rorer, Baxter, Astra, Cobe International, Nextar, Liposome, Imtix, Octapharma, Stem Cell Technologies, ICN Pharmaceuticals, and Bristol-Meyers Squibb.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alois Gratwohl, Kantonsspital Basel, Division of Hematology, Department of Internal Medicine, CH-4031 Basel, Switzerland; e-mail: hematology@uhbs.ch.