Abstract
Fetal/neonatal immune responses generally are considered to be immature and weaker than that of adults. We have studied the cord-blood T cells of newborns congenitally infected with Trypanosoma cruzi, the protozoan agent of Chagas disease. Our data demonstrate a predominant activation of CD8 T cells expressing activation markers and armed to mediate effector functions. The analysis of the T-cell receptor beta chain variable repertoire shows the oligoclonal expansion of these T lymphocytes, indicating that activation was driven by parasite antigens. Indeed, we have detected parasite-specific CD8 T cells secreting interferon-γ after coincubation with live T cruzi. This response is enhanced in the presence of recombinant interleukin-15, which limits the T-cell spontaneous apoptosis. These findings point out that the fetal immune system is more competent than previously appreciated, since fetuses exposed to live pathogens are able to develop an adultlike immune CD8 T-cell response.
Introduction
The morbidity and mortality rates of infectious diseases are highest among neonates and young children. This increased susceptibility has been related to the immaturity of the neonatal immune system and a bias toward a Th2-type response generally not best suited to fight intracellular pathogens.1 The majority of cord-blood T cells present the CD45RA+ naive phenotype and a decreased capacity to produce interferon-γ (IFN-γ) compared with adult peripheral blood cells.2,3 However, several studies showed the ability of uninfected fetuses to mount an immune response following transplacental exposure to parasite antigens.4,5IFN-γ–secreting cord-blood CD4 T cells have been recently detected following prenatal sensitization to Plasmodium falciparum blood stage antigens.6 Nevertheless, relatively little is known about the development of CD8 T-cell responses in cord blood. Indeed, most of the current knowledge comes from studies on virus-specific cytotoxic T-lymphocyte responses that claim the induction of a CD8 T-cell response as being age dependent and much weaker in infants than in adults.1
Trypanosoma cruzi, the protozoan agent of Chagas disease, is transmitted to humans congenitally or by blood-suckling vector insects or blood transfusions. After a generally mild acute illness, infected people (16 to 18 million in Latin America) enter a chronic phase, and 10 to 20 years later nearly 30% of them develop cardiac and/or gastrointestinal lesions, which can lead to death.7Congenital transmission of T cruzi occurs in 1% to 10% of chronically infected mothers.8 Since mechanisms implied in protective immunity in Chagas disease likely involve CD8 T cells,9 we have addressed the issue of a possible cord-blood CD8 T-cell response in congenitally T cruzi–infected neonates. We found that fetuses are able to generate a massive and oligoclonal expansion of effector/memory CD8 T cells directed toward a live pathogen.
Patients, materials, and methods
Patients
We obtained cord blood from newborns delivered at the maternity German Urquidi (Universidad Mayor de San Simon [UMSS], Cochabamba, Bolivia). We assessed maternal infection by T cruzi–specific serology using indirect hemagglutination and immunofluorescence as previously described.10 We detected congenital T cruzi infection in newborns by 2 classical parasitological tests: hemoculture and microscopic examination of heparinized microhematocrit tubes.11 12 The scientific/ethic committees of UMSS and Université Libre de Bruxelles (ULB) have approved this study, and we obtained informed written consent from the mothers before blood collection.
Cell sample isolation and cultures
We isolated cord-blood mononuclear cells (CBMCs) from heparinized blood by Nycoprep density gradient centrifugation (Nycomed Pharma AS, Oslo, Norway), and we cryopreserved cells in the presence of 50% heat-inactivated autologous plasma and 10% dimethyl sulfoxide until use. For stimulation, we distributed thawed CBMCs (2 × 105) in 96-well plates (Nunc, Roskilde, Denmark) in RPMI containing 10% heat-inactivated fetal calf serum, 1%l-glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin (all from BioWhittaker Europe, Verviers, Belgium). For Elispot and intracellular IFN-γ content analysis, we stimulated CBMCs with live T cruzi trypomastigotes (Tulahuen strain) in the presence or absence of recombinant human interleukin-15 (IL-15) (1 ng/mL) (R&D Systems Europe, Abingdon, United Kingdom) in a 2:1 parasite-to-cell ratio for 24 hours at 37°C in 5% CO2atmosphere. CBMCs also were stimulated with phorbol-myristate-acetate (PMA) (25 ng/mL) and ionomycin (1 μg/mL) (both from Sigma, St Louis, MO) for 5 hours. To detect intracellular cytokines, we added the protein-secretion-inhibitor brefeldin A (10 μg/mL) (Sigma) for the last 4 hours of the culture.
Flow cytometry analysis
We performed 3-color flow cytometry analysis of CBMCs' surface by using various combinations of the following monoclonal antibodies (mAbs) and their matched control isotypes: CD3-PercP, CD4-fluorescein isothiocyanate (FITC); CD8-phycoerythrin (PE), CD8-FITC, HLA-DR-FITC; CD69-FITC, CD45RO-PE, Vβ3, Vβ5 (Becton Dickinson, Erembodegem, Belgium), Vβ11, Vβ13.1, Vβ14, Vβ16, Vβ17, Vβ18, Vβ22, T-cell receptor–γδ, and CD25 (Immunotech, Marseille, France), all conjugated to PE. We analyzed cells using a CD3+ cell gate, and we considered CD8− or CD4− T cells as CD4+ and CD8+ T lymphocytes, respectively, and TCR-γδ− T cells as TCR-αβ+ T cells. We performed data acquisition and analysis using a Becton Dickinson fluorescence-activated cell-sorter scanner (FACScan) flow cytometer and CELLQuest software (Becton Dickinson).
We examined intracellular effector molecules with PE-conjugated mAbs antihuman IFN-γ, tumor necrosis factor α (TNF-α, IL-2, IL-4, or perforin (Becton Dickinson). We first stained CBMCs with both anti-CD3–Percp and anti-CD8–FITC mAbs for 15 minutes at room temperature. We then fixed the cells for 10 minutes at room temperature with the FACS lysing solution (Becton Dickinson), washed them, and then permeabilized them for 10 minutes with the FACS permeabilizing solution (Becton Dickinson) prior to staining with antihuman cytokines or perforin in the dark for 30 minutes. We expressed positive cells as percentages among the different T-cell subsets.
Apoptosis assay
Following 24 hours in culture medium supplemented or not with IL-15 (1 ng/mL), we recovered and stained CBMCs with anti-CD8–PE and anti-CD3–Percp. We then determined the extent of spontaneous cell death by staining CBMCs with FITC-conjugated annexin-V (Becton Dickinson). We quantified the level of cell death as the percentage of annexin-V–positive T cells. We also analyzed apoptosis using the Tdt mediated Utp nick end labeling (TUNEL) assay as recommended by the manufacturer (Immunotech).
Enzyme-linked immunospot assay for single-cell IFN-γ release
We seeded CBMCs (2 × 105 cells/well) in triplicate on 96-well nitrocellulose plates (MultiScreen HA, Millipore, Bedford, MA) that had been coated with 1 μg/mL capture mouse anti–human IFN-γ mAb (Mabtech, Stockholm, Sweden). We cultured cells for 24 hours, and we performed the IFN-γ Elispot (enzyme-linked immunospot) assay as previously described.13 Spots were counted with an AID Elispot Reader System (Autoimmun Diagnostika GmbH, Strasberg, Germany). Responses were considered significant if (1) a minimum of 5 spot-forming cells (SFCs) were present per well, and (2) this number was at least 2-fold more than obtained with the negative control.
TCR repertoire analysis
We analyzed beta chain CDR3 size distributions using the reverse transcriptase–polymerase chain reaction (RT-PCR)–based Immunoscope technique previously described.14 Briefly, we extracted total RNA from approximately 10 million cells per sample using Trizol (Life Technologies [Gibco BRL], Cergy Pontoise, France). Twenty-five percent of the total RNA was reverse transcribed into cDNA, to be further amplified in 40-cycle PCR reactions using 24 BV-specific primers, each paired with one beta-chain constant region (BC) primer. We then copied the 24 PCR amplification products in a 5-cycle run-off reaction primed with a nested fluorescent BC primer. We loaded aliquots of the labeled BV-BC products on an Applied Biosystems (Foster City, CA) 373 DNA sequencer, and we determined their size distribution with the help of the Immunoscope software.
Fluorescence in situ hybridization assay
We evaluated the proportion of maternal cells in CBMCs from male newborns by fluorescence in situ hybridization (FISH) assay using centromeric probes for the chromosomes X (CEPX, Xp11.1-q11.1, locus DXZ1, spectrum green) and Y (CEPY, Yp11.1-q11.1, locus DYZ3, spectrum orange) (Vysis, Downers Grove, IL). The preparation of the slides and the FISH analysis have been described in detail elsewhere.15 We counted a total of 300 cells in each experiment using a fluorescent microscope (Zeiss Axioskop, Thornwood, NY) equipped with single-band pass filters (Vysis).
Results
CBMCs of congenitally T cruzi–infected newborns contain a high percentage of activated CD8 T cells
We initially observed a marked decrease of the CD4/CD8 mean ratio in congenitally infected newborns compared with uninfected neonates (1.6 ± 0.3, n = 11; vs 3.7 ± 0.3, n = 32; respectively, Student test: P < .01). As expected, analysis of the expression of memory/activation markers indicated T cells from uninfected newborns being naive, since few cells expressed CD45RO and HLA-DR markers (Table 1). By contrast, the percentage of both CD8+CD45RO+ and CD8+HLA-DR+ T cells was strongly and significantly increased in all congenitally infected neonates. We also observed a significant rise in the number of CD4+ T cells expressing CD45RO and HLA-DR, although at a level much lower than that observed within the CD8 T-cell subset. Conversely, we did not observe any difference in the percentage of T cells expressing CD69 or CD25 molecules between both groups. To further characterize the presence of highly differentiated CD8 T cells, we extended our analysis to the expression of the costimulatory CD28 molecule, since CD8+CD28− T cells are defined as end-stage effectors.16 Our results clearly showed a significant degree of CD28 loss in congenitally infected newborns, predominantly within the CD8 T-cell population (Table 1). Taken together, these data illustrate that congenital transmission of T cruzi is associated with a strong activation of CD8 T cells.
Phenotypic characterization of cord-blood T cells
| . | % positive CD8 T cells . | % positive CD4 T cells . | ||
|---|---|---|---|---|
| Uninfected newborns . | Congenitally infected newborns . | Uninfected newborns . | Congenitally infected newborns . | |
| Surface activation markers | ||||
| CD45RO+ | 4.5 ± 1.1‡ (n = 24) | 33.9 ± 4.0† (n = 14) | 9.9 ± 1.6 | 13.6 ± 1.4* |
| HLA-DR+ | 1.2 ± 0.3 (n = 12) | 53.7 ± 6.3† (n = 13) | 0.8 ± 0.2 | 6.2 ± 1.2† |
| CD25+ | 0.34 ± 0.13 (n = 20) | 0.25 ± 0.11 (n = 4) | 11.4 ± 1.3 | 9.1 ± 3.0 |
| CD69+ | 5.5 ± 2.0 (n = 13) | 5.8 ± 1.8 (n = 6) | 4.5 ± 1.3 | 4.0 ± 1.1 |
| CD28− | 2.9 ± 0.8 (n = 5) | 26.1 ± 8.2* (n = 5) | 0.1 ± 0.0 | 1.6 ± 0.5* |
| Intracellular expression of effector/regulating molecules | ||||
| IFN-γ | 3.1 ± 1.7 (n = 10) | 22.5 ± 4.4† (n = 9) | 1.1 ± 0.6 | 2.6 ± 0.5* |
| TNF-α | 5.7 ± 2.5 (n = 5) | 20.0 ± 7.1* (n = 5) | 36.5 ± 9.0 | 37.3 ± 9.0 |
| IL-2 | 7.2 ± 2.9 (n = 4) | 8.6 ± 1.7 (n = 5) | 22.7 ± 7.7 | 23.7 ± 6.2 |
| IL-4 | 2.1 ± 0.8 (n = 5) | 2.5 ± 0.3 (n = 5) | 1.2 ± 0.5 | 2.0 ± 0.8 |
| Perforin | 4.0 ± 1.7 (n = 14) | 12.1 ± 4.2* (n = 11) | 0.4 ± 0.2 | 2.0 ± 0.5† |
| . | % positive CD8 T cells . | % positive CD4 T cells . | ||
|---|---|---|---|---|
| Uninfected newborns . | Congenitally infected newborns . | Uninfected newborns . | Congenitally infected newborns . | |
| Surface activation markers | ||||
| CD45RO+ | 4.5 ± 1.1‡ (n = 24) | 33.9 ± 4.0† (n = 14) | 9.9 ± 1.6 | 13.6 ± 1.4* |
| HLA-DR+ | 1.2 ± 0.3 (n = 12) | 53.7 ± 6.3† (n = 13) | 0.8 ± 0.2 | 6.2 ± 1.2† |
| CD25+ | 0.34 ± 0.13 (n = 20) | 0.25 ± 0.11 (n = 4) | 11.4 ± 1.3 | 9.1 ± 3.0 |
| CD69+ | 5.5 ± 2.0 (n = 13) | 5.8 ± 1.8 (n = 6) | 4.5 ± 1.3 | 4.0 ± 1.1 |
| CD28− | 2.9 ± 0.8 (n = 5) | 26.1 ± 8.2* (n = 5) | 0.1 ± 0.0 | 1.6 ± 0.5* |
| Intracellular expression of effector/regulating molecules | ||||
| IFN-γ | 3.1 ± 1.7 (n = 10) | 22.5 ± 4.4† (n = 9) | 1.1 ± 0.6 | 2.6 ± 0.5* |
| TNF-α | 5.7 ± 2.5 (n = 5) | 20.0 ± 7.1* (n = 5) | 36.5 ± 9.0 | 37.3 ± 9.0 |
| IL-2 | 7.2 ± 2.9 (n = 4) | 8.6 ± 1.7 (n = 5) | 22.7 ± 7.7 | 23.7 ± 6.2 |
| IL-4 | 2.1 ± 0.8 (n = 5) | 2.5 ± 0.3 (n = 5) | 1.2 ± 0.5 | 2.0 ± 0.8 |
| Perforin | 4.0 ± 1.7 (n = 14) | 12.1 ± 4.2* (n = 11) | 0.4 ± 0.2 | 2.0 ± 0.5† |
n indicates the number of donors analyzed.
P < .05 vs uninfected newborns (Student test).
P < .01 vs uninfected newborns (Student test).
Mean percentage of positive cells ± SEM.
We next asked if activated T cells in cord blood of congenitally infected newborns might correspond to transferred maternal cells (materno-fetal microchimerism).17 To test this possibility, we used the dual-color FISH assay to measure the proportion of maternal cells within CBMCs from one congenitally infected and one uninfected male newborn. The FISH assay allowed detection of normal rates of materno-fetal microchimerism of 0.3% and 1.6% in cord blood from congenitally infected (containing 61.6% HLA-DR+ and 35.8% CD45RO+ CD8 T cells) and uninfected newborns, respectively. These data indicate that activated T cells in congenitally infected newborns are fetal and not maternally derived lymphocytes.
T cells in congenitally infected newborns display restricted TCR Vβ repertoire and CDR3 size patterns
Preliminary experiments showed a predominant expansion of TCR-αβ T cells over TCR-γδ T cells among CD3+CD45RO+-positive T lymphocytes from congenitally infected newborns (92.2 ± 1.6%, n = 9; vs 77.3 ± 3.0%, n = 25; respectively, Mann-Whitney test:P < .05). To investigate if activated cord-blood T cells had defined TCR-αβ repertoires, we analyzed by flow cytometry the proportions of CD8 and CD4 T cells expressing the TCR-Vβ3, 5, 11, 13.1, 14, 16, 17, 18, and 22 subtypes in 3 congenitally infected neonates and 6 to 9 control newborns. We have arbitrarily chosen this panel of TCR Vβ chains that covered around 30% of the CD8 Vβ repertoire and 25% of the CD4 Vβ repertoire in the control group. The percentages of CD8 T cells expressing Vβ5, Vβ13.1, and Vβ17 in congenitally infected newborn 1, Vβ13.1 and Vβ22 in newborn 2, and Vβ16 in newborn 3 were increased above the upper threshold (mean + 2SD) of control newborns (Table2). We also detected a low expansion of CD4 T cells expressing Vβ5 and Vβ13.1 in newborn 1, nearly 2 times less than those obtained within CD8 T cells. Furthermore, it is worth pointing out that newborns 1 and 2 displayed a low increase in the percentage of CD4 T cells expressing Vβ11 and/or Vβ18, whereas we did not detect up-regulation for this Vβ family within the CD8 T-cell subset. These results demonstrate that mainly the cord-blood CD8 T cells of congenitally infected newborns display a restricted TCR Vβ repertoire.
TCR-Vβ use in cord-blood mononuclear cells of uninfected (mean % ± SEM, n = 6-9) or congenitally infected newborns (individual data of 3 newborns)
| . | CD8+ T cells . | CD4+ T cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected newborns . | Congenitally infected newborns . | Uninfected newborns . | Congenitally infected newborns . | |||||
| 1 . | 2 . | 3 . | 1 . | 2 . | 3 . | |||
| Vβ3 | 5.8 ± 1.0* | 0.7 | 1.2 | 6.6 | 4.9 ± 0.8 | 7.6 | 4.2 | 2.5 |
| Vβ5 | 3.2 ± 0.2 | 7.5† | 3.5 | 2.4 | 2.2 ± 0.1 | 3.4† | 2.6 | 1.6 |
| Vβ11 | 0.8 ± 0.1 | 1.1 | 0.6 | 0.8 | 0.8 ± 0.0 | 2.0† | 1.0† | 0.6 |
| Vβ13.1 | 3.4 ± 0.3 | 13.5† | 13.5† | 2.8 | 3.4 ± 0.3 | 7.1† | 3.4 | 2.8 |
| Vβ14 | 6.8 ± 0.7 | 9.0 | 4.5 | 4.1 | 2.9 ± 0.1 | 2.4 | 3.0 | 2.5 |
| Vβ16 | 0.8 ± 0.1 | 0.8 | 0.6 | 1.2† | 0.7 ± 0.1 | 0.3 | 0.9 | 0.5 |
| Vβ17 | 5.6 ± 0.6 | 23.9† | 3.0 | 5.2 | 5.1 ± 0.2 | 5.3 | 4.6 | 3.8 |
| Vβ18 | 0.6 ± 0.2 | 1.4 | 0.3 | 0.2 | 0.8 ± 0.1 | 1.5† | 1.1 | 0.8 |
| Vβ22 | 4.5 ± 0.6 | 1.1 | 11.7† | 2.2 | 3.9 ± 0.4 | 5.5 | 5.1 | 4.0 |
| . | CD8+ T cells . | CD4+ T cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected newborns . | Congenitally infected newborns . | Uninfected newborns . | Congenitally infected newborns . | |||||
| 1 . | 2 . | 3 . | 1 . | 2 . | 3 . | |||
| Vβ3 | 5.8 ± 1.0* | 0.7 | 1.2 | 6.6 | 4.9 ± 0.8 | 7.6 | 4.2 | 2.5 |
| Vβ5 | 3.2 ± 0.2 | 7.5† | 3.5 | 2.4 | 2.2 ± 0.1 | 3.4† | 2.6 | 1.6 |
| Vβ11 | 0.8 ± 0.1 | 1.1 | 0.6 | 0.8 | 0.8 ± 0.0 | 2.0† | 1.0† | 0.6 |
| Vβ13.1 | 3.4 ± 0.3 | 13.5† | 13.5† | 2.8 | 3.4 ± 0.3 | 7.1† | 3.4 | 2.8 |
| Vβ14 | 6.8 ± 0.7 | 9.0 | 4.5 | 4.1 | 2.9 ± 0.1 | 2.4 | 3.0 | 2.5 |
| Vβ16 | 0.8 ± 0.1 | 0.8 | 0.6 | 1.2† | 0.7 ± 0.1 | 0.3 | 0.9 | 0.5 |
| Vβ17 | 5.6 ± 0.6 | 23.9† | 3.0 | 5.2 | 5.1 ± 0.2 | 5.3 | 4.6 | 3.8 |
| Vβ18 | 0.6 ± 0.2 | 1.4 | 0.3 | 0.2 | 0.8 ± 0.1 | 1.5† | 1.1 | 0.8 |
| Vβ22 | 4.5 ± 0.6 | 1.1 | 11.7† | 2.2 | 3.9 ± 0.4 | 5.5 | 5.1 | 4.0 |
Mean percentage of positive cells ± SEM.
Values higher than the normal upper range (mean ± 2 SD of uninfected newborns).
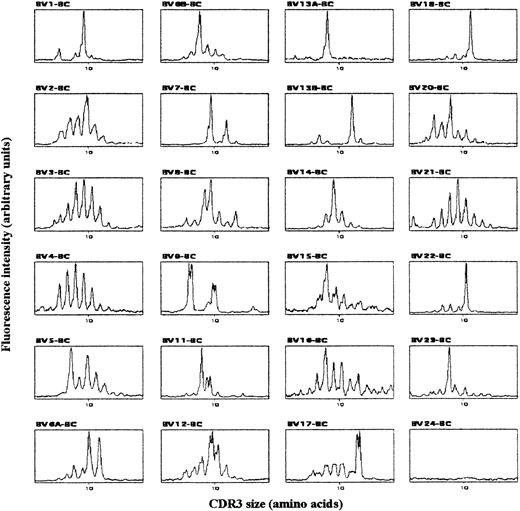
To characterize the clonality of the expanded T-cell populations, we analyzed CDR3 size patterns of TCR-BV chain transcripts. In agreement with previous work,18 cord-blood T cells from uninfected newborns displayed a gaussian CDR3 size pattern in all BV families (data not shown), revealing their diverse polyclonal TCR BV repertoires. By contrast, the analysis of CBMCs from 3 congenitally infected newborns showed nongaussian profiles, with the presence of dominant peaks in several BV subfamilies as well as in beta-chain junction region (BJ) families. Typical TCR BV pattern for newborn 2 is shown in Figure 1. Interestingly, we found the peak in BV13A from Figure 1 to correspond mainly to a BV13A-BJ1S5 rearrangement that could represent as little as one clone recognized by the BV13.1 antibody used in the flow cytometry studies (not shown). Thus, while the polyclonal repertoire of healthy newborns reflects the lack of T-cell priming by previous antigenic exposure, both the clear clonal T-cell expansion and the presence of activated and expanded CD8 T cells in congenitally infected newborns suggest that fetal CD8 T-lymphocyte activation was driven by parasite antigens.
Typical TCR BV-BC CDR3 size pattern from congenitally infected newborn 2.
cDNA made from total RNA extracted from CBMCs was amplified in 24 PCR reactions, each primed by a BV- and the common BC-specific oligonucleotide. The amplification products were copied in 24 run-off reactions primed by a nested fluorescent BC primer, and the labeled DNA copies were analyzed on a sequencing gel by an automated DNA sequencer. The fluorescent profiles of the 24 BV subfamilies are expressed as fluorescence intensity on the y-axis (arbitrary unit) and BV-BC size on the x-axis.
Typical TCR BV-BC CDR3 size pattern from congenitally infected newborn 2.
cDNA made from total RNA extracted from CBMCs was amplified in 24 PCR reactions, each primed by a BV- and the common BC-specific oligonucleotide. The amplification products were copied in 24 run-off reactions primed by a nested fluorescent BC primer, and the labeled DNA copies were analyzed on a sequencing gel by an automated DNA sequencer. The fluorescent profiles of the 24 BV subfamilies are expressed as fluorescence intensity on the y-axis (arbitrary unit) and BV-BC size on the x-axis.
CD8 and CD4 T cells from congenitally infected newborns are differentiated into potential effector T cells
We sought for a relationship between the activated T-cell phenotype and effector functions. For this purpose, we investigated by flow cytometry the ability of both CD8 and CD4 T cells to produce intracellular effector cytokines upon short in vitro restimulation with PMA and ionomycin. The percentage of cord-blood CD8 T cells positive for intracellular IFN-γ and TNF-α was significantly higher in congenitally infected newborns than in the control group (Table 1). We also detected a greater mean fluorescence intensity (MFI) for TNF-α expression within CD8 T cells from congenitally infected neonates (MFI ± SEM, 120.6 ± 22.4 vs 26.1 ± 3.4 in the control group), indicating a better ability to produce this cytokine. The percentage of CD4 T cells producing IFN-γ and the MFI of TNF-α expression within this cell subset (MFI, 46.3 ± 9.0 vs 29.6 ± 2.6 in the control group) were also significantly, albeit slightly, increased in congenitally infected newborns. In contrast, the proportions of IL-2– or IL-4–producing T cells were similar in both groups of newborns.
The ability of fetal T cells from infected newborns to exhibit effector cytokines prompted us to measure the intracellular expression of the lytic molecule perforin in unstimulated CBMCs. We observed a significant enrichment in the percentage of both CD8 and CD4 T cells positive for perforin in congenitally infected newborns as compared with the control group (Table 1). Altogether, these results show that activated T cells of congenitally infected newborns are armed to exert effector functions.
Congenitally T cruzi–infected newborns suffer a high level of CD8 T-cell spontaneous apoptosis
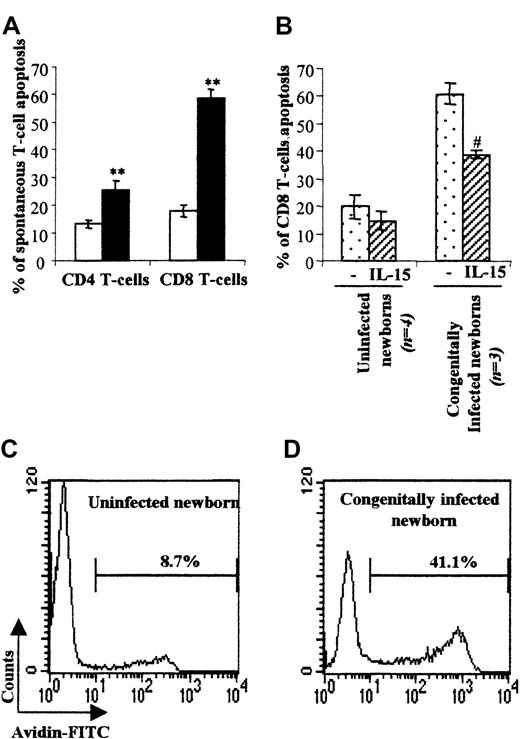
Generally, the overwhelming numbers of activated CD8 T cells generated following infection are associated with T-cell death.19 We therefore evaluated the level of spontaneous T-cell death using annexin-V staining after CBMC culture for 24 hours in the presence or absence of IL-15 known to improve T-cell survival20 (Figure 2). CD8 T cells from congenitally infected newborns underwent a marked level of spontaneous cell death (57.4 ± 4.0%) as compared with uninfected neonates (19.4 ± 3.0%). We also found a significant and higher cell death level within the CD4 T-cell subset (22.2 ± 2.1% vs 13.7 ± 1.9% in control group). The TUNEL assay applied on CBMCs from 2 patients confirmed that T cells from congenitally infected newborns were prone to die by apoptosis (Figure 2C-D). Moreover, addition of IL-15 (Figure 2B) to the cell culture prolonged the survival of CD8 T cells, which is consistent with a mitochondrial/cytokine rescuable pathway of apoptotic cell death.20
Spontaneous T-cell apoptosis and IL-15 rescue of cord-blood CD8 T cells.
(A) Mean percentage ± SEM of spontaneous T-cell death defined by annexin-V–FITC staining within the CD4+ and CD8+ subsets in uninfected (■, n = 17) and congenitally infected (▪, n = 12) newborns. **P < .01 vs uninfected newborns (Student test). (B) Mean percentage ± SEM of spontaneous T-cell death following cell culture for one day in the presence or absence of IL-15. (C, D) Two representative experiments, in one uninfected (C) and one congenitally infected neonate (D) of the TUNEL assay analysis of spontaneous T-cell death are shown. Percentages of cellular spontaneous apoptosis are indicated in bold. #P < .05 vs unstimulated CBMCs (Student test).
Spontaneous T-cell apoptosis and IL-15 rescue of cord-blood CD8 T cells.
(A) Mean percentage ± SEM of spontaneous T-cell death defined by annexin-V–FITC staining within the CD4+ and CD8+ subsets in uninfected (■, n = 17) and congenitally infected (▪, n = 12) newborns. **P < .01 vs uninfected newborns (Student test). (B) Mean percentage ± SEM of spontaneous T-cell death following cell culture for one day in the presence or absence of IL-15. (C, D) Two representative experiments, in one uninfected (C) and one congenitally infected neonate (D) of the TUNEL assay analysis of spontaneous T-cell death are shown. Percentages of cellular spontaneous apoptosis are indicated in bold. #P < .05 vs unstimulated CBMCs (Student test).
Cord blood from congenitally infected newborns contains parasite-specific CD8 T cells
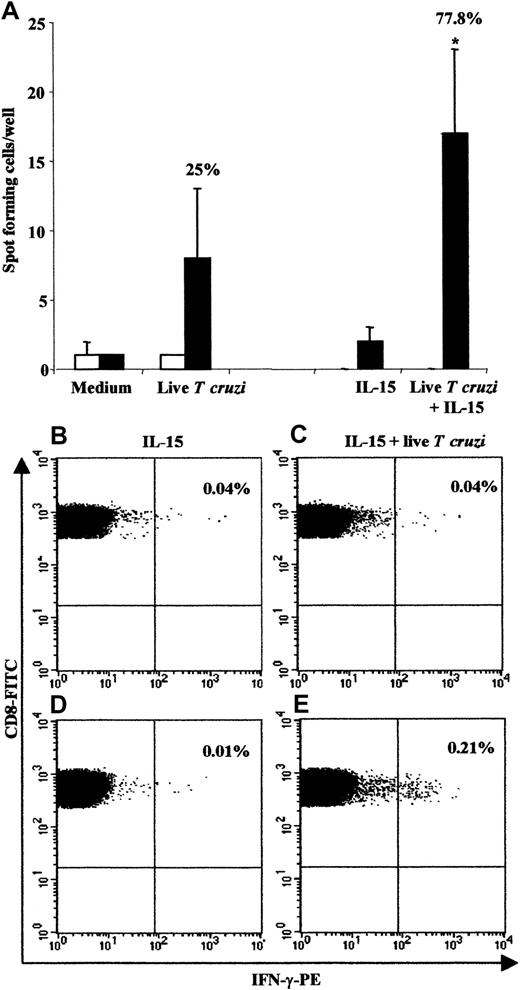
Given the vigorous CD8 T-cell response in congenitally infected newborns, we analyzed the presence of parasite-specific cord-blood T cells using the ex vivo IFN-γ Elispot assay (Figure3). We used live parasites to further enhance CD8 T-cell stimulation through a major histocompatibility complex (MHC) class I presentation. CBMCs were costimulated or not with IL-15 since this cytokine is known to: (1) improve T-cell responses in intracellular infection,21 (2) prevent apoptosis (see above), and (3) enhance the division of CD8+ memory pool.22 We observed IFN-γ–secreting cells from 7 of 9 (77.8%) congenitally infected newborns when IL-15 was added, while 3 of 12 (25%) displayed positive responses following stimulation with live T cruzi alone (Figure 3A). These responses could not be related to an unspecific production of IFN-γ, since cells from uninfected newborns failed to produce this cytokine, even after addition of IL-15. Likewise, flow cytometry analysis of CBMCs exposed to both IL-15 and live parasites showed that CD8 T cells from congenitally infected newborns expressed detectable amount of intracellular IFN-γ in 2 of 3 donors tested (Figure 3B-E). These results provide evidence that parasite-specific CD8 T cells are present in cord blood from congenitally infected newborns.
Frequency of responding cells measured by the IFN-γ Elispot assay and the intracellular IFN-γ content in CD8 T cells.
(A) For Elispot analysis, CBMCs from 17 uninfected (■) and 12 congenitally infected (▪) newborns were stimulated or not with liveT cruzi in the presence or absence of IL-15 (n = 12 and n = 9 in uninfected and infected neonates, respectively). The percentage of positive responses in congenitally infected newborns is indicated above the histograms. *P < .05 vs IL-15 alone (Student test). Visualization of intracellular IFN-γ content in CD8 T cells in one uninfected (B and C) and one congenitally infected newborn (D and E) is also shown. CBMCs were stimulated with IL-15 alone (B and D) or with both IL-15 and liveT cruzi (C and E). Analyses were performed within the CD8 T-cell subset. The percentage of IFN-γ–positive cells is indicated in the upper right quadrant. Two other congenitally infected newborns were tested, resulting in 1 of 2 positive responses.
Frequency of responding cells measured by the IFN-γ Elispot assay and the intracellular IFN-γ content in CD8 T cells.
(A) For Elispot analysis, CBMCs from 17 uninfected (■) and 12 congenitally infected (▪) newborns were stimulated or not with liveT cruzi in the presence or absence of IL-15 (n = 12 and n = 9 in uninfected and infected neonates, respectively). The percentage of positive responses in congenitally infected newborns is indicated above the histograms. *P < .05 vs IL-15 alone (Student test). Visualization of intracellular IFN-γ content in CD8 T cells in one uninfected (B and C) and one congenitally infected newborn (D and E) is also shown. CBMCs were stimulated with IL-15 alone (B and D) or with both IL-15 and liveT cruzi (C and E). Analyses were performed within the CD8 T-cell subset. The percentage of IFN-γ–positive cells is indicated in the upper right quadrant. Two other congenitally infected newborns were tested, resulting in 1 of 2 positive responses.
Discussion
The present work shows for the first time a massive and oligoclonal development of fetal CD8 T cells following congenital infection with an intracellular pathogen. These cells display a phenotype of activated effector T cells, and a substantial number of them are parasite specific. This in utero immune response of congenitally T cruzi–infected newborns mimics the strong antigen-specific CD8 T-cell expansion previously described in adults during acute viral infections.23,24 Furthermore, while a CD8+ T-cell–mediated response contributes to the control of acute parasitemia in experimental Chagas disease,25 no data are currently available concerning the role of CD8 T cells during acute human infection with T cruzi.
Increased T-cell apoptosis is a classical feature of T-cell activation following immune response in vivo.19 In our study, IL-15 rescued activated T cells from spontaneous apoptosis and improved the ability of parasite-specific CD8 T cells to produce IFN-γ. The strong disturbance in the TCR repertoire of CBMCs from congenitally infected newborns, with a clonal dominance in several BV and BJ families, also strongly argues for a predominant expansion of antigen-experienced CD8 T cells. Interestingly, we observed that the markedly disturbed TCR BV repertoire of infected newborns varied from one neonate to another, ruling out the possibility that superantigens were responsible for fetal T-cell activation.
The tremendous expansion of CD8 T cells was associated with major changes in surface phenotypes, which are closely related to acquired effector functions. Along this line, the expression of effector molecules such as IFN-γ , TNF-α, and perforin within CD8 T cells from congenitally infected newborns demonstrates that these cells are endowed with potent effector machinery. It is also of interest to note that CD8+CD28 T cells as well as CD8+CD45RO+ and CD8+HLA-DR+ lymphocytes have been shown to mediate memory and cytotoxic functions.16,26,27 The accumulation of CD8+CD28− T cells can be related to in vivo chronic antigenic stimulation and extensive rounds of cell division. Indeed, this CD8+CD28−subset has been reported to be end-stage T cells, which could explain the lack of increased IL-2 production in our work as observed in adults during viral infection.16 Altogether, these findings confirm a recent report showing that the CD8+ T-cell component of neonatal host defenses may be relatively competent compared with its CD4+ counterparts.28 Indeed, the authors demonstrated that the IFN-γ promoter was hypermethylated in neonatal CD4+CD45RO− T cells at CpG sites, but, conversely, was hypomethylated in CD8+CD45RO− T cells in adults.28Finally, the trends in the immune response of congenitally infected newborns, including the oligoclonal expansion of parasite-specific and activated CD8 T cells in addition to their probable clonal exhaustion, as demonstrated by the amount of spontaneous apoptosis, strengthen the view that fetuses are able to generate an adultlike CD8 T-cell response in utero.
Although we observed a preponderant CD8 T-cell response, we also detected low levels of activated CD4 T cells in cord blood from congenitally infected newborns, and the analysis of Vβ subfamilies suggested that some of the CD4 T-cell clones might be expanded. These observations might mirror T-helper function requirements for efficient primary CD8 T-lymphocyte activation.29 These activated cells also contained perforin and were primed to produce IFN-γ and TNF-α that might correspond to a subset of CD4 T lymphocytes with cytotoxic potential toward cells bearing MHC class II molecules29 and the appropriate parasite antigen.
However, our results do not support a previous report about a preferential modulation of Vβ5-expressing CD4 T cells rather than CD8 T cells in infants acutely infected with T cruzi.30 Indeed, these children came from the same endemic area of Bolivia as the newborns we have presently studied. Whether these surprising discrepancies relate to differences in the age or the mode of parasite transmission (vectorial versus congenital) remains an open question.
To date, the correlation between the observed CD8 T cells inT cruzi congenitally infected newborns and an immunoprotective role later in life remains to be determined. However, our data support the idea that the fetal immune system is more competent than previously appreciated. The demonstration that maturation of a CD8 T-cell immune response may occur in utero looks promising in the field of neonatal vaccination to control early infections with intracellular pathogens.
We thank Eduardo Suarez and Marisol Cordova and the staff of the maternity German Urquidi (Cochabamba, Bolivia) for the management of patients; Rudy Parrado and Myriam Huanca (Centro Universitario de Medicina Tropicale/Laboratorio de Biologica Medical [CUMETROP/LABIMED], Universidad Mayor de San Simón, Cochabamba, Bolivia) for T cruzi diagnosis of patients; and Samira Benyoucef Dahmani (Cytokine Profile [CYPRO SA]) for helping with the Elispot analysis. We are grateful to Michel Goldman and Eric Muraille for their comments on the manuscript.
Supported by the Centre de Recherche Interuniversitaire en Vaccinologie (CRIV) and sponsored by the Région Wallonne and Glaxo-Smithkline biologicals (Belgium) and by the Conseil Interuniversitaire de la Communauté française de Belgique (CIUF).
E.H. and C.A.-V. are research fellows of CRIV and Association pour la promotion de l'éducation et la formation àl'étranger (APEFE, Communauté Française de Belgique), respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yves Carlier, Laboratoire de Parasitologie, Faculté de Médecine, U.L.B. Route de Lennick, 808, CP 616, B-1070 Brussels, Belgium; e-mail: ycarlier@ulb.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal