Erythropoietin (Epo) and its receptor (EpoR) are indispensable to erythropoiesis. Although roles besides angiogenesis, such as neuroprotection and heart development, have been reported for the Epo-EpoR system, the precise contribution of Epo-EpoR to these nonhematopoietic tissues requires clarification. Exploiting aGATA-1 minigene cassette with hematopoietic regulatory domains, we established 2 lines of transgene-rescued EpoR-null mutant mice expressing EpoR exclusively in the hematopoietic lineage. Surprisingly, despite the lack of EpoR expression in nonhematopoietic tissues, these mice develop normally and are fertile. As such, we could exploit them for analyzing the roles of the Epo-EpoR system in adult hematopoiesis and in nonhematopoietic tissues. These rescued lines showed a differential level of EpoR expression in erythroid cells; one expressed approximately 40%, and the other expressed 120% of the wild-type EpoR level. A colony formation assay showed that erythroid progenitors in the 2 mutant lines exhibit distinct sensitivity to Epo. The circulating Epo level was much higher in the transgenic line with a lower EpoR expression. In response to induced anemia, the plasma Epo concentrations increased in both lines. Notably, the timing of the peak of plasma Epo concentration was delayed in both lines of rescued mice compared with wild type, suggesting that, in wild-type mice, nonhematopoietic EpoR contributes to the regulation of plasma Epo concentration. We thus conclude that nonhematopoietic expression of EpoR is dispensable to normal mouse development and that the expression level of EpoR regulates erythropoiesis by controlling the sensitivity of erythroid progenitors to Epo.
Introduction
Erythropoietin (Epo) stimulates the proliferation and differentiation of erythroid progenitors.1 Its receptor, EpoR, is a member of the type 1 cytokine receptor family characterized by a single transmembrane domain.2 Recently, the importance of the Epo-EpoR system in primitive and definitive erythropoiesis was determined by generating lines of mutant mice lacking either the Epo or EpoRgene.1,3,4 Both Epo and EpoRhomozygous mutant mice died of severe anemia between embryonic day 13 (E13) and E15. Existence of the erythroid progenitors erythroid colony-forming unit (CFU-E) and erythroid burst-forming unit (BFU-E) in the livers of these mutants indicated that Epo-EpoR is not required for the commitment of hematopoietic progenitors to the erythroid lineage.5,6 However, primitive erythropoiesis is partially impaired by the absence of the Epo-EpoR pathway, as shown by the smaller number of primitive erythrocytes observed in the yolk sac blood islands of homozygous mutant embryos compared with heterozygous mutant and wild-type embryos.1,3,4 In contrast, contribution of the Epo-EpoR system is crucial during definitive erythropoiesis, namely for proliferation, survival, and differentiation of erythroid progenitors in the later stages of differentiation.1,3 4
Expression of EpoR is not restricted to the hematopoietic lineage, but it can be identified in various nonhematopoietic tissues. For example, EpoR is expressed in epicardium and pericardium.7 In addition, Epo has been shown to promote the proliferation of endothelial cells, fetal liver stromal cells, and skeletal muscle satellite cells.8-11 Epo can also assist in the recovery of neurons from injury.12 13 This widespread expression profile of EpoR strongly suggests that a lack of Epo-EpoR may affect various biologic aspects. However, because germ-lineEpo-EpoR mutant embryos die in utero, it is unknown whether the Epo-EpoR pathway is actually required for the development and activity of cells in nonhematopoietic tissues.
GATA-1 has been shown to regulate the expression of the Epo receptor14 and most other erythroid genes. Transgenic expression of GATA-1 under the transcriptional control of theGATA-1 locus hematopoietic regulatory domain (GATA-1–HRD) rescued GATA-1 knockdown mice from embryonic lethality by restoring GATA-1 expression in hematopoietic tissues.15 16 Therefore, we exploited theGATA-1–HRD to rescue EpoR gene knockout mice from embryonic lethality by expressing the EpoR transgene specifically in erythroid cells.
The GATA-1–HRD-EpoR transgene recovered erythropoiesis in EpoR-null mice, rescuing the mutants from embryonic lethality to give fertile mice with a normal phenotype throughout their lives. We assume, therefore, that nonhematopoietic expression of EpoR is dispensable to normal mouse development. We generated 2 lines of EpoR-rescued mice and, compared with wild-type levels, one line expresses EpoR at 40%, whereas the other expresses EpoR at 120% in bone marrow cells. These mice do not suffer from polycythemia or anemia because the in vivo plasma Epo level is stringently regulated. Importantly, though, erythroid progenitors in the bone marrow of the low and high EpoR-expressing lines showed different sensitivity to Epo in colony-formation assays. Thus, the expression level of EpoR regulates erythropoiesis by controlling the sensitivity of erythroid progenitor cells to Epo.
Materials and methods
Mice and construction of EpoR transgene
EpoR-deficient (C57Bl/6) mice4 were supplied by Jackson Laboratories (Bar Harbor, ME). EpoR cDNA was ligated to a genomic fragment containing GATA-1–HRD.15Transgene constructs were injected into fertilized eggs derived from BDF1 parents, and 6 independent lines of transgenic mice were obtained. Two lines of mice, Tg-A and Tg-B, were mated withEpoR+/− mice to establish the compound mutant mice EpoR+/−::HG1-EpoR. EpoR−/−::HG1-EpoR mice were obtained by crossing the former mice withEpoR+/− mice. Resultant mice and embryos were genotyped by polymerase chain reaction (PCR), Southern blot analysis, or both. Genomic tail DNA was prepared and digested withAvrII. Southern membranes were hybridized with a32P-labeled probe, as indicated in Figure1A. PCR analysis with genomic DNA was also performed to amplify endogenous EpoR alleles (387 bp) and transgenes (303 bp). The PCR primer pair used was primer EpoR3S (5′-GGTGAGTCACGAAAGTCATG-3′) and primer EpoR4AS (5′-ACACGTCCACTTCATATCGG-3′), corresponding to the exon III and the exon IV sequence, respectively, of the EpoR gene. All mice were treated according to the regulations of the Standards for Human Care and Use of Laboratory Animals of the University of Tsukuba.
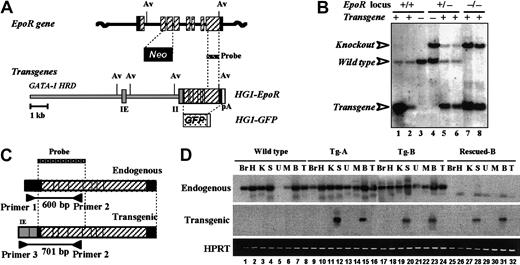
Establishment of rescued EpoR mutant mouse lines.
(A) Structure of the wild-type and mutant EpoRlocus4 and design of the HG1-EpoR andHG1-GFP transgenes. The GATA-1–HRD minigene containing exons IE and II of the mouse GATA-1 gene was ligated to either EpoR or GFP cDNA to give HG1-EpoR andHG1-GFP, respectively. The translated and untranslated exons of the EpoR gene are shown as solid and hatched boxes, respectively. Neo, pA, and Av represent the neomycin resistance gene cassette, polyadenylation signal, and AvrII sites, respectively. (B) Genotyping of transgenic and rescued mice by Southern blot analysis. Tail DNA samples digested with AvrII were hybridized to the EpoR probe shown in panel A. Tg-A (lane 1) and Tg-B (lane 2) mice contain 40 and 4 copies of theEpoR transgene, respectively. Note that the wild-typeEpoR band (4.2 kb) is absent in lanes 7 and 8 (Tg-B rescued mice), and a knockout allele band is present in lanes 4 to 8. (C) Expected mRNA structures of endogenous and transgenic EpoR. Transgene-derived EpoR mRNA includes exons IE and II of theGATA-1–HRD, so that its amplicon can be distinguished from endogenous EpoR-derived mRNA using the primer sets shown. (D) Endogenous and transgenic EpoR mRNA expression determined by RT-PCR analysis. Samples of total RNA from various tissues of wild-type (lanes 1-8), Tg-A (lanes 9-16), Tg-B (lanes 17-24), and Rescued-B (lanes 25-32) mice were analyzed. PCR was performed using primer sets Primer 1 with 2 and Primer 3 with 2 to detect endogenous (600 bp) and transgenic (701 bp) EpoR transcripts, respectively. HPRT was used as an internal control. Br indicates brain; H, heart; K, kidney; S, spleen; U, uterus; M, muscle; B, bone marrow; and T, testis.
Establishment of rescued EpoR mutant mouse lines.
(A) Structure of the wild-type and mutant EpoRlocus4 and design of the HG1-EpoR andHG1-GFP transgenes. The GATA-1–HRD minigene containing exons IE and II of the mouse GATA-1 gene was ligated to either EpoR or GFP cDNA to give HG1-EpoR andHG1-GFP, respectively. The translated and untranslated exons of the EpoR gene are shown as solid and hatched boxes, respectively. Neo, pA, and Av represent the neomycin resistance gene cassette, polyadenylation signal, and AvrII sites, respectively. (B) Genotyping of transgenic and rescued mice by Southern blot analysis. Tail DNA samples digested with AvrII were hybridized to the EpoR probe shown in panel A. Tg-A (lane 1) and Tg-B (lane 2) mice contain 40 and 4 copies of theEpoR transgene, respectively. Note that the wild-typeEpoR band (4.2 kb) is absent in lanes 7 and 8 (Tg-B rescued mice), and a knockout allele band is present in lanes 4 to 8. (C) Expected mRNA structures of endogenous and transgenic EpoR. Transgene-derived EpoR mRNA includes exons IE and II of theGATA-1–HRD, so that its amplicon can be distinguished from endogenous EpoR-derived mRNA using the primer sets shown. (D) Endogenous and transgenic EpoR mRNA expression determined by RT-PCR analysis. Samples of total RNA from various tissues of wild-type (lanes 1-8), Tg-A (lanes 9-16), Tg-B (lanes 17-24), and Rescued-B (lanes 25-32) mice were analyzed. PCR was performed using primer sets Primer 1 with 2 and Primer 3 with 2 to detect endogenous (600 bp) and transgenic (701 bp) EpoR transcripts, respectively. HPRT was used as an internal control. Br indicates brain; H, heart; K, kidney; S, spleen; U, uterus; M, muscle; B, bone marrow; and T, testis.
RNA and RT-PCR
After isolation from various mouse tissues using ISOGEN (Nippon-Gene, Osaka, Japan), total RNA was incubated with DNase I (RQ1; Promega, Madison, WI). Samples were reverse-transcribed by Super-Script II, and random hexamers (both from Gibco BRL, Rockville, MD) and the PCR reaction (40 cycles) were performed as follows: 40 seconds at 95°C, 40 seconds at 60°C, and 60 seconds at 72°C. The PCR primers used were primer-1, 5′-ACGAAACAGGGGCGCTGGAG-3′; primer-2, 5′-ACACGTCCACTTCATATCGG-3′; and primer-3, 5′-TCCTCTGCATCAACAAGCCC-3′ (Figure 1C). Hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as an internal control; the sense primer was 5′-GCTGGTGAAAAGGACCTCT-3′, and the antisense primer was 5′-CACAGGACTAGAACACCTGC-3′. PCR products were electrophoresed on an agarose gel and were transferred to a nylon membrane (Zeta-Probe; BioRad, Hercules, CA) for hybridization with a 32P-labeled probe. The expression of endogenous and transgenic EpoR was detected independently to avoid contamination of genomic DNA in such a highly sensitive detection method.
Histologic analysis and TUNEL assay
Embryos and tissues were fixed in 4% paraformaldehyde for 30 minutes and embedded in polyester wax (BDH Laboratory, Poole, England). Sections (5 μm) were incubated with rabbit anti-GFP antibody (diluted 1:1000; Molecular Probes, Eugene, OR), rat anti–PECAM-1 monoclonal antibody (1:500; BD PharMingen, San Diego, CA) and mouse anti–alpha sarcomeric muscle actin monoclonal antibody. Specific antibody binding was visualized using either horseradish peroxidase (HRP)–conjugated goat anti-mouse immunoglobulin that was polymerized by dextran (EnVision; DAKO, Carpinteria, CA) or HRP-conjugated anti-rabbit immunoglobulin (Biosource, Camarillo, CA) secondary antibody. TUNEL assay was performed using an in situ apoptosis detection kit (TAKARA, Osaka, Japan). Color detection was performed using diaminobenzidine (250 mg/mL), H2O2 (0.01%), and NiCl2 (0.05%) as a chromogen. Kernechtrot solution was used for counterstaining in all sections.
Flow cytometry
FACS analysis was performed with the CellQuest program (Becton Dickinson, San Jose, CA). Single-cell suspensions from E12.5 livers were prepared and incubated with CD16/CD32 (2.246) antibody (1:200) on ice for 15 minutes. Subsequently, cells were stained with allophycocyanin (APC)-conjugated anti-Ter119, fluorescein isothiocyanate (FITC)–conjugated anti-CD41, phycoerythrin (PE)-conjugated anti-CD44, and PE-conjugated anti–c-Kit antibodies for 30 minutes. After the final wash, viable cells were selected by propidium iodide (PI) staining. APC-, FITC-, and PE-conjugated rat IgG2b were used as isotype-matched controls. The antibodies were all obtained from BD PharMingen.
RT-PCR analysis with sorted bone marrow cells
Mononuclear cells from adult mouse bone marrow were prepared using Histopaque1082 (Sigma, St Louis, MO). Cell suspensions were stained with PI- and PE-conjugated streptavidin after incubation with biotin-conjugated anti-Terl19, anti-CD34 or anti–c-Kit antibodies. Fluorescence intensity of the cells was analyzed, and 5 × 104 green fluorescence protein (GFP)+ or GFP− cells in PI− fractions were sorted. RNA was extracted by RNeasy (QIAGEN, Basel, Switzerland) and reverse-transcribed by Sensiscript (QIAGEN). To detect GATA-1 mRNA, these samples were rendered for PCR amplification (35 cycles) using the primer pair 5′-ACTCGTCATACCACTAAGGT-3′ and 5′-AGTGTCTGTAGGCCTCAGCT-3′.
Epo binding assay
The number of EpoR on the hematopoietic cell surfaces was measured, as described previously.17 For each genotype, mononuclear cells were obtained from the bone marrow of 4 to 8 mice of 10 to 12 weeks of age.
Colony assays
Fetal liver cells (1 × 104) or bone marrow mononucleated cells (2 × 104) were cultured in 1 mL 0.8% methylcellulose medium containing 30% fetal bovine serum (FBS), 1% bovine serum albumin (BSA), 0.1 mM 2-mercaptoethanol and various concentrations of recombinant human Epo (Chugai Pharmaceutical, Tokyo, Japan). After 2 days (fetal liver) or 3 days (bone marrow) of culturing, cells were stained with benzidine, and positive colonies were counted. Bone marrow mononucleated cells (1 × 105) were cultured in the same medium supplemented with 100 ng/mL stem cell factor (SCF; R&D Systems, Minneapolis, MN) and various concentrations of Epo for 7 days, and benzidine-positive erythroid bursts were counted. These assays were performed in triplicate and repeated 3 times, and the results are shown with standard deviations.
Induction of anemia
Six-week-old mice were anesthetized with diethylether. After withdrawing 0.4 mL blood from the retro-orbital venous plexus using heparin-coated microtubes, an aliquot of the collected blood sample was used for determining the hematopoietic indices and leukocyte and platelet numbers using an automatic counter (Nihon-Kouden, Tokyo, Japan). Plasma was also isolated, and the Epo concentration was measured by radioimmunoassay.17
Results
Rescue of EpoR-null mutant mice from embryonic lethality byGATA-1–HRD-EpoR transgene
We generated 6 lines of transgenic mice expressing full-length EpoR under the control of GATA-1–HRD (HG1-EpoR) (Figure 1A). Genomic Southern blot analysis revealed that the Tg-A line (Figure 1B, lane 1) contains approximately 40 copies of theHG1-EpoR transgene, whereas the Tg-B line (lane 2) has only 4 copies. To monitor the expression of the EpoR transgene, line Tg-A was coinjected with a GATA-1–HRD-GFPexpression construct (HG1-GFP, Figure 1A). In the anticipation that the expression level of the EpoR transgene will vary according to the transgene copy number, we selected lines Tg-A and Tg-B for use in a genetic rescue experiment of EpoR-deficient mice.
EpoR+/− mice possessing either Tg-A or Tg-B were crossed with EpoR+/− mice to yieldEpoR−/−::Tg mice. Resultant pups were genotyped by PCR, Southern blot analysis, or both, and, according to Mendelian inheritance, an expected number of mice were found to have anEpoR−/−::Tg-A orEpoR−/−::Tg-B genotype (Table1). Remarkably, noEpoR−/− mice lacking the EpoRtransgene were found even among as many as 139 mice. We named the rescued EpoR mutant pups resulting from the crosses with lines Tg-A and Tg-B as Rescued-A and Rescued-B lines of mice, respectively. Genomic Southern blot analysis of tail DNA from theEpoR−/−::Tg-A (data not shown) and EpoR−/−::Tg-Bmice (Figure 1B, lanes 7, 8) clearly indicated that these rescued mice contained the knockout allele and the EpoR transgene. These results thus demonstrate that transgenic expression of EpoR cDNA under the influence of GATA-1–HRD rescued the germ-line EpoR-deficient mice from embryonic lethality.
Genotype of transgene-rescued EpoR mutant mouse offspring
| Crossing . | Litters . | Pups . | Transgene . | EpoRgenotype . | ||
|---|---|---|---|---|---|---|
| +/+ (%) . | +/− (%) . | −/− (%) . | ||||
| EpoR+/−∷Tg-A andEpoR+/− | 8 | 90 | + | 15 (24) | 28 (45) | 19 (31) |
| − | 6 (21) | 22 (79) | 0 (0) | |||
| EpoR+/−∷Tg-B andEpoR+/− | 7 | 49 | + | 5 (17) | 16 (53) | 9 (30) |
| − | 4 (21) | 15 (79) | 0 (0) | |||
| EpoR−/−∷Tg-B andEpoR−/−∷Tg-B | 4 | 23* | + | — | — | 23 (100) |
| − | — | — | 0 (0) | |||
| Crossing . | Litters . | Pups . | Transgene . | EpoRgenotype . | ||
|---|---|---|---|---|---|---|
| +/+ (%) . | +/− (%) . | −/− (%) . | ||||
| EpoR+/−∷Tg-A andEpoR+/− | 8 | 90 | + | 15 (24) | 28 (45) | 19 (31) |
| − | 6 (21) | 22 (79) | 0 (0) | |||
| EpoR+/−∷Tg-B andEpoR+/− | 7 | 49 | + | 5 (17) | 16 (53) | 9 (30) |
| − | 4 (21) | 15 (79) | 0 (0) | |||
| EpoR−/−∷Tg-B andEpoR−/−∷Tg-B | 4 | 23* | + | — | — | 23 (100) |
| − | — | — | 0 (0) | |||
Two lines of mice containing the HG1-EpoR transgene (Tg-A and Tg-B) were used for the rescue experiments. The genotypes of pups derived from each parent were determined 3 to 4 weeks after birth by PCR, Southern blot analysis, or both.
Twelve male and 11 female pups were derived from the interbreeding of Rescued-B mice.
Expression of transgenic EpoR in various mouse tissues and interbreeding experiments
Expression of the EpoR transgene was examined in various tissues and organs of the rescued mice, particularly in hematopoietic tissues. We used highly sensitive RT-PCR analysis using a radiolabeled probe, and the primer sets used are shown in Figure 1C. Analysis revealed specific expression of EpoR mRNA from theHG1-EpoR transgene in the spleen and bone marrow of Tg-A, Tg-B, and Rescued-B mice (Figure 1D, middle panel). In agreement with previous reports,7,8,11,12,18 19 endogenous expression of EpoR mRNA was detected in most of the tissues examined in wild-type, Tg-A and Tg-B mice, but not in Rescued-B mice (Figure 1D, top panel). Importantly, as we generated transgenic mouse lines with full-length EpoR cDNA, we expected that the transgene-rescued mice would express the full-length EpoR mRNA exclusively. Indeed, we did not detect any truncated form of EpoR mRNA by Northern blot and RT-PCR analyses (data not shown). These results demonstrate that hematopoietic lineage-specific expression of EpoR rescued the EpoRgerm-line mutant mice from embryonic lethality.
We then performed interbreeding experiments with the rescued male and female mice. We expected that 3 quarters of F1 embryos would survive because of the HG1-EpoR transgene. Indeed, the intercross resulted in 23 viable pups, all of which possessed theHG1-EpoR transgene, whereas no pups lacking the transgene were born (Table 1). The male-to-female ratio was approximately 1:1, and the pups lived for more than 1 year. Development, growth, mating, pregnancy, and childbirth of the rescued mice were within a normal range, and no apparent morphologic abnormality was observed (data not shown).
Rescue of primitive and definitive erythropoiesis in EpoR-null mutant embryos
Primitive erythropoiesis was also affected by a lack of EpoR,20 as seen in the paleness of the yolk sac of the E10.5 EpoR−/− embryo shown in Figure2C. In comparison, the yolk sacs of Rescued-B embryos (Figure 2D) were not anemic and displayed a color similar to those of wild-type and Tg-B embryos (Figure 2A-B).
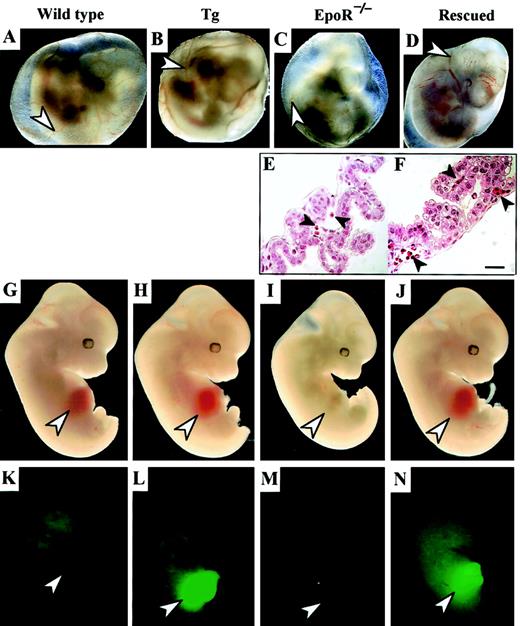
EpoR-null embryos were rescued from severe anemia and embryonic death by hematopoietic lineage-specific expression of the EpoRtransgene.
A single litter obtained by crossingEpoR+/−::Tg withEpoR+/−mice was used in this study. Wild-type (A), Tg-B (B), EpoR−/−(C, E), and Rescued-B (D, F) embryos are shown. Panels A to D show whole E10.5 embryos, whereas panels E and F show sections of the yolk sac of E11.5 embryos. Note that the yolk sac blood vessels of the rescued embryo were filled by nucleated erythrocytes (arrowheads in panel F), whereasEpoR−/− embryo contained only a small number of erythrocytes (E). Scale bar, 50 μm. Whole E12.5 embryos are shown in panels G to J. In contrast to theEpoR−/−embryo (I), the size and redness of the liver (arrowhead) in the Rescued-B embryo (J) were similar to those of the Tg-B (H) and wild-type (G) embryos. The E12.5 embryos shown in the fluorescence images K to N were from a single litter obtained by crossing EpoR+/−::Tg-Awith EpoR+/−mice. Because HG1-EpoRand HG1-GFP transgenes were coinjected, transgenic EpoR expression could be monitored by the intensity of green fluorescence. Green fluorescence was detected only in the livers (arrowheads) of Tg-A (L) and Rescued-A (N) embryos.
EpoR-null embryos were rescued from severe anemia and embryonic death by hematopoietic lineage-specific expression of the EpoRtransgene.
A single litter obtained by crossingEpoR+/−::Tg withEpoR+/−mice was used in this study. Wild-type (A), Tg-B (B), EpoR−/−(C, E), and Rescued-B (D, F) embryos are shown. Panels A to D show whole E10.5 embryos, whereas panels E and F show sections of the yolk sac of E11.5 embryos. Note that the yolk sac blood vessels of the rescued embryo were filled by nucleated erythrocytes (arrowheads in panel F), whereasEpoR−/− embryo contained only a small number of erythrocytes (E). Scale bar, 50 μm. Whole E12.5 embryos are shown in panels G to J. In contrast to theEpoR−/−embryo (I), the size and redness of the liver (arrowhead) in the Rescued-B embryo (J) were similar to those of the Tg-B (H) and wild-type (G) embryos. The E12.5 embryos shown in the fluorescence images K to N were from a single litter obtained by crossing EpoR+/−::Tg-Awith EpoR+/−mice. Because HG1-EpoRand HG1-GFP transgenes were coinjected, transgenic EpoR expression could be monitored by the intensity of green fluorescence. Green fluorescence was detected only in the livers (arrowheads) of Tg-A (L) and Rescued-A (N) embryos.
Only a small number of primitive erythrocytes were observed in the E11.5 EpoR−/− embryonic yolk sacs, whereas the endothelium surrounding the blood islands appeared normal (Figure 2E). In contrast, significantly more primitive erythrocytes were observed in the blood islands of Rescued-B yolk sacs (Figure 2F). Thus, EpoR expressed from the EpoR transgene effectively recovered primitive erythropoiesis in the yolk sacs ofEpoR−/−embryos.
On gross examination, embryonic livers of E12.5 wild-type (Figure2G) and Tg-B (Figure 2H) mice were red, indicating active definitive erythropoiesis. In contrast, the EpoR−/−embryonic livers were pale (Figure 2I), and the number of erythrocytes was markedly decreased (data not shown). Erythropoiesis in EpoR-null mutant livers recovered on transgenic expression of EpoR, as indicated by the liver color, which was similar to that found in wild-type embryos (Figure 2J).
Because Tg-A mice were injected with HG1-EpoR andHG1-GFP transgenes, we examined the embryonic livers under a fluorescence microscope. As can be seen by the green fluorescence, theHG1-GFP transgene was specifically expressed in the livers of E12.5 transgenic (Figure 2L) and rescued (Figure 2N) embryos, but not in the livers of wild-type (Figure 2K) andEpoR−/− (Figure 2M) embryos. These results indicate that the HG1-EpoR transgene was efficiently expressed in the embryonic liver.
Rescue of hematopoietic cells from apoptotic cell death by transgenic EpoR
GFP-positive erythrocytes (dark purple) and GFP-positive megakaryocytes (arrowheads) were observed in Tg-A (Figure3B) and Rescued-A (Figure 3D) embryonic livers, but not in the livers of wild-type orEpoR−/− embryos (Figure 3A,C). Histologic examination thus revealed that the EpoR-transgene was expressed in hematopoietic cells of the liver.
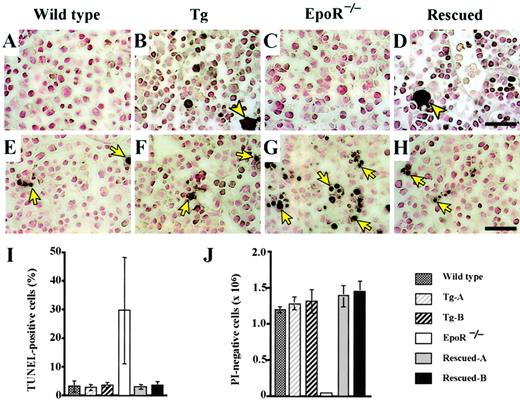
Rescue of hematopoietic cells from apoptotic cell death by transgenic expression of EpoR in E12.5 embryonic livers.
(A-D) Sections of E12.5 embryonic livers were stained with anti-GFP antibody. GFP+ cells (stained purple) are expected to coexpress the EpoR transgene. Megakaryocytes (arrowheads) and erythroid cells were stained positive in Tg-A (B) and Rescued-A (D) embryos only. (E-H) TUNEL (terminal transferase-mediated dUTP nick-end labeling) assay of E12.5 embryonic liver cells. Nuclei of TUNEL-positive cells were stained purple (arrows). Although most cells in the EpoR−/−liver stained TUNEL positive (G), livers of the rescued (H), wild-type (E), and Tg-A (F) embryos contained only a small number of positive cells. Scale bar equals 50 μm (A-H). (I) TUNEL-positive cells as a percentage of the total liver cells counted in each section. (J) Number of PI-negative cells assessed by FACS analysis.
Rescue of hematopoietic cells from apoptotic cell death by transgenic expression of EpoR in E12.5 embryonic livers.
(A-D) Sections of E12.5 embryonic livers were stained with anti-GFP antibody. GFP+ cells (stained purple) are expected to coexpress the EpoR transgene. Megakaryocytes (arrowheads) and erythroid cells were stained positive in Tg-A (B) and Rescued-A (D) embryos only. (E-H) TUNEL (terminal transferase-mediated dUTP nick-end labeling) assay of E12.5 embryonic liver cells. Nuclei of TUNEL-positive cells were stained purple (arrows). Although most cells in the EpoR−/−liver stained TUNEL positive (G), livers of the rescued (H), wild-type (E), and Tg-A (F) embryos contained only a small number of positive cells. Scale bar equals 50 μm (A-H). (I) TUNEL-positive cells as a percentage of the total liver cells counted in each section. (J) Number of PI-negative cells assessed by FACS analysis.
TUNEL staining allowed a clearer evaluation of the extent of apoptosis in the embryonic livers (Figure 3E-H). Rescued embryonic livers (Figure3H) harbored a significantly lower number of apoptotic cells thanEpoR−/− embryonic livers (Figure 3G). Although the frequency of TUNEL-positive cells varied among the knockout embryos, the number of TUNEL-positive cells was always several-fold higher on average than that of wild-type, transgenic, and rescued embryos (Figure 3I). We also examined the number of PI staining-negative cells, which represent living cells, in the knockout and rescued embryonic livers. EpoR−/− embryos contained 10-fold fewer PI-negative cells than wild-type, transgenic, and rescued embryos (Figure 3J). These results demonstrate that apoptosis is prevalent in the livers of EpoR−/−embryos, where most cells are erythroid. In addition, the transgenic expression of EpoR transduces signals that effectively protect hematopoietic cells from apoptotic cell death.
Hematopoietic cell populations in EpoR-deficient and transgene-rescued fetal livers
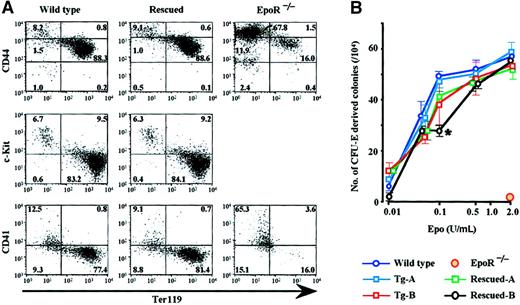
We assessed the population of hematopoietic cells in the livers of transgenic and rescued E12.5 embryos by determining the amount of cells positive for various markers of hematopoietic differentiation. FACS analysis showed that, whereas most of the cells in the wild-type and Rescued-B livers were c-Kit− (Figure4A, middle panel), c-Kit+hematopoietic progenitors were predominant in theEpoR−/− livers, albeit the total number of the cells was low (data not shown). In the FACS analysis, CD44low/Ter119+ cells represent a population of an erythroid cell lineage. Compared with wild type (88.3%) and Rescued-B (88.6%), this fraction of erythroid cells was less abundant in the EpoR−/− liver (16.0%; Figure 4A, top), suggesting that erythroid differentiation is suppressed by the absence of EpoR.
Fetal liver hematopoiesis in rescued embryos.
(A) Analysis of E12.5 fetal liver hematopoietic cell populations. Single cell suspensions were stained with anti–c-Kit, -Ter119, -CD41, and -CD44 antibodies, and these cells (1 × 104) were analyzed by FACS. PI-negative cells were gated, and the intensity of fluorescence was measured. The ratio of cells in each quadrangle is shown. (B) Fetal liver cells were cultured for 2 days in methylcellulose medium containing various concentrations of Epo, and erythroid colonies were counted after benzidine staining. *P < .01 compared with wild-type mouse.
Fetal liver hematopoiesis in rescued embryos.
(A) Analysis of E12.5 fetal liver hematopoietic cell populations. Single cell suspensions were stained with anti–c-Kit, -Ter119, -CD41, and -CD44 antibodies, and these cells (1 × 104) were analyzed by FACS. PI-negative cells were gated, and the intensity of fluorescence was measured. The ratio of cells in each quadrangle is shown. (B) Fetal liver cells were cultured for 2 days in methylcellulose medium containing various concentrations of Epo, and erythroid colonies were counted after benzidine staining. *P < .01 compared with wild-type mouse.
To elucidate the function of transgenic EpoR in erythroid cell development, an in vitro colony formation assay was undertaken. Embryonic liver cells were cultured in a methylcellulose medium containing various concentrations of Epo for 2 days. Benzidine-positive colonies were scored as CFU-E–derived colonies. At all Epo concentrations used, fetal liver cells from Tg-A, Tg-B, Rescued-A, and wild-type embryos showed a comparable number of erythroid colonies (Figure 4B), indicating a similar sensitivity of CFU-E progenitors to Epo among these embryos. Although liver cells from Rescued-B embryos formed a smaller number of erythroid colonies in the presence of 0.1 U/mL Epo, the erythroid colony number recovered on incubation with high concentrations of Epo. These observations suggest that livers from wild-type, Rescued-B, and other genotypes of embryos contain a similar number of CFU-E (approximately 50 CFU-E per 1 × 104fetal liver cells). In contrast, no benzidine-positive colony was detected in the EpoR−/− embryonic liver cells, indicating that transgenic expression of EpoR efficiently rescued CFU-E activity in the EpoR−/−embryonic liver.
EpoR has been reported to play a functional role in the formation of megakaryocytes.21 Furthermore, our study showed theEpoR transgene to be expressed in megakaryocytes in the livers of transgenic and rescued embryos (Figure 3B-D). We therefore examined the frequency of megakaryocytes in E12.5 embryonic livers. Despite overexpression of the EpoR transgene in megakaryocytes of Tg-B embryos and no expression at all in megakaryocytes of EpoR−/− embryos, the frequency of CD41+ megakaryocytes in these livers was comparable to that of the wild type (Figure 4A, bottom panel, and data not shown). The number of circulating platelets did not change in Tg-B or transgene-rescued mice (below). These results suggest that EpoR is dispensable in megakaryocyte development.
Rescue of heart development by hematopoietic expression of EpoR
Because Epo activates angiogenesis,22 we examined the distribution of endothelial cells in E12.5 embryos. After whole-mount staining with PECAM-1 antibody, embryos were sectioned and histologic examination was performed. The lateral sides of somites (Figure 5A-D) and livers (Figure 5E-H) are shown. In somites and liver, the endothelial cells inEpoR−/− and rescued embryos appeared to be similar to those in the wild-type embryos, suggesting that the Epo-EpoR signaling pathway is not necessary for endothelial cell development.
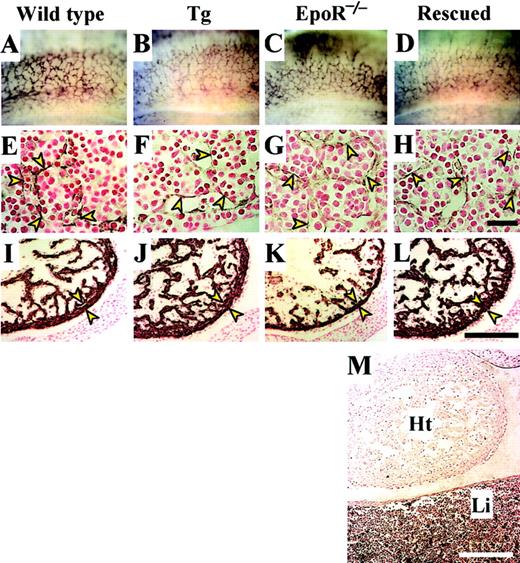
Analyses of nonhematopoietic embryogenesis.
Whole E12.5 embryos and sections were stained with anti–PECAM-1 antibody. The morphology of the blood vessels (stained purple) in the lateral sides of somites (A-D, upside is dorsal) and fetal livers (E-H, arrowheads) of wild-type, transgenic (Tg), EpoR-null, and rescued embryos were compared. No differences were found among the embryos. In the embryonic heart sections (I-L), the smooth muscles of the left ventricles were stained with anti–alpha sarcomeric muscle actin antibody. Although the compact layer (between the arrowheads) of the rescued embryo (L) was normal, the compact layer of theEpoR−/−embryo (K) was considerably thinner than that of wild-type (I) and transgenic (J) embryos. (M) A section including the heart (Ht) and liver (Li) of a Rescued-A embryo was stained with anti-GFP antibody. GFP+ cells were not detected in the heart, but most of cells in the liver were GFP+, as seen by the purple staining. Scale bar, 50 μm (E-H) and 200 μm (I-M).
Analyses of nonhematopoietic embryogenesis.
Whole E12.5 embryos and sections were stained with anti–PECAM-1 antibody. The morphology of the blood vessels (stained purple) in the lateral sides of somites (A-D, upside is dorsal) and fetal livers (E-H, arrowheads) of wild-type, transgenic (Tg), EpoR-null, and rescued embryos were compared. No differences were found among the embryos. In the embryonic heart sections (I-L), the smooth muscles of the left ventricles were stained with anti–alpha sarcomeric muscle actin antibody. Although the compact layer (between the arrowheads) of the rescued embryo (L) was normal, the compact layer of theEpoR−/−embryo (K) was considerably thinner than that of wild-type (I) and transgenic (J) embryos. (M) A section including the heart (Ht) and liver (Li) of a Rescued-A embryo was stained with anti-GFP antibody. GFP+ cells were not detected in the heart, but most of cells in the liver were GFP+, as seen by the purple staining. Scale bar, 50 μm (E-H) and 200 μm (I-M).
It has been reported that the Epo-EpoR signaling pathway plays an important role in cardiac morphogenesis.7 Indeed, in E12.5EpoR−/− embryonic hearts, epicardium detachment and a lack of defined capillary structures in the endocardium were observed (Figure 5K). However, the epicardium and capillary development in transgene-rescued mice were normal (Figure 5L) and were similar to those in wild-type (Figure 5I) and transgenic embryonic hearts (Figure 5J).
Because Rescued-A embryos were coinjected with the GFP transgene, embryos were examined by specific staining with anti-GFP antibody. The results clearly revealed that transgenic GFP, hence transgenic EpoR, was expressed in fetal liver (Figure 5M) but not in heart. Transgenic GFP expression was not detectable in the endocardium, epicardium, or pericardium of the embryos, where EpoR expression is usually detected, even at higher magnifications (data not shown). These results suggest that the Epo-EpoR system is not a crucial signal transduction pathway for normal development of the heart but rather that severe anemia might be pertinent to the blockage of heart development, perhaps through a reduction in the oxygen supply to the developing heart. Thus, we propose that the Epo-EpoR system does not relate directly to epicardium or capillary development in the embryonic heart.
Analysis of hematopoietic cells in transgene-rescued adult mouse bone marrow
Although EpoR expression has been demonstrated in hematopoietic stem cells and progenitors of the bone marrow,23 we envisage that expression of theHG1-EpoR transgene cannot recapitulate EpoR expression in such cells. To address the question as to whether EpoR is indispensable for the differentiation and maintenance of stem cells and progenitors, we performed FACS and RT-PCR analyses of Rescued-A mice containing Tg-A and HG1-GFP transgenes. Approximately 10% of bone marrow cells from the Rescued-A animals expressed GFP, and the population and intensity of fluorescence was similar to that of Tg-A mice (Figure 6A and data not shown).
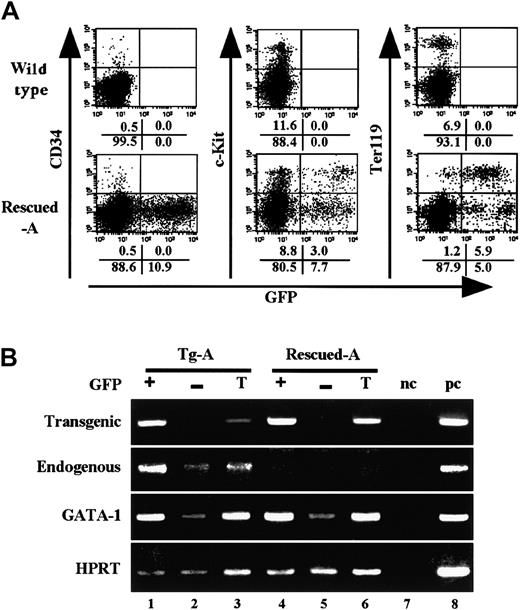
Analyses of bone marrow hematopoiesis in transgenic-EpoR rescued mice.
(A) FACS analysis of the mononucleated cells in Rescued-A mice. PI-negative cells were gated, and fluorescence intensities were analyzed. (B) RT-PCR analysis of sorted bone marrow cells from Tg-A and Rescued-A mice. GFP-positive (+), GFP-negative (−), and total (T) fractions were sorted, and RNA was extracted. Endogenous EpoR, transgenic EpoR, and GATA-1 transcripts were detected by RT-PCR. Negative (nc) and positive (pc) controls were loaded in lanes 7 and 8, respectively.
Analyses of bone marrow hematopoiesis in transgenic-EpoR rescued mice.
(A) FACS analysis of the mononucleated cells in Rescued-A mice. PI-negative cells were gated, and fluorescence intensities were analyzed. (B) RT-PCR analysis of sorted bone marrow cells from Tg-A and Rescued-A mice. GFP-positive (+), GFP-negative (−), and total (T) fractions were sorted, and RNA was extracted. Endogenous EpoR, transgenic EpoR, and GATA-1 transcripts were detected by RT-PCR. Negative (nc) and positive (pc) controls were loaded in lanes 7 and 8, respectively.
CD34+ progenitors, which were shown to express EpoR,24 did not express GFP at all (Figure 6A, left). Thus, EpoR expression in the CD34+ hematopoietic progenitors does not appear to be essential for normal hematopoiesis. Importantly, a GFPhigh fraction was included in the c-Kit+ fraction (Figure 6A, middle), which contains BFU-E and CFU-E erythroid progenitors (N. Suwabe, N.S., M.Y., unpublished observation, December 2001). We conclude that transgenic expression of EpoR in the CD34−/c-Kit+fraction rescued the differentiation of EpoR-null erythroid progenitors to give rise to Ter119+ mature erythroid cells.
RT-PCR analysis was performed on the GFP-positive and -negative fractions of bone marrow from Tg-A and Rescued-A mice. Although the Rescued-A mouse did not express endogenous EpoR mRNA at all, GFP− cells of the Tg-A mouse expressed endogenous EpoR mRNA weakly (Figure 6B). As expected, the GFP− fraction of the Rescued-A mouse did not express transgenic EpoR mRNA. GATA-1 mRNA was also enriched in the GFP+ fraction compared with the GFP− fraction of Tg-A and Rescued-A mice. Interestingly, GFP− cells from the Rescued-A mouse express endogenous GATA-1, albeit at a low level, suggesting that GATA-1–HRDregulation is still missing in some population of the cells in which the endogenous GATA-1 gene is expressed.
Expression level of EpoR determines the sensitivity of erythroid progenitors to Epo
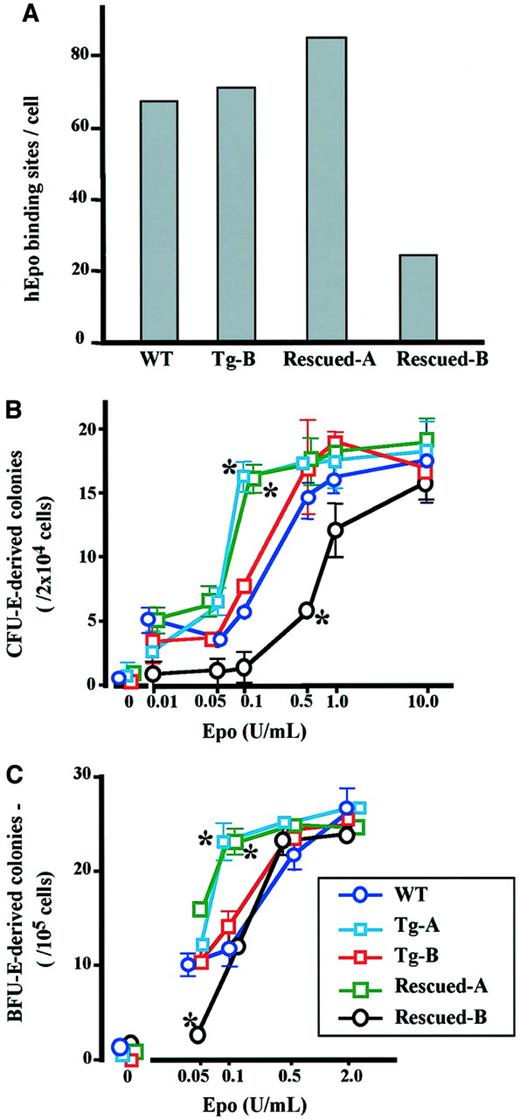
The expression of Epo binding sites in adult mouse bone marrow cells was investigated using sodium iodide I 125-labeled recombinant human Epo (Figure 7A), which has been shown to bind to mouse and human EpoR with comparable efficiency. The number of Epo binding sites in a bone marrow cell of the Tg-B mouse is approximately 71, almost comparable to that of the wild type (67 binding sites). In contrast, Rescued-B mice contain only 27 binding sites per cell, which represents a purely Tg-B–derived EpoR number. Rescued-A mice have 83 binding sites per cell, which is 1.2-fold that of the wild type. Transgenic and endogenous EpoR showed comparable dissociation constants against human Epo (Kdvalues of 201 pM and 270 pM for rescued and wild-type mice, respectively), indicating that the transgene-derived EpoR is synthesized and transferred normally to the cell surface.
Number of EpoR at the bone marrow cell surface and erythroid colony formation assay in differential concentrations of Epo.
(A) Number of radiolabeled recombinant human Epo binding sites at the surface of a bone marrow cell. Total mononuclear cells from the bone marrow of 4 to 8 mice at 10 to 12 weeks old were analyzed for each line. (B, C) Bone marrow cells were cultured for 3 days with different concentrations of Epo (B) or for 7 days with 50 ng/mL SCF and different concentrations of Epo (C) in methylcellulose medium. Erythroid colonies were counted by benzidine staining. *P < .01 compared with wild-type mouse.
Number of EpoR at the bone marrow cell surface and erythroid colony formation assay in differential concentrations of Epo.
(A) Number of radiolabeled recombinant human Epo binding sites at the surface of a bone marrow cell. Total mononuclear cells from the bone marrow of 4 to 8 mice at 10 to 12 weeks old were analyzed for each line. (B, C) Bone marrow cells were cultured for 3 days with different concentrations of Epo (B) or for 7 days with 50 ng/mL SCF and different concentrations of Epo (C) in methylcellulose medium. Erythroid colonies were counted by benzidine staining. *P < .01 compared with wild-type mouse.
The sensitivity of each erythroid progenitor to Epo was examined by erythroid colony formation assays in a methylcellulose medium containing various concentrations of Epo. For each EpoR genotype, the number of CFU-E–derived colonies in the bone marrow cells increased in a dose-dependent manner with an increasing Epo concentration (Figure7B). Erythroid progenitors from Tg-A and Rescued-A mice (high EpoR expressors) were in plateau at 0.1 U/mL Epo, indicating that these types of erythroid progenitors were more sensitive to Epo than other types of mouse progenitors. On the other hand, erythroid progenitors from Rescued-B mice (low EpoR expressor) showed the lowest number of CFU-E–derived colonies, and those from wild-type and Tg-B mice (medium EpoR expressors) showed an intermediate number of CFU-E–derived colonies at 0.1 U/mL Epo. An important finding was that, with a higher concentration of Epo (1-10 U/mL), the colony number derived from Rescued-B mice increased and became similar to that of the wild-type and Tg-A mice. Thus, first we conclude that a comparable number of CFU-E progenitors exists among wild-type, Tg-A, Rescued-A, Tg-B, and Rescued-B mice. Second, we conclude that the ability of progenitors to differentiate toward an erythroid lineage differs among the various EpoR genotypes and depends on the expression level of EpoR.
We also examined BFU-E–derived colony formation. The number of colonies from Tg-A and Rescued-A bone marrow cells reached a plateau at a lower concentration of Epo (0.1U/mL) than did the other genotypes (Figure 7C). Thus, at a low concentration of Epo, both CFU-E– and BFU-E–derived colony numbers directly correlate with the number of Epo binding sites on the surface of erythroid progenitors, which were produced distinctly by the germ-line EpoR-mutation andEpoR-transgene. Intriguingly, though Rescued-B bone marrow cells had the lowest number of BFU-E–derived colonies at 0.05 U/mL Epo, the BFU-E number did not differ significantly from that of the other genotypes between 0.5 and 2.0 U/mL EpoR (Figure 7C). This is in clear contrast to the CFU-E case at 0.5 U/mL EpoR, in which Rescued-B bone marrow cells formed only one third the number of CFU-E colonies formed in the other genotypes (Figure 7B).
Response of transgenic EpoR-rescued mice to anemia induced by bleeding
So far, this study has demonstrated that the number of Epo binding sites in erythroid cells from Rescued-A and Rescued-B mice are approximately 120% and 40% that of the wild type, respectively. Nevertheless, both lines of transgenic mice are viable and fertile. Importantly, the erythroid progenitors of Rescued-B mice are much less sensitive to Epo than those of the wild type. In contrast, the erythroid progenitors of Tg-A and Rescued-A mice are more sensitive to Epo than those of the wild type. We wanted to know whether red blood cell production is under the influence of circulating Epo levels in these genetically engineered mice.
To this end, we first analyzed the hematopoietic indices of these mice (Figure 8A, day 1). The hematocrit (HCT), hemoglobin concentration (Hb), mean corpuscular volume (MCV), number of white blood cells (WBCs), and platelet number (PLT) remained unaffected in the 2 rescued mouse lines. However, the total number of erythrocytes (RBCs) was slightly decreased in the Rescued-B mice than in the wild type. In spite of the fact that Rescued-A mice have 20% more Epo binding sites, polycythemia was not observed in this line of mice.
Effect of EpoR on recovery from anemia.
Anemia was induced in 4 different EpoR genotypes of mice by withdrawing blood from the retro-orbital venous plexus every day for 4 days (day 1 to 4). Recovery from anemia was observed over a period of 1 week. (A) Peripheral blood indices of the 4 lines of mice under normal (day 1) and anemic (day 4) conditions are shown in each panel. Hb, MCV, RBC, WBC, and PLT are shown. HCT values (B) and Epo concentrations in the plasma (C) were measured daily. Results are shown with SD; n = 5 for each line. * indicates P < .01; +,P < .05 compared with wild-type mouse.
Effect of EpoR on recovery from anemia.
Anemia was induced in 4 different EpoR genotypes of mice by withdrawing blood from the retro-orbital venous plexus every day for 4 days (day 1 to 4). Recovery from anemia was observed over a period of 1 week. (A) Peripheral blood indices of the 4 lines of mice under normal (day 1) and anemic (day 4) conditions are shown in each panel. Hb, MCV, RBC, WBC, and PLT are shown. HCT values (B) and Epo concentrations in the plasma (C) were measured daily. Results are shown with SD; n = 5 for each line. * indicates P < .01; +,P < .05 compared with wild-type mouse.
Anemia was induced in these mice by daily bleeding for 4 days, and HCT values (Figure 8B) and Epo concentrations (Figure 8C) in the blood were examined over a 10-day period, starting from the first day of bleeding. Other hematopoietic indices were measured before bleeding (day 1) and after 4 days (day 4) of bleeding (Figure 8A). Using this protocol for experimentally inducing anemia, the HCT values of all 4 genotypes reached as low as 25% (Figure 8B). Furthermore, the HCT, Hb, and RBC values decreased comparably in all 4 genotypes of mice (Figure 8A-B). Other blood cell lineages, such as WBC and PLT, were also similar among the 4 different lines of mice (Figure 8A).
In each line, the plasma Epo concentrations increased in response to induced anemia. Notably, the daily Epo concentration of Rescued-B mice was markedly higher than that of the wild type, Tg-B, and Rescued-A mice (Figure 8C), reflecting differences in the number of Epo-binding sites among these mice. It is noteworthy that, though the plasma Epo concentration peaked at day 3 in wild-type and Tg-B mice, the peak shifted to day 4 in Rescued-A and -B mice (Figure 8C). This time delay in peak plasma Epo concentration is most likely to reflect a lack of efficient internalization and subsequent metabolic turnover of Epo in nonhematopoietic tissues through binding to EpoR, considering that the Rescued-A and -B lines of mice lack the nonerythroid expression of EpoR. An alternative possibility is that Epo production may be regulated by a negative feedback mechanism, with the circulating Epo concentration sensed by Epo-producing cells through EpoR on the cell surface. Therefore, a low level of EpoR expression may transduce the low concentration signal less efficiently, giving rise to the delay in Epo production. These results suggest that the contribution of nonhematopoietic EpoR is important in the regulation of plasma Epo concentration.
Discussion
Gene ablation studies demonstrated that the Epo-EpoR signaling pathway is crucial for definitive erythropoiesis and revealed the embryonic lethal phenotype inherent in a deficiency in either Epo or EpoR.1,3 4 This embryonic lethality made it difficult to further analyze the Epo-EpoR system in the later stages of development. As a result, many uncertainties remain regarding the function of the Epo-EpoR system in embryonic development and adult hematopoiesis. Furthermore, it is unclear whether the Epo-EpoR pathway plays a crucial role in vivo in nonhematopoietic tissues. In this study, we resolved these issues by generating transgenic lines of mice expressing EpoR cDNA exclusively in the hematopoietic lineage.EpoR mutant pups were successfully rescued by crossing their EpoR-null parent with one of these EpoR transgenic mice. Because these EpoR transgene–rescued mice developed normally and were fertile, we exploited them for analyzing the roles of the Epo-EpoR system in adult hematopoiesis and in nonhematopoietic tissues. For the first time, our results exposed the absolute contribution of the Epo-EpoR signaling pathway to erythropoiesis in an integrated system in vivo. We demonstrated that nonhematopoietic expression of EpoR is dispensable in the birth, fertilization, and survival of mice and that the number of EpoR on the surface of erythroid progenitors determines the sensitivity of such progenitors to Epo stimuli.
All EpoR gene knockout mice reported to date showed that E12.5 EpoR−/− embryos are pale because of a significant reduction in circulating erythrocytes.1,3,4Our current analysis showed very good agreement with these results. In regard to definitive hematopoiesis, colony formation assays showed that supplementation of Epo and SCF is not sufficient to produce BFU-E– and CFU-E–derived colonies from EpoR−/− embryonic hematopoietic cells. However, a combination of SCF and thrombopoietin (Tpo) or of interleukin-3 (IL-3), IL-11, and Tpo was found to efficiently support BFU-E colony formation fromEpoR−/− embryonic cells.4 In contrast, colony formation of these erythroid progenitors was completely rescued by the transgenic expression of EpoR. To identify the expression profile of GATA-1,15 25 we generatedGATA-1–HRD-GFP transgenic lines and identified GFP expression in a fraction containing enriched BFU-E and CFU-E (N. Suwabe, N.S., M.Y., unpublished observation, December 2001). These results thus support our contention that the target cells rescued by the EpoR transgene include BFU-E and CFU-E progenitors in the definitive erythroid lineage.
Closer examination of E12.5 EpoR−/− embryos revealed a defect in cardiac development because of ventricular hypoplasia.7 In EpoR−/− embryonic hearts, the epicardial walls were detached and the capillary structures lacked definition. In contrast, transgene-rescued embryos exhibited no apparent defect or abnormality in their myocardial walls, indicating that the Epo-EpoR system is unnecessary for the initial development of the myocardial layer. Indeed, EpoR−/−embryonic stem cells have been shown to contribute to heart development in chimeric mice.7 These results strongly argue that impaired heart development results from a decrease of circulating mature erythrocytes, which are responsible for supplying oxygen to cells. We therefore propose that transgenic expression of EpoR in the knockout embryos rescued primitive and definitive erythropoiesis, which in turn rescued cardiac cell development.
Several reports have described the expression of EpoR in other nonhematopoietic tissues. For instance, Epo expression is induced by hypoxia and ischemia in astrocytes, and Epo protects neurons from ischemia, trauma, and the toxicity of kainate.12,13,26Binding of Epo to EpoR on the surfaces of neural cells induces the expression of antiapoptotic genes after the activation of Jak2 and NF-κB.27 EpoR is also expressed in skeletal muscle, kidney, and intestine, and Epo induces cell proliferation in these tissues.11,18 28 These findings led to the hypothesis that the Epo-EpoR system may play important roles when cells suffer from damage or stress. Although our study demonstrated that the Epo-EpoR system is not required for normal mouse development, these broad observations suggest that further analyses under pathological conditions are necessary for a comprehensive understanding of the contribution of the Epo-EpoR signaling system in nonhematopoietic tissues.
Alternative splicing of the EpoR transcript produces soluble and truncated isoforms that are assumed to negatively regulate erythropoiesis in immature erythroid progenitors.29Therefore, we examined whether the GATA-1–HRD transgene bearing a truncated form of EpoR could rescue germ-line EpoR mutant mice from embryonic lethality. However, the transgenic expression of truncated EpoR could not rescue erythroid cell development in EpoR-deficient mice (N.S., N.Y., unpublished observation, June 2000). The HG1-EpoR transgene expresses full-length EpoR exclusively. Because the transgene rescues EpoR germ-line mutant mice from embryonic lethality and recovers the mice from experimentally induced anemia, we conclude that these splicing variants are not essential for erythroid cell formation and recovery from anemia. On the contrary, because the truncated isoform is highly expressed in cells from patients with myelodysplastic syndrome and causes ineffective erythropoiesis,30 it is of interest to test the contribution of the EpoR transgene to blood cell differentiation in these patients.
Compared with wild-type mice, the amount of EpoR on the surface of bone marrow cells from Rescued-B mice is approximately 40%, and the sensitivity of erythroid progenitors to Epo in Rescued-B mice is much lower. This is consistent with the observation that CFU-E progenitors are more sensitive to Epo than BFU-E progenitors given that CFU-E progenitors contain more EpoR than BFU-E progenitors.5.6Nonetheless, Rescued-B mice have normal hematopoietic activity and can recover from anemia induced by bleeding. We believe that, because of the overproduction of Epo in vivo, a small number of receptors may be sufficient to sustain erythropoiesis. It should also be noted that though the erythroid progenitors in these lines of mice showed different sensitivity to Epo, it did not result in polycythemia or anemia, indicating that the in vivo Epo level is strictly regulated. Based on these results, we conclude that the expression level of EpoR controls the sensitivity of erythroid progenitors to Epo. To our knowledge, this is the first demonstration in vivo that the number of cytokine receptors on the surface of target cells controls the sensitivity of the target cells to the cytokine ligand.
In contrast to adult mice, there was no significant difference in CFU-E colony formation in fetal livers from the different lines of mice bearing different numbers of EpoR. We speculate that erythroid progenitors in fetal liver have a higher potential for producing erythroid cells and a higher sensitivity to the Epo signaling cascade than progenitors in adult bone marrow. The notion that fetal liver erythropoiesis is under a different regulation than adult bone marrow erythropoiesis requires further examination.
In summary, we presented the usefulness of the HG1-EpoRtransgene-rescued lines of mice for the analysis of EpoR function both in erythroid progenitors and in nonhematopoietic tissues. This study also implies that the GATA-1–HRD-EpoR minigene may be applicable to therapeutic use in that we anticipate an EpoR transgene under the regulation of this minigene to be expressed specifically in erythroid progenitors derived from patients with defective erythropoiesis.
We thank N. Kaneko and Y. Kikuchi for help and Drs F. Sugiyama, T. Nagasawa, and T. O'Connor for discussion and advice.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-01-0124.
Supported by grants from the Ministry of Education, Science, Sports and Culture (M.Y.), JSPS-RFTF and CREST (M.Y.), and PROBRAIN (S.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masayuki Yamamoto, Center for TARA, University of Tsukuba, 1-1-1 Tennoudai, Tsukuba 305-8577, Japan; e-mail:masi@tara.tsukuba.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal