Human T-cell leukemia virus type I (HTLV-I) is the causative agent of an aggressive form of leukemia designated adult T-cell leukemia (ATL). We have previously demonstrated that all T-cell lines infected with HTLV-I and primary leukemic cells from ATL patients display constitutively high activity of transcription factor NF-κB. In this study we showed that Bay 11-7082, an inhibitor of NF-κB, induced apoptosis of HTLV-I–infected T-cell lines but only negligible apoptosis of HTLV-I–negative T cells. Bay 11-7082 rapidly and efficiently reduced the DNA binding of NF-κB in HTLV-I–infected T-cell lines and down-regulated the expression of the antiapoptotic gene, Bcl-xL, regulated by NF-κB, whereas it had little effect on the DNA binding of another transcription factor, AP-1. Although the viral protein Tax is an activator of NF-κB, Bay 11-7082–induced apoptosis of HTLV-I–infected cells was not associated with reduced expression of Tax. Furthermore, Bay 11-7082– induced apoptosis of primary ATL cells was more prominent than that of normal peripheral blood mononuclear cells, and apoptosis of these cells was also associated with down-regulation of NF-κB activity. Our results indicate that NF-κB plays a crucial role in the pathogenesis and survival of HTLV-I–infected leukemic cells and that it is a suitable target for the prevention and treatment of ATL.
Introduction
Adult T-cell leukemia (ATL) is an aggressive malignancy of CD4+ T cells, and its development is closely associated with human T-cell leukemia virus type I (HTLV-I) infection.1-3 In vitro, HTLV-I transforms primary human CD4+ T cells in interleukin-2 (IL-2)–dependent and IL-2–independent manners. Although the mechanism of transformation and leukemogenesis is not fully elucidated, there is evidence to suggest that the viral Tax protein plays a crucial role in these processes. For instance, like HTLV-I, Tax immortalizes primary human CD4+T cells in vitro and transforms rat fibroblast cell lines.4,5 In addition, Tax inhibits apoptosis induced by various stimuli in T-cell lines.6
Tax activates viral gene expression through the binding sequence for cAMP response element binding protein (CREB)/activating transcription factor (ATF) in the 21-bp repeats of the HTLV-I long terminal repeat (LTR). Tax has been shown to activate the expression of a number of cellular genes through several distinct transcription factors, such as NF-κB/Rel, AP-1, and serum response factor.7-9 The cellular genes induced by Tax include cytokines/chemokines (IL-1α, IL-6, IL-8, tumor necrosis factor-β, monocyte chemoattractant protein-1, granulocyte macrophage-colony stimulating factor, and granulocyte colony-stimulating factor), their receptors (the α chain of IL-2 receptor [IL-2R] and IL-15R), cell adhesion molecule (OX40), matrix metalloproteinase (MMP-9), apoptosis inhibitor (Bcl-xL), and G1-cyclins (cyclin D1 and cyclin D2).7 10-21 The transcriptional activation of cellular genes, including those listed above, by Tax is thought to contribute to deregulated proliferation of HTLV-I–infected cells.
Accumulating evidence suggests that activation of cellular genes by Tax through NF-κB is a critical process in the transforming activity as well as the inhibition of apoptosis. For instance, a Tax mutant inactive for NF-κB cannot immortalize primary human T cells in vitro.22 NF-κB2, a member of the IκB protein family, abrogates the transformation of a rat fibroblast cell line by Tax.5 Tax inhibits apoptosis induced by IL-2 withdrawal in mouse factor–dependent T-cell lines, and such inhibition correlates with the activation of NF-κB and induction of the antiapoptotic gene Bcl-xL through the NF-κB pathway.23 24
In resting T cells, NF-κB is sequestered in the cytoplasm in an inactive form by association with an inhibitory IκB subunit. Activation of NF-κB generally involves phosphorylation and degradation of IκBs, followed by nuclear translocation of NF-κB and subsequent activation of the genes containing NF-κB binding sites. IκBs are phosphorylated by a multisubunit IκB kinase (IKK) complex, which is composed of 2 catalytic subunits, IKKα and IKKβ, and a noncatalytic subunit, IKKγ/NEMO. Tax interacts with IKKγ/NEMO to activate the catalytic activity of the IKK complex.25
ATL continues to have a poor prognosis, mainly because of its resistance to conventional as well as high-dose chemotherapy. Therefore, the establishment of new therapeutic strategies for ATL is important. Tax is a candidate for such strategies because it is necessary for the transformation of HTLV-I–infected T cells. However, the expression level of Tax in leukemic cells of ATL patients is extremely low; expression can be detected only by reverse transcriptase–polymerase chain reaction.26 Furthermore, leukemic cells from several ATL patients possess mutation and truncation of the Tax coding region, which inactivates its functions.27 28 Thus, Tax may not be essential in the maintenance of leukemic phenotype in the last stage of the leukemogenesis, indicating that Tax may not be a good therapeutic target for ATL.
In a previous study, we showed that the NF-κB pathway is constitutively activated in primary ATL cells of all patients, even in the presence of low Tax expression levels.29 In the present study, we demonstrate that chemical inhibition of NF-κB activity resulted in a marked increase of apoptosis not only of HTLV-I–infected T-cell lines but also of primary ATL cells. We also show that apoptosis was specifically associated with inhibition of NF-κB activity and that inhibition of NF-κB down-regulates the expression of the antiapoptotic gene, Bcl-xL, regulated by NF-κB. These findings will be discussed in the context of the role of NF-κB in leukemogenesis and the potential therapeutic role of NF-κB inhibitors in ATL.
Materials and methods
Cell lines and human specimens
Four HTLV-I–infected T-cell lines, MT-4,30SLB-1,31 C5/MJ,32 and HUT-102,1were maintained in culture with RPMI 1640, supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, UT) and 50 U/mL penicillin and 50 μg/mL streptomycin (Gibco BRL, Grand Island, NY) at 37°C in 5% CO2. HTLV-I–negative T-cell lines Jurkat and CCRF-CEM were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and the above antibiotics. JPX-9 and JPX/M (kindly provided by Dr M. Nakamura, Tokyo Medical and Dental University, Tokyo, Japan) are subclones of Jurkat cells expressing Tax wild type or a nonfunctional Tax mutant, respectively, under the control of the metallothionein promoter.33 34Leukemic cells from 9 patients diagnosed with either acute-type (cases 1-5, 8, and 9) or chronic-type (cases 6 and 7) ATL were analyzed. The diagnosis of ATL was based on clinical features, hematological findings, and the presence of anti–HTLV-I antibodies in the sera. Monoclonal HTLV-I provirus integration into the DNA of leukemic cells was confirmed by Southern blot hybridization in all cases (data not shown). Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and patients with ATL were purified by Ficoll-Hypaque gradient centrifugation (Pharmacia LKB, Uppsala, Sweden) and washed with phosphate-buffered saline. Each patient sample contained more than 90% leukemic cells at the time of analysis. All samples were obtained after informed consent.
Growth inhibition assay
The effect of Bay 11-7082 (Calbiochem, La Jolla, CA) on cell growth was assayed by the WST-1 method as described previously.35 The WST-1 cell counting kit was obtained from Wako Chemicals (Osaka, Japan). Briefly, 2 × 104(cell lines) or 2 × 105 (PBMCs) cells were incubated in a 96-well microculture plate under the above conditions in the absence or presence of various concentrations of Bay 11-7082. After 48 hours of culture, 10 μL WST-1 solution was added and the cells were further incubated for another 2 hours. The number of surviving cells was measured with a microplate reader (Bio-Rad, Richmond, CA) at a reference wavelength of 655 nm and test wavelength of 450 nm. Cell viability was determined as percentage of the control (ie, absence of Bay 11-7082).
Apo2.7 immunostaining
Quantification of apoptosis was performed by immunostaining cells with Apo2.7, which specifically detects the 38-kDa mitochondrial membrane antigen 7A6, which is present only on the mitochondrial membrane of apoptotic cells and can be used as a late apoptotic marker in nonpermeabilized cells.36 37 ATL cells cultured for 48 hours with Bay 11-7082 or media were labeled with the Apo2.7-phycoerythrin–conjugated monoclonal antibody (Beckman-Coulter/Immunotech, Miami, FL) or mouse IgG1 isotype control (Beckman-Coulter/Immunotech) and subsequently analyzed by flow cytometry.
Plasmids and transfections
The κB-LUC construct, which was kindly provided by Dr J. Fujisawa (Kansai Medical University, Osaka, Japan),38contains 5 tandem repeats of an NF-κB binding site from the IL-2R α-chain gene. HTLV-I LTR-LUC and IL-8 AP-1-LUC39constructs were generously provided by Dr I. Futsuki (Nagasaki University School of Medicine, Japan) and Dr N. Mukaida (Kanazawa University, Kanazawa, Japan), respectively. Transient transfections were performed in JPX-9 cells by electroporation, using 5 × 106 cells and 5 μg of appropriate reporter plasmids. To normalize transfection efficiencies, a thymidine kinase (TK) promoter–driven Renilla luciferase plasmid (pRL-TK, 1 μg; Promega, Madison, WI) was cotransfected as an internal control plasmid. Then, 16 hours after transfection, CdCl2was added to the cultures at a concentration of 20 μM to induce Tax expression and the cells were further cultured for 48 hours for assay of luciferase activity. Transfected cells were collected by centrifugation, washed with phosphate-buffered saline, and lysed in reporter lysis buffer (Promega). Lysates were assayed for reporter gene activity with the dual luciferase reporter assay system (Promega).
Electrophoretic mobility shift assay
Cells were placed in culture at 1 × 106cells/mL (cell lines) or 5 × 106 cells/mL (PBMCs) and examined for inhibition of NF-κB after exposure to Bay 11-7082 (0, 1.25, 2.5, and 5 μM) for 1, 3, and 24 hours at 37°C . Nuclear proteins were extracted and NF-κB and AP-1 binding activities to κB or AP-1 elements were examined by electrophoretic mobility shift assay (EMSA) as described previously.29 40 In brief, 5 μg nuclear extracts were preincubated in a binding buffer containing 1 μg poly-deoxy-inosinic-deoxy-cytidylic acid (Pharmacia, Piscataway, NJ) followed by addition of α32P-labeled oligonucleotide probes containing κB or AP-1 elements (approximately 50 000 cpm). These mixtures were incubated for 15 minutes at room temperature. The DNA-protein complexes were separated on a 4% polyacrylamide gel and visualized by autoradiography. To examine the specificity of the κB element probe, unlabeled competitor oligonucleotides were preincubated with nuclear extracts for 15 minutes before incubation with probes. The probes or competitors used were prepared by annealing the sense and antisense synthetic oligonucleotides as follows: a typical κB element from the IL-2R α-chain gene, 5′-gatcCGGCAGGGGAATCTCCCTCTC-3′; κB mutant, 5′-gatcCGGCAGatctATCTCCCTCTC-3′; and AP-1 element of the IL-8 gene, 5′-gatcGTGATGACTCAGGTT-3′. (Underlined sequences represent the NF-κB or AP-1 binding sites, and mutations are indicated in lowercase.) To identify NF-κB proteins in the DNA protein complex revealed by EMSA, we used antibodies specific for various NF-κB family proteins, including p50, p65, c-Rel, and p52 (Santa Cruz Biotechnology, Santa Cruz, CA), to elicit a supershift DNA protein complex formation. These antibodies were incubated with the nuclear extracts for 45 minutes at room temperature before incubation with radiolabeled probes.
Western blot analysis
Cells from treatment were solubilized at 4°C in lysis buffer (0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 66 μg/mL aprotinin, 100 μg/mL phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate). Cell lysates (50 μg) were resolved by electrophoresis on a 10% polyacrylamide gel and transferred to polyvinylidine difluoride membrane. After blocking of the membrane in 3% skimmed milk and 0.05% Tween 20 in Tris-buffered saline, the blots were incubated with the mouse monoclonal antibody to Tax, Lt-4,41 p53 (NeoMarkers, Fremont, CA), or Bcl-xL (Transduction Laboratories, Lexington, KY) or the rabbit polyclonal antibody to cyclin D1 or cyclin D2 (Santa Cruz Biotechnology). Phosphospecific antibodies to P-Ser15 and P-Ser392 (Oncogene Research Products, Boston, MA) were used in the detection of phosphorylated residues of p53. After several washes, the protein bands recognized by the antibodies were visualized by means of the enhanced chemiluminescence Western blotting detection system (Amersham, Arlington Heights, IL).
Northern blot analysis
Total RNA (20 μg) was subjected to electrophoresis through a formaldehyde-agarose gel and transferred to a nylon filter. Filters were prehybridized (in 0.5 M sodium phosphate, 0.1% bovine serum albumin, 7% sodium dodecyl sulfate, 100 μg/mL salmon testis DNA, and 100 μg/mL yeast RNA) for 2 hours at 65°C. Hybridization was then carried out overnight in a prehybridization buffer containing the following α32P-radiolabeled probes: cDNA of cyclin D1, cyclin D2, Bcl-xL, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Radiolabeled probes were generated by means of a Megaprime DNA labeling system (Amersham).
Results
Bay 11-7082 specifically inhibits NF-κB in HTLV-I–infected T-cell lines
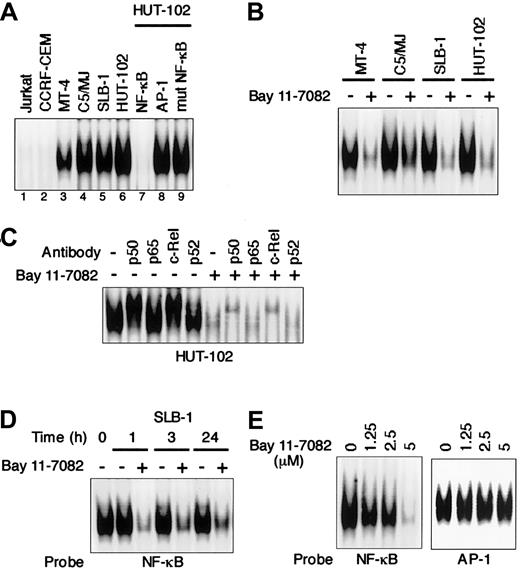
We first used EMSA to examine the effect of an IκB phosphorylation inhibitor, Bay 11-7082, on NF-κB activity in HTLV-I–infected T-cell lines. As shown in Figure1A, all 4 HTLV-I–infected T-cell lines (MT-4, SLB-1, C5/MJ, and HUT-102) displayed significantly greater NF-κB/DNA binding than the uninfected T-cell lines (Jurkat and CCRF-CEM). The observed protein/DNA binding was specific for NF-κB, since the binding was effectively competed and abrogated by excess unlabeled NF-κB oligonucleotide, but not by AP-1 and mutated NF-κB oligonuleotides. Bay 11-7082 inhibited NF-κB/DNA binding in all 4 HTLV-I–infected T-cell lines (Figure 1B). To determine the subunit composition of NF-κB complex, we used p50-, p65-, c-Rel–, or p52-specific antibody to induce a supershift. In HUT-102 cells, p50 and c-Rel led to supershifting of a fraction of the NF-κB complex. Complex containing p50 and c-Rel was decreased by Bay 11-7082 (Figure1C). NF-κB/DNA binding in SLB-1 cells was reduced within 1 hour after the addition of Bay 11-7082, and the inhibition was maintained until 24 hours (Figure 1D). To assess the specificity of NF-κB inhibition by Bay 11-7082, the effect of Bay 11-7082 on another transcription factor, AP-1, was evaluated in SLB-1 cells. Bay 11-7082 at concentrations of 1.25 μM to 5 μM did not significantly affect the binding of AP-1 to DNA in SLB-1 cells, whereas it reduced that of NF-κB, especially at the 5 μM concentration, suggesting that the inhibitory effects of Bay 11-7082 are specific to NF-κB (Figure 1E). Therefore, we used a 5-μM concentration of Bay 11-7082 in subsequent experiments to evaluate the activity of Bay 11-7082.
Specific inhibition of constitutive NF-κB activity in HTLV-I–infected T-cell lines treated with Bay 11-7082.
(A) Nuclear proteins extracted from cultures of T-cell lines were examined for DNA binding by EMSA with a radiolabeled NF-κB–specific probe. All HTLV-I–infected T-cell lines (lanes 3-6) exhibited protein/DNA binding with complex formation, compared with uninfected negative control cell lines (lanes 1 and 2). Cold competition using 100-fold excess of unlabeled NF-κB, AP-1, or mutated NF-κB oligonucleotides demonstrated the specificity of the protein/DNA binding complex (lanes 7-9). (B) Inhibition of constitutive NF-κB activity in HTLV-I–infected T-cell lines. HTLV-I–infected T-cell lines placed in culture at 1 × 106 cells/mL were treated for 1 hour with 5 μM Bay 11-7082 and assessed for NF-κB binding. Additional studies in which each cell line was analyzed for up to 24 hours after treatment yielded the same results. (C) NF-κB subunit specificity was determined by using antibodies to the NF-κB components p50, p65, c-Rel, and p52, resulting in supershift. (D) Time course of NF-κB inhibition in SLB-1 cells treated with Bay 11-7082. SLB-1 cells were treated with 5 μM Bay 11-7082. After the indicated times, nuclear proteins were extracted and EMSA was performed. (E) Specific inhibition of NF-κB by Bay 11-7082. SLB-1 cells were treated with the indicated concentrations of Bay 11-7082. After 1 hour, nuclear proteins were extracted and EMSA was performed with NF-κB– or AP-1–specific radiolabeled oligonucleotide probes. The specificity of the NF-κB inhibition was maintained throughout the 24-hour assay in SLB-1 cells.
Specific inhibition of constitutive NF-κB activity in HTLV-I–infected T-cell lines treated with Bay 11-7082.
(A) Nuclear proteins extracted from cultures of T-cell lines were examined for DNA binding by EMSA with a radiolabeled NF-κB–specific probe. All HTLV-I–infected T-cell lines (lanes 3-6) exhibited protein/DNA binding with complex formation, compared with uninfected negative control cell lines (lanes 1 and 2). Cold competition using 100-fold excess of unlabeled NF-κB, AP-1, or mutated NF-κB oligonucleotides demonstrated the specificity of the protein/DNA binding complex (lanes 7-9). (B) Inhibition of constitutive NF-κB activity in HTLV-I–infected T-cell lines. HTLV-I–infected T-cell lines placed in culture at 1 × 106 cells/mL were treated for 1 hour with 5 μM Bay 11-7082 and assessed for NF-κB binding. Additional studies in which each cell line was analyzed for up to 24 hours after treatment yielded the same results. (C) NF-κB subunit specificity was determined by using antibodies to the NF-κB components p50, p65, c-Rel, and p52, resulting in supershift. (D) Time course of NF-κB inhibition in SLB-1 cells treated with Bay 11-7082. SLB-1 cells were treated with 5 μM Bay 11-7082. After the indicated times, nuclear proteins were extracted and EMSA was performed. (E) Specific inhibition of NF-κB by Bay 11-7082. SLB-1 cells were treated with the indicated concentrations of Bay 11-7082. After 1 hour, nuclear proteins were extracted and EMSA was performed with NF-κB– or AP-1–specific radiolabeled oligonucleotide probes. The specificity of the NF-κB inhibition was maintained throughout the 24-hour assay in SLB-1 cells.
Bay 11-7082 specifically inhibits Tax-induced NF-κB activation
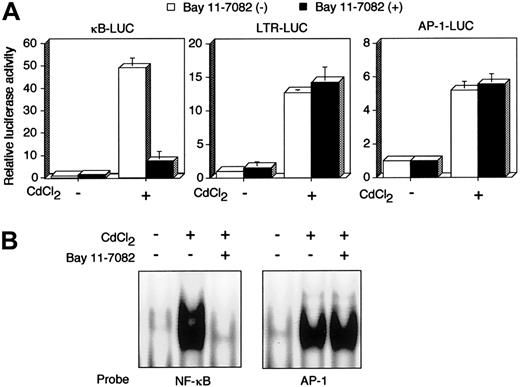
Tax is a viral transactivator and has been shown to activate a number of cellular genes through multiple distinct transcription factors, including NF-κB. JPX-9 is a derivative of the human HTLV-I–negative T-cell line Jurkat, and has an inducibletax gene under the control of metallothionein promoter.33,34 Using JPX-9, we next examined whether Bay 11-7082 inhibits Tax-induced activation of NF-κB. Luciferase expression plasmids regulated by 3 Tax-inducible enhancers were transfected into JPX-9 cells, and the cells were then treated with CdCl2. Treatment with CdCl2 efficiently activated 3 distinct transcription factor pathways—NF-κB, CREB/ATF, and AP-1—in JPX-9 (Figure 2A), whereas the same treatment failed to activate the same pathways in JPX/M (data not shown), as reported previously.12 33 Activation of NF-κB by Tax in JPX-9 cells was significantly inhibited by culture with 5 μM Bay 11-7082 for 48 hours, whereas the compound had little if any effect on activation of CREB/ATF and AP-1. We confirmed NF-κB inactivation by the use of EMSA. EMSA showed that the culture of CdCl2-treated JPX-9 cells with Bay 11-7082 for 48 hours mostly abrogated NF-κB/DNA binding. However, EMSA with the AP-1 binding site as a probe did not reveal any change of the binding of AP-1 to DNA in CdCl2-treated JPX-9 cells (Figure 2B). These results indicate that Bay 11-7082 selectively inhibits Tax-induced NF-κB activity in a human T-cell line.
Bay 11-7082 specifically inhibits Tax-induced NF-κB activation.
(A) κB-LUC, LTR-LUC, or AP-1-LUC was transfected into JPX-9 cells. JPX-9 cells were pretreated for 1 hour with 5 μM Bay 11-7082 followed by 48 hours of incubation with 20 μM CdCl2 in the continued presence of Bay 11-7082. Relative luciferase activity is expressed relative to the basal level measured in cells transfected with the reporter plasmid without further treatment. To normalize variations, the construct containing the TK promoter-drivenRenilla luciferase (pRL-TK) was cotransfected, and the activities of firefly and Renilla luciferases were measured sequentially from a single sample by means of a dual-luciferase reporter assay system. Data are the mean ± SD of 3 separate transfections. (B) Inhibition of NF-κB/DNA binding in CdCl2-treated JPX-9 cells. Nuclear proteins of JPX-9 cells treated without or with CdCl2 in the absence or presence of Bay 11-7082 were incubated with radiolabeled oligonucleotide containing NF-κB or AP-1 binding site, and DNA binding activity was examined by EMSA.
Bay 11-7082 specifically inhibits Tax-induced NF-κB activation.
(A) κB-LUC, LTR-LUC, or AP-1-LUC was transfected into JPX-9 cells. JPX-9 cells were pretreated for 1 hour with 5 μM Bay 11-7082 followed by 48 hours of incubation with 20 μM CdCl2 in the continued presence of Bay 11-7082. Relative luciferase activity is expressed relative to the basal level measured in cells transfected with the reporter plasmid without further treatment. To normalize variations, the construct containing the TK promoter-drivenRenilla luciferase (pRL-TK) was cotransfected, and the activities of firefly and Renilla luciferases were measured sequentially from a single sample by means of a dual-luciferase reporter assay system. Data are the mean ± SD of 3 separate transfections. (B) Inhibition of NF-κB/DNA binding in CdCl2-treated JPX-9 cells. Nuclear proteins of JPX-9 cells treated without or with CdCl2 in the absence or presence of Bay 11-7082 were incubated with radiolabeled oligonucleotide containing NF-κB or AP-1 binding site, and DNA binding activity was examined by EMSA.
Bay 11-7082 selectively induces apoptosis of HTLV-I–infected T-cell lines
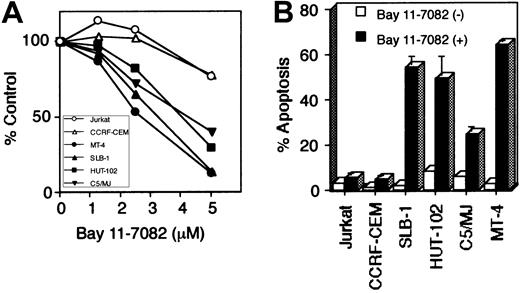
To evaluate the role of NF-κB in the cell growth of HTLV-I–infected T-cell lines, we cultured these cells in the presence of Bay 11-7082 for 48 hours. WST-1 cell growth assay showed that Bay 11-7082 reduced cell growth of 4 HTLV-I–infected T-cell lines in a dose-dependent manner, whereas it only weakly affected the cell growth of uninfected T-cell lines (Jurkat and CCRF-CEM) even when used at high concentration (Figure 3A). To examine whether the induction of apoptosis accounts for the cell growth inhibition observed in HTLV-I–infected T-cell lines, we used anti-7A6 antibody (Apo2.7, a mitochondrial membrane antigen expressed in late-stage apoptosis) conjugated with phycoerythrin to stain cells treated with Bay 11-7082 and then analyzed the stained cells by flow cytometry (Figure 3B). Significant apoptosis of HTLV-I–infected T-cell lines, compared with uninfected cells, was noted. DNA extracted from HTLV-I–infected cells treated with Bay 11-7082 revealed marked DNA fragmentation, another marker of apoptosis, compared with uninfected and untreated T-cell lines (data not shown). Considered together, these data suggest that constitutively high NF-κB activity in HTLV-I–infected T cells is required for their survival, and that specific inhibition of this activity results in the induction of apoptosis.
Bay 11-7082 reduces cell growth and induces apoptosis of HTLV-I–infected T-cell lines.
Various T-cell lines were placed in culture at 1 × 105 cells/mL without or with Bay 11-7082 for 48 hours. (A) Cell growth was assessed by the WST-1 method, is expressed as percentage of control (untreated cells), and represents the mean of 3 independent experiments. (B) Induction of apoptosis of HTLV-I–infected T-cell lines following inhibition of NF-κB. 1 × 106 cells were labeled with phycoerythrin-conjugated Apo2.7 and analyzed by flow cytometry. Data represent the mean ± SD percentages of apoptotic cells from 3 independent experiments for both untreated cells (open bars) and cells treated with 5 μM Bay 11-7082 (solid bars).
Bay 11-7082 reduces cell growth and induces apoptosis of HTLV-I–infected T-cell lines.
Various T-cell lines were placed in culture at 1 × 105 cells/mL without or with Bay 11-7082 for 48 hours. (A) Cell growth was assessed by the WST-1 method, is expressed as percentage of control (untreated cells), and represents the mean of 3 independent experiments. (B) Induction of apoptosis of HTLV-I–infected T-cell lines following inhibition of NF-κB. 1 × 106 cells were labeled with phycoerythrin-conjugated Apo2.7 and analyzed by flow cytometry. Data represent the mean ± SD percentages of apoptotic cells from 3 independent experiments for both untreated cells (open bars) and cells treated with 5 μM Bay 11-7082 (solid bars).
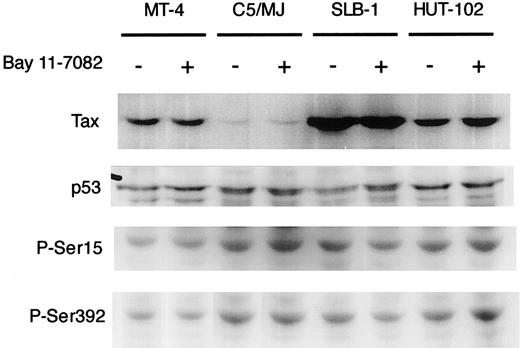
To clarify the mechanisms of induced apoptosis of HTLV-I–infected T cell lines, we examined the expression level of viral Tax and cellular proapoptotic protein p53 by Western blot analysis. Tax was detected in all 4 HTLV-I–infected cell lines (MT-4, SLB-1, C5/MJ, and HUT-102) as a specific band of about 40 kDa (Figure4), but it was not detected in HTLV-I–negative cells (Jurkat and CCRF-CEM; data not shown). The expression of Tax in HTLV-I–infected T-cell lines was not affected by treatment with Bay 11-7082 for 48 hours (Figure 4). Northern blot analysis also showed that Bay 11-7082 did not induce any significant change in the levels of the 3 major HTLV-I mRNAs in infected T-cell lines (data not shown). In addition, p53 expression in cell lines was not altered by treatment with Bay 11-7082. It has been shown that the activation of NF-κB by Tax directly correlated with phosphorylation-dependent inhibition of p53 transactivation function.42 One might expect that Bay 11-7082 treatment would cause a reactivation of p53 by reversing phosphorylation-induced inhibition. However, we did not see a change in phosphorylation of serines 15 and 392 in Bay 11-7082–treated HTLV-I–infected cell lines (Figure 4). These results suggest that Bay 11-7082 does not alter the expression of viral Tax and the expression and phosphorylation of proapoptotic protein p53 in HTLV-I–infected T-cell lines.
Effect of Bay 11-7082 on the expression of Tax and the expression and phosphorylation of p53 in HTLV-I–infected T-cell lines.
HTLV-I–infected T-cell lines were treated with 5 μM Bay 11-7082 for 48 hours and assessed by immunoblot for Tax and p53. Phosphospecific antibodies to P-Ser15 and P-Ser392 were used in the detection of phosphorylated residues of p53.
Effect of Bay 11-7082 on the expression of Tax and the expression and phosphorylation of p53 in HTLV-I–infected T-cell lines.
HTLV-I–infected T-cell lines were treated with 5 μM Bay 11-7082 for 48 hours and assessed by immunoblot for Tax and p53. Phosphospecific antibodies to P-Ser15 and P-Ser392 were used in the detection of phosphorylated residues of p53.
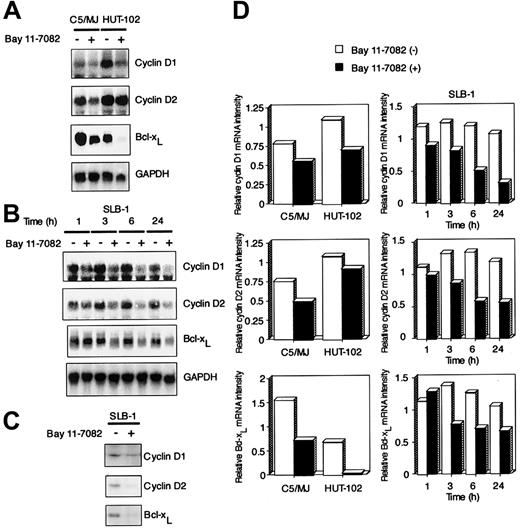
Bay 11-7082 down-regulates the expression of cyclin D1, cyclin D2, and Bcl-xL in HTLV-I–infected cells
To investigate the molecular mechanism through which Bay 11-7082 inhibits cell growth of HTLV-I–infected T-cell lines, we examined the expression of cell cycle–associated genes cyclin D1 and cyclin D2 and antiapoptotic gene Bcl-xL by Northern blot analysis. These 3 genes contain NF-κB enhancer elements and are undetectable in uninfected T-cell lines Jurkat and CCRF-CEM (data not shown). We and others have previously shown that Tax induces the expression of these genes through NF-κB binding sites.19-21 24 High expression levels of cyclin D1 and cyclin D2 were detected in 3 exponentially growing HTLV-I–infected T-cell lines (C5/MJ, HUT-102, and SLB-1), but the expression of cyclin D3 was not detected in these cell lines (partial data are shown in Figure5A,B). Signals were quantitated by densitometry, and values were normalized to those for GAPDH. On the basis of quantitations from the Northern blots, we deduced that cyclin D1, cyclin D2, and Bcl-xL mRNA levels in these 3 HTLV-I–infected T-cell lines were down-regulated by exposure to Bay 11-7082 (Figure 5D). Western blots with antibodies specific for cyclin D1, cyclin D2, and Bcl-xL confirmed that Bay 11-7082 treatment of SLB-1 cells had a markedly suppressed expression of these proteins (Figure 5C).
Effect of Bay 11-7082 on the expression of cyclin D1, cyclin D2, and Bcl-xL in HTLV-I–infected T-cell lines.
(A) Total RNA (20 μg) was extracted from HTLV-I–infected T-cell lines following treatment without or with 5 μM Bay 11-7082 for 24 hours. Northern blot analysis was performed with cyclin D1, cyclin D2, and Bcl-xL cDNA probes. The GAPDH probe was used to demonstrate equivalent RNA levels in each sample. (B) Time course of cyclin D1, cyclin D2, and Bcl-xL mRNA expression in SLB-1 cells treated with Bay 11-7082. Total RNA (20 μg) was extracted from SLB-1 cells following treatment without or with Bay 11-7082 for the indicated times. (C) Expression of cyclin D1, cyclin D2, and Bcl-xL at the protein level in SLB-1 cells. SLB-1 cells were treated without or with Bay 11-7082 for 48 hours. After treatment, the expression of these proteins was examined by Western blot. (D) Graphic quantitation of relative levels of cyclin D1, cyclin D2, and Bcl-xL mRNA expression in cells. GAPDH bands were measured by densitometry and the values were used to normalize for densitometric quantitations of cyclin D1, cyclin D2, and Bcl-xL.
Effect of Bay 11-7082 on the expression of cyclin D1, cyclin D2, and Bcl-xL in HTLV-I–infected T-cell lines.
(A) Total RNA (20 μg) was extracted from HTLV-I–infected T-cell lines following treatment without or with 5 μM Bay 11-7082 for 24 hours. Northern blot analysis was performed with cyclin D1, cyclin D2, and Bcl-xL cDNA probes. The GAPDH probe was used to demonstrate equivalent RNA levels in each sample. (B) Time course of cyclin D1, cyclin D2, and Bcl-xL mRNA expression in SLB-1 cells treated with Bay 11-7082. Total RNA (20 μg) was extracted from SLB-1 cells following treatment without or with Bay 11-7082 for the indicated times. (C) Expression of cyclin D1, cyclin D2, and Bcl-xL at the protein level in SLB-1 cells. SLB-1 cells were treated without or with Bay 11-7082 for 48 hours. After treatment, the expression of these proteins was examined by Western blot. (D) Graphic quantitation of relative levels of cyclin D1, cyclin D2, and Bcl-xL mRNA expression in cells. GAPDH bands were measured by densitometry and the values were used to normalize for densitometric quantitations of cyclin D1, cyclin D2, and Bcl-xL.
Effect of NF-κB inhibition in primary ATL cells on cell survival
We previously showed that primary leukemic cells of ATL patients express constitutively high levels of the NF-κB/DNA complex in the nucleus.29 Thus, we examined whether Bay 11-7082 inhibits the cell growth of primary ATL cells such as HTLV-I–infected T-cell lines. As described in our previous study,29 high NF-κB/DNA binding was detected in nuclear extracts from all primary ATL cells, but treatment with 5 μM of Bay 11-7082 severely reduced their NF-κB/DNA binding activity (Figure6A). Supershift assay showed a dramatic decrease in NF-κB/DNA binding, previously shown to correspond to p50-p65 heterodimers (Figure 6B).29 Concomitantly, the growth of these ATL cells was significantly inhibited compared with that of normal PBMCs treated with Bay 11-7082 (Figure 6C). Apo2.7 staining detected a high level of apoptosis in 7 ATL specimens, although the degree of apoptosis varied among the specimens (Figure6D). These data further indicate that NF-κB inhibition effectively and preferentially targeted primary ATL cells to induce apoptosis.
Bay 11-7082 reduces cell growth and induces apoptosis of primary ATL cells.
ATL cells were cultured at 1 × 106 cells/mL without or with 5 μM Bay 11-7082 for 48 hours. (A) Effect of Bay 11-7082 in ATL cells on the activation of NF-κB assessed by EMSA with NF-κB probe. (B) NF-κB subunit specificity was determined by using antibodies to the NF-κB components p50, p65, c-Rel, and p52, resulting in supershift. (C) ATL cell growth was assessed by the WST-1 method. (D) Induction of apoptosis of ATL cells following inhibition of NF-κB. Data represent the mean ± SD percentages of apoptotic cells from 3 independent experiments for both untreated cells (open bars) and cells treated with 5 μM Bay 11-7082 (solid bars).
Bay 11-7082 reduces cell growth and induces apoptosis of primary ATL cells.
ATL cells were cultured at 1 × 106 cells/mL without or with 5 μM Bay 11-7082 for 48 hours. (A) Effect of Bay 11-7082 in ATL cells on the activation of NF-κB assessed by EMSA with NF-κB probe. (B) NF-κB subunit specificity was determined by using antibodies to the NF-κB components p50, p65, c-Rel, and p52, resulting in supershift. (C) ATL cell growth was assessed by the WST-1 method. (D) Induction of apoptosis of ATL cells following inhibition of NF-κB. Data represent the mean ± SD percentages of apoptotic cells from 3 independent experiments for both untreated cells (open bars) and cells treated with 5 μM Bay 11-7082 (solid bars).
Discussion
Kitajima et al43 showed that NF-κB antisense treatment inhibits the cell growth of a Tax-transformed fibroblast cell line as well as an HTLV-I–infected T-cell line, MT-2, in vitro. We previously showed that wild-type Tax as well as a Tax mutant active for NF-κB inhibits apoptosis induced by IL-2 withdrawal in a mouse T-cell line.23 Here we extended these observations and demonstrated that inhibition of NF-κB suppressed cell growth of at least 4 human HTLV-I–infected T-cell lines by induction of apoptosis (Figure 3). The specificity of Bay 11-7082 to the NF-κB pathway has been extensively characterized,44 and we also confirmed the specificity of the drug to NF-κB. For instance, among Tax-inducible pathways, Bay 11-7082 selectively inhibited NF-κB but not AP-1 or CREB/ATF in a human T-cell line (Figure 2). Bay 11-7082 inhibited the growth and induced apoptosis of cells possessing high NF-κB activity, but not the ones with low activity. NF-κB is known to have antiapoptotic activity and is therefore considered to be a key survival factor for several types of cancer. Taken together, these results indicate that NF-κB plays crucial roles in the cell growth and inhibition of apoptosis of HTLV-I–infected cell lines. It should, however, be noted that the other NF-κB–dependent activities, especially cell cycle promotion, may also be a target of Bay 11-7082 in the cell growth of HTLV-I–infected cells, as discussed below.
NF-κB inhibitors that are currently available are divided into 3 groups based on the mechanism of action: inhibitors of proteasome-mediated degradation of IκB, inhibitors of IκB phosphorylation, and inhibitors of NF-κB translocation from the cytoplasm to the nucleus.45 In general, the most specific inhibitors are those that inhibit IκB phosphorylation and NF-κB translocation. Aspirin (another IκB phosphorylation inhibitor) and pyrrolidine dithiocarbamate (PDTC, a nuclear translocation inhibitor) did not inhibit constitutive NF-κB/DNA binding in either HTLV-I–infected T-cell lines or primary ATL cells at concentrations that inhibit inducible activation of NF-κB after stimulation by inflammatory cytokines such as tumor necrosis factor and IL-1 (data not shown). Furthermore, Kawakami et al46 showed that PDTC did not affect NF-κB/DNA binding in CdCl2-treated JPX-9 cells, although the incubation time of PDTC after the addition of CdCl2 was short (3 hours). Thus, Bay 11-7082 is the most specific and effective NF-κB inhibitor for HTLV-I–infected cells among the 3 compounds.
To determine the exact mechanisms by which NF-κB provides a survival advantage to HTLV-I–infected T cells, we examined the effects of Bay 11-7082 on the expression of Bcl-xL, cyclin D1, cyclin D2, and p53. Inhibition of NF-κB activity in HTLV-I–infected T-cell lines resulted in a significant reduction of expression of Bcl-xL, cyclin D1, and cyclin D2 (Figure 5). In contrast, the expression and phosphorylation of p53 were not modulated by Bay 11-7082 (Figure 4). Similar results have recently been reported by Mahieux et al, who treated HTLV-I–infected T cells with arsenic trioxide, which down-regulated NF-κB translocation.47These results are consistent with those of previous studies showing that Bcl-xL, cyclin D1, and cyclin D2 genes were induced by Tax through NF-κB enhancer elements in human and mouse T-cell lines.19-21,24 On the contrary, Kawakami et al46 reported that X-chromosome–linked inhibitor of apoptosis (XIAP) protein expression by Tax-mediated NF-κB activation, but not Bcl-xL, inhibited apoptosis of T cells by NF-κB inhibitors. Indeed, the expression of XIAP is regulated by NF-κB, and its expression is important to inhibit apoptosis in non–T cells such as endothelial cells.48 However, it is not apparent that its expression is regulated by NF-κB in T cells, because its expression level is high in JPX-9 cells untreated with CdCl2 and is not changed by the addition of CdCl2.46 Because Bcl-xL is an apoptotic inhibitor, and cyclin D1 and cyclin D2 are critical regulators of G1-S cell cycle progression in T cells, these genes may contribute to the aberrant cell growth and resistance to apoptosis of HTLV-I–infected T cells. These results also suggest that inhibition of NF-κB may affect not only survival but also cell cycle promotion of HTLV-I–infected cells, and that both mechanisms involve the growth inhibition of HTLV-I–infected cells in the presence of anti–NF-κB agent.
The other major finding of the present study was that Bay 11-7082 induced apoptosis of fresh primary leukemic cells from ATL patients. Like HTLV-I–infected T-cell lines, primary ATL cells display the constitutive NF-κB/DNA complex in the nucleus and overexpress a number of genes regulated by NF-κB. Thus, these results indicate that the activated NF-κB in primary leukemic cells inhibits apoptosis and its activation plays a crucial role in the inhibition of apoptosis, thereby the deregulated leukemic cell growth. It is questionable whether Tax alone could explain the in vivo activation of NF-κB in ATL cells, given that the expression of Tax in primary ATL cells is extremely low. Furthermore, Tax expression level does not correlate with the level of NF-κB/DNA binding in HTLV-I–infected T-cell lines (Figures 1 and 4). It is reasonable to assume that transactivation by Tax is not the only mechanism underlying NF-κB activation in T cells infected with HTLV-I. Thus, it is likely that Bay11-7082 inhibited Tax-independent NF-κB activation in ATL cells.
NF-κB is aberrantly activated in various types of malignancies, most frequently in hematopoietic malignancies such as chronic lymphocytic leukemia,49 multiple myeloma,50Hodgkin disease,51-53 acute lymphoblastic leukemia,54 acute myelogenous leukemia,55cutaneous T-cell lymphoma,56 and primary effusion lymphoma.44 Here we showed that an NF-κB inhibitor Bay 11-7082 induced apoptosis of primary leukemic cells of ATL patients. In addition, this compound is also known to induce apoptosis of lymphoma cells from patients with primary effusion lymphoma or cutaneous T-cell lymphoma.44,56 These results suggest that NF-κB could be considered a general therapeutic target for hematopoietic malignancies. Bay 11 was evaluated in vivo and shown to be a potent anti-inflammatory drug in animal models of inflammation.57 This compound may be useful clinically as a therapeutic agent. Thus, further analysis of the action of Bay11-7082 on ATL cells will advance the search for new therapies not only for ATL but also for other hematopoietic malignancies.
We are deeply indebted to the many patients with ATL and the control subjects who donated blood for these studies. We thank Drs J. Fujisawa, I. Futsuki, and N. Mukaida for providing luciferase reporter constructs κB-LUC, HTLV-I LTR-LUC, and IL-8 AP-1-LUC, respectively. We also thank Drs M. Hinz, H. Matsushime, and Y. Tsujimoto for providing cDNAs of cyclin D1, cyclin D2, and Bcl-xL, respectively. We are very grateful to Dr M. Nakamura for providing JPX-9 and JPX/M, and to Fujisaki Cell Center, Hayashibara Biomedical Laboratories (Okayama, Japan) for providing Jurkat, HUT-102, and C5/MJ cell lines. We also thank M. Yamamoto and M. Sasaki for their excellent technical assistance.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-01-0151.
Supported in part by a grant-in-aid for scientific research (category C) from the Japan Society for the Promotion of Science and the Japan Leukemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Naoki Mori, Department of Virology, Faculty of Medicine, University of the Ryukyus, 207, Uehara, Nishihara, Okinawa 903-0215, Japan; e-mail: n-mori@med.u-ryukyu.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal