Monoclonal chronic lymphocytic leukemia (CLL)–phenotype cells are detectable in 3.5% of otherwise healthy persons using flow cytometric analysis of CD5/CD20/CD79b expression on CD19-gated B cells. To determine whether detection of such CLL-phenotype cells is indicative of an inherited predisposition, we examined 59 healthy, first-degree relatives of patients from 21 families with CLL. CLL-phenotype cells were detected in 8 of 59 (13.5%) relatives, representing a highly significant increase in risk (P = .00002). CLL-phenotype cell levels were stable with time and had the characteristics of indolent CLL. Indolent and aggressive clinical forms were found in family members, suggesting that initiation and proliferation involves distinct factors. The detection of CLL-phenotype cells provides a surrogate marker of carrier status, potentially facilitating gene identification through mapping in families and direct analysis of isolated CLL-phenotype cells.
Introduction
The etiology of chronic lymphocytic leukemia (CLL) is largely unknown; however, several studies have reported families with an increased risk for CLL and other non-CLL lymphoproliferative disorders, indicative of an inherited predisposition.1-4 The detection of a CLL-associated marker in healthy relatives of patients with familial CLL may provide a surrogate marker of inherited predisposition, assisting in the identification of causative gene mutations. It is possible to identify markers of some B-cell malignancies in healthy persons, including the follicular lymphoma-associated t(14;18) translocation5,6and the neoplastic plasma cells common to myeloma and monoclonal gammopathy of undetermined significance (MGUS).7,8 We have previously reported a flow cytometry technique for quantifying CLL cells when they represent as few as 0.5% of B cells, using the higher CD5 and lower CD20/CD79 expression by the neoplastic cells.9 Application of this technique to 910 control subjects with normal hematologic parameters and no evidence of malignant disease detected CLL-phenotype cells in 3.5%.10We report here the frequency of CLL-phenotype cells in 59 healthy, first-degree relatives of affected patients in 21 CLL families.
Study design
Twenty-one families with 2 or more members who had CLL were ascertained through clinicians in the United Kingdom. Diagnoses of CLL were based on standard criteria.11 Fifty-nine healthy, first-degree relatives of a family member with CLL were studied, with repeat samples assessed in 38 of 59 relatives. Median age of relatives was 47 years (range, 23-86 years). Samples were also examined from 23 healthy spouses whose median age was 45 years (range, 23-79 years). The prevalence of CLL-phenotype cells in familial CLL relatives was compared to a sample of 910 persons reported previously.10 The age-adjusted odds ratio was derived from logistic regression analysis, and distribution of continuous variables was compared with Wilcoxon or Mann-Whitney U test using STATA version 6.0 (Stata, College Station, TX). Samples were obtained with informed consent and Ethical Review Board approval from the Royal Marsden National Health Service (NHS) Trust.
CLL-phenotype cells were enumerated as reported.10Briefly, 106 leukocytes isolated from peripheral blood were incubated with: (1) CD20 fluorescein isothiocyanate (FITC), CD79b phycoerythrin (PE), CD19 Cy5/PE, and CD5 allophycocyanin (APC); (2) anti-kappa FITC, anti-lambda PE, CD19 Cy5/PE, and CD5 APC. Total B lymphocytes were identified by their CD19 and light-scatter characteristics. Samples containing cells that represented more than 50 events in all 3 so-called CLL regions were subjected to extended phenotyping. Cells were incubated with CD19 Cy5/PE, CD20 APC, CD5 PE or FITC, and either CD11a FITC, CD22 PE, CD23 PE, CD27 FITC, CD38 PE, kappa FITC, lambda PE, or FMC7 FITC. Antibodies were either prepared in-house or were supplied by BD Biosciences (Oxford, United Kingdom), Immunotech (Marseilles, France), Serotec (Oxford, United Kingdom), or Chemicon (Harrow, United Kingdom). Samples were only classified as having a population of CLL-phenotype cells if the phenotype was consistent with clinical CLL for the antigens: CD5 (positive), CD20 (weak or negative), CD79b (weak or negative), CD22 (weak), FMC7 (weak).12
Results and discussion
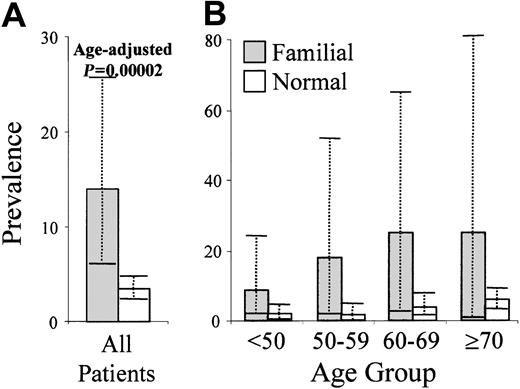
CLL-phenotype cells were detected in 8 of the 59 relatives from 7 families (Table 1). Absolute numbers were, on average, 1000-fold lower than the levels required for a clinical diagnosis of CLL (median, 5 cells/μL; range, 3-127 cells/μL) and were similar to the levels detected in the outpatient survey (median, 13 cells/μL; range, 2-1458 cells/μL;P = .07).10 The observation that 13.5% of relatives harbor CLL-phenotype cells translates to a 7-fold increase in risk for subclinical disease (odds ratio [OR], 6.6; 95% confidence interval [CI], 2.7-16.0; P = .00002, Figure1). CLL-phenotype cells were only detected in 1 of 23 unrelated family members, a prevalence not significantly different from that in the outpatient group (P > .1). This patient came from a different family than the affected relatives. The highly significant increase in risk for family members indicates that CLL-phenotype cells represent a surrogate marker of carrier status in CLL families.
Age, sex, and hematologic parameters of relatives with detectable B-cell expansions
| ID . | Sex . | Phenotype . | Age, y . | Monoclonal B-cell count (cells/μL) . | Leukocyte count (109/L) . | Hemoglobin (g/dL) . | Platelet count (109/L) . |
|---|---|---|---|---|---|---|---|
| 110205 | F | CLL | 62 | 2.5 | 10.8 | 13.1 | 267 |
| 267204 | M | CLL | 53 | 2.7 | 6.1 | 15.1 | 223 |
| 267207 | M | CLL | 49 | 2.7 | 9.1 | 15.3 | 269 |
| 001202 | M | CLL | 87 | 4.5 | 8.2 | 12.8 | 749 |
| 096203 | F | CLL | 57 | 6.3 | 8.8 | 12.5 | 244 |
| 227302 | F | CLL | 35 | 6.6 | 8.5 | 13.0 | 208 |
| 019303 | M | CLL | 29 | 9.2 | 7.3 | 16.4 | 281 |
| 060205 | F | CLL | 68 | 126.9 | 7.7 | 12.3 | 446 |
| 18203 | M | Non-CLL | 81 | 54.0 | 5.4 | 12.7 | 163 |
| Spouse | M | CLL | 65 | 20.8 | 6.8 | 15.0 | 244 |
| ID . | Sex . | Phenotype . | Age, y . | Monoclonal B-cell count (cells/μL) . | Leukocyte count (109/L) . | Hemoglobin (g/dL) . | Platelet count (109/L) . |
|---|---|---|---|---|---|---|---|
| 110205 | F | CLL | 62 | 2.5 | 10.8 | 13.1 | 267 |
| 267204 | M | CLL | 53 | 2.7 | 6.1 | 15.1 | 223 |
| 267207 | M | CLL | 49 | 2.7 | 9.1 | 15.3 | 269 |
| 001202 | M | CLL | 87 | 4.5 | 8.2 | 12.8 | 749 |
| 096203 | F | CLL | 57 | 6.3 | 8.8 | 12.5 | 244 |
| 227302 | F | CLL | 35 | 6.6 | 8.5 | 13.0 | 208 |
| 019303 | M | CLL | 29 | 9.2 | 7.3 | 16.4 | 281 |
| 060205 | F | CLL | 68 | 126.9 | 7.7 | 12.3 | 446 |
| 18203 | M | Non-CLL | 81 | 54.0 | 5.4 | 12.7 | 163 |
| Spouse | M | CLL | 65 | 20.8 | 6.8 | 15.0 | 244 |
Prevalence of monoclonal CLL-phenotype cells in relatives of familial CLL index cases compared with the general population.
(A) Highly significant overall difference (P is age-adjusted). (B) Increase in prevalence in the familial relatives is seen at all age groups, but it is not significant because of the numbers of relatives available for assessment.
Prevalence of monoclonal CLL-phenotype cells in relatives of familial CLL index cases compared with the general population.
(A) Highly significant overall difference (P is age-adjusted). (B) Increase in prevalence in the familial relatives is seen at all age groups, but it is not significant because of the numbers of relatives available for assessment.
Repeat analysis of most of the relatives was performed in a masked fashion to examine whether aberrant cells were persistently detectable. Median time between sampling was 18 weeks (range, 13-25 weeks). Six of the 8 relatives with detectable CLL-phenotype cells were reassessed, and all were positive at second assessment. The levels of CLL-phenotype cells were not different between the 2 time points (P = .1), with the second assessment level representing a median 85% of the initial level (range, 30%-112%). Long-term follow-up is required to determine whether the relationship between the CLL-phenotype cells and clinical disease follows a pattern similar to that seen in MGUS and myeloma.13 14 However, the fact that the levels are stable with time suggests that the generation of CLL-phenotype B cells is governed by genetic mechanisms different from their proliferative potential.
CLL-phenotype cells were not detectable in 38 of the relatives assessed at both time points. However, in 2 relatives, CLL-phenotype cells were detectable at either first or second assessment only. Levels were low in each (1.4/μL and 1.9/μL, respectively), below the range found in the outpatient study. As these relatives were not categorized as having CLL-phenotype cells, our estimate of the prevalence of this phenotype in relatives is likely to represent an underestimate of the true risk.
Indolent forms of clinical CLL are characterized by a high degree of immunoglobulin H (IgH) hypermutation and a low level of CD38 expression.15-18 CLL-phenotype cells present in otherwise healthy persons show these characteristics.10 CD38 expression was also undetectable on the CLL-phenotype cells from the affected relatives. CD38+ CLL-phenotype cells are presumably not seen in subclinical form because such clones would expand rapidly and present as clinical disease. However, aggressive CD38+ and indolent CD38− forms of clinical disease were present in the familial patients with clinical disease. This also indicates that proliferative potential is regulated separately from the oncogenic process and may have to do with the stage of differentiation in the B cell undergoing neoplastic transformation. Recent data have shown that CLL cells are most similar to memory B cells.19 As in clinical disease, the CLL-phenotype cells in healthy persons and familial CLL relatives all express CD27, which is normally restricted to postgerminal–center B cells.20In addition, the level of bcl-2 expression in the CLL-phenotype cells is equivalent to that of normal circulating memory B cells, a level approximately twice that of naive B cells (median, 1.9-fold higher;P = .018). This supports the hypothesis that all CLL-phenotype cells are derived from memory B cells. Additional studies are warranted to determine whether clinical features relate to particular memory B-cell subsets from which the neoplastic cells are derived.
In addition to detecting a CLL-phenotype in 8 relatives, a non–CLL-phenotype monoclonal B-cell population was detected in a relative from another family, with an extended phenotype suggestive of marginal zone lymphoma. Non–CLL-phenotype monoclonal B-cell expansions were also detected in 9 of 910 outpatients. The detection of subclinical disease in myeloma, follicular lymphoma, and now CLL, as well as other B-cell disorders, suggests that all common chronic lymphoproliferative disorders have a premalignant counterpart. Furthermore, the finding that all these lymphoproliferative disorders appear within certain families at the clinical and the subclinical levels raises the possibility of a common genetic predisposition to the development of B-cell malignancies. As in MGUS and myeloma, comparison of cells from patients with subclinical, indolent, and progressive disease should allow identification of some of the genetic factors responsible for oncogenesis and disease progression.21-23Application of our observation should facilitate identification of CLL genes through mapping in families and direct analysis of isolated CLL-phenotype populations.
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-03-0892.
Supported by grants from the Leukaemia Research Fund and Yorkshire Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard S. Houlston, Section of Cancer Genetics, Institute of Cancer Research, Surrey, United Kingdom; e-mail:r.houlston@icr.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal