Abstract
Notch signaling plays a critical role in cell fate determination in many developmental systems, including the hematopoietic system. We and others have recently cloned a novel Notch ligand called Delta4. In this study, we show the effect of retrovirus-mediated ectopic expression of Delta4 in hematopoietic cells. Lethally irradiated mice transplanted with bone marrow cells expressing Delta4 initially suffered from leukopenia and thrombocytopenia. Although all lineages were affected, the deficit in B cells and platelets was the most durable and profound. A rapid expansion of CD4+CD8+ cells occurred shortly after transplantation. CD4+CD8+ cells progressively invaded all tissues analyzed except the thymus, which surprisingly was atrophic. CD4+CD8+cells were mainly non–Delta4-transduced cells, strongly suggesting that the disease was not cell autonomous. Around 15 weeks after transplantation, mice died from this severe lymphoproliferative disorder, which was not transplantable in late-stage disease into secondary recipients. Mice transduced with a soluble form of Delta4 behaved like control mice. Characterization of early hematopoietic development revealed that Delta4 expression impaired formation of day-12 spleen colony-forming units (CFU-Ss) and, to a greater extent, pre–CFU-Ss. No effect was observed on myeloid colony-forming cells (CFU-Cs), indicating that Delta4 specifically acted on the earliest hematopoietic stem cell compartment. These results show that constitutive expression of Delta4 in hematopoietic cells impairs the development of B cells, platelets, and early stem cells and induces a lethal lymphoproliferative disease.
Introduction
The Notch family of proteins comprises a group of highly conserved, single-pass transmembrane cell surface receptors.1 Four Notch homologs, Notch1 through Notch4, have been identified in mammals.2-4 Notch signaling is triggered by interaction with a family of membrane-bound ligands. Notch ligands belong to the Delta, Serrate, Lag-2 (DSL) family of proteins. Several mammalian DSL family members have now been described.5-11 All ligands are characterized by 2 conserved motifs: the DSL domain, important for Notch binding,12,13 and a series of epidermal growth factor (EGF) repeats. The ligands are grouped into 2 subfamilies on the basis of the presence of a cysteine-rich domain in the extracellular region; ligands with the cysteine-rich region belong to the Serrate/Jagged family whereas those without belong to the Delta family.1 Engagement of Notch by its ligand results in 2 proteolytic cleavage events14-17 that result in the nuclear translocation of the Notch intracellular domain (NICD). NICD then activates transcription by associating with a DNA-binding protein known as C-promoter binding factor, Suppressor of hairless, Lag-1 (CSL).18-20 NICD can also associate with a number of other transcription factors, including myocyte enhancer factor2C (MEF-2C), Nur77, and nuclear factor κB (NF-kB).21-24
Notch signaling plays a critical role in cell fate determination and maintenance of progenitors in many developmental systems.25 In the hematopoietic system, the involvement of Notch in the cell fates of various cell lineages has been extensively studied.26 Notch receptors have been shown to be expressed on hematopoietic progenitors cells as well as to various degrees in peripheral blood T and B lymphocytes, monocytes, and neutrophils.26 Notch ligands have been shown to be expressed by bone marrow and fetal liver stroma cells, thymic epithelial cells, and hematopoietic cells.27-33 A number of recent studies have provided evidence that ligand-induced Notch signaling promotes the survival and proliferation of hematopoietic progenitors. Jagged-1, Jagged-2, and Delta-like1 have been shown to stimulate an increase in the formation of mouse primitive precursor cell populations.27-30 Jagged-1 and Delta have been shown to have similar effects on human hematopoietic stem cells.31,33,34 Karanu et al32,33demonstrated recently that preincubation of purified primitive human blood cells with Jagged-1, Delta-1, and Delta-4 and subsequent transplantation into immunodeficient mice resulted in the survival and expansion of human stem cells with pluripotent repopulating capacity. The expansion of progenitors has been shown to be paralled by a delay in myeloid diffferentiation.31 34
We and others have recently identified a novel Notch ligand, Delta4, and have shown that it interacts with and signals through Notch1, 2, and 4.10,11 We have previously shown that Delta4 is expressed in several hematopoietic and immune tissues, including the thymus, lymph nodes, bone marrow, and, to a lesser extent, spleen but not in peripheral blood cells.10 To better understand the function of Delta4 in these organs, we investigated Delta4 gene dysregulation in these tissues using in vivo retroviral gene delivery in hematopoietic cells.
Materials and methods
Construction and production of retroviruses
The retroviral vectors pMSCVpac and MigR are both based on the murine stem cell virus (MSCV 2.2) vector containing a murine embryonic stem cell virus long terminal repeat (LTR).35 The pMSCV carries the puromycin N-acetyl transferase (pac) gene driven by an internal murine phosphoglycerase kinase promoter. MigR carries the enhanced green fluorescent protein (GFP) expressed from an internal ribosomal entry site.36 The cloning of human Delta4 into MigR, resulting in MigR Delta4, was described previously.10 For the cloning into pMSCVpac, Delta4 cDNA (2.1 kb) was amplified by polymerase chain reaction (PCR) to introduce a unique 5′ XhoI and a 3′ EcoRI restriction site and a Kozak consensus sequence (ACCGCC) in the original cDNAs (Advantage-HF kit; Clontech Laboratories, Palo Alto, CA). The PCR product was ligated into MSCVpac, and clones were sequenced and selected for base-perfect match with the original cDNA. The extracellular domain of human Delta4 (1.6 kb), encoding a secreted form of Delta4, was also cloned into pMSCVpac.
Viral supernatants were generated in 293-EBNA cells (Invitrogen, Carlsbad, CA) by cotransfecting MSCVpacDelta4, MigRDelta4, MSCVpac, or MigR with pN8epsilon vector containing the gag/polgenes from the murine Moloney leukemia virus and the pN8epsilon vector containing the vesicular stomatitis virus envelope glycoprotein G gene as described previously.37Concentrated viral supernatants were prepared by centrifugation for 2 hours at 50 000g (SW28 rotor at 25 000 rpm) at 4°C. Pellets were resuspended in 1.5 mL Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS) (Stem Cell Technologies, Vancouver, BC, Canada), shaken at 4°C for 24 hours, filtered, and then frozen at −80°C.
Infection procedure
Bone marrow cells were collected from C57/B6 SJL mice (Taconic, Germantown, NY) 4 days after 5-fluorouracil treatment, 150 mg/kg administered intravenously. Enriched progenitor cells were selected by means of a magnetic cell-sorting depletion column (type BS; Miltenyi Biotech, Auburn, CA) as described previously.37 Briefly, cells were labeled with a mixture of 4 fluorescein isothiocyanate (FITC)–conjugated antibodies, against CD3ε, CD11b, CD45R, and Ly-6G (Pharmingen, San Diego, CA). Cells were washed and incubated with anti-FITC microbeads (Miltenyi Biotech). Labeled cells were removed with the use of depletion columns as per manufacturer's instructions. After separation, flow-through cells lacking the lineage markers (Lin−) were washed and resuspended in DMEM containing 10% FCS. Before infection, Lin− cells (106/mL) were prestimulated with 10 ng/mL recombinant mouse interleukin-3 (rmIL-3) (Endogen, Woburn, MA); 10 ng/mL recombinant mouse interleukin-6 (rmIL-6) (Endogen); 100 ng/mL recombinant mouse stem cell factor (rmSCF) (R&D Systems, Minneapolis, MN); 100 ng/mL recombinant mouse fms-like tyrosine kinase-3 ligand (rmFlt-3L) (R&D Systems) and 102 U/mL mouse thrombopoietin (mTPO) (conditioned medium) for 2 days. Cells were centrifuged; resuspended in DMEM, 10% FCS, and viral supernatant (1/1 vol/vol) in the presence of rmIL-3, rmIL-6, rmSCF, rmFlt-3L, and mTPO; and incubated at 37°C, 10% CO2. This infection procedure was repeated 24 hours later, and 4 hours after this second infection, the cells were collected, washed twice, and injected into lethally irradiated C57BL6 mice (Taconic). NIH3T3 cells were infected with MSCVpac, MSCVpac-Delta4, MigR, or MigRDelta4. Stable cell lines were generated by selection in puromycin (MSCVpac) or sorting of GFP-expressing cells (MigR).
Progenitor cell, CFU-S, and pre–CFU-S assays
Progenitor cell assays were carried out in conventional 1 mL methylcellulose cultures maximally stimulated by recombinant mIL-3, mSCF, and erythropoietin (Stem Cell Technologies). Duplicate or quadruplicate cultures were scored for colonies (more than 50 cells) after 7 to 14 days of incubation at 37°C in a humidified atmosphere of 10% CO2. Cultures were initiated with 3000 infected Lin− cells on the day of the bone marrow transplantation (BMT).
The spleen colony-forming unit (CFU-S) assay was carried out by means of standard methods. Lethally irradiated mice were grafted with 1 to 10 × 103 infected Lin− cells, and individual spleen colonies were scored 7 or 14 days after transplantation. The pre–CFU-S assay was initiated with bone marrow cells collected from mice grafted with 6000 Lin− cells 12 days after the transplantation. Secondary lethally irradiated recipients were injected with 1 × 105 bone marrow cells, and individual colonies were scored 12 days after transplantation.
Analysis of mice
Peripheral blood was used for flow cytometry analysis (FACS) and to analyze nucleated cells and platelet numbers by means of a hemocytometer after red blood cell lysis with ammonium chloride. Differential counts and hematocrit were also determined in some experiments by means of a blood analyzer (Hemavet 1500; CDC Technology, Oxford, CT).
For histological analysis, organs were harvested and fixed in 10% buffered formalin stained with hematoxylin and eosin. Tissue examined included skin, kidneys, sternum, uterus, thymus, bladder, heart, ovaries, lungs, skeletal muscle, thyroid/parathyroid, femur, brain, brown and white fat, pituitary, head, eyes, diaphragm, aorta, spleen, stomach, intestines, liver, and adrenal glands.
For FACS analysis, the spleen, thymus, and bone marrow (femur) were harvested and single-cell suspensions prepared. Blood was collected from the tail vein bleeds or at necropsy by heart puncture. Red blood cells were lysed and FACS was carried out with the use of FITC, phycoerythrin, and CyC directly conjugated antibodies (Pharmingen) as per manufacturer's instructions. All FACS analyses were gated for viable leukocytes on the basis of forward and side scatter.
RNA slot blot and Southern blot analysis
RNA expression of Delta4 was confirmed in the spleens of the transduced mice by means of a slot blot analysis. Total RNA (5 μg per sample) was loaded onto duplicate membranes by means of a slot blot apparatus (Slot Blot Manifold; Amersham Pharmacia Biotech, Piscataway, NJ). The membranes were washed with 10 × SSC and irradiated with a UV transilluminator. Hybridization was carried out by means of 75 ng 32P (deoxycytidine triphosphate [dCTP]) randomly labeled (Rediprime; Amersham Pharmacia Biotech) Delta4 (corresponding to the extracellular domain of Delta4) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probes. After washing, signals were analyzed by means of a phosphoimager (Fujifilm BAS-2500; Fuji Medical Systems, Stamford, CT).
Southern blots were carried out with DNA from the spleen of reconstituted animals. DNA was isolated, and the digests (20 μg) were sized-fractionated by electrophoresis in 1% agarose gels, which were then blotted onto Hybond N+ membranes (Amersham Pharmacia Biotech). The blots were hybridized with a32P-labeled Delta4 cDNA fragment. Blots were washed 4 times in 2 × SSC/0.1% sodium dodecyl sulfate (SDS) at room temperature and 3 times in 0.1% SSC/0.1% SDS at 65°C. Autoradiography was performed with the use of Kodak (Rochester, NY) X-OMAT AR film, and the bands were quantified with a phosphoimager system.
Western blot analysis
Cell extracts were prepared and Western blot analysis was performed as described previously.38 Anti–myc antibodies (clone 9E10) were purchased from Calbiochem (La Jolla, CA). Rabbit polyclonal antibodies to Delta4 were raised against a peptide in the intracellular domain (Leu-Lys-Asn-ThrAsn-Gln-Lys-Lys-Glu-Leu-Glu-Val-Asp-Cys-Gly), and peptide affinity purified by Research Genetics (Huntsville, AL).
Results
Retroviral transduction and expression of Delta4
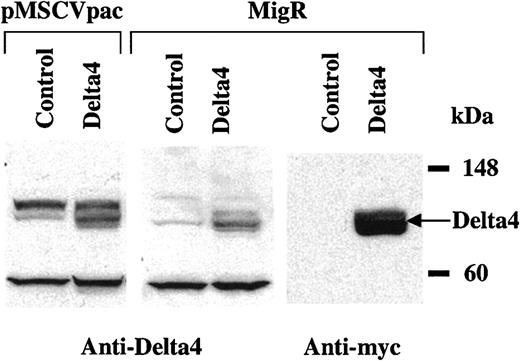
Full-length Delta4 was cloned into the retroviral expression vectors MSCVpac and MigR. To confirm that the protein was produced, NIH3T3 cells were infected with MSCVpac-Delta4, MigR-Delta4, or empty MSCVpac and MigR viruses. Expression of Delta4 protein (predicted molecular weight [MW], 74 kDa) was shown by Western blot analysis with the use of polyclonal antibodies to Delta4 or anti-myc epitope. A band corresponding to the predicted molecular weight was detected in cells transduced with Delta4-carrying virus (Figure1). This band was much less intense but present in cells transduced with control virus when probed with anti-Delta4 and most likely represents endogenous Delta4. The antibody also detected several background bands of equal intensity in control and Delta4-transduced cells (Figure 1, left and middle panels). Delta4 in MigR is fused to a C-terminal myc tag and could also be detected with anti-myc antibodies (Figure 1, right panel). The full-length membrane-bound form of Delta4 has been previously shown to be a functional Notch ligand.10 A retroviral vector containing a secreted form of Delta4 (MSCVpac-Delta4sec) was also used in some experiments. This construct contained the extracellular domain of Delta4 and has been shown to encode a functionally active protein that increased the expansion of human CD34+ cells (E. Lauret et al, unpublished data, 2001).
Retroviral expression of Delta4 protein.
Cell extracts were prepared from NIH3T3 cells transduced with pMSCVpac, pMSCVpacDelta4, MigR, or MigRDelta4. Expression of Delta4 was detected by Western blot analysis with polyclonal anti-Delta4 antibodies in cells transduced with Delta4-carrying virus but not control virus. Delta4 in MigR was fused to a C-terminal myc tag and could also be detected with anti-myc monoclonal antibodies (mAbs).
Retroviral expression of Delta4 protein.
Cell extracts were prepared from NIH3T3 cells transduced with pMSCVpac, pMSCVpacDelta4, MigR, or MigRDelta4. Expression of Delta4 was detected by Western blot analysis with polyclonal anti-Delta4 antibodies in cells transduced with Delta4-carrying virus but not control virus. Delta4 in MigR was fused to a C-terminal myc tag and could also be detected with anti-myc monoclonal antibodies (mAbs).
To determine the gene transfer efficiency to progenitor cells, infected Lin− cells were plated in methylcellulose cultures in the presence or absence of puromycin. The percentage of infection was first estimated from the number of puromycin-resistant colonies (MSCVpac virus) or from the number of GFP+ (MigR virus) colonies counted from 7 to 14 days after plating. From 7 independent experiments using either MigR (n = 2) or MSCVpac (n = 5), the average percentage of infected progenitor cells forming colonies was 74% ± 12% for the control viruses and 76% ± 9% for the viruses containing full-length Delta4. The virus containing Delta4sec was used in 2 experiments, and the percentage of infected cells was 60% ± 39%.
Effects of Delta4 on peripheral blood cell repopulation
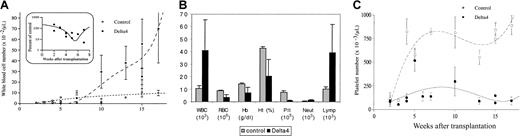
We studied the ability of Delta4-transduced Lin− bone marrow cells to reconstitute the hematopoietic system of lethally irradiated mice. White blood cell (WBC) numbers were determined at various time points between 2 and 17 weeks following BMT (Figure2A-C). Leucocytopenia was observed in Delta4-transduced mice as well as in control mice when WBC counts were analyzed 2 to 3 weeks after transplantation (Figure 2A-B). Control mice recovered normal nucleated blood cell levels (6600 ± 1500 cells per microliter; n = 9) 6 to 8 weeks after transplantation, while Delta4-transduced mice suffered from leucocytopenia during this period (32% ± 8% of WBC count in control mice; n = 8). Between 7 and 10 weeks after transplantation, nucleated blood cells in full-length Delta4-transduced mice increased, reaching 70 000 ± 32 000 cells per microliter, 17 weeks after transplantation (Figure 2A-B). Differential blood counts at week 15 (Figure 2C) showed that the increase in WBC counts was paralleled by a similar increase in lymphocytes, suggesting that lymphocytes were responsible for the elevated WBC count in Delta4 animals. Neutrophil counts were only slightly affected, being normal or slightly elevated.
Effect of Delta4 expression on blood cell formation.
Delta4 expression alters blood cell formation. WBC (panels A,B), hemoglobin, hematocrit (panel B), and platelet (panels B,C) levels in mice transplanted with bone marrow cells infected with the control viruses or the viruses carrying Delta 4. These data (mean values ± SE) resulted from independent experiments using the pMSCVpac or the MigR viruses. Inset: the ratio, in early time points after transplantation, between the WBC levels in control mice and in mice transduced with Delta 4 is shown. In panel B, differential blood cells counts, platelets, and hemoglobin and hematocrit levels were determined 15 weeks after BMT from mice transduced with control viruses (MSCVpac, n = 4; MigR, n = 2) or Delta4 viruses (MSCVpacDelta4, n = 2; MigRDelta4, n = 2). The cell number per microliter is indicated. Multiplication factors differ for different cell types and are shown in brackets. Hemoglobin and hematocrit are shown as grams per deciliter and percentages, respectively.
Effect of Delta4 expression on blood cell formation.
Delta4 expression alters blood cell formation. WBC (panels A,B), hemoglobin, hematocrit (panel B), and platelet (panels B,C) levels in mice transplanted with bone marrow cells infected with the control viruses or the viruses carrying Delta 4. These data (mean values ± SE) resulted from independent experiments using the pMSCVpac or the MigR viruses. Inset: the ratio, in early time points after transplantation, between the WBC levels in control mice and in mice transduced with Delta 4 is shown. In panel B, differential blood cells counts, platelets, and hemoglobin and hematocrit levels were determined 15 weeks after BMT from mice transduced with control viruses (MSCVpac, n = 4; MigR, n = 2) or Delta4 viruses (MSCVpacDelta4, n = 2; MigRDelta4, n = 2). The cell number per microliter is indicated. Multiplication factors differ for different cell types and are shown in brackets. Hemoglobin and hematocrit are shown as grams per deciliter and percentages, respectively.
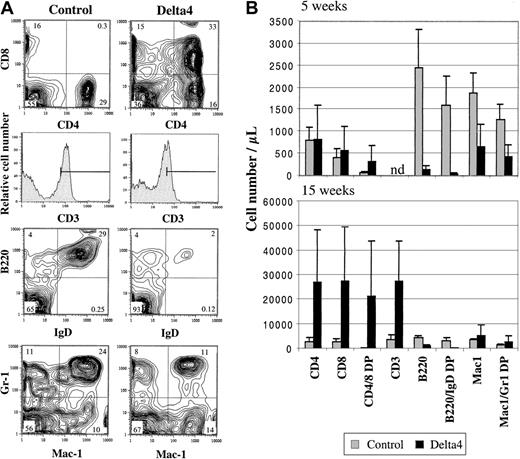
Peripheral blood cells were analyzed at various time points after transplantation for expression of T-cell, B-cell, and myeloid markers by flow cytometry (Figure 3A-B). Significant differences were observed in both the B- and T-cell lineage at 5 and 15 weeks after transplantation, respectively (Figure 3A-B). The number of total B cells (B220+) and mature B cells (B220+/IgD+) was strongly reduced in Delta4 mice. The number of mature B cells was reduced approximately 40-fold 5 weeks after transplantation and 12-fold 15 weeks after transplantation compared with control animals. A population of CD4+CD8+ cells that is normally found only in the thymus was detected in the peripheral blood of Delta4 mice but not control mice. This population was already clearly present 5 weeks after transplantation and constituted the predominant cell population in the peripheral blood 15 weeks after transplantation. After 15 weeks, the number of CD4+CD8+ cells was highly variable in individual Delta4-transduced animals, probably reflecting variability in the disease time course, and ranged from 8000 to 58 000 cells per microliter (average was 21 000 ± 20 000/μL). The increase in CD4+CD8+ cells in Delta4 mice was paralleled by a similar increase in the number of CD3+ cells in Delta4 mice. CD3 expression levels were lower on T cells from Delta4-transduced mice compared with controls (mean fluorescence intensity shifted from 95 to 40) (Figure 3A). This is probably because the circulating T cells in Delta4 mice are CD4+CD8+ cells, which express lower levels of CD3. Analysis of the CD4+CD8+population at week 15 showed that about 85% are CD3+; 84% are T-cell receptor–β+ (TCR-β+); and 99% are Thy1.2+ (data not shown). CD45.1, a marker expressed by donor but not recipient mice, was expressed on 99% of CD4+CD8+, indicating that they were donor derived.
Effect of Delta4 on B-cell and T-cell development.
Expression of Delta4 impairs B-cell development and leads to occurrence of CD4+CD8+ T cells in the peripheral blood. White blood cells were isolated and stained with fluorochrome-conjugated antibodies to CD4, CD8, CD3, B220, immunoglobulin D (IgD), Mac1, Gr-1, and CD45.1. (A) Representative FACS contour blots and histograms are shown for staining of WBC cells from one Delta4-transduced and one control mouse 15 weeks after bone marrow transplantation. The numbers indicate the percentage of cells within each quadrant. (B) The average number of positive cells for the cell populations indicated is shown for Delta4-transduced and control mice at week 5 (MSCVpac, n = 5; MSCVpac-Delta4, n = 5) and 15 (MSCVpac, n = 4; MigR, n = 2; MSCVpacDelta4, n = 2; MigRDelta4, n = 2) after BMT. Error bars represent the SD.
Effect of Delta4 on B-cell and T-cell development.
Expression of Delta4 impairs B-cell development and leads to occurrence of CD4+CD8+ T cells in the peripheral blood. White blood cells were isolated and stained with fluorochrome-conjugated antibodies to CD4, CD8, CD3, B220, immunoglobulin D (IgD), Mac1, Gr-1, and CD45.1. (A) Representative FACS contour blots and histograms are shown for staining of WBC cells from one Delta4-transduced and one control mouse 15 weeks after bone marrow transplantation. The numbers indicate the percentage of cells within each quadrant. (B) The average number of positive cells for the cell populations indicated is shown for Delta4-transduced and control mice at week 5 (MSCVpac, n = 5; MSCVpac-Delta4, n = 5) and 15 (MSCVpac, n = 4; MigR, n = 2; MSCVpacDelta4, n = 2; MigRDelta4, n = 2) after BMT. Error bars represent the SD.
The effect of Delta4 on myeloid cells (Mac1+ and Mac1+/Gr-1+) was variable. A 3-fold reduction in the number of myeloid cells was observed at week 5, while a slight increase (1.5-fold) was observed at week 15 in Delta4-transduced mice (Figure 3A-B). This is also reflected in the 2-fold increase of neutrophils at week 15 (Figure 2C).
Importantly, the overall reconstitution of hematopoiesis following transplantation was identical 3 weeks after transplantation in Delta4 and control mice as analyzed by the percentage (around 80%) and the total number of cells expressing CD45.1 (a marker that is expressed on donor but not recipient cells). However, at 5 and 15 weeks after transplantation, this percentage was slightly decreased in Delta4 mice (with individual variations from 55% to 70%) compared with controls (with individual variations from 85% to 90%). In total numbers of cells, this represents a significant increase at week 15 in endogenous reconstitution.
Platelet numbers were determined at various time points between 2 and 17 weeks following BMT (Figure 2C-D). Platelet numbers in mice reconstituted with control-infected bone marrow recovered to normal levels (7.5 × 105 cells per microliter) by week 7 after BMT. However, platelet numbers in mice reconstituted with Delta4-transduced cells were only 20% of control levels (Figure 2D). The Delta4-transduced mice suffered from thrombocytopenia during the 17-week analysis with a mean number of 1.5 ± 0.4 × 105 per microliter (n = 15). Delta4-transduced mice also showed some signs of anemia (about 2-fold reduction in red blood cells and hemoglobin) (Figure 2C). However, this could be the result of hemorrhages observed in mice with advanced disease.
In contrast to the phenotype observed with the full-length membrane-bound Delta4, mice transduced with soluble Delta4 behaved like control mice, displaying normal WBC, red blood cell, and platelet counts (data not shown).
In summary, analysis of the peripheral blood of Delta4-transduced and control mice revealed a significant effect of Delta4 expression on hematopoietic development. Leukopenia and thrombocytopenia was observed after BMT in Delta4-transduced animals. While the thrombocytopenia persisted at later time points, the leukopenia turned into a leukocytosis starting around weeks 7 to 9. FACS analysis revealed a severe impairment in the B-cell compartment and the aberrant appearance of a CD4+CD8+ T-cell population in the blood of Delta4-transduced mice. The increase in WBC cells at later time points was due to CD4+CD8+ cells.
Delta4 expression causes severe lymphoproliferative disease
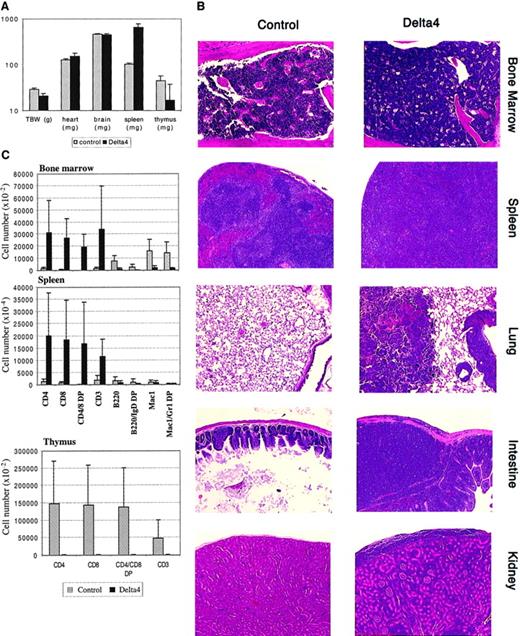
Expression of Delta4 severely affected the viability of mice. Delta4 mice started to die around 10 weeks after transplantation, and only 40% survived more than 15 weeks after transplantation. All of the control mice survived up to 15 weeks (data not shown). Surviving Delta4 and control mice were killed 15 weeks after transplantation and analyzed extensively (Figure 4). Delta4-transduced mice suffered from severe cachexia. Their total body weight was reduced 25% compared with control mice. A striking splenomegaly was observed, with spleens being enlarged up to 8-fold compared with controls. The thymus was strongly reduced in size and weight (up to 30-fold). Heart and brain weights were not affected (Figure 4A). The number of nucleated cells in the femoral bone marrow was increased about 2-fold compared with controls, whereas red blood cells were nearly absent in bone marrow of Delta4 mice (data not shown). Bone marrow from Delta4 and control mice was analyzed for the presence of myeloid progenitor cells in myeloid colony-forming cell (CFU-C) assays. Whereas control bone marrow yielded the expected number of colonies, no colony formation was observed in Delta4-transduced bone marrow (data not shown), indicating a severe defect in medullary myelopoiesis.
Effect of Delta4 expression on lymphoproliferative disease.
Delta4 expression causes severe lymphoproliferative disease. Animals were killed at week 15, and tissues were collected for histology and FACS analysis; MSCVpac, n = 4; MigR, n = 2; MSCVpacDelta4, n = 2; MigRDelta4, n = 2. (A) The mean values ± SD for total body and organ weights are shown. (B) Representative paraffin sections stained with hematoxylin and eosin are shown for bone marrow, spleen, lung, intestine, and kidney from control animals and animals transduced with Delta4. Magnification × 200. (C) Single-cell suspensions were prepared from bone marrow, spleen, and thymus and stained with the antibodies indicated. Mean values (± SD) for the numbers of positive cells are given with the use of gating as shown in Figure3A.
Effect of Delta4 expression on lymphoproliferative disease.
Delta4 expression causes severe lymphoproliferative disease. Animals were killed at week 15, and tissues were collected for histology and FACS analysis; MSCVpac, n = 4; MigR, n = 2; MSCVpacDelta4, n = 2; MigRDelta4, n = 2. (A) The mean values ± SD for total body and organ weights are shown. (B) Representative paraffin sections stained with hematoxylin and eosin are shown for bone marrow, spleen, lung, intestine, and kidney from control animals and animals transduced with Delta4. Magnification × 200. (C) Single-cell suspensions were prepared from bone marrow, spleen, and thymus and stained with the antibodies indicated. Mean values (± SD) for the numbers of positive cells are given with the use of gating as shown in Figure3A.
Histological analysis of a large number of organs revealed aggressive infiltration of nearly all organs with lymphoblasts. Organs analyzed and affected include liver, kidney, uterus, lamina propria of the stomach, small and large intestines, pancreas, salivary glands, brain and spinal meninges, lymph nodes, spleen, and bone marrow. Representative pictures are shown in Figure 4B. The splenic architecture was destroyed in Delta4 mice by the lymphoblastic infiltration. Red and white pulp were indistinguishable, and lymphoblasts constituted the majority of the cells detectable. Similarily, most of the bone cavities were taken over by lymphoblasts, and very few cells of the myeloid or erythroid lineage were detectable. Destruction of basic architecture by aggressive lymphoblastic infiltration is also shown for lung, intestine, and kidney (Figure 4B).
Nucleated cells from spleen, bone marrow, and thymus were isolated at week 15 and stained with antibodies to the lymphoid and myeloid lineages (Figure 4C). Spleen and bone marrow from Delta4 mice showed a staining pattern similar to what was observed for peripheral blood. B cell numbers (B220+ and B220+/IgD+) were strongly reduced in Delta4 animals. CD4+CD8+ T cells constituted the predominant population in spleen and bone marrow of Delta4 animals. As expected, in control mice the CD4+CD8+ population was not detectable in spleen and bone marrow. The number of Mac1+and Mac1+/Gr-1+ cells was strongly reduced in bone marrow (about 10-fold) but was comparable to control animals in the spleen.
Surprisingly, analysis of the thymus from Delta4 mice revealed that this organ was atrophic. We observed an approximately 30-fold reduction in thymus size in Delta4-transduced animals (Figure 4A). The thymus from Delta4 mice was virtually devoid of T cells (Figure 4C). This shows that the thymus was not only spared from the infiltration of invading CD4+CD8+ T cells but also depleted of its normal T-cell population.
The percentage of CD45.1+ was only slightly lower in the bone marrow, spleen, and thymus of Delta4 mice compared with controls, indicating that the overall donor reconstitution after bone marrow cell transplantation was comparable.
In summary, these results show that Delta4-transduced animals develop a fatal lymphoproliferative disorder that results in the death of animals between 10 and 17 weeks after BMT. CD4+CD8+ T cells aggressively infiltrated all organs analyzed, destroying their architecture.
To determine if the disease was transplantable into secondary recipients, we injected spleen cells (comprising about 60% CD4+CD8+) from 15-week posttransplantation Delta4 mice into nonirradiated or sublethally irradiated syngenic animals. These animals were monitored for longer than 1 year. None of them developed the disease, indicating that the disease caused by Delta4 expression was nontransplantable into nonlethally irradiated recipients. This suggested that the CD4+CD8+cells were not transformed and the observed phenotype was due to a lymphoproliferative disorder rather than a lymphoma.
Proviral integration and expression of Delta4
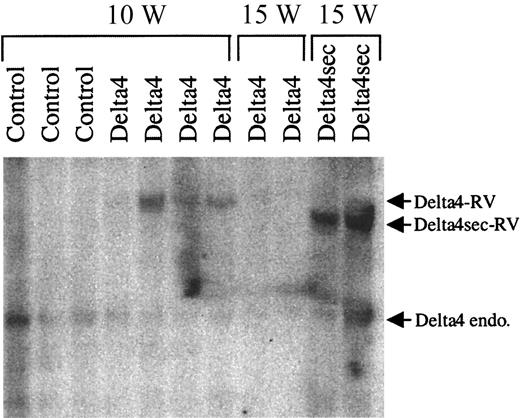
To assess expression levels of Delta4 in retrovirally transduced cells, RNA was isolated from spleens of Delta4 and control animals. Slot blot analysis showed that full-length Delta4 was expressed, albeit at very low levels (data not shown). The level of transcription was only about 4% of the level of endogenous GAPDH mRNA. For comparison, the secreted form of Delta4 was expressed at 48% of the level of GAPDH mRNA in a parallel experiment. No expression of Delta4 was detectable in controls. To estimate the proportion of transduced cells and the integrity of the provirus, Southern blot analysis was carried out on genomic DNA isolated from spleen 10 and 15 weeks after BMT (Figure5). Genomic DNA was digested withXbaI, which cuts in the retroviral LTR and releases a 4.6-kb fragment containing Delta4. A band of the expected size was clearly detected after 10 weeks in 3 of 4 Delta4 samples, indicating no gross rearrangement of the Delta4 provirus. The intensity of the Delta4 proviral band was several-fold higher than endogenous Delta4 in 3 samples, indicating multiple integration events. However, the Delta4 proviral band was nearly undetectable after 15 weeks. In contrast, the intensity of the proviral band encoding secreted Delta4 was very strong after 15 weeks. Considering the high level of retroviral transduction of the initial graft and the fact that the dominant spleen cell population at 15 weeks was CD4+CD8+, these results strongly suggest that non–Delta4-transduced cells were mainly responsible for the lymphoproliferative disease. This conclusion was reinforced when the MigR vector, which contains a GFP reporter gene, was used in 2 separate experiments. In Delta4-transduced mice only 8% percent of the spleen cells were GFP+ 15 weeks after transplantation. This was especially surprising given the higher percentage (45%) of GFP+ donor cells during the first several weeks after transplantation and the initial high rate of progenitor cell infection in the graft of this experiment (85%). The percentage of GFP-expressing cells (70%) was stable over the course of 1 year in mice transduced with control MigR virus and correlated well with the infection rate before transplantation (76%).
Decrease of Delta4-transduced spleen cells in late-stage disease.
Spleens were harvested 10 (10 W) and 15 (15 W) weeks after transplantation from animals transduced with control virus or viruses carrying either full-length (Delta4) or secreted Delta4 (Delta4sec). Southern blot analysis for proviral integration was generated from genomic DNA digested with XbaI. A cDNA fragment corresponding to the extracellular domain of Delta4 was used as a probe. XbaI cuts in the retroviral LTR and releases a 4.75-kb fragment for full-length Delta4 and a 4.25-kb fragment for secreted Delta4. The endogenous (endo) and retroviral (RT) Delta4 bands are indicated.
Decrease of Delta4-transduced spleen cells in late-stage disease.
Spleens were harvested 10 (10 W) and 15 (15 W) weeks after transplantation from animals transduced with control virus or viruses carrying either full-length (Delta4) or secreted Delta4 (Delta4sec). Southern blot analysis for proviral integration was generated from genomic DNA digested with XbaI. A cDNA fragment corresponding to the extracellular domain of Delta4 was used as a probe. XbaI cuts in the retroviral LTR and releases a 4.75-kb fragment for full-length Delta4 and a 4.25-kb fragment for secreted Delta4. The endogenous (endo) and retroviral (RT) Delta4 bands are indicated.
In summary, these data show that the ectopic expression of Delta4 preferentially induces the proliferation of non–Delta4-transduced CD4+CD8+ cells compared with Delta4-transduced CD4+CD8+. This suggests that the underlying mechanism for the aggressive expansion of CD4+CD8+ cells is of a paracrine rather than autocrine nature and might also explain the observation that the lymphoproliferative disease is not transplantable. A relatively low number of Delta4-expressing spleen cells were used for secondary transplantation experiments, and this might not have been sufficient to support the disease in the recipient mice.
Effect of Delta4 on the development of CFU-Cs, CFU-Ss, and pre–CFU-Ss
To evaluate the effect of Delta4 expression on the early stage of hematopoiesis, we determined the number of CFU-Cs, CFU-Ss, and pre–CFU-Ss present after transduction with the control or Delta4 viruses (Figure 6). CFU-Cs and day-7 CFU-Ss represent similar developmental stages, ranging from late to early progenitor cells, whereas day 12 CFU-Ss represent the stage from early progenitors to cells capable of long-term reconstitution. Pre–CFU-S cells are closely related to cells with long-term repopulating activity.
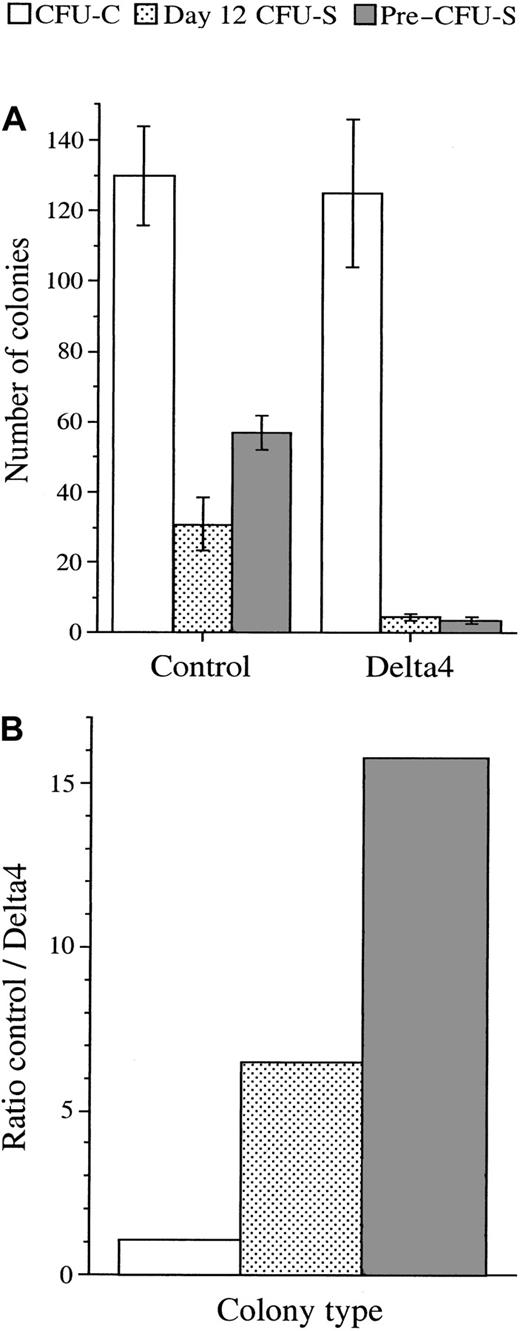
Effect of Delta 4 expression on the development of early stem cells.
Delta 4 expression impairs the development of early stem cells (day-12 CFU-Ss and pre–CFU-Ss). (A) The CFU-C, day 12 CFU-S, and pre–CFU-S colonies were generated from the Lin− bone marrow cells infected with the control viruses or the viruses carrying Delta4. The CFU-C colony numbers are the mean values (± SE) resulting from 7 independent experiments using the control viruses or the viruses carrying the full-length Delta4 (n = 2 for pMSCV; n = 5 for MigR). The average percentage of puromycin-resistant or GFP+colonies was 74% ± 12% for the control viruses and 76% ± 9% for the Delta4 viruses. Only puromycin-resistant or GFP+colonies were counted. The numbers of CFU-S colonies are the mean values (± SE) obtained from 2 independent experiments, each using 5 mice injected with 6000 Lin− bone marrow cells transduced with pMSCVpac (n = 5) or MigR (n = 5) viruses. The number of GFP+ CFU-S colonies assayed in one experiment (data not shown) and the total number of CFU-S colonies (data shown) gave similar results. The pre–CFU-S colony numbers are the mean values (± SE) generated from the injection of 8 mice with 100 000 bone marrow cells collected from the femurs of 5 recipient mice 12 days after being injected with 6000 Lin− cells infected with the pMSCVpac viruses. (B) The ratios between the number of colonies generated from the Lin− bone marrow cells infected with the control viruses or the Delta4 viruses are generated from panel A. They indicate the effect of Delta4 expression on the number of colonies generated from CFU-Cs, CFU-Ss, and pre–CFU-Ss.
Effect of Delta 4 expression on the development of early stem cells.
Delta 4 expression impairs the development of early stem cells (day-12 CFU-Ss and pre–CFU-Ss). (A) The CFU-C, day 12 CFU-S, and pre–CFU-S colonies were generated from the Lin− bone marrow cells infected with the control viruses or the viruses carrying Delta4. The CFU-C colony numbers are the mean values (± SE) resulting from 7 independent experiments using the control viruses or the viruses carrying the full-length Delta4 (n = 2 for pMSCV; n = 5 for MigR). The average percentage of puromycin-resistant or GFP+colonies was 74% ± 12% for the control viruses and 76% ± 9% for the Delta4 viruses. Only puromycin-resistant or GFP+colonies were counted. The numbers of CFU-S colonies are the mean values (± SE) obtained from 2 independent experiments, each using 5 mice injected with 6000 Lin− bone marrow cells transduced with pMSCVpac (n = 5) or MigR (n = 5) viruses. The number of GFP+ CFU-S colonies assayed in one experiment (data not shown) and the total number of CFU-S colonies (data shown) gave similar results. The pre–CFU-S colony numbers are the mean values (± SE) generated from the injection of 8 mice with 100 000 bone marrow cells collected from the femurs of 5 recipient mice 12 days after being injected with 6000 Lin− cells infected with the pMSCVpac viruses. (B) The ratios between the number of colonies generated from the Lin− bone marrow cells infected with the control viruses or the Delta4 viruses are generated from panel A. They indicate the effect of Delta4 expression on the number of colonies generated from CFU-Cs, CFU-Ss, and pre–CFU-Ss.
In 7 separate experiments using MigR (n = 2) or the MSCVpac (n = 5), the average number of CFU-Cs generated from 3000 infected Lin− cells was 130 ± 14 for the control virus and 125 ± 21 for the viruses containing full-length Delta4 (Figure 6A-B). In 2 experiments using the MSCVpac-Delta4sec, an average of 138 ± 61 colonies per 3000 cells plated was observed. The type and size of colonies did not appear to differ between control and Delta4-carrying viruses. The effect of Delta4 expression on spleen colony formation was then analyzed in lethally irradiated animals grafted with limited numbers of transduced cells. The number of day-7 CFU-Ss was evaluated in one experiment. The number of colonies as well as the size of the spleen was similar with the control or the Delta4 viruses (data not shown). In contrast, in 3 independent day-12 CFU-S experiments, the number of colonies and the size of the spleens was consistently smaller in the animals grafted with Delta4-transduced cells compared with the control cells. Of the 3 experiments performed, the 2 that gave sufficient colony resolution to score individual CFU-Ss showed a drop in colony numbers varying from 3.8- to 8.8-fold, with a mean drop of 6.4-fold in mice grafted with Delta4-transduced cells compared with control animals (Figure 6A-B). In one experiment, the percentage of GFP+/− CFU-S colonies was read in situ with the use of an inverted microscope on the intact spleen. This percentage was similar to the percentage of GFP+/−colonies derived from the progenitor cells of the same Lin− cell population. This result suggests that both Delta4-transduced and nontransduced CFU-S populations equally failed to develop. To evaluate if differences were also present at earlier differentiation stages than the ones represented by day-12 CFU-Ss, bone marrow cells from animals grafted with virus-infected cells were collected 12 days after transplantation and grafted into secondary recipients to analyze pre–CFU-Ss. The total number of colonies derived from pre–CFU-Ss was decreased approximately 16-fold (Figure 6A-B) in the Delta4-transduced animals compared with controls. This reduction was the result of a 4-fold reduction in marrow cellularity observed in primary recipients (1 × 105 versus 4 × 105 cells per femur from 6 × 103injected cells) and a 4-fold reduction in the number of pre–CFU-S colonies counted in secondary recipients (3.6 ± 0.8 versus 14.2 ± 1.2 colonies from 105 injected cells).
These results showed that Delta4 expression did not affect CFU-C or day-7 CFU-S colony formation but had an inhibitory effect on day-12 CFU-Ss, and an even more pronounced effect on pre–CFU-S colony formation. This indicated that Delta4 impaired the development of the most immature hematopoietic cells but did not directly act on the later stages. It is therefore possible that this impairment is partially responsible for the blood cell deficit observed in the Delta4-transduced mice.
Discussion
In this study, we show that ectopic expression of membrane-bound Delta4 in hematopoietic cells severely impaired hematopoietic development in mice and induced a lymphoproliferative disease. To our knowledge, this is the first report to describe the consequences of constitutive expression of a Notch ligand on in vivo hematopoiesis. Transplanted recipients initially suffered from profound leukopenia and thrombocytopenia. Although initially all lineages were involved, platelets and circulating B cells were more affected than other lineages, and their numbers remained low in the blood up to 4 months after transplantation. In contrast, circulating granulocytes and monocytes slowly reached normal levels. T-cell numbers not only recovered but eventually exceeded normal levels, leading to a lymphoproliferative disease. CD4+CD8+ T cells constituted the majority of the expansive T-cell population in the periphery. These cells were extremely invasive and infiltrated all tissues examined except the thymus. Ultimately, all mice died from this lymphoproliferative syndrome 3 to 4 months after transplantation.
These results are similar to studies describing constitutive active Notch expression in hematopoietic cells.39-41 Similar to the results in our work, mice receiving bone marrow cells transduced with activated Notch1 displayed aggressive expansion of immature CD4+CD8+ T cells and exhibited a simultaneous block in early B-cell development in the bone marrow.39 Granulocytes or monocytes were only moderately, if at all, affected. The similarity of the phenotypes suggests that constitutive Delta4 expression mimics constitutive Notch activation. Indeed, we showed that expression of HES, one of the main target of Notch, is strongly induced by Delta4 in human hematopoietic cells (E. Lauret et al, unpublished data, 2001).
However, in contrast to constitutive expression of Notch1, which led to lymphoblastic T-cell leukemias/lymphomas,40 constitutive expression of Delta4 did not have transforming activity. The disease was not transplantable into nonirradiated or sublethally irradiated mice with the use of splenic CD4+CD8+ T cells. This result can be compared to others studies showing that expression of constitutive activated receptors displayed oncogenic activities in mice,42,43 whereas constitutive expression of ligands induces nontransforming proliferative diseases.44-46
Delta4 expression impaired platelet formation and stem cell development. These observations were not reported in studies describing the effect of constitutive active Notch expression in hematopoietic cells.39-41 The prolonged thrombocytopenia could be due to Delta4's selectively blocking platelet development or to the fact that platelets, the last hematopoietic cells to recover from BMT, are more sensitive to stem cell impairment than other blood cell types. Alternatively, the aggressive expansion of CD4+CD8+ T cells could interfere with megakaryopoiesis. Further studies are needed to address this point. The long-lasting deficit in blood cells indicated that constitutive expression of Delta4 profoundly impaired hematopoiesis. We showed that this was not due to a defect in the development of late progenitor cells like CFU-Cs and day-7 CFU-Ss. However, the observed 6- and 16-fold reduction in the formation of day-12 CFU-Ss and pre–CFU-Ss, respectively, suggested that the graft impairment was due to a profound and selective defect in the development of the most primitive hematopoietic stem cells. Several studies have shown that Notch ligands preferentially affect the earliest stage of hematopoiesis. Notch ligands had no, or only a modest, effect on the number or the type of mature cells generated from in vitro stem cell expansion, including the number of late progenitor cells.28,31 In contrast, in vitro exposure of hematopoietic cells to Notch ligands has been shown to induce an expansion of early progenitor cells. including the day-12 CFU-Ss,30 mixed colony-forming units (CFU-Mix's),31 high proliferative potential colony-forming cells (HPP-CFCs),28 and stem cells scored for cobblestone-area formation or multilineage reconstitution in severe combined immunodeficiency recipient mice.28 In contrast to these studies, which indicate that Notch ligands positively regulate stem cell expansion, our results showed that Delta4 expression negatively regulates stem cell expansion. This unexpected result may be explained by the duration of the stimulus or the use of other Notch ligands. However, overall, our result is in agreement with the general concept that Notch blocks development of several cell types.25
A remarkable feature of the proliferative disease induced by forced expression of Delta4 is the absence of invading Delta4-transduced cells. This was demonstrated 15 weeks after transplantation in the enlarged spleen by the disappearance of the retrovirally transduced cells (Figure 5). Furthermore, with the use of GFP as a reporter gene, despite a high percentage of transduced cells in the graft, only a low percentage of GFP+ cells were found in spleen of Delta4-transduced mice 15 weeks after transplantation. One possible explanation for this observation was that only nontransduced cells responded to the Delta4 signal, and cells that express Delta4 were unable to respond to the stimulus in a similar manner. It has been observed previously in the Drosophilasystem that cells expressing Notch ligands exhibit a reduced response to Notch signaling.47,48 Similarly, in a more recent study, it was shown that Delta1 expression in keratinocytes can cell-autonomously block Notch activation in the expressing cells, thereby preventing these cells from differentiating.49Only neighboring cells that did not express Delta1 responded to the signal, indicating that the Delta1 signal had to be provided intrans, in a paracrine fashion, to non–Delta1-expressing cells. A similar requirement for trans-activation by Delta4 could account for the preferential expansion of Delta4-negative T cells. In our experiment, this signal of activation could be provided by hematopoietic cells, but also by stromal cells derived from the transduced Lin− bone marrow cell population. An abnormal extrathymic development of the CD4+CD8+ cells was reported in models of Notch constitutive activation.39 Interestingly, we noticed a profound atrophy of the thymus, which contained fewer CD4+CD8+ cells than the control. It is therefore possible that constitutive expression of Delta4, or Notch activation, induces abnormal differentiation of these cells in organs unable to provide a correct signal for their development and therefore leading to their expansion.
In conclusion, we show that constitutive expression of Delta4 profoundly modified in vivo differentiation of hematopoietic cells. The effects are multiple and include impairment of stem cell, B cells, and platelet development, and amplification of immature T cells. Our model shares several similarities with models of constitutive Notch activation in hematopoietic cells, including the inhibition of B-cell formation and the dramatic amplification of CD4+CD8+ cells. However, inhibition of stem cell and platelet development has not been reported in mice expressing constitutive active Notch1. Further studies will be necessary to address the mechanism of stem cell inhibition, the platelet defect, but also the selective proliferation of non-Delta4–expressing CD4+CD8+ cells induced by Delta4 in these mice.
During the review of this manuscript, Yan et al50 also published that Delta4 induces T-cell leukemia/lymphoma in mice with the use of retroviral-mediated gene transfer. In contrast to our study, the disease was transplantable in 4 cases out of 5. Transplantation failure may be the consequence of the low percentage of Delta4-transduced cells in late stages of the disease, also reported by Yan et al, and the concept that the syndrome is secondary rather than primarily driven by Delta4.
The authors are grateful to Dr J. Morgenstern and K. Theriault, from Millennium Pharmaceuticals, and Dr W. Pear from the University of Pennsylvania Medical School (Philadelphia), for providing the vectors used in the retroviral infection; S. Calandra and James Boden from Millennium Pharmaceuticals for excellent technical assistance; Prof R. Bronson from Tufts University School of Veterinary Medicine (Boston, MA) for the excellent histological analysis; Prof K. Humphries from the Terry Fox Laboratory, BC Cancer Research Centre (Vancouver, British Columbia, Canada); Dr W. Vainchenker from INSERM U362 (Villejuif, France); and Prof T. Kadesch, from the University of Pennsylvania School of Medicine (Philadelphia), for critically reviewing the manuscript.
M.D., G.Z., D.Y., Y.W., Q.S., C.M., X.X., Q.S., J.-C.G.-R., C.F. and J.-L.V. are employed by Millenium Pharmaceuticals, Inc., whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marion Dorsch, Inflammation, Millennium Pharmaceuticals, 75 Sidney St, Cambridge MA 02139; e-mail:dorsch@mpi.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal