Abstract
Natural killer (NK) cells have held great promise for the immunotherapy of cancer for more than 3 decades. However, to date only modest clinical success has been achieved manipulating the NK cell compartment in patients with malignant disease. Progress in the field of NK cell receptors has revolutionized our concept of how NK cells selectively recognize and lyse tumor and virally infected cells while sparing normal cells. Major families of cell surface receptors that inhibit and activate NK cells to lyse target cells have been characterized, including killer cell immunoglobulinlike receptors (KIRs), C-type lectins, and natural cytotoxicity receptors (NCRs). Further, identification of NK receptor ligands and their expression on normal and transformed cells completes the information needed to begin development of rational clinical approaches to manipulating receptor/ligand interactions for clinical benefit. Indeed, clinical data suggest that mismatch of NK receptors and ligands during allogeneic bone marrow transplantation may be used to prevent leukemia relapse. Here, we review how NK cell receptors control natural cytotoxicity and novel approaches to manipulating NK receptor-ligand interactions for the potential benefit of patients with cancer.
Introduction
Over the past 2 decades, a number of different immune-based strategies aimed at eradication or suppression of residual malignant disease have been proposed for the clearance of leukemic cells. T-cell–mediated graft-versus-leukemia (GVL) has been shown to be the most efficacious following allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions.1,2 Although specific antileukemic responses have been documented, most T-cell–mediated alloreactions are thought to be directed against minor or major histocompatibility antigens shared by both leukemic and normal cells with potential for widespread host tissue damage. Successful T-cell–based immunotherapy will, therefore, ultimately require better definition of tumor-specific antigens that will allow the direction of the immune response to the tumor cells.3 Unfortunately, tumor-specific antigens have only been identified on a minority of cancers.4
In contrast to antigen-specific T cells, effector cells of the innate immune system lack the ability to rearrange the genes in the germ line that encode receptor components, and, hence, they cannot recognize a multitude of antigens in the context of classical major histocompatibility complex (MHC) molecules. Natural killer (NK) cells are innate immune lymphocytes that held early clinical promise because of their ability to lyse tumor cells without specific antigen recognition.5,6 Clinical trials attempting to harness the antitumor effect of NK cells, either through in vivo or in vitro activation, have met with only modest success to date.7-9However, over the past decade our knowledge of how NK cells recognize target cells using an integration of activating and inhibitory receptors now points toward potential clinical utility for the treatment of leukemia and other malignancies. In this review we summarize our current understanding of the receptors involved in NK cell recognition of tumor targets and discuss their potential clinical role in immunotherapy of leukemia with and without hematopoietic stem cell transplantation.
Human NK cells
NK cells are innate immune lymphocytes critical to host defense against invading infectious pathogens and malignant transformation through elaboration of cytokines and cytolytic activity.5,6,10 Human NK cells comprise approximately 10% of all blood lymphocytes and are identified by the expression of the CD56 surface antigen and the lack of CD3. Functionally, NK cells are an important source of innate immunoregulatory cytokines (eg, interferon-γ [IFN-γ], tumor necrosis factor-α [TNF-α], granulocyte macrophage colony-stimulating factor [GM-CSF]) that co-orchestrate the early immune response and contribute to the delayed T-cell response following infection.10 NK cells also have direct or natural cytotoxic activity against some virus-infected, leukemic, and other tumor cells, and they also mediate antibody-dependent cellular cytotoxicity (ADCC) of targets through FcγRIII (CD16), a receptor that binds the Fc portion of antibody.11-14 The receptors that regulate NK cell recognition and lysis of tumor targets are discussed below.
Human NK cell subsets
Two distinct subsets of human NK cells are identified according to cell surface density of CD56 expression as recently reviewed elsewhere.10 The majority (90%) of human NK cells are CD56dim and express high levels of CD16, whereas a minority (10%) is CD56bright and CD16dim/neg. These NK subsets are functionally distinct with the immunoregulatory CD56bright cells producing abundant cytokines and the cytotoxic CD56dim cells likely functioning as efficient effectors of natural and antibody-dependent target cell lysis.15 CD56bright NK cells constitutively express the high and intermediate affinity interleukin (IL)-2 receptors and expand in vitro and in vivo in response to low (picomolar) doses of IL-2.8,16 In contrast, resting CD56dim NK cells express only the intermediate-affinity IL-2 receptor and proliferate weakly in response to high doses of IL-2 (1-10 nM) in vitro, even after induction of the high-affinity IL-2 receptor.16,17 Resting CD56dim NK cells are more cytotoxic against NK-sensitive targets (K562 and COLO205 cell lines) than CD56bright NK cells.18 However, after activation with IL-2 or IL-12, CD56bright cells exhibit similar or enhanced cytotoxicity against NK targets compared with CD56dim cells.18-20 In addition, resting CD56bright and CD56dim NK cell subsets show differences in their NK receptor repertoires.21,22 Resting CD56bright NK cells are large agranular cells and express high levels of the C-type lectin CD94/NKG2 family with only very small fractions expressing killer-cell immunoglobulin receptor (KIR) family.18 Resting CD56dim NK cells, however, express both KIR and C-type lectin NK receptors at relatively high surface density along with an abundance of cytolytic granules packaged in the cytoplasm.18
Human NK cell development
NK cells originate in the bone marrow from human CD34+hematopoietic progenitor cells (HPCs) and require the bone marrow microenvironment for complete maturation. Bone marrow stroma–derived cytokines, including IL-15 in cooperation with c-kit ligand (KL) and flt-3 ligand (FL), are critical physiologic factors for NK cell development, as recently reviewed.23 Human NK cell development can be divided into an early phase of development in which a NK progenitor responds to early acting stromal cell growth factors, KL and FL, and develops into an NK cell precursor intermediate with the phenotype CD34+IL-2/ IL-15Rβ+CD56−. This precursor is then responsive to IL-15 for maturation into a functional CD56+ NK cell. The NK cells resulting from adult CD34+ HPCs in stroma-free cultures following addition of IL-15, however, resemble the CD56bright NK cell population both functionally and phenotypically (CD56brightCD16dim/neg), including their NK receptor repertoire.24 This finding suggests that other soluble or cell-contact signals are required for CD56dim NK cell characteristics and KIR acquisition, or alternatively the CD56dim population of NK cells may arise from a different precursor. Additional studies are required to understand the regulation of CD56dim NK cell differentiation, and if a developmental relationship exists between these 2 NK subsets. The events and factors that regulate NKR repertoire acquisition during NK cell differentiation is currently unclear and an active area of investigation.25 26
Human NK cell recognition of target cells: NK receptors
Unlike T and B lymphocytes, NK cells do not rearrange genes encoding receptors for antigen recognition, but they have developed the ability to recognize self-MHC class I or class I–like molecules through a unique class of receptors, NK cell receptors (NKRs), that can inhibit or activate NK cell killing. Initially, Ljunggren and Karre27 proposed the “missing-self” hypothesis, wherein the function of NK cells is to recognize and destroy autologous cells that have lost or altered self-MHC class I molecules. Although tolerant to normal autologous cells, NK cells can recognize and attack virus-infected and transformed cells that have down-regulated MHC class I molecules. Human NK cells lyse class I–deficient Epstein-Barr virus (EBV)–transformed B-lymphoblastoid cell lines, whereas transfection of class I alleles into target cells inhibits NK lysis.28 29Accordingly, over the past decade, a number of inhibitory NK receptors specific for classical (eg, HLA-A, -B, or -C) or nonclassical (eg, HLA-E, -G) class I molecules have been recognized.
However, MHC class I is not always necessary for protection from lysis by NK cells, and inhibition by MHC class I is not always sufficient to prevent NK cytotoxicity. For example, NK cells are unable to reject MHC class I–deficient nonhematopoietic tissues, such as skin grafts, and in vitro they fail to lyse fibroblasts, even from β2-microglobulin null mice that lack class I expression.30 Conversely, some virus-infected cells that maintain expression of MHC class I at the cell surface can still be killed by autologous NK cells.31,32 Furthermore, IL-2–activated NK cells have increased lytic activity compared with circulating NK cells and are able to lyse otherwise NK cell–resistant targets.16 These observations point to the additional importance of activating receptors in regulating NK cell effector function. Ligation of NK-activating receptors with membrane-bound molecules of target cells results in NK cell blastogenesis, cytokine production, cytotoxicity, and migration. Each NK cell, therefore, appears to express its own repertoire of activating and inhibitory receptors, and cytotoxicity is ultimately regulated by a balance of signals from these activating and inhibitory receptors that interact with MHC class I and class I–like molecules on target cells (Figure1). Later, we provide an overview of some of the inhibitory and activating NKRs whose ligands or target molecules have been identified. This overview will not comprehensively examine NKR biology, as several reviews have recently been published.10 33-35 We will focus on some potential clinical applications in the therapy of hematologic malignancies based on known NKR biology.
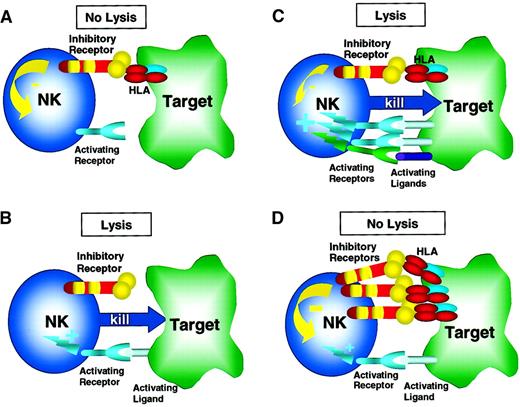
Regulation of NK cell response by activating and inhibitory receptors.
Inhibitory receptors (eg, inhibitory KIR, CD94/NKG2A) recognize and engage their ligands, MHC class I molecules (HLA), on the surface of the target tumor cell, thereby initiating an inhibitory signal. Activating receptors (eg, activating KIR, CD94/NKG2C, NKG2D) bind ligands on the target cell surface and trigger NK cell activation and target cell lysis. (A) When inhibitory receptors engage HLA in the absence of an activating receptor/ligand interaction, a net negative signal is generated, resulting in no target cell lysis. (B) Conversely, when activating receptors engage their ligands on target cells in the absence of inhibitory receptor/ligand interaction, a net activation signal is generated, resulting in target cell lysis. This scenario is likely operative in NK alloreactivity in the setting of KIR epitope mismatch (see Figure 4 and text for more details). More complex physiologic scenarios are shown in C and D with both inhibitory and activating receptor/ligand signals being generated when an NK cell interacts with a target cell. (C) Here, the activating receptor/ligand interactions predominate over weaker inhibitory receptor/ligand signals with the net result of NK cell activation and target cell lysis. This net result may occur when activation receptors and ligands are up-regulated, thereby amplifying the net activation signal to exceed the inhibitory signal. For example, the activating ligands MICA/B and ULBPs are expressed highly in stressed or transformed cells, thereby activating NKG2D/PI3K pathways that are not susceptible to inhibitory signals (see text for details). Alternatively, when expression of self-MHC class I ligands is decreased in the setting of viral infection or transformation, the net signal may be positive, also resulting in target cell lysis. (D) Here, inhibitory receptor/ligand interactions result in a net negative signal that prevents NK cell lysis of the target cell. This process may occur constantly as NK cells survey normal host tissues. Not shown is the scenario of absence of both inhibitory and activating signals that results in no NK cell activation.
Regulation of NK cell response by activating and inhibitory receptors.
Inhibitory receptors (eg, inhibitory KIR, CD94/NKG2A) recognize and engage their ligands, MHC class I molecules (HLA), on the surface of the target tumor cell, thereby initiating an inhibitory signal. Activating receptors (eg, activating KIR, CD94/NKG2C, NKG2D) bind ligands on the target cell surface and trigger NK cell activation and target cell lysis. (A) When inhibitory receptors engage HLA in the absence of an activating receptor/ligand interaction, a net negative signal is generated, resulting in no target cell lysis. (B) Conversely, when activating receptors engage their ligands on target cells in the absence of inhibitory receptor/ligand interaction, a net activation signal is generated, resulting in target cell lysis. This scenario is likely operative in NK alloreactivity in the setting of KIR epitope mismatch (see Figure 4 and text for more details). More complex physiologic scenarios are shown in C and D with both inhibitory and activating receptor/ligand signals being generated when an NK cell interacts with a target cell. (C) Here, the activating receptor/ligand interactions predominate over weaker inhibitory receptor/ligand signals with the net result of NK cell activation and target cell lysis. This net result may occur when activation receptors and ligands are up-regulated, thereby amplifying the net activation signal to exceed the inhibitory signal. For example, the activating ligands MICA/B and ULBPs are expressed highly in stressed or transformed cells, thereby activating NKG2D/PI3K pathways that are not susceptible to inhibitory signals (see text for details). Alternatively, when expression of self-MHC class I ligands is decreased in the setting of viral infection or transformation, the net signal may be positive, also resulting in target cell lysis. (D) Here, inhibitory receptor/ligand interactions result in a net negative signal that prevents NK cell lysis of the target cell. This process may occur constantly as NK cells survey normal host tissues. Not shown is the scenario of absence of both inhibitory and activating signals that results in no NK cell activation.
Human killer cell immunoglobulin receptors
KIRs belong to the immunoglobulin superfamily and are characterized structurally by 2 or 3 extracellular immunoglobulinlike domains. KIRs specifically recognize MHC class I alleles, including groups of HLA-A,36,37 HLA-B,38-40 and HLA-C.38,41,42 There are 2 functionally distinct sets of KIRs: inhibitory and activating. Each set has an identical extracellular domain, and, consequently, each set binds to identical ligands. However, because of differences in their transmembrane and intracellular or cytoplasmic domains, one set of KIRs signals an inhibitory response and one set signals an activating response following their binding to identical MHC class I alleles (Figure2).43 44
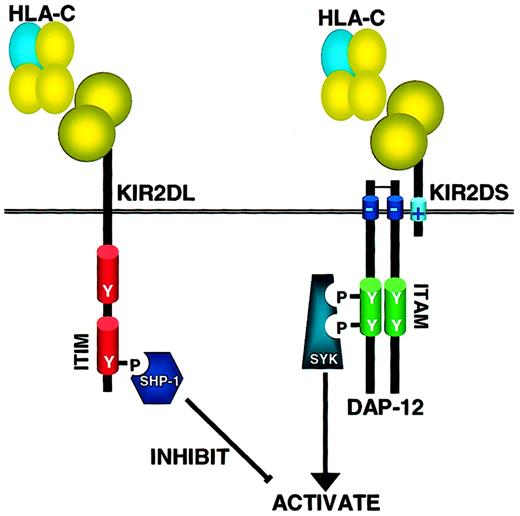
How inhibitory and activating KIR operate on NK cells.
KIR receptors have either 2 or 3 immunoglobulin domains (2D or 3D) and a long (L) cytoplasmic tail containing ITIM motifs or a short (S) cytoplasmic tail and positively charged transmembrane domain that interacts with the ITAM-containing adaptor molecule DAP-12. When inhibitory KIRs bind their HLA ligand, src family kinases phosphorylate the ITIM, allowing binding of the tyrosine phosphatase SHP-1 (and possibly SHP-2) through its SH2 domain. SHP-1 is able to dephosphorylate multiple targets in the ITAM-activating pathway, thereby mediating its negative signal. In contrast, when activating KIRs are ligated, src family kinases phosphorylate the ITAM-containing adaptor molecule DAP-12 that, in turn, binds to and activates the Syk family tyrosine kinases that trigger the downstream activation cascade. As inhibitory KIRs have higher affinity for the MHC class I ligands, coligation of both activating and inhibitory receptors results in a net negative signal, and no activation of the NK cell's cytotoxic or cytokine-secreting programs.
How inhibitory and activating KIR operate on NK cells.
KIR receptors have either 2 or 3 immunoglobulin domains (2D or 3D) and a long (L) cytoplasmic tail containing ITIM motifs or a short (S) cytoplasmic tail and positively charged transmembrane domain that interacts with the ITAM-containing adaptor molecule DAP-12. When inhibitory KIRs bind their HLA ligand, src family kinases phosphorylate the ITIM, allowing binding of the tyrosine phosphatase SHP-1 (and possibly SHP-2) through its SH2 domain. SHP-1 is able to dephosphorylate multiple targets in the ITAM-activating pathway, thereby mediating its negative signal. In contrast, when activating KIRs are ligated, src family kinases phosphorylate the ITAM-containing adaptor molecule DAP-12 that, in turn, binds to and activates the Syk family tyrosine kinases that trigger the downstream activation cascade. As inhibitory KIRs have higher affinity for the MHC class I ligands, coligation of both activating and inhibitory receptors results in a net negative signal, and no activation of the NK cell's cytotoxic or cytokine-secreting programs.
The KIR family of NKR, located on chromosome 19p13.4,45 46includes 12 members and a number of allelic variants, of which 6 receptors are inhibitory and 6 are activating. These are monomeric (single chain) receptors with either 2 (KIR2D) or 3 immunoglobulinlike domains (KIR3D), which can be further subdivided into those with long (L) cytoplasmic tails (KIR2DL and KIR3DL) and short (S) cytoplasmic tails (KIR2DS and KIR3DS) (Figure 2). The long tail KIRs generate an inhibitory signal, whereas the short tail KIRs generate an activation signal. The inhibitory signal results from the presence of immunoreceptor tyrosine-based inhibition motifs (ITIMs) in the cytoplasmic domains of the long tail receptors. The short tail receptors owe their activating signals to their association with adaptor proteins bearing immunoreceptor tyrosine-based activating motifs (ITAMs) (Figure 2). Whereas KIRs are specific for a number of MHC class I molecules, HLA-C is the predominant class I isotype involved in the inhibitory and activating regulation of human NK cells to provide either protection from or induction of target cell lysis and cytokine production. For the purposes of our discussion, we will focus on KIR recognition of HLA-C class I ligands. A current listing of known KIRs and their known ligands can be found in Table1.
Human inhibitory NK cell receptors
| Receptor . | Ligand specificity . |
|---|---|
| KIR | |
| KIR2DL1 (CD158a) | Group 2 HLA-C Asn77Lys80 (w2, w4, w5, w6, and related alleles) |
| KIR2DL2 (CD158b) | Group 1 HLA-C Ser77Asn80 (w1, w3, w7, w8, and related alleles) |
| KIR2DL3 (CD158b) | Group 1 HLA-C Ser77Asn80 (w1, w3, w7, w8, and related alleles) |
| KIR2DL5 | Unknown |
| KIR3DL1 | HLA-Bw4 |
| KIR3DL2 | HLA-A3, -A11 |
| KIR3DL7 | Unknown |
| C-type lectin receptors | |
| CD94/NKG2A/B* | HLA-E (loaded with HLA-A, -B, -C, -G leader peptides) |
| Immunoglobulinlike transcripts | |
| ILT-2 (LIR-1) | Unknown |
| Others | |
| P75/AIRM | Unknown (sialic acid dependent) |
| IRp60 | Unknown |
| LAIR-1 | Ep-CAM |
| Receptor . | Ligand specificity . |
|---|---|
| KIR | |
| KIR2DL1 (CD158a) | Group 2 HLA-C Asn77Lys80 (w2, w4, w5, w6, and related alleles) |
| KIR2DL2 (CD158b) | Group 1 HLA-C Ser77Asn80 (w1, w3, w7, w8, and related alleles) |
| KIR2DL3 (CD158b) | Group 1 HLA-C Ser77Asn80 (w1, w3, w7, w8, and related alleles) |
| KIR2DL5 | Unknown |
| KIR3DL1 | HLA-Bw4 |
| KIR3DL2 | HLA-A3, -A11 |
| KIR3DL7 | Unknown |
| C-type lectin receptors | |
| CD94/NKG2A/B* | HLA-E (loaded with HLA-A, -B, -C, -G leader peptides) |
| Immunoglobulinlike transcripts | |
| ILT-2 (LIR-1) | Unknown |
| Others | |
| P75/AIRM | Unknown (sialic acid dependent) |
| IRp60 | Unknown |
| LAIR-1 | Ep-CAM |
Asn, asparagine; Lys, lysine; Ser, serine; LAIR, leukocyte-associated immunoglobulinlike receptor; Ep-CAM, epithelial cellular adhesion molecule.134
NKG2A and NKG2B are splice transcripts.
A single KIR recognizes determinants that are shared between members of a group of HLA-C alleles. Two HLA-C allotype groups are identified according to amino acid residues present at positions 77 and 80 in the α1 helix of the HLA-C molecule. Group 1 HLA-C alleles each have Ser77 and Asn80 and include HLA-Cw1, -Cw3, -Cw7, and -Cw8. The inhibitory KIR2DL2 and KIR2DL3 NKR recognize the group 1 HLA-C alleles (Table 1). Of course, activating KIR2DS2 and KIR2DS3 bear the same extracellular domains as their inhibitory counterparts and, therefore, also recognize the group 1 HLA-C alleles. The group 2 HLA-C alleles each have Asn77 and Lys80 in the α1 helix and include those of HLA-Cw2, -Cw4, -Cw5, and -Cw6. The inhibitory KIR2DL1 and activating KIR2DS1 NKR recognize the group 2 HLA-C alleles. In general, it appears that the inhibitory KIRs have a greater affinity or attraction for the corresponding group of HLA-C alleles than do the activating KIRs.47 Therefore, a human NK cell that expresses both inhibitory and activating KIRs recognizing a single allele will generally be inhibited from killing.
Importantly, the number and role of inhibitory KIRs in NK cell biology are still evolving.48 A few additional inhibitory KIRs are listed in Table 1 with their respective ligands, when known. It appears clear that corresponding inhibitory KIRs to many HLA-A and HLA-B alleles do not exist, indicating that the KIR repertoire is not all inclusive for human classical class I allotypes.49 One long cytoplasmic tail KIR, KIR2DL4, recognizes the nonclassical MHC class I allele HLA-G.50,51 HLA-G is a molecule that displays limited polymorphism, and its expression has a unique restricted tissue distribution on fetal extravillous trophoblasts that invade the maternal decidua during pregnancy.52 In contrast to other KIRs that are clonally distributed, KIR2DL4 is thought to be expressed by all NK cells,51,53 although one study found that only decidual NK cells (all of which are CD56bright) expressed this receptor and that peripheral blood NK cells did not express KIR2DL4.54 Although initially classified as an inhibitory receptor because of the presence of ITIM in its cytoplasmic domain, recent evidence indicates that ligation of KIR2DL4 on resting NK cells results in activation with the unique property of inducing IFN-γ production without lytic activity.55 This process appears to depend on an intact transmembrane domain and not the ITIM.55 The precise function of this receptor is unclear, although it has been speculated that it may mediate tolerance of the hemi-allogeneic fetus, or possibly vascularization at the implanting site through IFN-γ secretion.56
Another group of inhibitory receptors belongs to the immunoglobulin superfamily and is represented by the immunoglobulinlike transcripts (ILTs), also referred to as leukocyte immunoglobulinlike receptors (LIRs).57,58 These receptors are encoded by a series of genes on chromosome 19, close to the region encoding KIR. ILT receptors are expressed primarily on myeloid cells, dendritic cells, and B cells.57 ILT-2 (LIR-1) is also expressed on NK cells and interacts directly with a broad spectrum of HLA class I molecules, including HLA-G.57-59
C-type lectin family of human NK receptors
A second family of human NK receptors is structurally characterized by C-type lectin extracellular domains and is expressed as heterodimers composed of a common subunit (CD94) covalently bonded to a distinct chain encoded by a gene of the C-type lectin NKG2 family.49,60-63 CD94 is a product of a single nonpolymorphic gene and essentially lacks a cytoplasmic domain for intrinsic signal transduction capacity.64 The extracellular and cytoplasmic domains of the NKG2 molecules are structurally diverse, consistent with differences in ligand recognition and signal transduction.65-67 Homodimers of CD94 exist and are of uncertain physiologic function.68 Four closely related transcripts of the NKG2 family, with corresponding genes, have been identified: NKG2A (and its splice variant NKG2B), NKG2C, NKG2E (and its splice variant NKG2H), and NKG2F.65-67,69 NKG2D is a fifth distantly related member that displays only a low sequence similarity with the other NKG2 members and does not interact with CD94 (discussed later). The CD94 and NKG2genes are all closely linked on chromosome 12p12.3-p13.1 in the human NK gene complex.69 70
CD94/NKG2 heterodimers are selectively expressed by NK cells and cytotoxic T lymphocytes.71 Of the C-type lectin NK receptors, only CD94/NKG2A is inhibitory, whereas other heterodimers are activating receptors (Tables 1 and2). NK clones may selectively bear either inhibitory or activating CD94/NKG2 receptors, yet NKG2A and NKG2C can be detected by revere transcription polymerase chain reaction (RT-PCR) in some NK clones, and indirect functional data suggest that a subset of NK cells may coexpress both receptors.68 The inhibitory receptor CD94/NKG2A complex binds the nonclassical class I molecule HLA-E.72-74 Interestingly, HLA-E binds leader peptides derived from HLA-A, -B, -C, and -G, and, therefore, CD94/NKG2A functionally senses overall expression of HLA class I molecules at the cell surface. For the same peptide/HLA-E complex, binding to the inhibitory receptor CD94/NKG2A is stronger than binding to the activating receptor CD94/NKG2C.75
Human activating NK cell receptors
| Receptor . | Ligand specificity . |
|---|---|
| MHC class I–specific | |
| (a) Killer immunoglobulin receptors | |
| KIR2DS1 | Group 2 HLA-C Asn77Lys80 (w2, w4, w5, w6, and related alleles) |
| KIR2DS2 | Group 1 HLA-C Ser77Asn80 (w1, w3, w7, w8, and related alleles) |
| KIR2DL4 | HLA-G |
| KIR2DS4 | Unknown |
| KIR2DS5 | Unknown |
| KIR3DS1 | Unknown |
| (b) C-type lectin receptors | |
| CD94/NKG2C | HLA-E (loaded with HLA-A, -B, -C leader peptides) |
| CD94/NKG2E/H* | Unknown |
| Non-MHC class I–specific | |
| (a) Natural cytotoxicity receptors | |
| NKp46 | Unknown |
| NKp44 | Unknown |
| NKp30 | Unknown |
| (b) C-type lectin receptor | |
| NKG2D | MICA, MICB, ULBP-1, -2, -3 |
| Other (coreceptors) | |
| CD16 (FcγRIII) | Fc of IgG |
| CD2 | CD58 (LFA-3) |
| LFA-1 | ICAM-1 |
| 2B4 | CD48 |
| NKp80 | Unknown |
| CD69 | Unknown |
| CD40 ligand | CD40 |
| Receptor . | Ligand specificity . |
|---|---|
| MHC class I–specific | |
| (a) Killer immunoglobulin receptors | |
| KIR2DS1 | Group 2 HLA-C Asn77Lys80 (w2, w4, w5, w6, and related alleles) |
| KIR2DS2 | Group 1 HLA-C Ser77Asn80 (w1, w3, w7, w8, and related alleles) |
| KIR2DL4 | HLA-G |
| KIR2DS4 | Unknown |
| KIR2DS5 | Unknown |
| KIR3DS1 | Unknown |
| (b) C-type lectin receptors | |
| CD94/NKG2C | HLA-E (loaded with HLA-A, -B, -C leader peptides) |
| CD94/NKG2E/H* | Unknown |
| Non-MHC class I–specific | |
| (a) Natural cytotoxicity receptors | |
| NKp46 | Unknown |
| NKp44 | Unknown |
| NKp30 | Unknown |
| (b) C-type lectin receptor | |
| NKG2D | MICA, MICB, ULBP-1, -2, -3 |
| Other (coreceptors) | |
| CD16 (FcγRIII) | Fc of IgG |
| CD2 | CD58 (LFA-3) |
| LFA-1 | ICAM-1 |
| 2B4 | CD48 |
| NKp80 | Unknown |
| CD69 | Unknown |
| CD40 ligand | CD40 |
Asn indicates asparagine; Lys, lysine; Ser, serine; LAIR, leukocyte-associated immunoglobulinlike receptor; Ep-CAM, epithelial cellular adhesion molecule.
NKG2E and NKG2H are splice variants.
Only when loaded with the appropriate nonamer peptides derived from the signal sequences of classic class I MHC molecules can the HLA-E molecule be transported to the cell surface.72-74 The detection of HLA-E by the CD94/NKG2 receptors may therefore be a sensitive mechanism for the immunosurveillance for normal biosynthesis of HLA class I molecules, a process that can be altered in virally infected or tumor cells. In addition, the ability of CD94/NKG2 receptors to discriminate among different peptide/HLA-E complexes might also influence reactivity against allogeneic cells. The spectrum of HLA molecules covered by KIRs and, indirectly, by CD94/NKG2 receptors, is only partially overlapping, suggesting that both systems play a complementary role for monitoring the biosynthesis/expression of most HLA class I molecules.76 The relative importance of each system and their interaction in modulating the reactivity of NK cells against autologous virus-infected and transformed cells, or allogeneic cells, remains to be elucidated.
The biologic significance for the existence of paired inhibitory and activating receptors for MHC class I remains unclear. In both cases of KIRs and CD94/NKG2 receptors, the affinity of the activating receptor is lower than that of the corresponding inhibitory receptor,47,77 ensuring a predominance of the inhibitory signal when both activating and inhibitory receptors recognizing HLA molecules are expressed on the same NK cells. However, only a minority of NK cell clones express both activating and inhibitory isoforms that recognize the same HLA allotype.48,53,78 Much more commonly, NK cell clones expressing an activating receptor coexpress at least one inhibitory receptor specific for a different HLA class I allele that, when engaged, predominates. Therefore, the MHC class I–specific activating receptors may only signal when target cells have lost the expression of an HLA allele recognized by the inhibitory receptor, thus allowing NK cells' activating receptor to engage its ligand. In this way, NK cell surveillance may be important for removal of cells that have down-regulated or lost a single MHC class I allele while normal cells would be left unaffected.79 For example, virus-infected or transformed cells may selectively down-regulate HLA-A and -B allotypes while leaving HLA-C and -E unaffected.80 81 In this and other instances, positive target cell recognition by activating receptors is also essential to triggering NK cell cytotoxicity, and the balance between opposite signals delivered by inhibitory and/or activating receptors regulates NK cell functions. Activating receptors can be broadly grouped into those that are counterparts of the inhibitory receptors that recognize MHC class I molecules (discussed earlier) and those that do not have inhibitory counterparts and recognize inducible non-MHC molecules on target cells (Table 2).
Non-MHC class I–specific activating NK cell receptors
Although the activating KIRs and CD94/NKG2 receptors may be important in mediating NK cytotoxicity against MHC class I–bearing targets, other activating receptors are important in mediating cytotoxicity against MHC class I–deficient or negative targets. A number of activating receptors with no apparent specificity for MHC class I molecules have been reported, although many act as coactivators rather than direct stimulators of NK cell function. In humans, a group of receptors called natural cytotoxicity receptors (NCRs)82-86 and the NKG2D receptor have emerged as activating receptors important in recognizing tumor cells in an MHC-independent manner. Three NCRs (NKp46, NKp44, and NKp30) have been identified. NKp46 and NKp30 are constitutively expressed by all peripheral blood NK cells and are not found on other immune cells.82,86 NKp44 is not expressed by resting NK cells but is up-regulated on NK cells after IL-2 stimulation84 and may be important for the cytotoxicity of IL-2–activated NK cells. NKp44 is also found on a proportion of γδ T cells.87These receptors signal through coupling with ITAM-containing CD3ζ and/or FcεRIγ adaptor proteins and are involved in the recognition of various tumor cells. However, the ligands for the NCR have yet to be elucidated. Thus, although they are likely to have an important role in some aspect of immune surveillance and target cell recognition, their biologic significance in NK cell interactions is currently unclear.
Human NKG2D receptor
The NKG2D is the best characterized activating receptor described on NK cells. It is a C-type lectin surface receptor that is misleadingly named as a member of the NKG2 family, encoded within the NK gene complex on human chromosome 12.66,69,70 Unlike the other NKG2 proteins presented earlier, NKG2D has little sequence homology and does not associate with CD94 but is expressed as a homodimer.88 The surface expression of NKG2D requires association with a newly described adaptor subunit designated DAP1088 or KAP10.89 The intracellular domain of NKG2D does not have any signaling motif, and, therefore, signaling is exclusively through its association with DAP10, which does not contain a cytoplasmic ITAM but recruits phosphatidylinositol (PI)-3 kinase after phosphorylation that, in turn, induces cytotoxicity. Importantly, because NKG2D has a signaling pathway that is distinct from the activating KIR and C-type lectin NKR described earlier, triggering via NKG2D is likely less susceptible to signals mediated by inhibitory receptors. NKG2D is constitutively expressed by all NK cells but is also expressed by almost all human γδ+ T cells and all CD8+ T cells.90
Ligands for human NKG2D: MIC and UL16 binding proteins
In contrast to the NCRs, target cell ligands for NKG2D have been identified and are induced by “stress” or neoplastic transformation, suggesting that NKG2D mediates the killing of cells that have been altered by these processes. The expression of these ligands may, therefore, be signals of “altered self” or “danger” to the innate immune system to promote NK and T-cell responses. In the human, these recently defined ligands belong to 2 distinct families, the MHC class Ichain-related (MIC) antigens, and the UL16binding proteins (ULBPs).91-94
The MIC antigens are encoded by a distinct family of genes stationed along the entire MHC class I region on human chromosome 6. They have a low degree of homology to other MHC-encoded class I genes, distinct transcriptional control elements, and a peculiar pattern of polymorphism.91 There are 7 MIC loci,A to G, but only the MICA andMICB genes are transcribed; MIC-C, -D, -E, -Fand -G are pseudogenes.91-93 The MIC glycoproteins contain 3 MHC-like α-domains but, in contrast to MHC class I molecules, do not require β2-microglobulin or peptide for stable surface expression.95 MICA and MICB have been shown to be specific ligands for human NKG2D.90Currently, 54 MICA alleles, of which 47 encode distinct putative glycoproteins, and 16 MICB alleles have been identified.91These alleles are defined by nucleotide substitutions throughout the α1, α2, and α3 domains.
Unlike MHC class I, MIC genes are not ubiquitously expressed. Bahram91 and Shiina et al92 have demonstrated that MIC transcripts are not expressed in the spleen and cells of the lympho-hematopoietic lineage, although they are expressed in fibroblast and epithelial cell lines as well as almost all tissues harboring these cell types. The expression of MICA and MICB are under the control of promoter elements similar to those of the heat shock protein gene PSP70.96 In this respect, exposure of MIC-expressing epithelial lines to heat shock was shown to increase expression of MIC transcripts and proteins.96 Furthermore, MIC expression on fibroblasts and epithelial cells was strongly up-regulated following infection with cytomegalovirus (CMV).97 Importantly, high MICA and MICB expression was detected on many human epithelial tumors98,99 and more recently on the JA3 and Raji leukemic cell lines,98 as well as on primary acute myeloid leukemia (AML) blasts (S.S.F., T.A.F., and M.A.C., unpublished observations, February 2002). Therefore, induction or up-regulation of MIC expression may occur with cellular stress, viral infection, or neoplastic transformation and may facilitate attack of these altered cells by NK cells and some T cells.
The second family of NKG2D ligands reported are human cellular proteins, initially identified by their ability to bind the human CMV protein UL16, which is a type I transmembrane protein known to be expressed by CMV-infected cells.100 UL16 binds to MICB and 2 proteins designated ULBP-1 and ULBP-2.94 By expression-cloning a third member of this family of proteins has been reported, ULBP-3, that, however, does not bind UL16.94 The 3 ULBPs possess α1 and α2 domains but differ from MIC and MHC class I molecules in lacking the α3 domain, and they are expressed as glycosylphosphatidyl inositol (GPI)–anchored cell surface proteins without a requirement for association with β2-microglobulin for surface expression. It is currently unknown if any polymorphisms exist within this gene family. It is also possible that other ULBPs exist that may function as ligands for NKG2D. Specificity for binding to human NKG2D has been demonstrated by the cross-competition of soluble ULBPs with MICs for binding to NK cells, the ability to bind to recombinantly expressed NKG2D/DAP10 complexes, and the complete blocking of ULBP binding to primary human NK cells by anti-NKG2D antibodies.94-101
The expression of ULBPs is more widespread than that of MIC proteins. With the use of RT-PCR, ULBP transcripts were detected in heart, lung, testis, brain, lymph nodes, thymus, tonsil, liver, and bone marrow.94 However, in some tissues where high ULBP mRNA expression was detected, no cell surface expression was found by monoclonal antibody,94 suggesting that surface expression of ULBPs may be regulated at a posttranscriptional level. This finding has important implications for the study of these ligands in tumor cell recognition by NK cells.
Roles of NCR and NKG2D in NK-mediated tumor cell killing
The relative role of NCR (NKp46, NKp44, and NKp30) and NKG2D in the lysis of various tumors by human NK cell clones has been recently analyzed. Pende et al98 demonstrated that, although lysis of certain tumor cell lines was exclusively NCR dependent, killing of other tumor types, including melanoma and leukemia cell lines, involved synergism between NCR and NKG2D.98 With the use of NCRdull NK cell clones, it was also demonstrated that in some cases NKG2D was the only triggering receptor responsible for tumor cell lysis. Surface density expression of NKG2D on both NCRbright and NCRdull NK cell clones was equivalent.98 Therefore, the functions of NCR, whose ligands are unknown, and NKG2D appear to be complementary and depend on ligand expression by the tumor cells. To date, most studies have been performed by using tumor cell lines, and little investigation of the interaction between activation receptors and primary tumor cells has been conducted.
The extent to which activation of NK cells through NKG2D can be prevented by inhibitory signals from MHC class I–recognizing inhibitory receptors is not yet fully resolved. As mentioned earlier, the affinity of inhibitory KIRs and CD94/NKG2 receptors for their MHC class I ligands is significantly higher that that of their activating counterparts, suggesting that inhibitory signals predominate. NCR-dependent lysis of autologous cells appears to require blocking of inhibitory receptors by anti-HLA class I monoclonal antibodies, similarly suggesting that the inhibitory signal may normally predominate.83 In contrast, the activating signal through NKG2D may override the inhibitory signaling mediated by MHC class I molecules both ex vivo90,94 and in vivo in a mouse model.102 The basis of the mechanism for this observation is not known but may be related to a higher affinity of NKG2D for its ligands.103 The overriding effect of NKG2D-mediated activating signal, however, was not confirmed in studies by Pende et al,98 who showed that the engagement of different HLA class I–specific inhibitory receptors by either specific antibodies or the appropriate HLA class I ligand led to inhibition of NKG2D-mediated NK cell triggering. Of note, this effect was observed when normal cells (phytohemagglutinin [PHA] blasts) or the ovarian tumor cell line, IGROV-1, expressing high densities of HLA class I molecules, were used.98 It is, therefore, likely that NK cell killing of tumor cells is the result of integration of signals from several activating and inhibitory receptors that are regulated by the density and selection of class I and class I–like ligands expressed by the target cell (Figure 1C,D). In this respect, it is not known to what extent exogenously administered cytokines, such as IL-2 and IL-12, may modulate effector cell NKR and ligand expression on target cells in vivo.
Other membrane receptors implicated in modulation of NK cell function
A number of other receptor molecules have been implicated in the process of triggering of NK cell-mediated cytotoxicity. In redirected killing assay using monoclonal antibodies that initiate killing of Fc receptor–bearing tumor cell lines, NK cell surface molecules that have been implicated in cell activation include CD2,104CD16,105 LFA-1,106 CD40 ligand,107 CD69,108 2B4,109-111and NKp80.112,113 These receptors, however, function as coreceptors in that their triggering function depends on the simultaneous ligation of main triggering receptors. For example, monoclonal antibody triggering via the 2B4 and NKp80 molecules induces strong cytolytic responses only in NKp46bright NK cells but not in NKp46dull cells, although the latter expressed comparable surface densities of 2B4 and NKp80.111 112Therefore, these coreceptors only result in activation if a first signal has been provided by the main receptor. The relative roles of these coreceptors in NK-mediated killing of tumor cells remains to be fully elucidated.
Clinical roles of NK cell receptors: stem cell transplantation and cellular therapy
Previous strategies using NK cells for the treatment of human malignancy have been largely restricted to the use of ex vivo generated lymphokine-activated killer (LAK) cells or activated NK cells114-116 or in vivo cytokine therapy to expand and activate NK cells against autologous cancer cells.9 117-120 In most studies, it has been difficult to convincingly demonstrate a clinical benefit, especially in small phase I and II clinical trials. Although it is not known to what extent cytokines, such as IL-2, may alter the balance between activating and inhibitory receptors on NK cells, it is likely that the inhibitory signals delivered by MHC class I molecules expressed on the autologous target cancer are a major limitation to successful treatment. Strategies aimed at either using the mismatch of receptors and ligands that is present in certain allogeneic settings, or blocking the interaction between inhibitory NK cell receptors and MHC class I molecules, may be more successful.
KIR-epitope mismatch in haplotype-mismatched stem cell transplantation
The cure of refractory leukemia and other hematologic malignancies following allogeneic stem cell transplantation has been largely attributable to the ability of donor immune cells in the graft to recognize and eliminate neoplastic cells that have escaped killing by high-dose chemotherapy. Moreover, following minimally myeloablative transplants or donor lymphocyte infusion (DLI), this GVL effect is the only mechanism by which leukemic cells are eliminated. Immune cells infused in the graft or the DLI exert a GVL effect through T-cell–mediated alloreactions directed against host minor or major histocompatibility antigens, depending on the extent of MHC mismatching between donor and recipient. In humans, it has been difficult to demonstrate a role for NK cells in mediating a GVL effect in MHC-matched transplants. T-cell depletion of stem cell grafts, to reduce the risk of graft-versus-host disease (GVHD), has been associated with a significantly increased risk of relapse, particularly in patients with chronic myeloid leukemia.1,121 In the full haplotype-mismatched setting, however, NK cells have recently been demonstrated to exert an important GVL effects.122
In full haplotype-mismatched stem cell transplantation, in which donors and recipient pairs are identical for one HLA haplotype and incompatible at the HLA class I and II loci of the unshared haplotype, all cases are at risk of T-cell–mediated alloreactions in the host-versus-graft (HvG) and graft-versus-host (GvH) direction. These T-cell–mediated reactions can be eliminated to a large extent by altering the immunosuppressive intensity of the conditioning regimen to prevent rejection by and extensive T-cell depletion of the graft to prevent GVHD. However, another level of alloreactivity is provided by mismatches at the KIR epitopes expressed by HLA class I alleles, as shown in Figure4. Recall from earlier that certain KIRs recognize a variety of HLA class I alleles belonging to a specific group, even if the NK cell itself does not express those alleles. Further, inhibitory KIRs will predominate over activating KIRs.
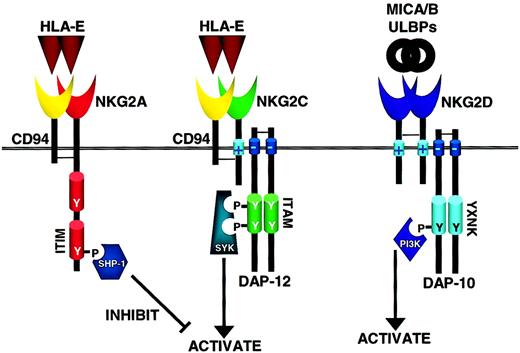
How inhibitory and activating C-lectin receptors operate in NK cells.
The C-type lectin receptors are disulfide-linked heterodimers of CD94 and NKG2 family members, either the inhibitory NKG2A or the activating NKG2C, and recognize the nonclassical MHC class I molecule HLA-E. Similar to KIRs with long cytoplasmic tails, ITIM-containing NKG2A signals through SHP-1/2 that mediate inhibitory signals. Likewise, NKG2C has a positively charged transmembrane domain that interacts with DAP-12 and transduces activating signals through Syk family members. In contrast, NKG2D is only distantly related to the other NKG2 family members, does not associate with CD94, and binds to the MHC-like ligands MICA, MICB, and ULBP family. Through its positively charged transmembrane domain NKG2D associates with the adaptor molecule DAP-10 that contains an YXNK motif to bind PI-3 kinase (PI3K) and send activating signals through this alternative pathway (see text for details). As the PI3K cascade is not inhibited by SHP-1/2, NKG2D may be able to mediate a dominant activation signal.
How inhibitory and activating C-lectin receptors operate in NK cells.
The C-type lectin receptors are disulfide-linked heterodimers of CD94 and NKG2 family members, either the inhibitory NKG2A or the activating NKG2C, and recognize the nonclassical MHC class I molecule HLA-E. Similar to KIRs with long cytoplasmic tails, ITIM-containing NKG2A signals through SHP-1/2 that mediate inhibitory signals. Likewise, NKG2C has a positively charged transmembrane domain that interacts with DAP-12 and transduces activating signals through Syk family members. In contrast, NKG2D is only distantly related to the other NKG2 family members, does not associate with CD94, and binds to the MHC-like ligands MICA, MICB, and ULBP family. Through its positively charged transmembrane domain NKG2D associates with the adaptor molecule DAP-10 that contains an YXNK motif to bind PI-3 kinase (PI3K) and send activating signals through this alternative pathway (see text for details). As the PI3K cascade is not inhibited by SHP-1/2, NKG2D may be able to mediate a dominant activation signal.
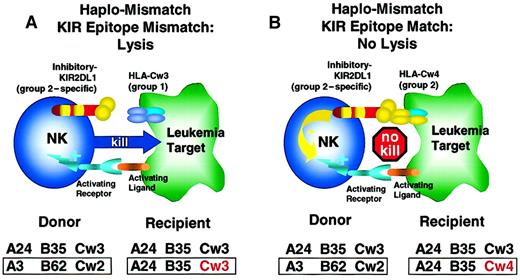
KIR-epitope mismatch in haplotype-mismatched stem cell transplantation.
(A) In this example, donor and recipient are HLA haplotype–mismatched and are KIR-epitope mismatched at the HLA-C locus. The donor NK cell clones expressing KIR2DL1 recognize and are inhibited by an epitope shared by the group 2 HLA-C alleles (HLA-Cw2, 4, 5, and 6). The recipient's leukemic blasts express HLA-Cw3, a member of the group 1 HLA-C alleles, and are, therefore, not recognized by the donor's KIR2DL1, and activation of donor NK cell occurs with leukemic cell lysis. (B) Here, donor and recipient are haplotype-mismatched, but express HLA-C alleles of the same supertype group 2 (HLA-Cw2, 4, 5, and 6). Therefore, donor NK cell clones expressing the inhibitory KIR2DL1 recognize a “self-epitope” (HLA-Cw4) on the recipient's cells with inhibition of lysis of leukemic blasts. KIR epitope mismatching exerts another level of graft alloreactivity and a potent GVL effect.122
KIR-epitope mismatch in haplotype-mismatched stem cell transplantation.
(A) In this example, donor and recipient are HLA haplotype–mismatched and are KIR-epitope mismatched at the HLA-C locus. The donor NK cell clones expressing KIR2DL1 recognize and are inhibited by an epitope shared by the group 2 HLA-C alleles (HLA-Cw2, 4, 5, and 6). The recipient's leukemic blasts express HLA-Cw3, a member of the group 1 HLA-C alleles, and are, therefore, not recognized by the donor's KIR2DL1, and activation of donor NK cell occurs with leukemic cell lysis. (B) Here, donor and recipient are haplotype-mismatched, but express HLA-C alleles of the same supertype group 2 (HLA-Cw2, 4, 5, and 6). Therefore, donor NK cell clones expressing the inhibitory KIR2DL1 recognize a “self-epitope” (HLA-Cw4) on the recipient's cells with inhibition of lysis of leukemic blasts. KIR epitope mismatching exerts another level of graft alloreactivity and a potent GVL effect.122
Individuals who are homozygous for group 2 HLA-C alleles generate NK cells with KIRs that recognize group 2 HLA-C alleles, eg, KIR2DL1 and KIR2DS1, and individuals who are homozygous for group 1 HLA-C alleles generate NK cells with KIRs that recognize group 1 HLA-C alleles, eg, KIR2DL3 and KIR2DS3.123 In this way, an individual's NK cell compartment inspects host tissues, engages HLA-C class I ligands through inhibitory KIRs with greater affinity than activating KIRs and, consequently, maintains natural immunologic tolerance and avoids autoimmunity. However, the same can be true of NK allorecognition, if the HLA-C allele on the allogeneic target cell belongs to the group with the same shared determinants in the α1 chain. For example, if a donor expresses the group 1 allele HLA-Cw3 and the group 2 allele HLA-Cw2, he or she will have some NK cells expressing KIRs that recognize the group 1 alleles (Cw1, Cw3, Cw7, and Cw8) and some NK cells expressing KIRs that recognize the group 2 alleles (eg, Cw2, Cw4, Cw5, and Cw6). If their NK cells are incubated with allogeneic cells from a haploidentical individual only expressing group 1 alleles (eg, HLA-Cw3), those donor clones expressing NK KIR2DL1 recognizing group 2 alleles will fail to recognize group 1 alleles, and lysis will occur if ligands binding to the activating NKRs are also present (Figure 4A, and discussed further later). However, if their same NK cells recognizing group 2 alleles are incubated with allogeneic cells from a haploidentical individual expressing a group 2 allele (eg, HLA-Cw4), the donor NK cells will recognize the group 2 allele, and no lysis will occur (Figure 4B). These 2 scenarios illustrate that even though the NK cell is incubated with a HLA-C mismatched allogeneic target cell in both circumstances, only the former has the potential for activation of NK cell cytotoxicity. It is precisely this principle of dimorphic allorecognition that needs to be carefully explored when patients with leukemia undergo T-cell–depleted haplo-mismatched allogeneic bone marrow transplantation, as is discussed later.
Note that the donor in Figure 4A has one HLA-C haplotype encoding a group 2 HLA-C allele (eg, HLA-Cw2) and another haplotype encoding a group 1 HLA-C allele (eg, HLA-Cw3), such that all nucleated cells in this individual express a group 1 and group 2 HLA-C class I allele. Can there be autologous NK killing of normal tissue? KIRs are clonally distributed, so, in this instance, each of that individual's NK cells will have at least one inhibitory KIR recognizing either the group 1 (eg, KIR2DL2) or group 2 (eg, KIR2DL1) HLA-C allele but not necessarily both.53 Self-tolerance is preserved, because the NK cell always engages at least one ligand for its inhibitory KIR as it performs immune surveillance of its own normal cells, each of which will express the full complement of the genotype (in this case, both a group 1 and a group 2 HLA-C allele). Thus, some NK clones from this individual will express KIR2DL1 and will have cytolytic potential against allogeneic target cells from a patient homozygous for group 1 HLA-C alleles (Figure 4A), whereas other NK clones from the same individual will express KIR2DL2 or KIR2DL3 and will have cytolytic potential against allogeneic target cells from a patient homozygous for group 2 HLA-C alleles (not shown). However, each of these NK clones will not be able to lyse allogeneic target cells that coexpressed group 1 and 2 HLA-C alleles (Figure 4B). These differences have been validated in vitro,123 124 lending additional insight into the potential role of NK cells in eradicating host lymphohematopoietic cells, including leukemia, following T-cell–depleted haplotype-mismatch hematopoietic transplantation.
In haplotype-mismatch hematopoietic transplantation, donor NK cell clones not engaging their class I inhibitory KIR ligands or members of their group class I alleles in the recipient, in the setting in which the donor and recipient are also mismatched at the HLA class I group, are expected to display alloreactivity against host leukemic cells. Conversely, if class I group mismatch is not present, despite the haplotype-mismatch, donor NK cells will be inhibited by their inhibitory KIR ligands and no alloreactivity is expected.
This finding has been recently confirmed by Ruggeri et al,122 who isolated donor-versus-recipient alloreactive NK cell clones from donor blood, capable of lysing recipients' PHA lymphoblasts or EBV-transformed B-lymphoblastoid cell line obtained prior to transplantation in all tested donor-recipient pairs KIR epitope-mismatched in the GvH direction.122 In addition, following transplantation of T-cell–depleted stem cells, the engrafted stem cells rapidly gave rise to a transient wave of reconstituting NK cells whose KIR repertoire was identical to that originally displayed by the donor, including high-frequency donor-versus-recipient alloreactive NK clones.122 Importantly, donor-derived alloreactive NK cell clones could not be isolated from the recipient's blood beyond 4 months after transplantation, indicating that deletion of “self-reactive” NK cells or induction of anergy in these cells occurred.122
Although the persistence of alloreactive NK cell clones was transient, a remarkable GVL effect was observed in patients with acute myeloid leukemia at high risk of relapse (15% of patients were in second complete remission, and 85% were in third or more complete remission or in relapse).125 Patients were conditioned with total body irradiation (800 cGy), thiotepa, antithymocyte globulin, and fludarabine (or cyclophosphamide) and were transplanted with T-cell–depleted granulocyte colony-stimulating factor-mobilized peripheral blood cells (15 ± 5 × 106CD34+ cells/kg and < 4 × 104CD3+ cells/kg). No posttransplantation GVHD prophylaxis was administered.
When KIR epitope-mismatch in the GvH direction was not present, the probability of relapse at 5 years was 75% in the face of intensive pretransplantation conditioning regimen, compared with 0% when KIR epitope-mismatch in the GvH direction was present.125 In addition, whereas rejection and grade II or greater acute GVHD rates were 15.5% and 13.7%, respectively, in the first group, they were 0% for both in the second. Thus, in AML the probability of survival at 5 years was 5% without KIR ligand incompatibility in the GvH direction and 60% with it. The latter is considerably better than survival reported by the National Marrow Donor Program after matched unrelated-donor transplant in adult patients in the same risk category (27% in second complete remission; 7% in third or more complete remission or in relapse). Remarkably, it is also better than the 35% survival reported in patients transplanted in first complete remission.126 In the remaining 40% of patients, death was caused by infection. The advanced stage of disease at transplantation largely accounted for such infection-related mortality. Indeed, the probability of survival of patients transplanted in remission, with KIR ligand incompatibility in the GvH direction, was 80% (A.V., unpublished observations, December 2001).
Although the data from Perugia emphasize the important role for KIR inhibitory receptors in haplotype-mismatched stem cell transplants,122 the role of activating NK cells receptors has not been studied. In this respect, it is important to note that, although myeloid leukemias were susceptible to NK lysis when KIR epitope mismatches were found, blasts from patients with acute lymphoblastic leukemia (ALL) were resistant, and a high relapse rate was observed in patients with ALL. Immunofluorescence analysis of several adhesion molecules showed that NK-resistant ALL blasts exhibited lower surface expression of lymphocyte function antigen-1 (LFA-1), an adhesion molecule instrumental to binding of NK cells to their targets, compared with NK-susceptible ALL blasts, and leukemic myeloblasts.122 However, it is also possible that a difference in expression of activating ligands between ALL and myeloid leukemia also exists. Systematic studies of the expression levels of the known activating ligands, MICA and MICB, and ULBP-1, -2, and -3 on primary leukemic cells are lacking. Although MICA and MICB expression have been demonstrated on the T-cell leukemia cell line JA3, and MICA on the Burkitt lymphoma cell line Raji,98 there are no published reports investigating the expression on primary lymphoblasts. We have begun investigating the expression of activating ligands on leukemic blasts and detected MICA and/or MICB transcripts in myeloblasts of 6 of 8 AML cases (S.S.F., T.A.F., and M.A.C., unpublished data, February 2002). This type of analysis, however, is limited by the fact that not all ligands for the activating NK receptors are known. Ligands for the NCRs have not yet been identified, and it is likely that there are more ligands for NKG2D than are currently known. The role of other MIC alleles as ligands for NK receptors is unknown, and the possibility exists that other proteins of the ULBP family may also act as activating ligands. The development of an NKG2D-immunoglobulin fusion protein should facilitate the investigation of the cellular distribution of ligands and the identification of other potential ligands for this receptor.
Another important result of the Perugia study122,125 is absence of GVHD despite the strong antileukemic effect by donor-versus-recipient alloreactive NK cell clones. A similar phenomenon of “hybrid resistance” is seen in murine transplantation models, in which host F1 mouse NK cells reject parental bone marrow cells but tolerate parental skin and organ grafts.127 It has been presumed that nonhematopoietic tissues lack the necessary ligands to bind and/or activate NK cells. In this respect, it is of note that the stress-inducible activating ligands for NKG2D, MICA, and MICB, are not highly expressed on normal epithelial tissues usually involved in GVHD. In contrast, although ULBP transcripts have been detected in epithelial cells, the extent of surface expression of these molecules is unknown. It is, therefore, possible that differential expression of these activating ligands on hematopoietic and nonhematopoietic tissues may explain the observed GVL in the absence of GVHD. Further evidence comes from mouse transplant models. Infusion of allogeneic NK cells (presumably containing NK cells not recognizing recipient MHC) has not been associated with the development, but sometime associated with suppression, of GVHD.128-130 The Perugia study125 directly addressed this point by infusing high numbers of purified host-reactive NK cells into lethally irradiated mice.125 No GVHD whatsoever was observed. On the contrary, the infusion of alloreactive NK cells actually prevented GVHD by killing host antigen-presenting cells, thus allowing the safe add-back of 20 times the lethal dose of allogeneic T cells for immune reconstitution. Indeed, in clinical hematopoietic stem cell transplantation KIR epitope incompatibility in the GvH direction not only did not cause GVHD but also was totally protected from it (see earlier). Should the pretransplantation infusion of alloreactive NK cells become feasible in humans, more T cells could safely be given with the graft, thus greatly improving the efficacy of haplotype-mismatched transplants. As is well known, the T-cell depletion, which is currently needed to prevent GVHD, leads to poor immune reconstitution and infection-related mortality.
KIR epitope-mismatch may facilitate bone marrow engraftment
Evidence that NK cell alloreactivity does not target tissues other than lymphohematopoietic cells suggested the pretransplantation infusion of alloreactive NK cells might be safely used to condition the host to transplantation. To test this finding, Ruggeri et al125 reversed the hybrid resistance transplantation partners, such that NK reactivity was now in the GvH direction. They showed that infusion of hybrid H-2d/b mouse NK cells, reactive against the homozygous host H-2b/b cells, into nonlethally irradiated H-2b/b recipients allowed the engraftment of H-2d/b bone marrow cells.125 In the absence of the alloreactive hybrid H-2d/b mouse NK cell infusion, nonlethally irradiated H-2b/b mice rejected the donor bone marrow and recovered uneventfully. These experiments indicate that alloreactive NK cells in the GvH direction can themselves promote sufficient immune suppression and myeloablation to allow engraftment of MHC disparate bone marrow, without development of GVHD, using a nonlethal preparative regimen. If this finding can be translated into the clinic, it would permit the extension of nonmyeloablative transplantation to the haplotype-mismatched setting without needing to severely immunosuppress the recipient to prevent rejection of donor stem cells. Given the relatively low dose of alloreactive NK cells required for this effect, translation of these experiments to the clinic should be considered, particularly in light of the retrospective clinical observation that transplantation with KIR epitope incompatibility in the GvH direction and the ensuing spontaneous generation of alloreactive NK cells totally controls rejection.
In vivo blockade of KIR inhibitory receptors may augment NK antileukemic activity
Another potential therapeutic strategy is blocking the interaction between KIR inhibitory receptors and their MHC class I ligands. In the autologous and HLA-matched setting, expression of MHC class I molecules by leukemic cells might confer resistance to NK cell-mediated lysis. Koh et al131 have recently used a murine acute leukemia model to demonstrate that in vivo NK cell inhibitory receptor blockade is feasible. Treatment of B6 mice, injected with C1498 (H2d) murine leukemia cells, with 5E6 F(ab′)2anti-Ly49C and I (murine counterparts of inhibitory NK receptors) protected them from leukemic death without toxicity or effects on hematologic parameters.131 In contrast, control mice treated with vehicle died of leukemia. In further experiments, adoptive transfer of IL-2–activated NK cells treated with 5E6 F(ab′)2 ex vivo protected mice from leukemic death, compared with transfer of untreated IL-2–activated NK cells. This study provides strong rationale to pursue translational research in inhibitory receptor blockade, noting the increased complexity in the human system.
As only certain subsets of NK cells express inhibitory receptors that recognize specific self-MHC molecules, simultaneous blockade of several receptors, including CD94/NKG2, may provide even greater antileukemic effects. In addition to in vivo therapy with blocking antibodies, extension of this approach to ex vivo purging of autologous stem cell products by coculture of stem cell preparations with activated NK cells in the presence of F(ab′)2 fragments directed toward inhibitory receptors has also been suggested.132 The susceptibility of human CD34+ cells to lysis by alloreactive NK cells, however, may limit this approach.133 Alternatively, NK inhibitory receptor blockade may be applied to enhance immune responses to viral infections, such as CMV, which encodes a MHC class I homologue to evade the innate immune system. Clinical trials of receptor blockade are awaited and will provide a novel approach to immunotherapy of leukemia.
Modulation of activating NK cell receptors and ligands
As discussed earlier, it appears that the response of NK cells depends on the balance between triggering and inhibitory signals mediated through corresponding activating and inhibitory receptors. An alternative approach to altering this balance in favor of activation may be the induction of activating receptor expression on NK cells through the use of cytokines. Few studies have examined this issue to date. It is known, for example, that IL-2 induces the natural cytotoxicity receptor, NKp44, which is not found on resting NK cells.84 The effect of cytokines on expression of other NK cell receptors is not well studied. Preliminary reports suggest that expression density of NCR may be stable and not influenced by IL-2, IL-15, IL-12, and IL-18.35 No data are available on the effect of cytokines on NKG2D expression density. However, as it is known that activated NK cells are more effective at lysing targets that are resistant to nonactivated NK cells, it would seem reasonable to more fully study the differences in the pattern of NK receptor and coreceptor expression with cytokine activation. An ability to manipulate the balance of activating and inhibitory receptors on NK cells may open new avenues in immunotherapy. Along the same lines, understanding the regulation of activating ligands on leukemic cells may allow development of strategies directed at increasing expression of these ligands, possibly in combination with activated NK cell therapy.
Conclusions
The past decade has witnessed a virtual explosion in our knowledge of NK cell receptors. It is now appreciated that NK cell responses are a result of competing signals mediated through inhibitory and activating receptors. Although in physiologic situations, inhibitory signals through the interaction of KIR and CD94/NKG2A receptors with MHC class I molecules appear to dominate, in other situations, the balance may be altered in favor of NK cell triggering. Appreciation of this concept has already led to the successful use of the antileukemic potential of NK cells through KIR-epitope mismatches in the setting of haplotype-mismatched stem cell transplantation. The benefits of NK cell alloreactivity, ie, ablation of leukemia and improved prevention of rejection and GVHD, are already a reality in high-risk patients with AML who receive HLA haplotype-mismatched transplants after high-intensity conditioning regimens. Preclinical models show NK cell alloreactivity could make reduced-intensity conditioning mismatched transplants available to patients with leukemia who cannot withstand the toxicity of current regimens. Clinical trials, using KIR-epitope mismatched NK cells to facilitate engraftment of mismatched stem cells with reduced intensity conditioning, and NK cell inhibitory receptor blockade are eagerly awaited. Over the forthcoming decade, it is expected that translation of our improved knowledge of NK cell biology and receptors will lead to advancements in cancer immunotherapy.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-02-0350.
Supported by grants CA68458, CA65670, and P30CA16058 from the National Institutes of Health. T.A.F. is the recipient of Medical Scientist Program (MSP) and Bennett fellowships from The Ohio State University College of Medicine and Public Health. A.V. is supported by grants from the Italian Association for Cancer Research (AIRC), the Italian Ministry of Research (MURST), the Italian Ministry of Health, and by a Translational Research Grant from the Leukemia & Lymphoma Society.
References
Author notes
Sherif S. Farag, The Ohio State University, A433A Starling Loving Hall, 320 W Tenth Ave, Columbus, OH 43210; e-mail: farag-1@medctr.osu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal