Abstract
Annexin V has phospholipid-binding capacity and plays a potent antithrombotic role. Recently, a C to T transition has been described in the Kozak region of this gene, affecting the nucleotide preceding the initiation ATG codon. We have developed a simple method to detect this genetic change, showing by analysis of 580 Mediterranean white subjects that the −1C to T transition (−1C>T) is a common polymorphism (allele frequency, 0.121). This polymorphism is in linkage disequilibrium with a new C>G polymorphism located 27 bp downstream in intron 2. We show that −1C/C carriers presented significantly lower plasma levels of annexin V than −1C/T subjects (0.45 ± 0.20 ng/mL versus 0.73 ± 0.28 ng/mL, respectively;P = .02). In vitro transcription/translation experiments support that the −1T allele increases translation efficiency. The clinical relevance of the −1C>T change was investigated in consecutive patients with nontraumatic spontaneous intracranial hemorrhage (n = 225), deep venous thrombosis (n = 151), and coronary heart disease (n = 101). Finally, we also studied 166 survivors of an acute myocardial infarction occurring at age of 45 or less. This polymorphism seems to have a minor effect in bleeding disorders, but to play a protective role against early myocardial infarction, reducing by 2-fold the risk of developing the disease (P = .006; odds ratio, 0.51; 95% confidence interval, 0.30-0.85).
Introduction
The annexin family is integrated by proteins that share structural similarities and have the functional property of binding to phospholipids in the presence of Ca++ions. Annexin V (ANV) is the most abundant member of this group of proteins, found in many tissues, including blood.1 The circulating ANV might be released from cells present in the vessel wall. Once ANV is secreted into plasma, it binds immediately to blood cells and probably also to endothelial cells, accounting for the low levels of free protein in plasma (1.1 ± 1.7 ng/mL; range, 0-8.2 ng/mL in citrated blood).2
ANV possesses a diverse range of functions, most of these related to its phospholipid-binding capacity. ANV preferentially binds to negatively charged phospholipids that are present predominantly at the inner leaflet of the plasma membrane of eukariotic cells. Under different circumstances, these phospholipids become surface exposed, being able to bind ANV. The activation of platelets and red blood cells significantly increases the number of binding sites for ANV on these cells.3,4 Moreover, apoptosis is another process associated with phospholipid exposure and, thus, with greater binding of ANV to the cell surface.5
The capacity of ANV to bind phospholipids is closely associated with its physiological relevance in the hemostatic system, since negatively charged phospholipids form the catalytic surface to which different coagulation complexes assemble. Thus, ANV prevents formation of prothrombinase and tenase complexes6 and can also interfere in activation of protein C on the surface of endothelial cells.7 Recently, ANV has been found to displace the majority of preadsorbed anticardiolipin antibodies—β-glycoprotein I complexes from coagulant membranes.8 Moreover, ANV inhibits platelet adhesion at venous blood flow conditions and diminishes aggregate formation under blood flow conditions, corresponding to medium-size arteries.9 Additionally, ANV binds to the highly thrombogenic core debris resulting from plaque rupture, and reduces the levels of tissue factor.10-12Finally, ANV has also been found to improve the effect of fibrinolytic drugs.13 All these effects account for the potent antithrombotic role of ANV.2
The ANV gene is 29 kb long and contains 12 exons that code for a single polypeptide chain of 319 amino acids.14 Recently, a preliminary report described a C to T transition in exon 2 of the ANV gene, affecting the nucleotide preceding the initiation ATG codon (position, −1).15 In higher eukaryotes, the expression of about 1 gene in 10 is strongly regulated at the level of mRNA translation into protein. Regulatory effects are often mediated by the 5′-untranslated region and rely on the fact that the 40S ribosomal subunit first binds to the cap structure at the 5′-end of mRNA and then scans for the first ATG codon. The sequences flanking the initiating ATG codons (Kozak sequences) modulate the ability of the 40S ribosomal subunits to stop and initiate translation, as elegantly demonstrated by classic in vitro experiments.16
The aims of the present study were to investigate (1) the prevalence of the −1 C to T transition (−1C>T) of the ANV gene; (2) the effect of this polymorphism in the plasma levels of this protein; (3) its role in translation efficiency; (4) the risk of developing thromboembolic or hemorrhagic disorders associated with this polymorphism.
Patients and methods
Selection of control subjects and case patients
Genetic analyses were performed on 580 white unrelated subjects representative of the general population of our Mediterranean area. These controls were blood donors and traumatology and ophthalmology patients admitted to the hospital who had neither documented history of vascular disease nor personal history of thromboembolic or hemorrhagic disease. Moreover, we studied 225 consecutive patients suffering from a first episode of nontraumatic spontaneous intracranial hemorrhage (SIH). Diagnosis was verified on admission in all cases by computed tomography performed within 24 hours of the beginning of the symptoms.17 We also included 151 consecutive patients with a first confirmed episode (by compression ultrasonography or contrast venography) of deep venous thrombosis (DVT). Additionally, we studied 101 consecutive patients admitted to the coronary unit, all of them with an established first diagnosis of coronary heart disease (CHD), in concordance with the World Health Organization criteria.18Finally, we studied from our cardiology outpatient clinic 166 consecutive patients who survived an acute myocardial infarction (MI) before the age of 45 years. The diagnosis of MI required the presence of at least 2 of these criteria: typical chest pain lasting more than 30 minutes; creatine kinase elevation exceeding twice the upper normal limit with a concomitant rise in the creatine kinase MB isoenzyme level; presence of new Q-waves, or new or presumedly new abnormal ST-T features.
All patients enrolled were unrelated white subjects from the same region as the control subjects. Clinical features of all subjects included in our study are summarized in Table 1.
Clinical features and genetic frequencies of Kozak ANV −1 C/T polymorphism, among patients and controls
| . | Control . | SIH . | DVT . | CHD . | MI before 45 y . |
|---|---|---|---|---|---|
| No. | 580 | 225 | 151 | 101 | 166 |
| Age, mean ± SD | 49.5 ± 19.6 | 67.4 ± 13.1* | 55.8 ± 17.7* | 62.9 ± 11.1* | 39.9 ± 4.6* |
| Sex, % males | 61.0 | 58.7 | 55.6 | 73.3* | 92.7* |
| Hypertension, % | 22.2 | 59.6* | — | 49.5* | 29.5 |
| Type I or II diabetes, % | 10.2 | — | — | 37.6* | 10.8 |
| Hypercholesterolemia, % | 12.6 | — | — | 42.6* | 78.9* |
| Smoking, % | 37.9 | 27.4* | — | 51.5* | 85.5* |
| Factor V Leiden +/−, % | 4.1 | — | 10.6* | — | — |
| Factor II 20210 A/G, % | 2.1 | — | 6.0* | — | — |
| Genotype | |||||
| −1 C/C, % | 446 (76.9) | 179 (79.6) | 123 (81.5) | 84 (83.2) | 144 (86.8) |
| −1 C/T + T/T, % | 128 + 6 (22.1 + 1.0) | 39 + 7 (17.3 + 3.1) | 28 + 0 (18.5 + 0) | 17 + 0 (16.8 + 0) | 20 + 2 (12.0 + 1.2) |
| P† | NA | .416 | .229 | .161 | .006 |
| OR (95% CI) | NA | 0.86 (0.58-1.27) | 0.76 (0.47-1.22) | 0.67 (0.37-1.21) | 0.51 (0.30-0.85) |
| Allele | |||||
| C | 0.879 | 0.882 | 0.907 | 0.916 | 0.928 |
| T | 0.121 | 0.118 | 0.093 | 0.084 | 0.072 |
| P† | NA | .872 | .175 | .134 | .013 |
| OR (95% CI) | NA | 0.97 (0.68-1.38) | 0.74 (0.47-1.16) | 0.67 (0.38-1.16) | 0.57 (0.35-0.91) |
| . | Control . | SIH . | DVT . | CHD . | MI before 45 y . |
|---|---|---|---|---|---|
| No. | 580 | 225 | 151 | 101 | 166 |
| Age, mean ± SD | 49.5 ± 19.6 | 67.4 ± 13.1* | 55.8 ± 17.7* | 62.9 ± 11.1* | 39.9 ± 4.6* |
| Sex, % males | 61.0 | 58.7 | 55.6 | 73.3* | 92.7* |
| Hypertension, % | 22.2 | 59.6* | — | 49.5* | 29.5 |
| Type I or II diabetes, % | 10.2 | — | — | 37.6* | 10.8 |
| Hypercholesterolemia, % | 12.6 | — | — | 42.6* | 78.9* |
| Smoking, % | 37.9 | 27.4* | — | 51.5* | 85.5* |
| Factor V Leiden +/−, % | 4.1 | — | 10.6* | — | — |
| Factor II 20210 A/G, % | 2.1 | — | 6.0* | — | — |
| Genotype | |||||
| −1 C/C, % | 446 (76.9) | 179 (79.6) | 123 (81.5) | 84 (83.2) | 144 (86.8) |
| −1 C/T + T/T, % | 128 + 6 (22.1 + 1.0) | 39 + 7 (17.3 + 3.1) | 28 + 0 (18.5 + 0) | 17 + 0 (16.8 + 0) | 20 + 2 (12.0 + 1.2) |
| P† | NA | .416 | .229 | .161 | .006 |
| OR (95% CI) | NA | 0.86 (0.58-1.27) | 0.76 (0.47-1.22) | 0.67 (0.37-1.21) | 0.51 (0.30-0.85) |
| Allele | |||||
| C | 0.879 | 0.882 | 0.907 | 0.916 | 0.928 |
| T | 0.121 | 0.118 | 0.093 | 0.084 | 0.072 |
| P† | NA | .872 | .175 | .134 | .013 |
| OR (95% CI) | NA | 0.97 (0.68-1.38) | 0.74 (0.47-1.16) | 0.67 (0.38-1.16) | 0.57 (0.35-0.91) |
Hypertension was defined as blood pressure exceeding 140 mm Hg (systolic) or exceeding 90 mm Hg (diastolic) on repeated observations during 3 months or, if no blood pressure values were available, by treatment for chronic antihypertensive therapy. Subjects were classified as “smoking” if they currently/formerly smoked more than 10 cigarettes per day. Hypercholesterolemia was defined as a total serum cholesterol level exceeding 5.72 mM (220 mg/dL).
SIH indicates spontaneous intracranial haemorrhage; DVT, deep venous thrombosis; CHD, coronary heart disease; MI, myocardial infarction; OR, odds ratio; CI, confidence interval, and NA, not applicable.
P < .05 between patients and controls.
Statistical comparison was performed between patients and controls.
Patients, controls, and family members of those patients with low level of consciousness at presentation were fully informed of the aim of this study. All subjects included gave their informed consent to enter the study, which had been approved by the local ethics committee and was performed in accordance with the declaration of Helsinki, as amended in Edinburgh in 2000.
Genotyping of the ANV polymorphisms
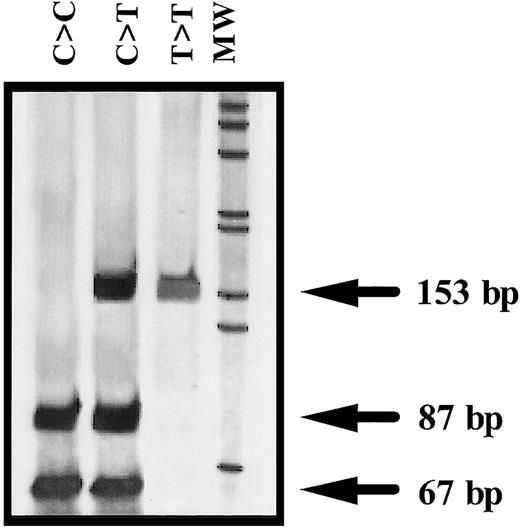
Amplification of exon 2 and flanking regions of ANV gene was performed by genomic polymerase chain reaction (PCR) with the use of 2 oligonucleotide primers: ANV forward primer (ANVF), 5′gggcacgagttgcaaatggcg3′ (502-522, nucleotide numbers according to Cookson et al14); and ANV backward primer–1(ANVB1), 5′gtcgcagcatacaaagttgtg3′ (634-654). Identification of the ANV −1C>T polymorphism was performed by restriction analysis of the PCR product with NcoI (New England Biolabs, Hertfordshire, United Kingdom). Briefly, 3 μL PCR product was completely digested with 1 U NcoI during 6 hours at 37°C. The restriction pattern was evaluated by electrophoresis in 7% acrylamide gels and by silver staining. The ANV −1C allele corresponded to a band of 87 bp, whereas the presence of a 153-bp band was distinctive of the −1T allele (Figure1).
Representative genotypes of the −1C>T polymorphism of the ANV gene.
MW indicates molecular weight marker (1-kb ladder) (GIBCO-BRL, Rockville, MD).
Representative genotypes of the −1C>T polymorphism of the ANV gene.
MW indicates molecular weight marker (1-kb ladder) (GIBCO-BRL, Rockville, MD).
A second endonuclease, BstXI (New England Biolabs), was employed for the identification of a previously unreported C>G change located at position 18 of intron 2 (ISV2 + 18). The PCR product was completely digested with 1 U BstXI during 6 hours at 55°C. A BstXI restriction pattern of 121- and 32-bp bands corresponded to the ISV2 + 18 C allele, while a fragment of 153 bp identified the ISV2 + 18 G variant (data not shown).
To confirm the results of the restriction study, we performed sequence analysis in selected individuals with different genotypes. PCR products were isolated and purified from 1.5% agarose gels by means of Ultraclean Gel Spin (MoBio, Solana Beach, CA). The sequence reaction was performed with the ABI Prism Big Dye Terminator Cycle sequencing kit on an automated sequencer type 377 (Perkin-Elmer Applied Biosystems, Warrington, Cheshire, United Kingdom) with forward and reverse primers used for amplification.
Free annexin V plasma levels
We selected 20 healthy blood donors, 10 with −1C/C genotype and 10 with −1C/T genotype, matched for age and sex. These subjects were negative for the anti–annexin V antibodies test (Diagnostica STAGO, Paris, France). Venous punctures were performed in the morning after 12-hour fasting by the donors. Blood samples were drawn atraumatically and without stasis into syringes preloaded with trisodium citrate (0.011 M, final concentration). After collection, platelet-poor plasma fractions were obtained by centrifugation for 20 minutes at 2200g and stored at −70°C. We determined ANV plasma levels by enzyme immunoassay (ELISA) technique (Annexin V, Diagnostica STAGO) following the manufacturer′s instructions.
Cell-free transcription/translation
Site-directed mutagenesis was performed following the manufacturer's instructions on the ANV complete cDNA (clone MGC:2261; IMAGE:3140878) with the use of ExSite PCR-Based Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The 2 forms of the ANV cDNA (−1C and −1T) were cloned into the vector pBluescript II KS (+/−) (Stratagene), in which transcription is driven from the T7 promoter. Plasmid from single colonies were purified by Wizard Plus SV Minipreps (Promega, Madison, WI) and sequenced.
The transcription/translation experiments were performed by means of the TNT quick-coupled transcription/translation system (Promega). One microgram of each DNA was added to 40 μL of the “master-mix,” which contains ribonucleoside triphosphates, rabbit reticulocyte lysate, T7 polymerase, all of the necessary amino acids except methionine, RNAse inhibitor, buffer, and 20 μCi (0.74 MBq) 35S-methionine (Amersham Life Science, Arlington Heights, IL). The volume was brought to 50 μL with nuclease-free water. The mixture was incubated at 30°C for 60 minutes. We always performed a parallel reaction with no DNA as a negative control.
Analysis of protein translation was performed by 2 methods: (1) Determination of incorporation of radioactive label. We precipitated 2 μL complete translation reaction with 900 μL ice-cold 25% trichloroacetic acid (TCA)/2% casamino acids (Sigma, St Louis, MO) during 30 minutes on ice. The precipitated translation product was collected by vacuum-filtering 250 μL TCA reaction mix with Whatman GF/C glass fiber filter (Whatman International, Kent, England) and washing with ice-cold 5% TCA and acetone. Then, the filter was allowed to dry. For determination of 35S incorporation, the filter was put into scintillation mixture and counted in a liquid scintillation counter (Wallac 1409; EG&G Instruments, Turku, Finland). Total counts present in the reaction were measured by spotting 5 μL TCA reaction mix directly onto a filter. (2) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). First, 5 μL translation reaction was analyzed on 10% SDS–polyacrylamide gels. The radioactive bands were detected by exposing the dried gel to Kodak X-OMAT film (Rochester, NY). The band density was quantified by means of Quantity One version 4.0.3 software (Biorad, Hercules, CA).
Statistical analysis
Discrete variables were expressed as percentages. Continuous variables were expressed as mean ± SD. The chi-square test was used to compare frequency distributions. The strength of the association of the polymorphisms with the occurrence of diseases was estimated by calculation of the odds ratio (OR) with the EpiInfo software and the use of the Cornfield method for the calculation of 95% confidence intervals (CIs). Comparison between continuous variables was performed by the Mann-Whitney-U test or the Wilcoxon signed rank test as appropriate. Multiple analysis was performed by means of logistic regression by the Forward Stepwise Method with SPSS (Chicago, IL) 8.0 software. The differences with a 2-tailed P < .05 were considered significant.
Results
Prevalence of the −1C>T polymorphism in a white population
We have developed a simple and reproducible PCR–allelic-specific restriction assay (ASRA) method that allows a rapid determination of the ANV −1C>T polymorphism. The genotype obtained by this method and by direct sequencing of the PCR product matched in all samples tested.
We have determined the prevalence of ANV −1C>T polymorphism in a population of 580 white control subjects from the Mediterranean area. The −1C/C genotype was the most represented in this group (76.9%); 22.1% carried the heterozygous −1C/T genotype, while the remaining 1.0% were −1T/T (Table 1). Therefore, the allelic frequencies of the −1C and −1T alleles were 0.879 and 0.121, respectively. This is the first report describing the frequency of this polymorphism in a normal population. Previously, a preliminary report indicated the presence of this polymorphism in 5 out of 65 French families with thrombotic complications.15 We did not detect age- or sex-dependent differences in the frequency of these alleles, and the distribution of genotypes was not significantly different from Hardy-Weinberg proportions (data not shown).
Identification of a new ANV polymorphism located in intron 2
The sequence analysis of the PCR product containing ANV exon 2 and flanking regions revealed a new nucleotide change in subjects carrying the −1T allele. We observed a C>G substitution at position 18 of the intron 2 sequence. These 2 nucleotide changes seemed to be linked, since the −1T allele was always associated with the ISV2 + 18 G allele. In order to confirm the existence of the new ISV2 + 18 C>G polymorphism and the linkage between both polymorphisms, we performed PCR-ASRA using BstXI in 65 subjects previously genotyped for the ANV −1C>T polymorphism (25 C/C, 25 C/T, and 15 T/T). All 25 −1C/C subjects were ISV2 + 18 C/C; all −1C/T individuals were also heterozygous for the ISV2 + 18 polymorphism, while the 15 −1T/T homozygous subjects carried the ISV2 + 18 G/G genotype. These data, together with the reduced distance between these 2 nucleotide changes (27 bp) suggest that the 2 polymorphisms could be in complete linkage disequilibrium.
Quantitative detection of free ANV in plasma by ELISA
We performed a quantitative determination of plasma levels of ANV in citrated plasma from 20 selected healthy white blood donors, 10 of them with −1C/C genotype and 10 with −1C/T genotype. The mean level of plasma ANV detected in this study (0.60 ± 0.27 ng/mL) did not differ from previous reports,2 with a wide range of values (0.23 to 1.12 ng/mL). Interestingly, carriers of the −1C/C genotype displayed a significantly lower plasma levels of ANV than subjects with −1C/T genotype (0.45 ± 0.20 ng/mL versus 0.73 ± 0.28 ng/mL;P = .02).
The −1C>T polymorphism affecting the Kozak sequence of the ANV gene influences translation efficiency
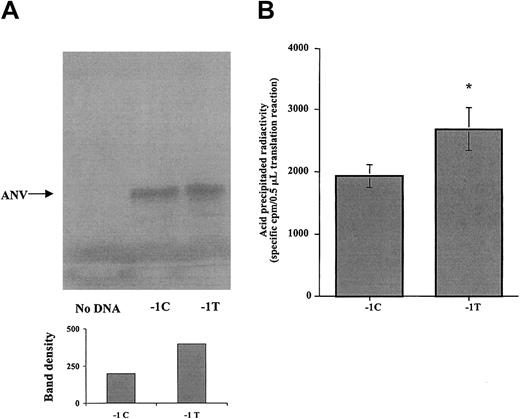
To investigate whether the −1C>T polymorphism in the Kozak sequence of the ANV gene affects the translation efficiency, ANV cDNA constructs differing only in the nucleotide at position −1 were created by site-directed mutagenesis and were cloned under the transcriptional control of T7 promoter in pBluescript II KS (+/−). Then, we evaluated the 2 allelic cDNA forms for their ability to produce protein in a cell-free transcription/translation system. As shown in Figure 2, SDS-PAGE analysis demonstrated the presence of a major protein band, with molecular weight corresponding to ANV (40 kDa), in those translation reactions containing the cDNA construct of ANV, either −1C or−1T, but not in negative control reactions without cDNA. Densitometric analysis of gel bands suggested higher protein production in reactions using the −1T cDNA construct (Figure 2A). For further quantitative comparison of the translation efficiency as a function of the −1C>T polymorphism, equivalent volumes of translation reaction were precipitated with trichloroacetic acid, and the amount of radioactive precipitated proteins was estimated by scintillation counting. Result from 3 different series of experiments demonstrated that −1T ANV cDNA produced a 1.4-fold increase in protein production compared with −1C ANV cDNA (Figure 2B).
Effect of the −1C>T polymorphism on the Kozak ANV sequence in an in vitro cell-free transcription/translation system.
(A) Representative autoradiogram of the SDS-PAGE analysis. A main band of 40 kDa is present both in −1C and in −1T plasmids, but not in the negative control. Densitometric analysis of ANV band shown in the gel (arbitrary units) indicates that more protein was synthesized from the −1T plasmid. (B) The 35S-methionine incorporated into acid-precipitable proteins in translation reactions containing −1C or −1T cDNA constructs of ANV. Results are mean (± SE) from 3 different series of experiments, each one including translation reactions with −1C or −1T cDNA or, as negative control, without cDNA. For each reaction, 6 replicates were analyzed, and the coefficient of variation between replicates was less than 30%. Precipitable radioactivity from negative control reaction without DNA was considered to be background and subtracted from corresponding values for −1C and −1T reactions. Significantly higher translation efficiency was associated with the −1T cDNA form (*P < .01).
Effect of the −1C>T polymorphism on the Kozak ANV sequence in an in vitro cell-free transcription/translation system.
(A) Representative autoradiogram of the SDS-PAGE analysis. A main band of 40 kDa is present both in −1C and in −1T plasmids, but not in the negative control. Densitometric analysis of ANV band shown in the gel (arbitrary units) indicates that more protein was synthesized from the −1T plasmid. (B) The 35S-methionine incorporated into acid-precipitable proteins in translation reactions containing −1C or −1T cDNA constructs of ANV. Results are mean (± SE) from 3 different series of experiments, each one including translation reactions with −1C or −1T cDNA or, as negative control, without cDNA. For each reaction, 6 replicates were analyzed, and the coefficient of variation between replicates was less than 30%. Precipitable radioactivity from negative control reaction without DNA was considered to be background and subtracted from corresponding values for −1C and −1T reactions. Significantly higher translation efficiency was associated with the −1T cDNA form (*P < .01).
Prevalence of the −1C>T polymorphism in thrombotic and hemorrhagic patients
To elucidate the role of the ANV −1C>T polymorphism in thrombotic or hemorrhagic disorders, we analyzed the prevalence of this polymorphism in patients with SIH, as well as in patients with venous or arterial thrombosis. Results were compared with those achieved in the control group (Table 1).
The ANV −1 genotype and allele frequencies detected in 225 patients with SIH were similar to those identified in 580 control subjects (Table 1). Intriguingly, homozygous T/T genotype was out of the Hardy-Weinberg equilibrium (3 expected versus 7 found), and thus, the prevalence of this genotype is higher than is found in the control group (3.5% versus 1%, respectively; P = .036).
By contrast, we observed a slightly lower percentage of −1C/T plus T/T subjects among 151 DVT patients than in controls (18.5% versus 23.1%, respectively) although the difference did not reach statistical significance (P = .229). This difference was more marked when we analyzed 101 consecutive patients with CHD (16.8% versus 23.1%), but was not statistically significant (P = .161).
Finally, the analysis of 166 selected patients who had suffered from acute MI before age 45 years revealed statistical significant differences (P = .006) in the percentage of subjects carrying the −1T allele (13.2%) compared with controls (23.1%). From these data, we conclude that the −1T allele reduces by 2-fold the risk of developing early acute myocardial infarction (OR, 0.51; 95% CI, 0.30-0.85) (Table 1). We also analyzed the prevalence of this polymorphism in 166 control subjects matched for age and sex with premature MI patients. The percentage of C/T plus T/T individuals of this group did not differ from that achieved in the general population (22.9% versus 23.1%, respectively), and it was statistically higher than that obtained among patients with premature MI (22.9% versus 13.2%, respectively; P = .022) (data not shown).
We evaluated the role of the ANV −1 genotype according to the type of MI. Only 18 patients suffered a non–Q-wave MI; 15 of them were ANV −1C/C and 3 were −1C/T.
Thus, the prevalence of the C/T genotype in the non–Q-wave MI group was similar to that achieved in the general population (16.7% versus 23.1%, respectively). Therefore, it seems that the Q-wave myocardial infarction might be the clinical situation where the C/T or T/T genotype played the most important protective role (10.2% versus 23.1%, respectively; P = .006; OR, 0.49; 95% CI, 0.28-0.84).
Finally, logistic regression analysis confirmed that the single nucleotide polymorphism had an independent protective effect in premature myocardial infarction (P = .039).
Discussion
The molecular basis of the variability among individuals affecting the level of different proteins is frequently associated with genetic variations located in regions with a key role in transcriptional control (promoters, enhancers, or silencers), as well as in the coding sequence.19 In the 1980s, Marilyn Kozak described the regulatory relevance of a region, highly conserved in eukaryotic genes, that precedes the initial methionine, called “Kozak sequence” in her honor. This sequence holds at least 6 nucleotides, and its integrity is directly associated with the regulation of translation by eukaryotic ribosome and, thus, with the level of protein synthesis.16 Few modifications altering the Kozak sequence have been described in patients in connection with the development of different pathologies.20-22 Nowadays, only 2 polymorphisms affecting the Kozak sequence have been described.23,24 A recent preliminary report has described a C to T change at position −1 of the Kozak sequence of the ANV gene.15 We have developed a simple and rapid method for identification of this genetic change, demonstrating that it represents a frequent polymorphism, present in the general population of our area with an allelic frequency of 0.121. Therefore, this becomes the third polymorphism being described in the Kozak region of a human gene. We also identified a new C>G polymorphism at position +18 of the ANV intron 2, located only 27 bp from the −1C>T polymorphism. These 2 polymorphisms seem to be in linkage disequilibrium, suggesting that they appeared simultaneously. The role of the new intronic polymorphism in the plasma level of ANV remains to be elucidated, but according to our cell-free transcription/translation experiments, the −1C>T polymorphism affecting the Kozak sequence of the ANV gene could directly contribute to the variation of plasma levels of ANV by increasing the translation efficiency. Thus, the −1T variant is associated with a 1.4-fold increase of the translation productiveness of the ANV protein. These data support the higher plasma levels of ANV detected in subjects carrying the −1C/T genotype.
ANV is a potent antithrombotic molecule with potential clinical usefulness in the future. Since variations in the levels of ANV could influence the thrombotic risk, we analyzed the relevance of this polymorphism in pathologies of the hemostatic system: hemorrhagic and thrombotic disorders.
Our results in spontaneous intracranial hemorrhage suggest that the ANV −1C>T polymorphism could play a minor role in the development of bleeding disorders. Interestingly, the homozygous −1T/T genotype seems to increase the risk of this disorder, although further studies that include more patients are required to support this conclusion. Similarly to our recent data obtained with other hemostatic polymorphisms,17 the annexin V −1C>T polymorphism could play a different role in thrombotic and hemorrhagic disorders. Thus, the −1T allele could play a protective role against thromboembolic diseases. This protective effect is not significant in DVT, but seems to have greater relevance in arterial thrombosis, especially in young patients (P = .006; OR, 0.51; 95% CI, 0.30-0.85), a situation where genetic risk factors may have a stronger influence than environmental risk factors. Moreover, multiple analysis by logistic regression supports the protective role of this polymorphism in premature MI (P = .039). Clinical and experimental data are consistent: greater levels of ANV, related to the −1T allele, might propitiate a stronger inhibitory blockade of phospholipid-dependent procoagulant complexes, platelet adhesion, and aggregation, thus reducing the risk of developing thrombotic episodes. This effect could be of greater relevance in arterial thrombosis, since platelet adhesion, activation (resulting in the flip-flop mechanism exposing negatively charged phospholipids on the cell surface), and aggregation play a key role in the development of the arterial thrombus. Finally, our results agree with the suggested antithrombotic role of ANV in situations of plaque rupture.10-12 Plaque rupture and deep erosion are often the underlying events in Q-wave MI, and therefore, elevated levels of ANV associated with the ANV −1T allele could play a strong protective role. By contrast, the increased thrombogenicity of non–Q-wave MI is usually due to elevated shear stress with minor changes in plaque geometry.25 26 These conditions could explain the minor relevance of the ANV polymorphism in non–Q-wave MI, although further studies that include more patients are required to address this question.
Since the antithrombotic mechanism of ANV is quite broad, the analysis of the −1C>T polymorphism could be relevant in other pathologies in which the level of ANV might play an important role, such as systemic lupus erythematosus, recurrent miscarriage, thrombocytopenia, or thrombosis associated with circulating antiphospholipid antibodies.8,27,28 Finally, additional studies are required to determine the role of the ANV −1C>T polymorphism in other tissues, such as in placenta, where ANV is more abundant.29
The study was performed in patients who survived an acute thrombotic event. Therefore, a survival bias cannot be avoided in the disease-association study, and the likelihood of early mortality in patients could lead to an underestimation of the −1C>T ANV polymorphism. Moreover, our study refers to the association between this polymorphism of ANV and hemostatic diseases, specifically in the Mediterranean white population. The relevance of this polymorphism should be investigated in other populations and with prospective and family studies.
We are grateful to L. Martinez (Centro Regional de Hemodonación de Murcia), Dr Sogorb, and Dr Pineda (Hospital General de Alicante) for their help in the recruitment of patients and controls. We acknowledge J. Yélamos for helpful discussion and M. C. Cánovas for secretarial support.
Supported by FIS 99/1091 and FIS 00/0328. R.G.-C. and J.C. are Contratados Ramon y Cajal of Universidad de Murcia. C.M. is Postdoctoral Fellow of Ministerio de Educacion y Ciencia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vicente Vicente, Centro Regional de Hemodonación, Ronda de Garay S/N, 30003 Murcia, Spain; e-mail:vvg@um.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal